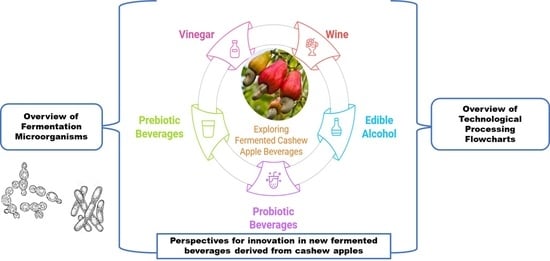
Fermented Cashew Apple Beverages: Current State of Knowledge and Prospects
Abstract
1. Introduction
2. Methodology
3. Chemical Composition of the Cashew Apple: An Asset for Fermentation
4. Cashew Apple Pre-Fermentation Treatments
4.1. A Strategy for the Reduction of Astringency
4.1.1. Use of Clarifying Agents
4.1.2. Thermal Methods
4.1.3. Membrane Processes
| Characteristics | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Methods of Elimination | Quantities of Clarifying Agent | Clarification Time (min) | Tannin Removal Mechanism | % Reduction in Condensed Tannin | % Reduction of Total Polyphenols | Impact of Clarification on the Sensory Quality of the Finished Product | Impact of Clarification on the Nutritional Quality of the Finished Product | Economic Aspect | Authors | |
| Chemical | Polyvinyl pyrrolidone (PVP) | 1.4 g/L | ND | Precipitation and sedimentation | ND | 97 | Negative | Negative | ++ | [51] |
| Ethanol vapor | 3.5 g/L | 720 | Tannin polymerization | ND | ND | Neutral | Neutral | - | [38] | |
| Natural adjuvant | Cassava starch | 2 g/L | ND | Flocculation and/or coagulation | 42.85 | ND | Neutral | Neutral | - | [11] |
| Rice groats | 10 g/L | 193 | Gelatinization and sedimentation | 42.14 | ND | Neutral | Neutral | - | [19] | |
| Gelatin | 0.67% (p/v) | 15 | Precipitation and sedimentation | 50 | ND | Neutral | Negative | + | [17] | |
| Moringa Oleifera seed powder | 10 g/0.25 L | 60 | Coagulation | 80 | ND | Negative | Neutral | - | [52] | |
| Dried okra powder | 0.3% | 30 | Sedimentation | 42.6 | ND | Neutral | Negative | - | [16] | |
| Defatted soya flour | 2% (p/v) | 240 | Precipitation and sedimentation | 34.3 | ND | Neutral | Neutral | - | [53] | |
| 2.4% (p/v) | 120 | Sedimentation | ND | ND | Neutral | Neutral | - | [54] | ||
| Sweet potato starch | 2.4% (p/v) | 120 | Sedimentation | ND | ND | Neutral | Neutral | - | [54] | |
| Enzymatic | Laccase modified by acid anhydrides (2-octenyl succinic anhydride) | ND | ND | Precipitation and sedimentation | 28.59 | 44.48 | Positive | Neutral | ++ | [15] |
| Use of thermal methods | Hot water treatment | ND | 20 | Thermal degradation of apple tannin compounds | 96 | ND | Neutral | Neutral | - | [39] |
| Use of membrane processes | 0.2 µm tubular ceramic membrane | ND | ND | Filtration | 97 | ND | Neutral | Neutral | ++ | [48] |
5. Fermented Cashew Apple Products
5.1. Food Beverages Obtained from the Alcoholic Fermentation of Cashew Apples
5.1.1. Cashew Apple Wine
5.1.2. Other Alcoholic Beverages from Cashew Apples: The Case of Feni
5.2. Food Beverages Derived from the Lactic Fermentation of Cashew Apples
5.2.1. Fermented Cashew Apple Juice Used as a Prebiotic Drink
5.2.2. Fermented Cashew Apple Juice Used as a Probiotic Drink
5.3. Food Products Derived from the Mixed Fermentation of Cashew Apples
Cashew Apple Vinegar
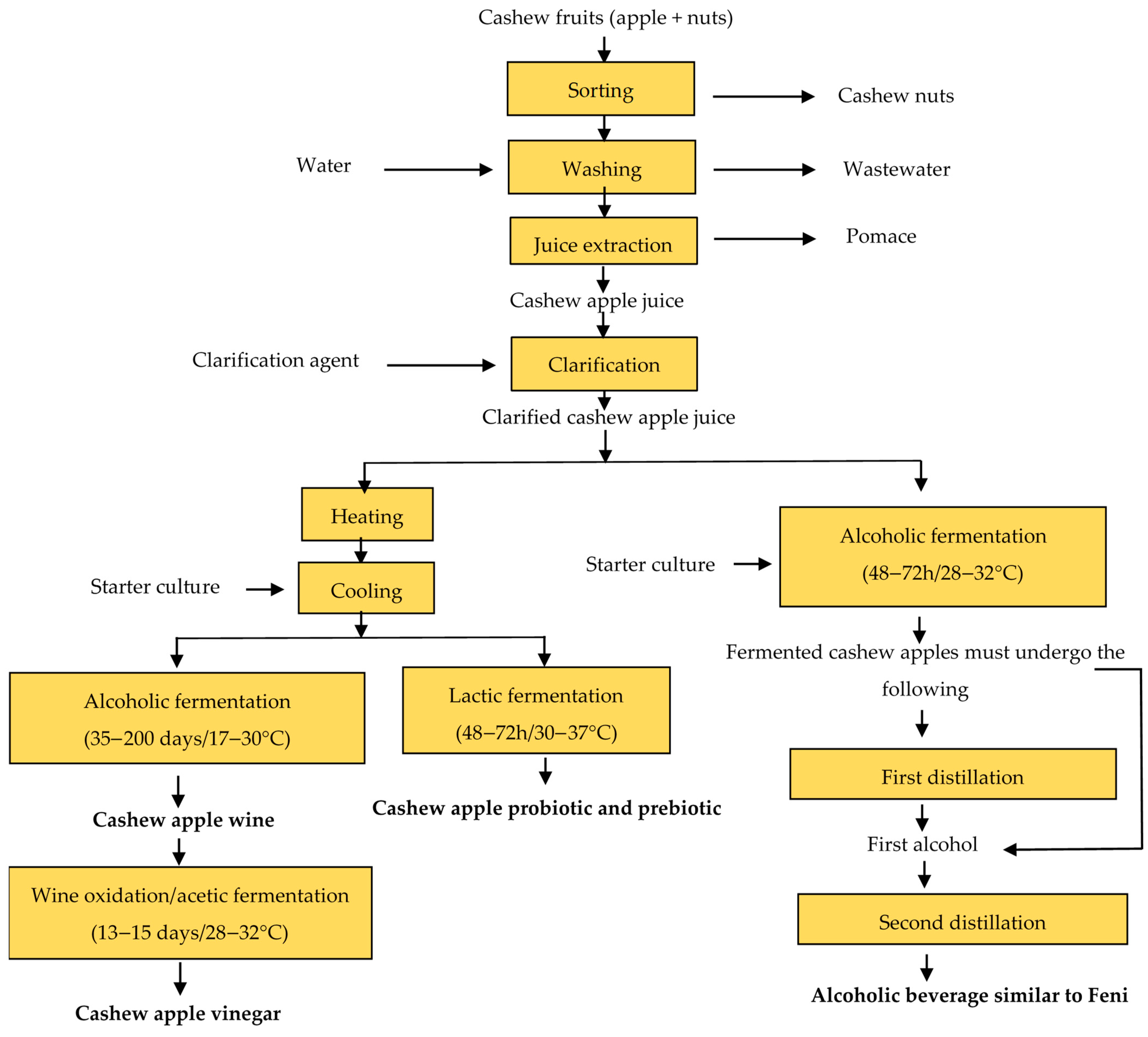
6. Microorganisms Involved in the Fermentation of Cashew Apple Beverages and the Overall Process of Their Production
6.1. Microorganisms Involved in the Fermentation of Fermented Cashew Apple Beverages
6.2. Overview of the Production Processes of Fermented Cashew Apple Beverages
7. Innovative Prospects of New Beverages Made from Cashew Apples
7.1. Cashew Apple Juice as a Substrate for Fermented Beverages like Kombucha
7.2. Cashew Apple Juice as a Substrate for Fermented Beverages like Kefir
8. Conclusions and Future Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Mothé, C.; Oliveira, N.; Freitas, J.; Mothé, M. Cashew Tree Gum: A Scientific and Technological Review. Int. J. Environ. Agric. Biotechnol. 2017, 2, 681–688. [Google Scholar] [CrossRef]
- Lautié, E.; Dornier, M.; De Souza Filho, M.; Reynes, M. Les produits de l’anacardier: Caractéristiques, voies de valorisation et marchés. Fruits 2001, 56, 235–248. [Google Scholar] [CrossRef]
- Oliveira, N.N.; Mothé, C.G.; Mothé, M.G.; De Oliveira, L.G. Cashew Nut and Cashew Apple: A Scientific and Technological Monitoring Worldwide Review. J. Food Sci. Technol. 2020, 57, 12–21. [Google Scholar] [CrossRef] [PubMed]
- Dendena, B.; Corsi, S. Cashew, from Seed to Market: A Review. Agron. Sustain. Dev. 2014, 34, 753–772. [Google Scholar] [CrossRef]
- Attri, B.L. Effect of Initial Sugar Concentration on the Physico-Chemical Characteristics and Sensory Qualities of Cashew Apple Wine. Nat. Prod. Radiance 2009, 8, 374–379. [Google Scholar]
- Sousa, J.M.S.; De Abreu, F.A.P.; Ruiz, A.L.T.G.; Da Silva, G.G.; Machado, S.L.; Garcia, C.P.G.; Filho, F.O.; Wurlitzer, N.J.; De Figueiredo, E.A.T.; Magalhães, F.E.A.; et al. Cashew Apple (Anacardium occidentale L.) Extract from a by-Product of Juice Processing: Assessment of Its Toxicity, Antiproliferative and Antimicrobial Activities. J. Food Sci. Technol. 2021, 58, 764–776. [Google Scholar] [CrossRef]
- Silveira, M.S.; Fontes, C.P.M.L.; Guilherme, A.A.; Fernandes, F.A.N.; Rodrigues, S. Cashew Apple Juice as Substrate for Lactic Acid Production. Food Bioprocess Technol. 2012, 5, 947–953. [Google Scholar] [CrossRef]
- Padonou, S.W.; Olou, D.; Houssou, P.; Karimou, K.; Todohoue, M.C.; Dossou, J.; Mensah, G.A. Comparaison de quelques techniques d’extraction pour l’amélioration de la production et de la qualité du jus de pommes d’anacarde. J. Appl. Biosci. 2016, 96, 9063. [Google Scholar] [CrossRef][Green Version]
- Talasila, U.; Shaik, K.B. Quality, Spoilage and Preservation of Cashew Apple Juice: A Review. J. Food Sci. Technol. 2015, 52, 54–62. [Google Scholar] [CrossRef]
- Rianda, L.; Syarief, R.; Budiastra, I.W. Studyon Characteristics and Shelf Life Prediction of Cashew Appels Using Modified Atmosphere Packaging System. J. Keteknikan Pertan. 2000, 14. [Google Scholar] [CrossRef]
- Talasila, U.; Vechalapu, R.R.; Shaik, K.B. Clarification, Preservation, and Shelf Life Evaluation of Cashew Apple Juice. Food Sci. Biotechnol. 2012, 21, 709–714. [Google Scholar] [CrossRef]
- de Souza, A.R.M.; Brazaca, S.G.C.; Arthur, V.; Oliveira, A.G.C.; Spoto, M.H.F.; Walder, J.M.M. Effect of Gamma Radiation and Storage on Cashew Apple (Anacardium occidentale L.) Quality. Ciênc. Agrotecnol. 2009, 33, 848–854. [Google Scholar]
- Moura, C.F.H.; de Figueiredo, R.W.; Alves, R.E.; de Oliveira Silva, E.; de Araújo, P.G.L.; Maciel, V.T. Cold Storage of Cashew Apple of the BRS 189, CCP 76, END 183 and END 189 Early Dwarf Clones under Different Modified Atmospheres. In Proceedings of the 3rd International Symposium of Tropical and Subtropical Fruits, Fortaleza Ceara, Brazil, 12–17 September 2004; pp. 12–17. [Google Scholar]
- Aluko, A.; Makule, E.; Kassim, N. Effect of Clarification on Physicochemical Properties and Nutrient Retention of Pressed and Blended Cashew Apple Juice. Food Sci. Nutr. 2023, 11, 1891–1903. [Google Scholar] [CrossRef]
- Danait-Nabar, S.; Singhal, R.S. Chemical Modification of Laccase Using Phthalic and 2-Octenyl Succinic Anhydrides: Enzyme Characterization, Stability, and Its Potential for Clarification of Cashew Apple Juice. Process Biochem. 2022, 122, 181–195. [Google Scholar] [CrossRef]
- Das, I.; Sasmal, S.; Arora, A. Effect of Thermal and Non-Thermal Processing on Astringency Reduction and Nutrient Retention in Cashew Apple Fruit and Its Juice. J. Food Sci. Technol. 2021, 58, 2337–2348. [Google Scholar] [CrossRef]
- Prommajak, T.; Leksawasdi, N.; Rattanapanone, N. Optimizing Tannin Precipitation in Cashew Apple Juice. Chiang Mai Univ. J. Nat. Sci. 2018, 17, 13–23. [Google Scholar] [CrossRef]
- Ukonze, J.A.; Ogu, E.; Onu, F.M.; Dimelu, I.; Ifeanyieze, F.O.; Ejiofor, T.E. Impact of Clarification Process on the Nutritional, Mineral and Vitamin Composition of Cashew (Anacardium occidentale) Apple Juice. Afr. J. Biotechnol. 2018, 17, 337–342. [Google Scholar] [CrossRef]
- Dedehou, E.S.C.A.; Dossou, J.; Ahohuendo, B.; Saidou, A.; Ahanchede, A.; Soumanou, M.M. Optimization of Cashew (Anacardium occidentale L.) Apple Juice’s Clarification Process by Using Cassava and Rice Starch. J. Appl. Biosci. 2016, 95, 8989. [Google Scholar] [CrossRef]
- Hashimoto, E.H.; De Cassia Campos Pena, A.; Da Cunha, M.A.A.; De Freitas Branco, R.; De Lima, K.P.; Couto, G.H.; Binder Pagnoncelli, M.G. Fermentation-Mediated Sustainable Development and Improvement of Quality of Plant-Based Foods: From Waste to a New Food. Syst. Microbiol. Biomanuf. 2024, 5, 69–100. [Google Scholar] [CrossRef]
- Reina, L.J.C.; Durán-Aranguren, D.D.; Forero-Rojas, L.F.; Tarapuez-Viveros, L.F.; Durán-Sequeda, D.; Carazzone, C.; Sierra, R. Chemical Composition and Bioactive Compounds of Cashew (Anacardium occidentale) Apple Juice and Bagasse from Colombian Varieties. Heliyon 2022, 8, e09528. [Google Scholar] [CrossRef]
- Lee, E.-R.; Kang, G.-H.; Cho, S.-G. Effect of Flavonoids on Human Health: Old Subjects but New Challenges. Available online: https://www.ingentaconnect.com/content/ben/biot/2007/00000001/00000002/art00003 (accessed on 3 April 2025).
- Mohanty, S.; Ray, P.; Swain, M.R.; Ray, R.C. Fermentation of cashew (Anacardium occidentale L.) “Apple” into wine. J. Food Process. Preserv. 2006, 30, 314–322. [Google Scholar] [CrossRef]
- Campos, D.C.P.; Santos, A.S.; Wolkoff, D.B.; Matta, V.M.; Cabral, L.M.C.; Couri, S. Cashew Apple Juice Stabilization by Microfiltration. Desalination 2002, 148, 61–65. [Google Scholar] [CrossRef]
- Olalusi, A.P.; Ajayi, O.C.; Erinle, O.C. Selected Physio-Chemical Properties for the Processing of Cashew Apple (Anacardium occidentale). IOP Conf. Ser. Earth Environ. Sci. 2020, 445, 012012. [Google Scholar] [CrossRef]
- Vergara, C.M.D.A.C.; Honorato, T.L.; Maia, G.A.; Rodrigues, S. Prebiotic Effect of Fermented Cashew Apple (Anacardium occidentale L.) Juice. LWT—Food Sci. Technol. 2010, 43, 141–145. [Google Scholar] [CrossRef]
- Abdullah, S.; Pradhan, R.C.; Pradhan, D.; Mishra, S. Modeling and Optimization of Pectinase-Assisted Low-Temperature Extraction of Cashew Apple Juice Using Artificial Neural Network Coupled with Genetic Algorithm. Food Chem. 2021, 339, 127862. [Google Scholar] [CrossRef]
- Adou, M.; Kouassi, D.A.; Tetchi, F.A.; Amani, N.G. Phenolic Profile of Cashew Apple Juice (Anacardium occidentale L.) from Yamoussoukro and Korhogo (Côte d’Ivoire). J. Appl. Biosci. 2012, 49, 3331–3338. [Google Scholar]
- Adou, M.; Tetchi Fabrice, A.; Gbané, M.; Kouassi Kouakou, N.; Amani N’Guessan, G. Physico-Chemical Characterization of Cashew Apple Juice (Anacardium occidentale, L.) from Yamoussoukro (Côte d’Ivoire). Rom. Food Biotechnol. 2012, 32–43. Available online: https://www.researchgate.net/publication/262918230_PHYSICO-CHEMICAL_CHARACTERIZATION_OF_CASHEW_APPLE_JUICE_ANACARDIUM_OCCIDENTALE_L_FROM_YAMOUSSOUKRO_COTE_D'IVOIRE (accessed on 9 April 2025).
- Leite, A.K.; Fonteles, T.V.; Miguel, T.B.; da Silva, G.S.; de Brito, E.S.; Alves Filho, E.G.; Fernandes, F.A.N.; Rodrigues, S. Atmospheric Cold Plasma Frequency Imparts Changes on Cashew Apple Juice Composition and Improves Vitamin C Bioaccessibility. Food Res. Int. 2021, 147, 110479. [Google Scholar] [CrossRef]
- de Brito, E.S.; Pessanha de Araújo, M.C.; Lin, L.-Z.; Harnly, J. Dosage Des Composants Flavonoïdes de La Pomme de Cajou (Anacardium occidentale) Par LC-DAD-ESI/MS. Food Chem. 2007, 105, 1112–1118. [Google Scholar] [CrossRef]
- Akyereko, Y.G.; Yeboah, G.B.; Wireko-Manu, F.D.; Alemawor, F.; Mills-Robertson, F.C.; Odoom, W. Nutritional Value and Health Benefits of Cashew Apple. JSFA Rep. 2023, 3, 110–118. [Google Scholar] [CrossRef]
- Michodjehoun-Mestres, L.; Souquet, J.-M.; Fulcrand, H.; Bouchut, C.; Reynes, M.; Brillouet, J.-M. Monomeric Phenols of Cashew Apple (Anacardium occidentale L.). Food Chem. 2009, 112, 851–857. [Google Scholar] [CrossRef]
- Wang, S.; Smyth, H.E.; Olarte Mantilla, S.M.; Stokes, J.R.; Smith, P.A. Astringency and Its Sub-Qualities: A Review of Astringency Mechanisms and Methods for Measuring Saliva Lubrication. Chem. Senses 2024, 49, bjae016. [Google Scholar] [CrossRef] [PubMed]
- Melis, M.; Yousaf, N.Y.; Mattes, M.Z.; Cabras, T.; Messana, I.; Crnjar, R.; Tomassini Barbarossa, I.; Tepper, B.J. Sensory Perception of and Salivary Protein Response to Astringency as a Function of the 6-n-Propylthioural (PROP) Bitter-Taste Phenotype. Physiol. Behav. 2017, 173, 163–173. [Google Scholar] [CrossRef]
- Obreque-Slíer, E.; Peña-Neira, Á.; López-Solís, R. Enhancement of Both Salivary Protein–Enological Tannin Interactions and Astringency Perception by Ethanol. J. Agric. Food Chem. 2010, 58, 3729–3735. [Google Scholar] [CrossRef]
- Soares, S.; Ferrer-Galego, R.; Brandão, E.; Silva, M.; Mateus, N.; Freitas, V.D. Contribution of Human Oral Cells to Astringency by Binding Salivary Protein/Tannin Complexes. J. Agric. Food Chem. 2016, 64, 7823–7828. [Google Scholar] [CrossRef]
- Tezotto-Uliana, J.V.; De Paula, J.T.; Tessmer, M.A.; Kluge, R.A. Ethanol Vapor Is Efficient for Reduction of Astringency Compounds in Cashew Apple. Postharvest Biol. Technol. 2018, 145, 117–124. [Google Scholar] [CrossRef]
- Hagerman, A.E. Tannin-Protein Interactions. In Phenolic Compounds in Food and Their Effects on Health. I-Analysis, Occurrence and Chemistry; Ho, P., Lee, C.Y., Huang, M.T., Eds.; ACS Symposium Series; American Chemical Society: Washington, DC, USA, 1992. [Google Scholar]
- Benitez, E.I.; Lozano, J.E. Effect of Gelatin on Apple Juice Turbidity. Lat. Am. Appl. Res. 2007, 37, 261–266. [Google Scholar]
- Jayalekshmy, V.G.; John, P.S. ‘Sago’—A Natural Product for Cashew Apple Juice Clarification. J. Trop. Agric. 2004, 42, 67–68. [Google Scholar]
- Dheeraj; Srivastava, A.; Mishra, A. Mitigation of Cashew Apple Fruits Astringency. Environ. Sustain. 2023, 6, 319–329. [Google Scholar] [CrossRef]
- Emelike, N.J.T.; Ebere, C.O. Effect of Treatments on the Tannin Content and Quality Assessment of Cashew Apple Juice and the Kernel. Eur. J. Food Sci. Technol. 2016, 4, 25–36. [Google Scholar]
- Soro, D.; Delalonde, M.; Wisniewski, C.; Dornier, M. Clarification of Cashew Apple Juice by Crossflow Microfiltration: Foulant Fractions and Interest of Filterability Tests. In Proceedings of the Article De Collogue, 2012. EFFoST Annual Conference, Montpellier, France, 20–23 November 2012. [Google Scholar]
- Conidi, C.; Castro-Muñoz, R.; Cassano, A. Membrane-Based Operations in the Fruit Juice Processing Industry: A Review. Beverages 2020, 6, 18. [Google Scholar] [CrossRef]
- Dornier, M.; Belleville, M.-P.; Vaillant, F. Membrane Technologies for Fruit Juice Processing. In Fruit Preservation; Rosenthal, A., Deliza, R., Welti-Chanes, J., Barbosa-Cánovas, G.V., Eds.; Food Engineering Series; Springer: New York, NY, USA, 2018; pp. 211–248. ISBN 978-1-4939-3309-9. [Google Scholar]
- Abreu, F.; Perez, A.M.; Dornier, M.; Reynes, M. Potentialités de La Microfiltration Tangentielle Sur Membranes Minérales Pour La Clarification Du Jus de Pomme de Cajou. Fruits 2005, 60, 33–40. [Google Scholar] [CrossRef]
- de Andrade, M.I.R.; de Souza, A.C.R.; de Abreu, F.A.P.; Ximenes, S.F.; dos Santos Garruti, D. Changes in Cashew Apple Juice Flavor after Tangential Microfiltration Process. Ann. Nutr. Food Sci. 2018, 2, 1029. [Google Scholar]
- Cianci, F.C.; Silva, L.F.M.; Cabral, L.; Matta, V.M. Clarification and Concentration of Cashew Apple Juice by Membrane Processes. Food Sci. Technol. 2005, 25, 579–583. [Google Scholar] [CrossRef]
- Abdullah, S.; Karmakar, S.; Pradhan, R.C.; Mishra, S. Pressure-driven Crossflow Microfiltration Coupled with Centrifugation for Tannin Reduction and Clarification of Cashew Apple Juice: Modeling of Permeate Flux Decline and Optimization of Process Parameters. J. Food Process. Preserv. 2022, 46, e16497. [Google Scholar] [CrossRef]
- Gyedu-Akoto, E. Utilization of Some Cashew By-products. Nutr. Food Sci. 2011, 41, 393–400. [Google Scholar] [CrossRef]
- Ugwuoke, C.U.; Ugwuoke, F.G.; Omeje, B.A.; Eze, G.E.; Monwuba, N.S.; Ifeanyieze, F.O.; Ukonze, J.A. Effect of Moringa oleifera Seed Powder on the Clarity, Colloidal Particles, and Nutritional Contents of Cashew Apple Juice. Scientifica 2020, 2020, 4380407. [Google Scholar] [CrossRef]
- Ra, D.; Janani, P.; Preethi, P.; Raichurkar, S.J.; Athawale, G.H. Removal of Tannins from Cashew Apple Juice by Using Low Cost Food Grade Materials. J. Pharmacogn. Phytochem. 2018, 7, 222–225. [Google Scholar] [CrossRef]
- Ngoko, J.T.; Ngatchic, J.; Saïdou, C.; Yanou, N.N. Amélioration du procédé traditionnel de production du jus d’anacarde (Anacardium occidentale L.). In Proceedings of the Conférence International LOREXP-2021, Ngaoundere, Cameroon, 20–23 April 2021; pp. 658–678. [Google Scholar]
- Hassan, A.N.; Nelson, B.K. Invited Review: Anaerobic Fermentation of Dairy Food Wastewater. J. Dairy Sci. 2012, 95, 6188–6203. [Google Scholar] [CrossRef]
- Katz, S.E. The Art of Fermentation: An in-Depth Exploration of Essential Concepts and Processes from Around the World; Chelsea Green Publishing: Junction, VT, USA, 2012; ISBN 978-1-60358-286-5. [Google Scholar]
- Dashko, S.; Zhou, N.; Compagno, C.; Piškur, J. Why, When, and How Did Yeast Evolve Alcoholic Fermentation? FEMS Yeast Res. 2014, 14, 826–832. [Google Scholar] [CrossRef]
- Steinkraus, K.H.; Hui, Y.H.; Meunier-Goddik, L.; Hansen, A.S.; Josephsen, J.; Nip, W.K.; Stanfeld, P.S.; Toldra, F. Origin and History of Food Fermentations. In Handbook of Food and Beverage Fermentation Technology; CRC Press: Boca Raton, FL, USA, 2004; pp. 22–30. [Google Scholar]
- Fleet, G.H.; Lafon-Lafourcade, S.; Rib reau-Gayon, P. Evolution of Yeasts and Lactic Acid Bacteria during Fermentation and Storage of Bordeaux Wines. Appl. Environ. Microbiol. 1984, 48, 1034–1038. [Google Scholar] [CrossRef] [PubMed]
- Joshi, V.K.; Panesar, P.S.; Rana, V.S.; Kaur, S. Chapter 1—Science and Technology of Fruit Wines: An Overview. In Science and Technology of Fruit Wine Production; Kosseva, M.R., Joshi, V.K., Panesar, P.S., Eds.; Academic Press: San Diego, CA, USA, 2017; pp. 1–72. ISBN 978-0-12-800850-8. [Google Scholar]
- Awe, S.; Sani, A.; Eniola, K.I.T.; Kayode, R.M.O. Toxicological assessment of locally produced cashew wine. IJRRAS 2013, 15, 125–131. [Google Scholar]
- Apine, O.A.; Jadhav, J.P. Fermentation of Cashew Apple (Anacardium occidentale) Juice into Wine by Different Saccharomyces Cerevisiae Strains: A Comparative Study. Indian J. Res. 2015, 4, 6–10. [Google Scholar]
- Koui, E.W.; Soro, D.; Gnoumou, J.I.K.; Assidjo, N.E.; Yao, K.B. Production of Cashew Apple Wine Enriched with Hibiscus Sabdariffa Extracts. Food Nutr. Sci. 2023, 14, 720–729. [Google Scholar]
- Lowor, S.; Yabani, D.; Winifred, K.; Agyente-Badu, C. Production of Wine and Vinegar from Cashew (Anacardium occidentale) “Apple”. Br. Biotechnol. J. 2016, 12, 1–11. [Google Scholar] [CrossRef]
- Rêgo, E.S.B.; Rosa, C.A.; Freire, A.L.; Machado, A.M.D.R.; Gomes, F.D.C.O.; Costa, A.S.P.D.; Mendonça, M.D.C.; Hernández-Macedo, M.L.; Padilha, F.F. Cashew Wine and Volatile Compounds Produced during Fermentation by Non-Saccharomyces and Saccharomyces Yeast. LWT 2020, 126, 109291. [Google Scholar] [CrossRef]
- Freire, C.O. Tropical and Subtropical Fruit Fermented Beverages. Microb. Biotechnol. Hortic. 2008, 3, 3. [Google Scholar]
- Rangnekar, D. Geographical Indications and Localisation: A Case Study of Feni. ESRC Rep. 2009. Available online: https://ssrn.com/abstract=1564624 (accessed on 9 April 2025).
- Kannan, V.; Rangarajan, V.; Manjare, S.D.; Pathak, P.V. Microbial Production of Value-Added Products from Cashew Apples-an Economical Boost to Cashew Farmers. J. Pure Appl. Microbiol. 2021, 15, 1816–1832. [Google Scholar] [CrossRef]
- Gibson, G.R.; Roberfroid, M.B. Dietary Modulation of the Human Colonic Microbiota: Introducing the Concept of Prebiotics. J. Nutr. 1995, 125, 1401–1412. [Google Scholar] [CrossRef]
- Gibson, G.R.; Scott, K.P.; Rastall, R.A.; Tuohy, K.M.; Hotchkiss, A.; Dubert-Ferrandon, A.; Gareau, M.; Murphy, E.F.; Saulnier, D.; Loh, G.; et al. Dietary Prebiotics: Current Status and New Definition. Food Sci. Technol. Bull. Funct. Foods 2010, 7, 1–19. [Google Scholar] [CrossRef]
- Khangwal, I.; Shukla, P. Potential Prebiotics and Their Transmission Mechanisms: Recent Approaches. J. Food Drug Anal. 2019, 27, 649–656. [Google Scholar] [CrossRef] [PubMed]
- Quigley, E.M.M. Prebiotics and Probiotics in Digestive Health. Clin. Gastroenterol. Hepatol. 2019, 17, 333–344. [Google Scholar] [CrossRef] [PubMed]
- Ashwini, A.; Ramya, H.N.; Ramkumar, C.; Reddy, K.R.; Kulkarni, R.V.; Abinaya, V.; Naveen, S.; Raghu, A.V. Reactive Mechanism and the Applications of Bioactive Prebiotics for Human Health: Review. J. Microbiol. Methods 2019, 159, 128–137. [Google Scholar] [CrossRef] [PubMed]
- Kao, A.C.-C.; Burnet, P.W.J.; Lennox, B.R. Can Prebiotics Assist in the Management of Cognition and Weight Gain in Schizophrenia? Psychoneuroendocrinology 2018, 95, 179–185. [Google Scholar] [CrossRef]
- Ewe, J.-A.; Wan-Abdullah, W.-N.; Liong, M.-T. Viability and Growth Characteristics of Lactobacillus in Soymilk Supplemented with B-Vitamins. Int. J. Food Sci. Nutr. 2010, 61, 87–107. [Google Scholar] [CrossRef]
- Frias, J.; Miranda, M.L.; Doblado, R.; Vidal-Valverde, C. Effect of Germination and Fermentation on the Antioxidant Vitamin Content and Antioxidant Capacity of Lupinus Albus L. Var. Multolupa. Food Chem. 2005, 92, 211–220. [Google Scholar] [CrossRef]
- Kaprasob, R.; Kerdchoechuen, O.; Laohakunjit, N.; Somboonpanyakul, P. B Vitamins and Prebiotic Fructooligosaccharides of Cashew Apple Fermented with Probiotic Strains Lactobacillus spp., Leuconostoc mesenteroides and Bifidobacterium longum. Process Biochem. 2018, 70, 9–19. [Google Scholar] [CrossRef]
- Naseem, Z.; Mir, S.A.; Wani, S.M.; Rouf, M.A.; Bashir, I.; Zehra, A. Probiotic-Fortified Fruit Juices: Health Benefits, Challenges, and Future Perspective. Nutrition 2023, 115, 112154. [Google Scholar] [CrossRef]
- Filannino, P.; Bai, Y.; Di Cagno, R.; Gobbetti, M.; Gänzle, M.G. Metabolism of Phenolic Compounds by Lactobacillus spp. during Fermentation of Cherry Juice and Broccoli Puree. Food Microbiol. 2015, 46, 272–279. [Google Scholar] [CrossRef]
- Saubade, F.; Hemery, Y.M.; Guyot, J.-P.; Humblot, C. Lactic Acid Fermentation as a Tool for Increasing the Folate Content of Foods. Crit. Rev. Food Sci. Nutr. 2017, 57, 3894–3910. [Google Scholar] [CrossRef] [PubMed]
- Worobo, R.W.; Splittstoesser, D.F. Microbiology of Fruit Products. Process. Fruit 2005, 2, 161–284. [Google Scholar]
- Sahu, A.; Samal, K. Potential Application of Cashew Apple (Anacardium occidentale L.) as a Probiotic Beverage Fermented with Lactobacillus. Vigyan Varta 2020, 1, 48–54. [Google Scholar]
- Ho, C.W.; Lazim, A.M.; Fazry, S.; Zaki, U.K.H.H.; Lim, S.J. Varieties, Production, Composition and Health Benefits of Vinegars: A Review. Food Chem. 2017, 221, 1621–1630. [Google Scholar] [CrossRef]
- Fynn, G.H. Biotechnology: A Textbook of Industrial Microbiology: W. Crueger and A. Creuger Sinauer Associates/Blackwell Scientific Publications; Oxford, 1984 308 Pages. £24.50. FEBS Lett. 1985, 180, 136. [Google Scholar] [CrossRef]
- Wareing, P.; Davenport, R.R. Microbiology of Soft Drinks and Fruit Juices. Chem. Technol. Soft Drink. Fruit Juices 2004, 279–299. [Google Scholar] [CrossRef]
- Houssou, P.; Padonou, S.W.; Dansou, V.; Todohoue, C.; Agbobatinkpo, P.; N’djolosse, K.; Gotoechan Hodonou, H.; Kodjo, S.; Yaï, A.C.; Bello, S. Production Du Vinaigre à Base de La Pomme d’anacardier; Fiche Technique, 4ème Trimestre; Bibliothèque Nationale (BN) Du Bénin—Recherche: Porto-Novo, Benin, 2016; Available online: https://www.bing.com/search?pglt=299&q=Houssou+P%2C+Padonou+SW%2C+Dansou+V%2C+Todohoue+C+et+al+(2016)+Production+du+vinaigre+%C3%A0+base+de+la+pomme+d%27anacardier.+Fiche+technique%2C+4%C3%A8me+trimestre+2016+ISBN+%3A+978-99919-2-546-2.+Biblioth%C3%A8que+Nationale+(BN)+du+B%C3%A9nin&cvid=ee774f5772294d17ac5f692326b95fbc&gs_lcrp=EgRlZGdlKgYIABBFGDkyBggAEEUYOdIBCDI4NzFqMGoxqAIAsAIA&FORM=ANNTA1&PC=DCTS (accessed on 20 January 2025)ISBN 978-99919-2-546-2.
- Desai, M.V.; Dubey, K.V.; Vakil, B.V.; Ranade, V.V. Isolation, Identification and Screening of the Yeast Flora from Indian Cashew Apple for Sugar and Ethanol Tolerance. Int. J. Biotechnol. Wellness Ind. 2012, 1, 259. [Google Scholar]
- Prabhu Khorjuvenkar, S.N.; Doijad, S.P.; Poharkar, K.; Dubal, Z.B.; Barbuddhe, S.B. Antimicrobial Activity of a Novel Pichia Membranifaciens Strain Isolated from Naturally Fermented Cashew Apple Juice. Proc. Natl. Acad. Sci. India Sect. B Biol. Sci. 2016, 86, 125–129. [Google Scholar] [CrossRef]
- Kannan, V.; Rangarajan, V.; Manjare, S.D. Investigating the Potential of Ethanol and Sugar Tolerant Yeast Strain Isolates from Fermented Cashew Apple Juice for Improved Feni Production. Waste Biomass Valorization 2024, 15, 4885–4898. [Google Scholar] [CrossRef]
- Barros, E.M.; Rodrigues, T.H.S.; Pinheiro, A.D.T.; Angelim, A.L.; Melo, V.M.M.; Rocha, M.V.P.; Gonçalves, L.R.B. A Yeast Isolated from Cashew Apple Juice and Its Ability to Produce First- and Second-Generation Ethanol. Appl. Biochem. Biotechnol. 2014, 174, 2762–2776. [Google Scholar] [CrossRef]
- Osho, A. Ethanol and Sugar Tolerance of Wine Yeasts Isolated from Fermenting Cashew Apple Juice. Afr. J. Biotechnol. 2005, 4, 660–662. [Google Scholar] [CrossRef]
- Geroyiannaki, M.; Komaitis, M.E.; Stavrakas, D.E.; Polysiou, M.; Athanasopoulos, P.E.; Spanos, M. Evaluation of Acetaldehyde and Methanol in Greek Traditional Alcoholic Beverages from Varietal Fermented Grape Pomaces (Vitis vinifera L.). Food Control 2007, 18, 988–995. [Google Scholar] [CrossRef]
- Hatta-Sakoda, B.; Guevara-Pérez, A.; Morales-Soriano, E. Evolution of Acetaldehyde, Methanol, and Furfural in Pisco Distillation. ACS Food Sci. Technol. 2024, 4, 889–894. [Google Scholar] [CrossRef]
- Kokkinakis, M.; Tsakiris, I.; Tzatzarakis, M.; Vakonaki, E.; Alegakis, A.; Papachristou, S.; Karzi, V.; Kokkinaki, A.; Goumenou, M.; Kallionakis, M.; et al. Carcinogenic, Ethanol, Acetaldehyde and Noncarcinogenic Higher Alcohols, Esters, and Methanol Compounds Found in Traditional Alcoholic Beverages. A Risk Assessment Approach. Toxicol. Rep. 2020, 7, 1057–1065. [Google Scholar] [CrossRef]
- Ohimain, E.I. Methanol Contamination in Traditionally Fermented Alcoholic Beverages: The Microbial Dimension. SpringerPlus 2016, 5, 1607. [Google Scholar] [CrossRef]
- Lachenmeier, D.W.; Kanteres, F.; Rehm, J. Carcinogenicity of Acetaldehyde in Alcoholic Beverages: Risk Assessment Outside Ethanol Metabolism. Addiction 2009, 104, 533–550. [Google Scholar] [CrossRef]
- Dini, I. 1—An Overview of Functional Beverages. In Functional and Medicinal Beverages; Grumezescu, A.M., Holban, A.M., Eds.; Academic Press: Cambridge, MA, USA, 2019; pp. 1–40. ISBN 978-0-12-816397-9. [Google Scholar]
- Verni, M.; Verardo, V.; Rizzello, C.G. How Fermentation Affects the Antioxidant Properties of Cereals and Legumes. Foods 2019, 8, 362. [Google Scholar] [CrossRef]
- Ávila, M.; Jaquet, M.; Moine, D.; Requena, T.; Peláez, C.; Arigoni, F.; Jankovic, I. Physiological and Biochemical Characterization of the Two α-l-Rhamnosidases of Lactobacillus plantarum NCC245. Microbiology 2009, 155, 2739–2749. [Google Scholar] [CrossRef]
- María Landete, J.; Hernández, T.; Robredo, S.; Duenas, M.; De Las Rivas, B.; Estrella, I.; Munoz, R. Effect of Soaking and Fermentation on Content of Phenolic Compounds of Soybean (Glycine max cv. Merit) and Mung Beans (Vigna radiata [L] Wilczek). Int. J. Food Sci. Nutr. 2015, 66, 203–209. [Google Scholar] [CrossRef]
- Ciani, M.; Comitini, F.; Mannazzu, I.; Domizio, P. Controlled Mixed Culture Fermentation: A New Perspective on the Use of Non- Saccharomyces Yeasts in Winemaking. FEMS Yeast Res. 2010, 10, 123–133. [Google Scholar] [CrossRef]
- Jayabalan, R.; Malbaša, R.V.; Sathishkumar, M. Kombucha. In Reference Module in Food Science; Elsevier: Amsterdam, The Netherlands, 2016; ISBN 978-0-08-100596-5. [Google Scholar]
- Saleem, K.; Ikram, A.; Saeed, F.; Afzaal, M.; Ateeq, H.; Hussain, M.; Raza, A.; Rasheed, A.; Asghar, A.; Asif Shah, M. Nutritional and Functional Properties of Kefir: Review. Int. J. Food Prop. 2023, 26, 3261–3274. [Google Scholar] [CrossRef]
- Diez-Ozaeta, I.; Astiazaran, O.J. Recent Advances in Kombucha Tea: Microbial Consortium, Chemical Parameters, Health Implications and Biocellulose Production. Int. J. Food Microbiol. 2022, 377, 109783. [Google Scholar] [CrossRef] [PubMed]
- Leonarski, E.; Guimarães, A.C.; Cesca, K.; Poletto, P. Production Process and Characteristics of Kombucha Fermented from Alternative Raw Materials. Food Biosci. 2022, 49, 101841. [Google Scholar] [CrossRef]
- Freitas, A.; Sousa, P.; Wurlitzer, N. Alternative Raw Materials in Kombucha Production. Int. J. Gastron. Food Sci. 2022, 30, 100594. [Google Scholar] [CrossRef]
- Laavanya, D.; Shirkole, S.; Balasubramanian, P. Current Challenges, Applications and Future Perspectives of SCOBY Cellulose of Kombucha Fermentation. J. Clean. Prod. 2021, 295, 126454. [Google Scholar] [CrossRef]
- Chakravorty, S.; Bhattacharya, S.; Chatzinotas, A.; Chakraborty, W.; Bhattacharya, D.; Gachhui, R. Kombucha Tea Fermentation: Microbial and Biochemical Dynamics. Int. J. Food Microbiol. 2016, 220, 63–72. [Google Scholar] [CrossRef]
- Marsh, A.J.; O’Sullivan, O.; Hill, C.; Ross, R.P.; Cotter, P.D. Sequence-Based Analysis of the Bacterial and Fungal Compositions of Multiple Kombucha (Tea fungus) Samples. Food Microbiol. 2014, 38, 171–178. [Google Scholar] [CrossRef]
- Sharifudin, S.A.; Ho, W.Y.; Yeap, S.K.; Abdullah, R.; Koh, S.P. Fermentation and Characterisation of Potential Kombucha Cultures on Papaya-Based Substrates. LWT 2021, 151, 112060. [Google Scholar] [CrossRef]
- Morales, D.; Gutiérrez-Pensado, R.; Bravo, F.I.; Muguerza, B. Novel Kombucha Beverages with Antioxidant Activity Based on Fruits as Alternative Substrates. LWT 2023, 189, 115482. [Google Scholar] [CrossRef]
- Emiljanowicz, K.E.; Malinowska-Pańczyk, E. Kombucha from Alternative Raw Materials—The Review. Crit. Rev. Food Sci. Nutr. 2020, 60, 3185–3194. [Google Scholar] [CrossRef]
- Grønnevik, H.; Falstad, M.; Narvhus, J.A. Microbiological and Chemical Properties of Norwegian Kefir during Storage. Int. Dairy J. 2011, 21, 601–606. [Google Scholar] [CrossRef]
- Paredes, J.L.; Escudero-Gilete, M.L.; Vicario, I.M. A New Functional Kefir Fermented Beverage Obtained from Fruit and Vegetable Juice: Development and Characterization. LWT 2022, 154, 112728. [Google Scholar] [CrossRef]
- Guzel-Seydim, Z.B.; Seydim, A.C.; Greene, A.K.; Taş, T. Determination of Antimutagenic Properties of Acetone Extracted Fermented Milks and Changes in Their Total Fatty Acid Profiles Including Conjugated Linoleic Acids. Int. J. Dairy Technol. 2006, 59, 209–215. [Google Scholar] [CrossRef]
- Gulitz, A.; Stadie, J.; Wenning, M.; Ehrmann, M.A.; Vogel, R.F. The Microbial Diversity of Water Kefir. Int. J. Food Microbiol. 2011, 151, 284–288. [Google Scholar] [CrossRef]
- Randazzo, W.; Corona, O.; Guarcello, R.; Francesca, N.; Germanà, M.A.; Erten, H.; Moschetti, G.; Settanni, L. Development of New Non-Dairy Beverages from Mediterranean Fruit Juices Fermented with Water Kefir Microorganisms. Food Microbiol. 2016, 54, 40–51. [Google Scholar] [CrossRef]
| Parameters | Values | Countries | References |
|---|---|---|---|
| pH | 3.40–4.61 | India, Colombia | [11,21,23] |
| Total soluble sugar | 7–23% | Colombia, Brazil | [21,24] |
| Potassium | 69–1210 mg·L−1 | Nigeria, Brazil | [25,26] |
| Calcium | <ND–42 mg·L−1 | ||
| Sodium | 0.4–90 mg·L−1 | ||
| Magnesium | 62–1170 mg·L−1 | ||
| Zinc | 0.06–11.20 mg·L−1 | ||
| Iron | 0.07–6.97 mg·L−1 | ||
| Total polyphenols | 1.11–2.45 gGAE.L−1 | India | [27] |
| Flavonoids | 0.29–0.48 g·L−1 | Ivory Coast | [28] |
| Tannins | 1.71–1.92 gTA.L−1 | Colombia | [21] |
| Glucose | 36.5–65.8 g·L−1 | Ivory Coast, Colombia | [21,29] |
| Fructose | 13.9–110.3 g·L−1 | ||
| Sucrose | 2.5–5.3 g·L−1 | ||
| Ascorbic acid | 0.49–2.56 g·L−1 | Colombia | [21,27,30] |
| Oxalic acid | 0.098–0.16 g·L−1 | [21] | |
| Citric acid | 0.53–0.56 g·L−1 | ||
| Tartaric acid | 0.67–0.75 g·L−1 | ||
| Fumaric acid | 0.12–0.18 g·L−1 |
| Compound | Cashew Apple (mg/g) |
|---|---|
| Myricetin 3-O-galactoside | 0.0532 |
| Myricetin 3-O-glucoside | 0.0274 |
| Myricetin 3-O-xylopyranoside | 0.0124 |
| Myricetin 3-O-arabinopyrannoside | 0.0104 |
| Myricetin 3-O-arabinofuranoside | 0.0097 |
| Myricetin 3-O-rhamnoside | 0.0400 |
| Total myricetin glycosides | 0.1511 |
| Quercetin 3-O-galactoside | 0.0465 |
| Quercetin 3-O-glucoside | 0.0144 |
| Quercetin 3-O-xylopyranoside | 0.0116 |
| Quercetin 3-O-arabinopyrannoside | 0.0108 |
| Quercetin 3-O-arabinofuranoside | 0.0079 |
| Quercetin 3-O-rhamnoside | 0.0227 |
| Total quercetin glycosides | 0.1139 |
| Kaempferol 3-O-glucoside | Trace amount |
| 5-Methylcyanidin 3-O-hexoside | 0.0197 |
| Total glycosylatedflavonoids | 0.2847 |
| Microorganism Strains Used | Fermentation Time | Fermentation Temperature | Ethanol Content | Wine Sensory Characteristics | Country of Production | References |
|---|---|---|---|---|---|---|
| S. cerevisiae var. bayanus | 36 to 51 days | 30 °C to 34 °C | 7% | Slightly yellowish color, moderate astringency and acidity | India | [23] |
| S. cerevisiae var. ellipsoidea | 6 months and 15 days | 28–30 °C | 8.25% | Color and taste accepted by panelists | India | [5] |
| S. cerevisiae | 48 days | 29 ± 2 °C | 10% | ND | Nigeria | [61] |
| S. cerevisiae from Lallemand | 35 days | 17 °C to 22 °C | ND | Color and taste similar to grape wine, unpleasant aroma | Ghana | [64] |
| S. cerevisiae, Torulaspora delbrueckii, H. opuntiae | 48 h | 28 °C | 7% | Excellent organoleptic quality | Brazil | [65] |
| Alcoholic Fermentation of Cashew Apples | Lactic Fermentation of Cashew Apples | Mixed Fermentation of Cashew Apples | |
|---|---|---|---|
| Native microorganisms involved in the fermentation process | T. delbrueckii [65], C. krusei, C. norvegica, C. magnoliae, C. parapsilosis, C. colliculosa, C. norvegica, C. parapsilosis [87], P. membranifaciens [88], P. kudriavzevii [89] Hanseniaspora spp. [90], S. cerevisiae, S. uvarum [91] | ND | ND |
| Non-native microorganisms involved in the fermentation process | S. cerevisiae var. bayanus [23], S. cerevisiae var. ellipsoidea [5], S. cerevisiae [60], S. cerevisiae from Lallemand [63], S. cerevisiae, H. opuntiae [65]. | L. acidophilus, L. casei, L. plantarum, L. mesenteroides, B. Longum [77], L. plantarum [82] | S. cerevisiae, acetic acid bacterial biofilm [86] |
| Fermented food products | Wine and edible spirits | Probiotic and prebiotic beverages | Vinegar |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Codjia, F.S.; Dabadé, D.S.; Agbobatinkpo, P.; Collombel, I.; Achir, N.; Azokpota, P.; Dossou, J. Fermented Cashew Apple Beverages: Current State of Knowledge and Prospects. Beverages 2025, 11, 49. https://doi.org/10.3390/beverages11020049
Codjia FS, Dabadé DS, Agbobatinkpo P, Collombel I, Achir N, Azokpota P, Dossou J. Fermented Cashew Apple Beverages: Current State of Knowledge and Prospects. Beverages. 2025; 11(2):49. https://doi.org/10.3390/beverages11020049
Chicago/Turabian StyleCodjia, Fabrice S., D. Sylvain Dabadé, Pélagie Agbobatinkpo, Ingrid Collombel, Nawel Achir, Paulin Azokpota, and Joseph Dossou. 2025. "Fermented Cashew Apple Beverages: Current State of Knowledge and Prospects" Beverages 11, no. 2: 49. https://doi.org/10.3390/beverages11020049
APA StyleCodjia, F. S., Dabadé, D. S., Agbobatinkpo, P., Collombel, I., Achir, N., Azokpota, P., & Dossou, J. (2025). Fermented Cashew Apple Beverages: Current State of Knowledge and Prospects. Beverages, 11(2), 49. https://doi.org/10.3390/beverages11020049