Exploring Metschnikowia pulcherrima as a Co-Fermenter with Saccharomyces cerevisiae: Influence on Wine Aroma during Fermentation and Ageing
Abstract
1. Introduction
2. Materials and Methods
2.1. Winemaking and Ageing in Bottles
2.2. Analysis of General Parameters
2.3. Analysis of Wines’ Volatile Compounds through GC-MS
2.4. Sensory Analysis
2.5. Statistical Analysis
3. Results and Discussion
3.1. Enological Parameters of the Wines
3.2. Volatile Compositions of the Wines
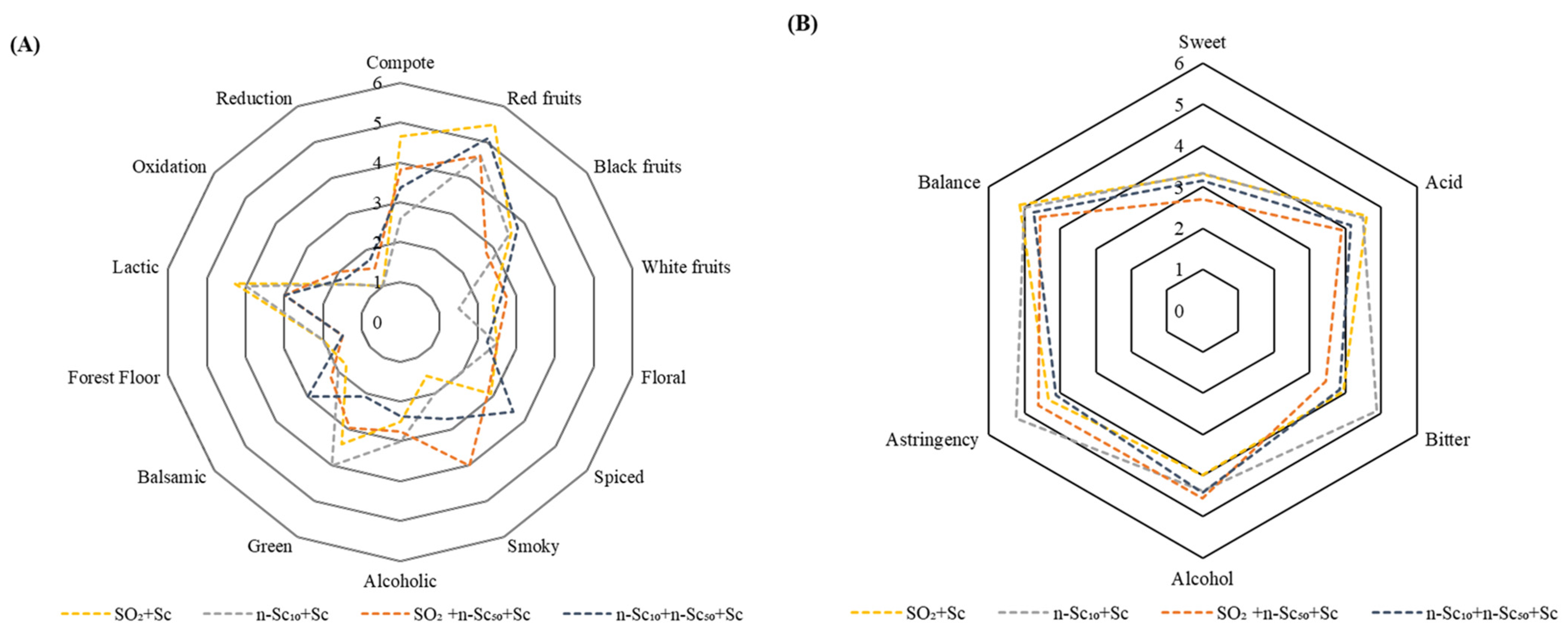
3.3. Sensory Analysis of the Wines
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Corrales, L.; Antolínez, D.; Bohórquez, J.A.; Corredor Vargas, A.M. Bacterias anaerobias: Procesos que realizan y contribuyen a la sostenibilidad de la vida en el planeta. Nova 2015, 13, 55–81. [Google Scholar] [CrossRef]
- Pretorius, I.S. Tailoring wine yeast for the new millennium: Novel approaches to the ancient art of winemaking. Yeast 2000, 16, 675–729. [Google Scholar] [CrossRef]
- Varela, C.; Sengler, F.; Solomon, M.; Curtin, C. Volatile flavour profile of reduced alcohol wines fermented with the non-conventional yeast species Metschnikowia pulcherrima and Saccharomyces uvarum. Food Chem. 2016, 209, 57–64. [Google Scholar] [CrossRef] [PubMed]
- De Filippis, F.; La Storia, A.; Blaiotta, G. Monitoring the mycobiota during Greco di Tufo and Aglianico wine fermentation by 18S rRNA gene sequencing. Food Microbiol. 2017, 63, 117–122. [Google Scholar] [CrossRef]
- Tedesco, F.; Siesto, G.; Pietrafesa, R.; Romano, P.; Salvia, R.; Scieuzo, C.; Falabella, P.; Capece, A. Chemical Methods for Microbiological Control of Winemaking: An Overview of Current and Future Applications. Beverages 2022, 8, 58. [Google Scholar] [CrossRef]
- Capece, A.; Pietrafesa, R.; Siesto, G.; Romano, P. Biotechnological Approach Based on Selected Saccharomyces cerevisiae Starters for Reducing the Use of Sulfur Dioxide in Wine. Microorganisms 2020, 8, 738. [Google Scholar] [CrossRef]
- García-Ruiz, A.; Cueva, C.; González-Rompinelli, E.; Yuste, M.; Torres, M.; Martín-Álvarez, P.J.; Bartolomé, B.; Moreno-Arribas, M. Antimicrobial phenolic extracts able to inhibit lactic acid bacteria growth and wine malolactic fermentation. Food Control 2012, 28, 212–219. [Google Scholar] [CrossRef]
- Christofi, S.; Malliaris, D.; Katsaros, G.; Panagou, E.; Kallithraka, S. Limit SO2 content of wines by applying High Hydrostatic Pressure. Inn. Food Sci. Emerg. Technol. 2020, 62, 102342. [Google Scholar] [CrossRef]
- Agarbati, A.; Canonico, L.; Ciani, M.; Comitini, F. Metschnikowia pulcherrima in Cold Clarification: Biocontrol Activity and Aroma Enhancement in Verdicchio Wine. Fermentation 2023, 9, 302. [Google Scholar] [CrossRef]
- Giménez, P.; Just-Borras, A.; Pons, P.; Gombau, J.; Heras, J.; Sieczkowski, N.; Canals, J.; Zamora, F. Biotechnological tools for reducing the use of sulfur dioxide in white grape must and preventing enzymatic browning: Glutathione; inactivated dry yeasts rich in glutathione; and bioprotection with Metschnikowia pulcherrima. Eur. Food Res. Technol. 2023, 249, 1491–1501. [Google Scholar] [CrossRef]
- Puyo, M.; Simonin, S.; Klein, G.; David-vaizant, V.; Quijada-mor, N.; Alexandre, H.; Tourdot-Maréchal, R. Use of Oenological Tannins to Protect the Colour of Rosé Wine in a Bioprotection Strategy with Metschnikowia pulcherrima. Foods 2023, 12, 735. [Google Scholar] [CrossRef] [PubMed]
- Howe, P.A.; Worobo, R.; Sacks, G.L. Conventional Measurements of Sulfur Dioxide (SO2) in Red Wine Overestimate SO2 Antimicrobial Activity. Am. J. Enol. Vitic. 2018, 69, 210–220. [Google Scholar] [CrossRef]
- Lisanti, M.T.; Blaiotta, G.; Nioi, C.; Moio, L. Alternative Methods to SO2 for Microbiological Stabilization of Wine. Compr. Rev. Food Sci. Food Saf. 2019, 18, 455–479. [Google Scholar] [CrossRef] [PubMed]
- Giacosa, S.; Río, S.; Cagnasso, E.; Caudana, A.; Rolle, L.; Gerbi, V. SO2 in Wines: Rational Use and Possible Alternatives. In Red Wine Technology; Morata, A., Ed.; Academic Press: London, UK, 2019; pp. 309–321. [Google Scholar] [CrossRef]
- Chacon-Rodriguez, L.; Joseph, C.; Nazaris, B.; Coulon, J.; Richardson, S.; Dycus, D.A. Innovative Use of Non-Saccharomyces in Bio-Protection: T. delbrueckii and M. pulcherrima Applied to a Machine Harvester. Catalyst 2020, 4, 82–90. [Google Scholar] [CrossRef]
- Windholtz, S.; Redon, P.; Lacampagne, S.; Farris, L.; Lytra, G.; Cameleyre, M.; Barbe, J.; Coulon, J.; Thibon, J.; Masneuf-Pomar, I. Non-Saccharomyces yeasts as bioprotection in the composition of red wine and in the reduction of sulfur dioxide. WT—Food Sci. Technol. 2021, 149, 111781. [Google Scholar] [CrossRef]
- Di Gianvito, P.; Englezos, V.; Rantsiou, K.; Cocolin, L. International Journal of Food Microbiology Bioprotection strategies in winemaking. Int. J. Food Microbiol. 2022, 364, 109532. [Google Scholar] [CrossRef] [PubMed]
- Canonico, L.; Solomon, M.; Comitini, F.; Ciani, M.; Varela, C. Volatile profile of reduced alcohol wines fermented with selected non-Saccharomyces yeasts under different aeration conditions. Food Microbiol. 2019, 84, 103247. [Google Scholar] [CrossRef]
- Windholtz, S.; Nioi, C.; Coulon, J.; Masneuf-Pomarede, I. Bioprotection by non-Saccharomyces yeasts in oenology: Evaluation of O2 consumption and impact on acetic acid bacteria. Int. J. Food Microbiol. 2023, 405, 110338. [Google Scholar] [CrossRef]
- Maicas, S. Advances in wine fermentation. Fermentation 2021, 7, 187. [Google Scholar] [CrossRef]
- Yao, M.; Wang, F.; Arpentin, G. Bioprotection as a tool to produce natural wine: Impact on physicochemical and sensory analysis. BIO Web Conf. 2023, 56, 02019. [Google Scholar] [CrossRef]
- Canonico, L.; Agarbati, A.; Galli, E.; Comitini, F.; Ciani, M. Metschnikowia Pulcherrima as Biocontrol Agent and Wine Aroma Enhancer in Combination with a Native Saccharomyces cerevisiae. LWT 2023, 181, 114758. [Google Scholar] [CrossRef]
- Liu, S.; Laaksonen, O.; Yang, B. Volatile composition of bilberry wines fermented with non-Saccharomyces and Saccharomyces yeasts in pure, sequential and simultaneous inoculations. Food Microbiol. 2019, 80, 25–39. [Google Scholar] [CrossRef] [PubMed]
- Minnaar, P.P.; du Plessis, H.W.; Jolly, N.P.; van der Rijst, M.; du Toit, M. Non-Saccharomyces yeast and lactic acid bacteria in Co-inoculated fermentations with two Saccharomyces cerevisiae yeast strains: A strategy to improve the phenolic content of Syrah wine. Food Chem. X 2019, 4, 100070. [Google Scholar] [CrossRef] [PubMed]
- Xu, A.; Xiao, Y.; He, Z.; Liu, J.; Wang, Y.; Gao, B.; Chang, J.; Zhu, D. Use of Non-Saccharomyces Yeast Co-Fermentation with Saccharomyces cerevisiae to Improve the Polyphenol and Volatile Aroma Compound Contents in Nanfeng Tangerine Wines. J. Fungi 2022, 8, 128. [Google Scholar] [CrossRef] [PubMed]
- Yılmaz, C.; Gökmen, V. Formation of amino acid derivatives in white and red wines during fermentation: Effects of non-Saccharomyces yeasts and Oenococcus oeni. Food Chem. 2021, 343, 128415. [Google Scholar] [CrossRef] [PubMed]
- Oro, L.; Ciani, M.; Comitini, F. Antimicrobial activity of Metschnikowia pulcherrima on wine yeasts. J. Appl. Microbiol. 2014, 116, 1209–1217. [Google Scholar] [CrossRef] [PubMed]
- Maurivin. Awri 796. AB Biotek 2017. Available online: http://wine.abbiotek.com/perch/resources/maurivin-awri-796-product-information-may-2107-spanish-web.pdf (accessed on 16 January 2024).
- Zhang, B.; Ivanova-Petropulos, V.; Duan, C.; Yan, G. Distinctive chemical and aromatic composition of red wines produced by Saccharomyces cerevisiae co-fermentation with indigenous and commercial non-Saccharomyces strains. Food Biosci. 2021, 41, 100925. [Google Scholar] [CrossRef]
- Azzolini, M.; Tosi, E.; Lorenzini, M.; Finato, F.; Zapparoli, G. Contribution to the aroma of white wines by controlled Torulaspora delbrueckii cultures in association with Saccharomyces cerevisiae. World J. Microbiol. Biotechnol. 2015, 31, 277–293. [Google Scholar] [CrossRef] [PubMed]
- Hu, K.; Jin, G.; Xu, Y.; Tao, Y. Wine aroma response to different participation of selected Hanseniaspora uvarum in mixed fermentation with Saccharomyces cerevisiae. Food Res. Int. 2018, 108, 119–127. [Google Scholar] [CrossRef]
- Ferreira, V.; Lopez, R. The actual and potential aroma of winemaking grapes. Biomolecules 2019, 9, 818. [Google Scholar] [CrossRef]
- Denat, M.; Dolores, P. The effects of Saccharomyces cerevisiae strains carrying alcoholic fermentation on the fermentative and varietal aroma profiles of young and aged Tempranillo wines. Food Chem. 2021, 9, 100116. [Google Scholar] [CrossRef] [PubMed]
- Echave, J.; Barral, M.; Fraga-Corral, M.; Prieto, M.A.; Simal-Gandara, J. Bottle Aging and Storage of Wines: A Review. Molecules 2021, 26, 713. [Google Scholar] [CrossRef] [PubMed]
- Benito, S.; Hofmann, T.; Laier, M.; Lochbühler, B.; Schüttler, A.; Ebert, K.; Fritsch, S.; Röcker, J.; Rauhut, D. Effect on quality and composition of Riesling wines fermented by sequential inoculation with non-Saccharomyces and Saccharomyces cerevisiae. Eur. Food Res. Technol. 2015, 241, 707–717. [Google Scholar] [CrossRef]
- Seguinot, P.; Ortiz-Julien, A.; Camarasa, C. Impact of Nutrient Availability on the Fermentation and Production of Aroma Compounds Under Sequential Inoculation with M. pulcherrima and S. cerevisiae. Front. Microbiol. 2020, 11, 305. [Google Scholar] [CrossRef] [PubMed]
- Vejarano, R.; Gil-Calderón, A. Commercially available non-Saccharomyces yeasts for winemaking: Current market, advantages over Saccharomyces, biocompatibility, and safety. Fermentation 2021, 7, 171. [Google Scholar] [CrossRef]
- Zhang, B.; Shen, J.; Duan, C.; Yan, G. Use of Indigenous Hanseniaspora vineae and Metschnikowia pulcherrima Co-fermentation With Saccharomyces cerevisiae to Improve the Aroma Diversity of Vidal Blanc Icewine. Front. Microbiol. 2018, 9, 2303. [Google Scholar] [CrossRef]
- Chen, K.; Escott, C.; Loira, I.; Del Fresno, J.; Morata, A.; Tesfaye, W.; Calderon, F.; Suarez-Lepe, J.A.; Han, S.; Benito, S.; et al. Use of non-Saccharomyces yeasts and oenological tannin in red winemaking: Influence on colour, aroma and sensorial properties of young wines. Food Microbiol. 2018, 69, 51–63. [Google Scholar] [CrossRef]
- Oliveira, I.; Ferreira, V. Modulating Fermentative, Varietal and Aging Aromas of Wine Using non-Saccharomyces Yeasts in a Sequential Inoculation Approach. Microorganisms 2019, 7, 164. [Google Scholar] [CrossRef] [PubMed]
- Yan, G.; Zhang, B.; Joseph, L.; Waterhouse, A.L. Effects of initial oxygenation on chemical and aromatic composition of wine in mixed starters of Hanseniaspora vineae and Saccharomyces cerevisiae. J. Food Microbiol. 2020, 90, 103460. [Google Scholar] [CrossRef]
- OIV. Compendium of Internationals Methods of Wine and Must Analysis; OIV: Paris, France, 2009. [Google Scholar]
- Garde-Cerdán, T.; Sáenz de Urturi, I.; Murillo-Peña, R.; Iribarren, M.; Marín-San Román, S.; Rubio-Bretón, P.; Pérez-Álvarez, E.P. Bottle Aging Affected Aromatic and Phenolic Wine Composition More Than Yeast Starter Strains. Appl. Sci. 2022, 12, 4478. [Google Scholar] [CrossRef]
- Garde-Cerdán, T.; Gutiérrez-Gamboa, G.; Ayestarán, B.; González-Lázaro, M.; Rubio-Bretón, P.; Pérez-Álvarez, E.P. Influence of seaweed foliar application to Tempranillo grapevines on grape and wine phenolic compounds over two vintages. Food Chem. 2021, 345, 128843. [Google Scholar] [CrossRef] [PubMed]
- 332a/2009; OIV Standard for International Wine and Spirituous Beverages of Vitivinicultural Origin Competitions. International Organisation of Vine and Wine: Paris, France, 2009.
- Prior, K.J.; Bauer, F.F.; Divol, B. The utilizationtion of nitrogenous compounds by commercial non-Saccharomyces yeasts associated with wine. Food Microbiol. 2019, 79, 75–84. [Google Scholar] [CrossRef] [PubMed]
- Escribano, R.; González-Arenzana, L.; Portu, J.; Garijo, P.; López-Alfaro, I.; López, R.; Santamaría, P.; Gutiérrez, A.R. Wine Aromatic Compound Production and Fermentative Behaviour within Different Non- Saccharomyces Species and Clones. J. Appl. Microbiol. 2018, 124, 1521–1531. [Google Scholar] [CrossRef] [PubMed]
- Garde-Cerdán, T.; Rubio-Bretón, P.; Marín-San Román, S.; Baroja, E.; Sáenz de Urturi, I.; Pérez-Álvarez, E.P. Study of wine volatile composition of Tempranillo versus Tempranillo Blanco, a new white grape variety. Beverages 2021, 7, 72. [Google Scholar] [CrossRef]
- Pérez-Álvarez, E.P.; Sáenz de Urturi, I.; Rubio-Bretón, P.; Marín-San Román, S.; Murillo-Peña, R.; Parra-Torrejón, B.; Ramírez-Rodríguez, G.B.; Delgado-López, J.M.; Garde-Cerdán, T. Application of Elicitors, as Conventional and Nano Forms, in Viticulture: Effects on Phenolic, Aromatic and Nitrogen Composition of Tempranillo Wines. Beverages 2022, 8, 56. [Google Scholar] [CrossRef]
- Garde-Cerdán, T.; Jarauta, I.; Salinas, M.R.; Ancín-Azpilicueta, C. Comparative study of the volatile composition in wines obtained from traditional vinification and from the Ganimede method. J. Sci. Food Agric. 2008, 88, 1777–1785. [Google Scholar] [CrossRef]
- Gambetta, J.M.; Elaine, S.; Bastian, P.; Cozzolino, D.; Jeffery, D.W. Factors Influencing the Aroma Composition of Chardonnay Wines. J. Agric. Food Chem. 2014, 62, 6512–6534. [Google Scholar] [CrossRef] [PubMed]
- Contreras, A.; Hidalgo, C.; Henschke, P.A.; Chambers, P.J.; Curtin, C.; Varela, C. Evaluation of Non-Saccharomyces Yeasts for the Reduction of Alcohol Content in Wine. Appl. Environm. Microbiol. 2014, 80, 1670–1678. [Google Scholar] [CrossRef]
- Varela, C.; Bartel, C.; Espinase Nandorfy, D.; Bilogrevic, E.; Tran, T.; Heinrich, A.; Balzan, T.; Bindon, K.; Borneman, A. Volatile aroma composition and sensory profile of Shiraz and Cabernet Sauvignon wines produced with novel Metschnikowia pulcherrima yeast starter cultures. Aust. J. Grape Wine Res. 2021, 27, 406–418. [Google Scholar] [CrossRef]
| End of MLF | 6 Months in Bottles | 9 Months in Bottles | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| SO2+Sc | n-Sc10+Sc | SO2+n-Sc50+Sc | n-Sc10+n-Sc50+Sc | SO2+Sc | n-Sc10+Sc | SO2+n-Sc50+Sc | n-Sc10+n-Sc50+Sc | SO2+Sc | n-Sc10+Sc | SO2+n-Sc50+Sc | n-Sc10+n-Sc50+Sc | |
| Alcohol degree (% v/v) | 13.75 ± 0.07 b | 13.23 ± 0.25 a | 13.05 ± 0.00 a | 13.20 ± 0.00 a | - | - | - | - | - | - | - | - |
| pH | 4.04 ± 0.06 a | 3.95 ± 0.01 a | 4.19 ± 0.03 a | 4.11 ± 0.04 a | - | - | - | - | - | - | - | - |
| Total acidity (g/L) * | 5.10 ± 0.11 b | 5.23 ± 0.13 b | 3.88 ± 0.19 a | 3.94 ± 0.11 a | - | - | - | - | - | - | - | - |
| Malic acid (g/L) | n.d. | n.d. | n.d. | n.d. | - | - | - | - | - | - | - | - |
| Lactic acid (g/L) | 2.62 ± 0.10 c | 2.81 ± 0.01 b | 2.29 ± 0.02 a | 2.35 ± 0.19 a | - | - | - | - | - | - | - | - |
| Volatile acidity (g/L) ** | 0.50 ± 0.02 a | 0.46 ± 0.06 a | 0.51 ± 0.06 a | 0.48 ± 0.11 a | 0.46 ± 0.0 a | 0.46 ± 0.06 a | 0.52 ± 0.00 a | 0.49 ± 0.15 a | 0.49 ± 0.04 a | 0.47 ± 0.10 a | 0.56 ± 0.08 a | 0.50 ± 0.16 a |
| YAN (mg N/L) | 9 ± 2 a | 8 ± 3 a | 31 ± 31 a | 11 ± 7 a | - | - | - | - | - | - | - | - |
| OD 420 nm | 0.24 ± 0.01 a | 0.23 ± 0.03 a | 0.21 ± 0.00 a | 0.21 ± 0.00 a | 0.28 ± 0.02 a | 0.27 ± 0.03 a | 0.25 ± 0.00 a | 0.25 ± 0.00 a | 0.30 ± 0.02 ab | 0.29 ± 0.03 b | 0.26 ± 0.01 a | 0.27 ± 0.01 a |
| OD 520 nm | 0.30 ± 0.02 a | 0.30 ± 0.05 a | 0.23 ± 0.01 a | 0.25 ± 0.01 a | 0.36 ± 0.04 a | 0.35 ± 0.06 a | 0.30 ± 0.00 a | 0.31 ± 0.01 a | 0.37 ± 0.05 b | 0.37 ± 0.06 b | 0.30 ± 0.01 a | 0.33 ± 0.00 a |
| OD 620 nm | 0.07 ± 0.00 a | 0.06 ± 0.01 a | 0.05 ± 0.00 a | 0.05 ± 0.00 a | 0.11 ± 0.01 a | 0.10 ± 0.02 a | 0.10 ± 0.00 a | 0.10 ± 0.00 a | 0.12 ± 0.01 ab | 0.12 ± 0.01 b | 0.11 ± 0.00 a | 0.11 ± 0.00 a |
| Colour intensity (CI) | 6.11 ± 0.27 a | 5.85 ± 0.88 a | 4.92 ± 0.11 a | 5.10 ± 0.06 ab | 7.51 ± 0.74 a | 7.21 ± 1.10 a | 6.48 ± 0.08 a | 6.64 ± 0.16 a | 8.00 ± 0.74 a | 7.73 ± 1.01 a | 6.75 ± 0.20 a | 7.15 ± 0.01 a |
| TPI | 43.70 ± 0.69 a | 41.66 ± 4.02 a | 42.45 ± 0.66 a | 41.40 ± 0.93 a | 43.40 ± 0.42 a | 41.50 ± 3.54 a | 42.65 ± 0.78 a | 40.75 ± 1.06 a | 42.04 ± 0.49 a | 40.15 ± 3.36 a | 41.58 ± 0.75 a | 39.96 ± 1.05 a |
| Total anthocyanins (mg/L) | 553.3 ± 15.2 a | 499.4 ± 67.1 a | 574.8 ± 27.4 a | 538.2 ± 6.1 a | 226.1 ± 11.1 a | 204.4 ± 35.9 a | 255.0 ± 24.4 a | 235.5 ± 5.5 a | 73.1 ± 0.3 ab | 66.1 ± 8.5 a | 87.3 ± 1.9 c | 84.5 ± 0.9 bc |
| Total phenols (mg/L) | 1718.6 ± 63.7 a | 1626.5 ± 48.9 a | 1812.2 ± 130 a | 1676.2 ± 70.4 a | 1625.3 ± 15.3 a | 1626.5 ± 146.1 a | 1656.3 ± 52.2 a | 1570.8 ± 70.2 a | 1536.8 ± 11.2 a | 1458.2 ± 122.8 a | 1505.3 ± 26.1 a | 1451.8 ± 19.9 a |
| Assay (A) | Moment (M) | |||||||
|---|---|---|---|---|---|---|---|---|
| SO2+Sc | n-Sc10+Sc | SO2+n-Sc50+Sc | n-Sc10+n-Sc50+Sc | MLF | 6 Months | 9 Months | Interaction (A × M) | |
| Alcohols | ||||||||
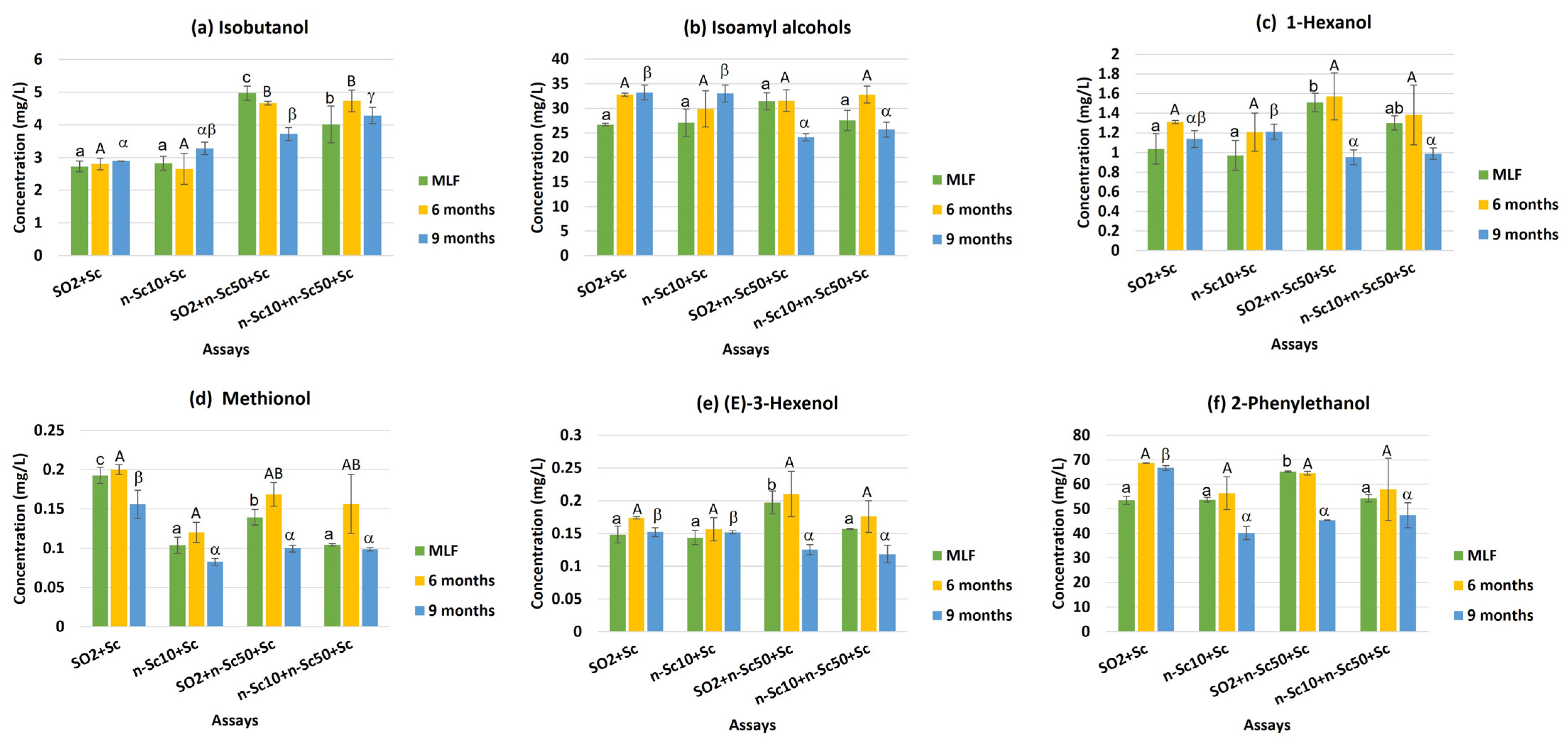
| Isobutanol | 2.81 a | 2.91 a | 4.45 b | 4.34 b | 3.63 a | 3.71 a | 3.54 a | ** |
| Isoamyl alcohols | 30.90 a | 30.00 a | 29.05 a | 28.67 a | 28.18 a | 31.76 b | 29.02 a | ** |
| 1-Hexanol | 1.16 ab | 1.13 a | 1.34 b | 1.22 ab | 1.20 a | 1.37 b | 1.07 a | * |
| Methionol | 0.18 c | 0.10 a | 0.14 b | 0.12 ab | 0.14 b | 0.16 c | 0.11 a | N.S. |
| (E)-3-Hexenol | 0.16 ab | 0.15 a | 0.18 b | 0.15 a | 0.16 b | 0.18 c | 0.14 a | * |
| 2-Phenylethanol | 62.96 c | 50.09 a | 53.34 bc | 53.20 ab | 56.66 b | 61.88 c | 49.90 a | ** |
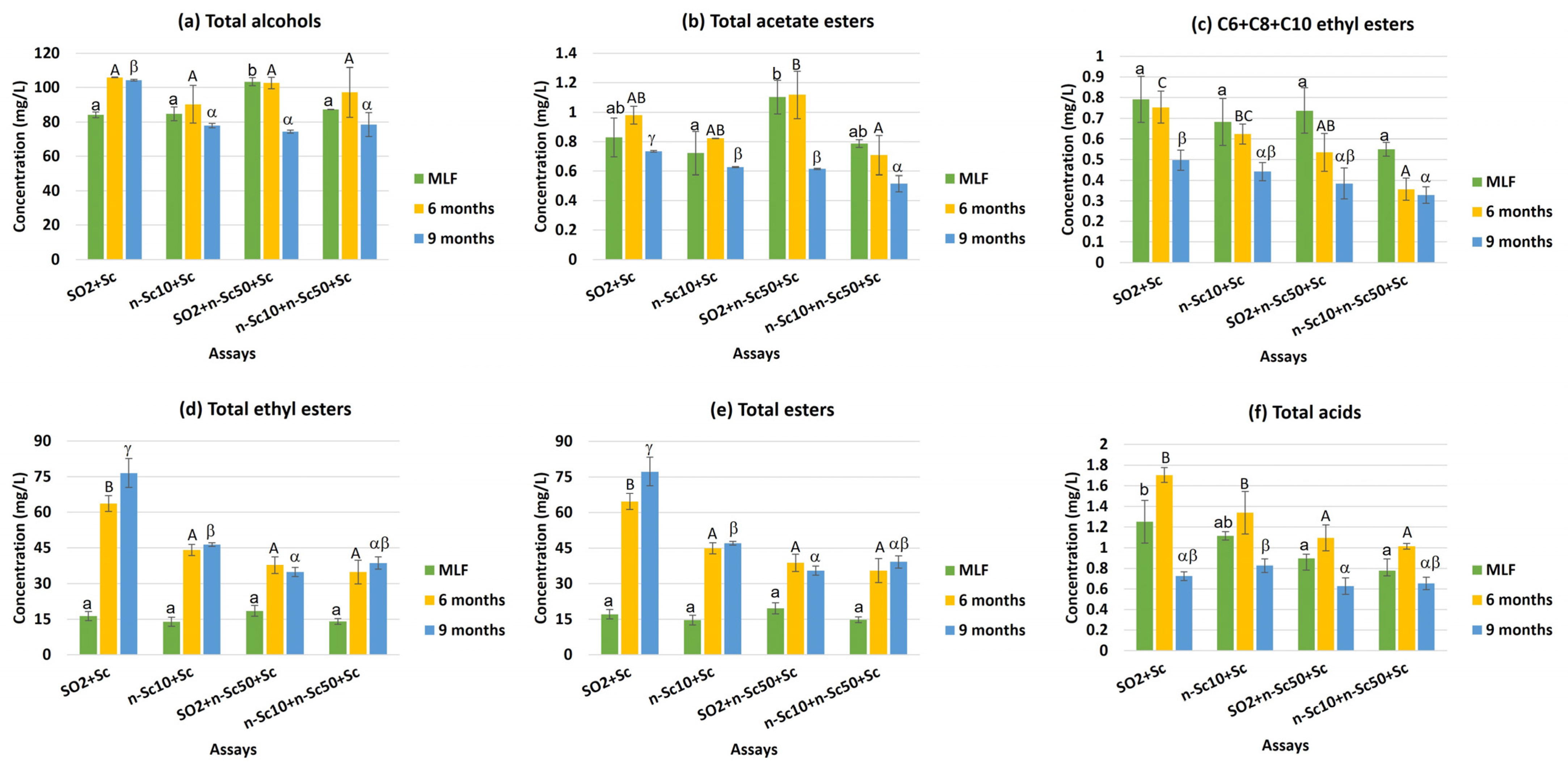
| Total alcohols | 98.16 c | 84.39 a | 93.50 bc | 87.71 ab | 89.97 a | 99.06 b | 83.78 a | ** |
| Esters | ||||||||
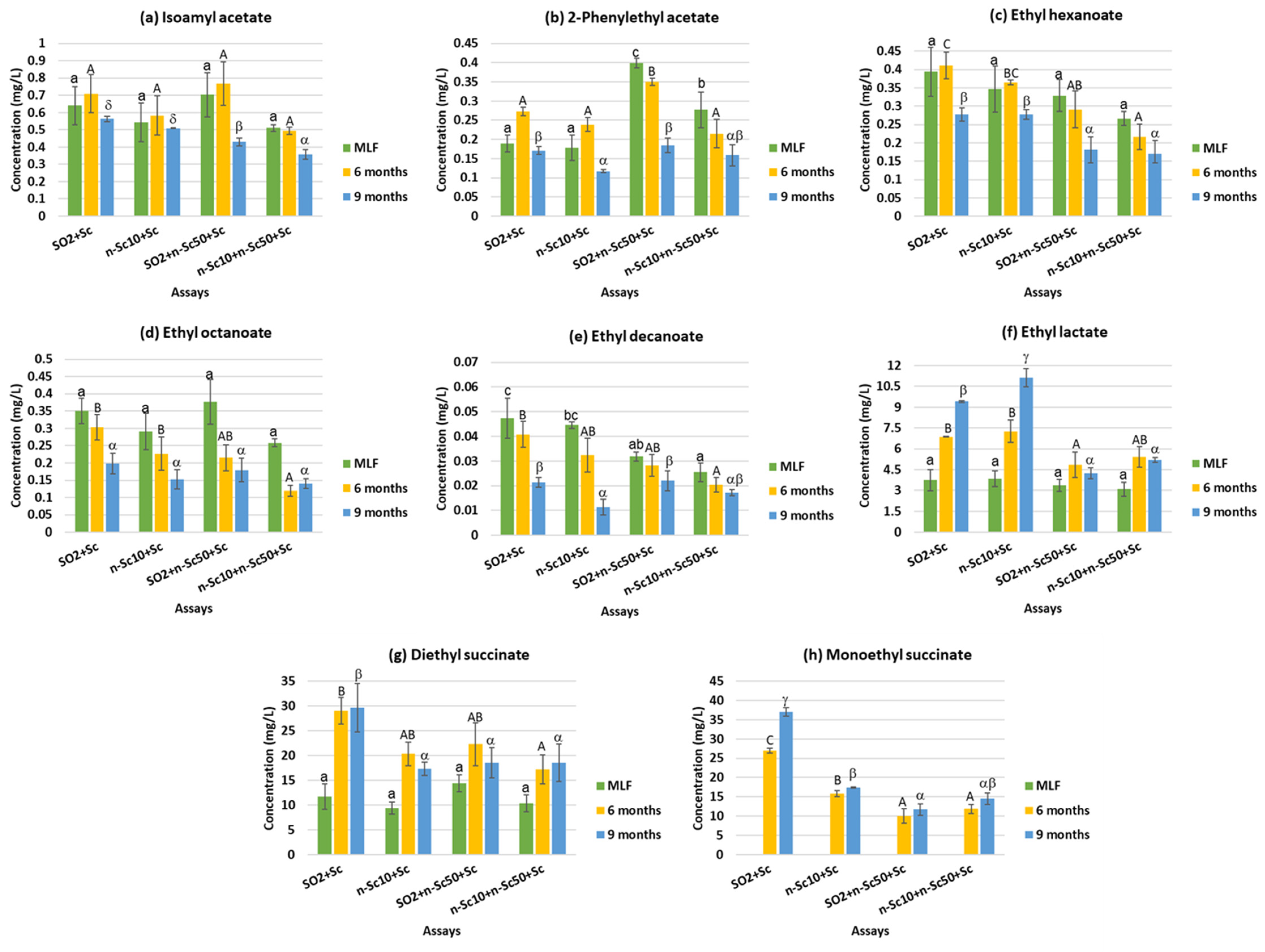
| Isoamyl acetate | 0.64 b | 0.54 ab | 0.63 b | 0.45 a | 0.60 b | 0.64 b | 0.46 a | N.S. |
| 2-Phenylethyl acetate | 0.21 b | 0.18 a | 0.31 c | 0.22 b | 0.26 b | 0.27 b | 0.16 a | *** |
| Total acetate esters | 0.85 b | 0.72 a | 0.94 b | 0.67 a | 0.86 b | 0.91 b | 0.62 a | N.S. |
| Ethyl hexanoate | 0.36 c | 0.33 c | 0.27 b | 0.22 a | 0.33 b | 0.32 b | 0.23 a | N.S. |
| Ethyl octanoate | 0.28 c | 0.22 b | 0.26 bc | 0.17 a | 0.32 c | 0.22 b | 0.17 a | N.S. |
| Ethyl decanoate | 0.04 c | 0.03 b | 0.03 b | 0.02 a | 0.04 c | 0.03 b | 0.02 a | ** |
| C6 + C8 + C10 ethyl esters | 0.68 c | 0.58 b | 0.55 b | 0.41 a | 0.69 c | 0.57 b | 0.41 a | N.S. |
| Ethyl lactate | 6.67 b | 7.42 c | 4.15 a | 4.58 a | 3.52 a | 6.10 b | 7.50 c | *** |
| Diethyl succinate | 23.47 b | 15.66 a | 18.41 a | 15.38 a | 11.47 a | 22.20 b | 21.02 b | N.S. |
| Monoethyl succinate | 32.00 d | 16.68 c | 10.87 a | 13.18 b | n.d. a | 16.20 b | 20.17 c | ** |
| Total ethyl esters | 52.16 c | 34.79 b | 30.36 a | 29.15 a | 15.67 a | 45.07 b | 49.10 c | *** |
| Total esters | 53.00 c | 35.51 b | 31.31 a | 29.82 a | 16.53 a | 45.98 b | 49.72 c | *** |
| Acids | ||||||||
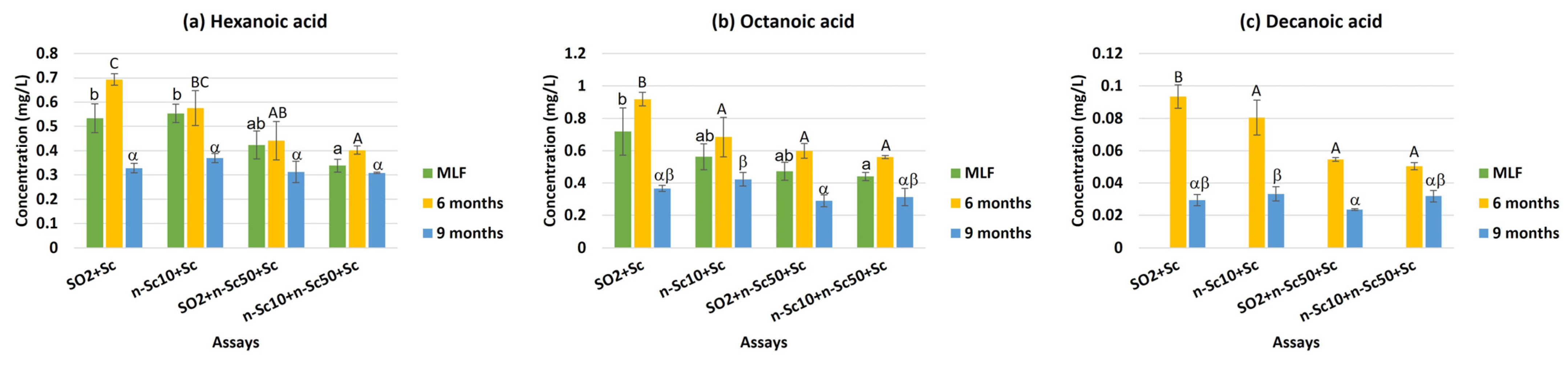
| Hexanoic acid | 0.52 b | 0.50 b | 0.39 a | 0.35 a | 0.46 b | 0.53 c | 0.33 a | * |
| Octanoic acid | 0.67 c | 0.56 b | 0.45 a | 0.44 a | 0.55 b | 0.69 c | 0.35 a | N.S. |
| Decanoic acid | 0.06 b | 0.06 b | 0.04 a | 0.04 a | n.d. a | 0.07 c | 0.03 b | *** |
| Total acids | 1.23 b | 1.09 b | 0.87 a | 0.82 a | 1.01 b | 1.29 c | 0.71 a | * |
| SO2+Sc | n-Sc10+Sc | SO2+n-Sc50+Sc | n-Sc10+n-Sc50+Sc | ||
|---|---|---|---|---|---|
| View | Cleannes | 3.95 ± 0.79 a | 4.13 ± 0.87 a | 3.85 ± 0.75 a | 4.04 ± 0.82 a |
| Colour | 7.62 ± 1.71 a | 7.65 ± 1.56 a | 6.99 ± 2.00 a | 7.73 ± 1.74 a | |
| Smell | Intensity | 6.06 ± 1.43 a | 6.31 ± 0.97 a | 6.17 ± 0.95 a | 6.00 ± 1.24 a |
| Frankness | 4.14 ± 0.77 a | 4.36 ± 0.71 a | 3.72 ± 0.98 a | 3.88 ± 1.10 a | |
| Quality | 12.55 ± 1.65 a | 12.80 ± 1.78 a | 11.43 ± 2.06 a | 12.02 ± 2.56 a | |
| Taste | Intensity | 6.03 ± 1.11 a | 6.05 ± 0.77 a | 5.99 ± 1.21 a | 6.06 ± 1.15 a |
| Frankness | 4.16 ± 0.89 a | 4.17 ± 0.65 a | 3.96 ± 0.83 a | 4.01 ± 1.00 a | |
| Quality | 16.15 ± 2.71 a | 16.37 ± 2.29 a | 14.96 ± 2.79 a | 16.01 ± 2.86 a | |
| Persistence | 6.17 ± 1.04 a | 6.34 ± 0.83 a | 6.22 ± 0.95 a | 6.31 ± 0.93 a | |
| Harmony | 8.98 ± 0.90 a | 9.20 ± 0.59 a | 8.67 ± 0.93 a | 8.97 ± 0.88 a | |
| Total valuation | 75.61 ± 10.04 a | 76.97 ± 9.10 a | 71.97 ± 10.41 a | 75.02 ± 11.52 a | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Torres-Díaz, L.L.; Murillo-Peña, R.; Iribarren, M.; Sáenz de Urturi, I.; Marín-San Román, S.; González-Lázaro, M.; Pérez-Álvarez, E.P.; Garde-Cerdán, T. Exploring Metschnikowia pulcherrima as a Co-Fermenter with Saccharomyces cerevisiae: Influence on Wine Aroma during Fermentation and Ageing. Beverages 2024, 10, 26. https://doi.org/10.3390/beverages10020026
Torres-Díaz LL, Murillo-Peña R, Iribarren M, Sáenz de Urturi I, Marín-San Román S, González-Lázaro M, Pérez-Álvarez EP, Garde-Cerdán T. Exploring Metschnikowia pulcherrima as a Co-Fermenter with Saccharomyces cerevisiae: Influence on Wine Aroma during Fermentation and Ageing. Beverages. 2024; 10(2):26. https://doi.org/10.3390/beverages10020026
Chicago/Turabian StyleTorres-Díaz, Lesly L., Rebeca Murillo-Peña, Miquel Iribarren, Itziar Sáenz de Urturi, Sandra Marín-San Román, Miriam González-Lázaro, Eva P. Pérez-Álvarez, and Teresa Garde-Cerdán. 2024. "Exploring Metschnikowia pulcherrima as a Co-Fermenter with Saccharomyces cerevisiae: Influence on Wine Aroma during Fermentation and Ageing" Beverages 10, no. 2: 26. https://doi.org/10.3390/beverages10020026
APA StyleTorres-Díaz, L. L., Murillo-Peña, R., Iribarren, M., Sáenz de Urturi, I., Marín-San Román, S., González-Lázaro, M., Pérez-Álvarez, E. P., & Garde-Cerdán, T. (2024). Exploring Metschnikowia pulcherrima as a Co-Fermenter with Saccharomyces cerevisiae: Influence on Wine Aroma during Fermentation and Ageing. Beverages, 10(2), 26. https://doi.org/10.3390/beverages10020026







