Brewing Mainly from Stale Bread: A Pale Ale Case Study
Abstract
1. Introduction
2. Materials and Methods
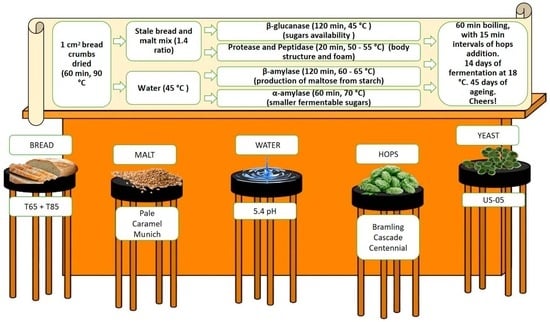
2.1. Production and Materials
2.1.1. Equipment
2.1.2. Ingredients
2.1.3. Process
2.2. Analytic Methods
2.2.1. Soluble Solid Content
2.2.2. pH Measurement
2.2.3. Analysis of Volatile Compounds
2.2.4. Analysis of Sugars and Main Metabolites
2.3. Qualitative Methods
2.3.1. Panel Theoretical Background
2.3.2. Sensorial Analysis
2.4. Statistical Analysis
3. Results and Discussion
3.1. °Brix and pH
3.2. Volatile Compounds
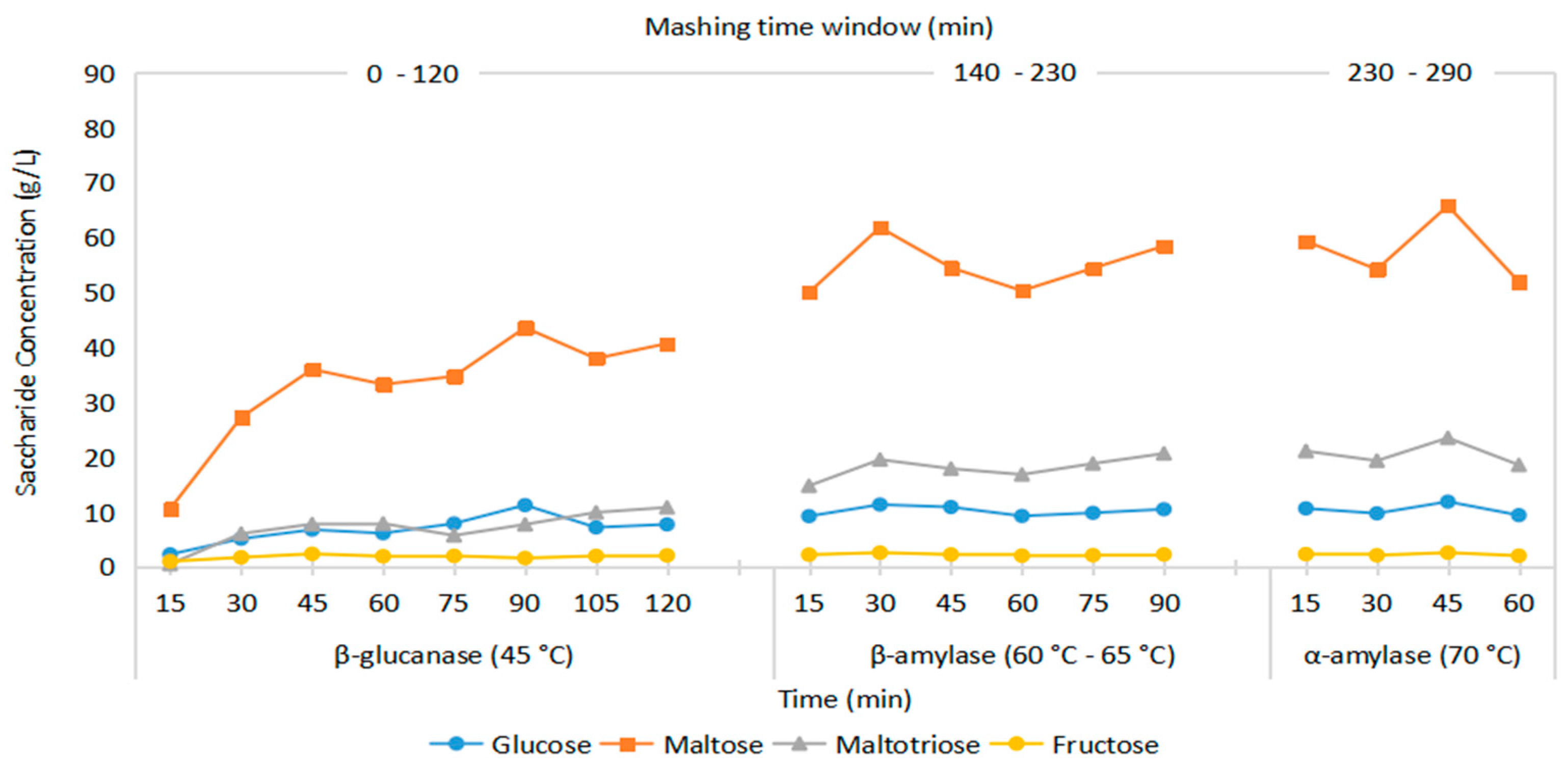
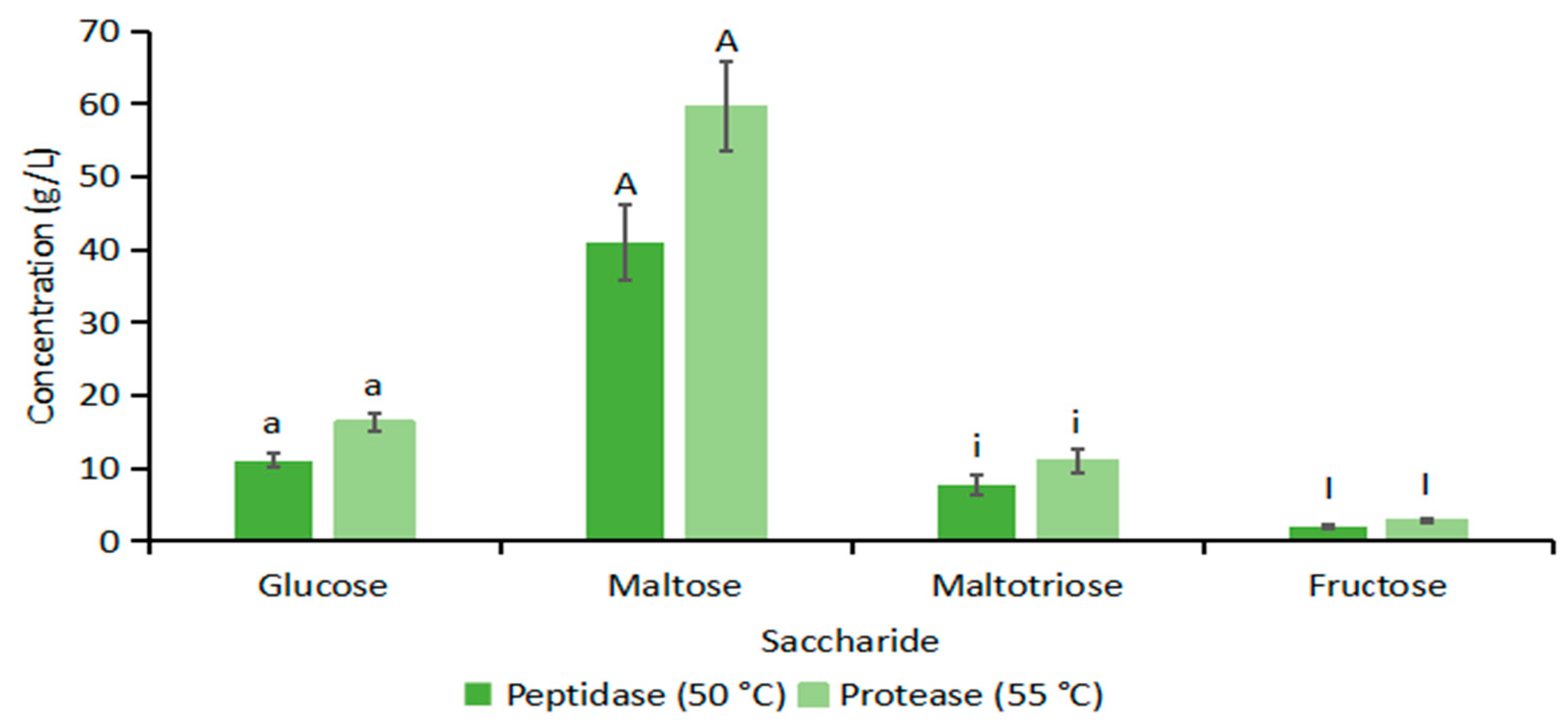
3.3. Saccharide and Fermentation Metabolite Level
3.3.1. Glucanases and Penteases
3.3.2. Amylases
3.3.3. Wort, Green Beer, and Final Beer
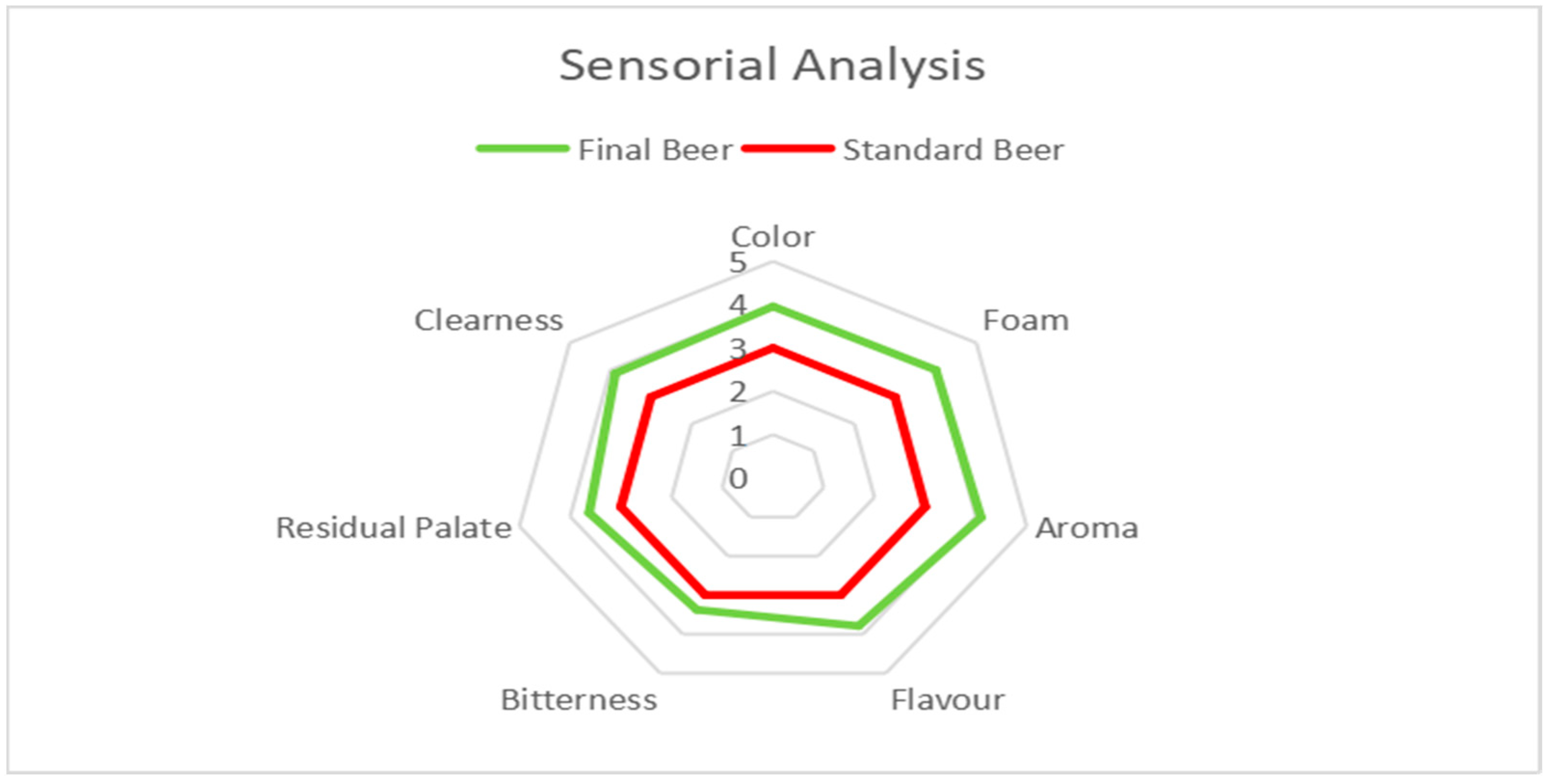
3.4. Sensory Analysis
3.4.1. Theoretical Knowledge Data
3.4.2. Sensory Analysis Feedback
4. Final Remarks
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ellen Macarthur Foundation. Reinventing a Regenerative Food System. In Growth Within: A Circular Economy Vision for a Competitive Europe; McKinsey Center for Business and Environment: New York, NY, USA, 2015. [Google Scholar]
- European Commission. A New Circular Economy Action Plan for a Cleaner and More Competitive Europe; European Commission: Brussels, Belgium, 2020. [Google Scholar]
- Carocho, M.; Morales, P.; Ciudad-Mulero, M.; Fernández-Ruiz, V.; Ferreira, E.; Heleno, S.; Rodrigues, P.; Barros, L.; Ferreira, I. Comparison of different bread types: Chemical and physical parameters. Food Chem. 2020, 310, 125954. [Google Scholar] [CrossRef] [PubMed]
- Gebski, J.; Zychowicz, M.; Szlachciuk, J.; Gebska, M. Impact of nutritional claims on consumer preferences for bread with varied fiber and salt content. Food Qual. Prefer. 2019, 76, 91–99. [Google Scholar] [CrossRef]
- Riaukaite, J.; Basinskiene, L.; Syrpas, M. Bioconversion of Waste Bread to Glucose Fructose Syrup as a Value-Added Product. In Foodbalt 2019, Proceedings of the 13th Baltic Conference on Food Science and Technology “FOOD, NUTRITION, WELL-BEING”, Jelgavia, Latvia, 2–3 May 2019; Straumite, E., Ed.; Latvia University of Life Sciences and Technologies: Jelgavia, Latvia, 2019; Volume 12, pp. 120–124. [Google Scholar]
- Torabi, S.; Satari, B.; Beygi, S. Process optimization for dilute acid and enzymatic hydrolysis of waste wheat bread and its effect aflatoxin rate and ethanol production. Biomass Convers. Biorefinery 2020, 11, 2617–2625. [Google Scholar] [CrossRef]
- Narisetty, V.; Cox, R.; Willoughby, N.; Aktas, E.; Tiwari, B.; Matharu, A.S.; Salonitis, K.; Kumar, V. Recycling bread waste into chemical building blocks using a circular biorefining approach. Sustain. Energy Fuels 2021, 5, 4842–4849. [Google Scholar] [CrossRef] [PubMed]
- Demirci, A.S.; Palabriyik, I.; Gümüs, T.; Özalp, Ş. Waste Bread as a Biomass Source: Optimization of Enzymatic Hydrolysis and Relation between Rheological Behavior and Glucose Yield. Waste Biomass Valor. 2017, 8, 775–782. [Google Scholar] [CrossRef]
- Lemaire, A. Circular Economy: Best Practices and Future Perspectives for the Beer Brewing Industry. Master’s Thesis, Louvain School of Management Université catholique de Louvain, Louvain-la-Neuve, Wallonia, Belgium, 2020. [Google Scholar]
- Schmidt, C.; Mouritsen, O. The Solution to Sustainable Eating Is Not a One-Way Street. Front. Psychol. 2020, 11, 531. [Google Scholar] [CrossRef] [PubMed]
- TOAST Here’s to Change. Impact Report 2022; Toast Ale: London, UK, 2023. [Google Scholar]
- Pietrzak, W.; Kawa-Rygielska, J. Simultaneous saccharification and ethanol fermentation of waste wheat-rye bread at very high solids loading: Effect of enzymatic liquefaction condition. Fuel 2017, 147, 236–242. [Google Scholar] [CrossRef]
- Sigüenza-Andrés, T.; Pando, V.; Gómez, M.; Rodríguez-Nogales, J.M. Optimization of a Simultaneous Enzymatic Hydrolysis to Obtain a High-Glucose Slurry from Bread Waste. Foods 2022, 11, 1793. [Google Scholar] [CrossRef] [PubMed]
- Dymchenko, A.; Geršl, M.; Gregor, T. The Perspective of Circular Food Waste Management in the Combined Case of Bakery and Brewery. In Zero Waste Management and Circular Economy, Proceedings of the International Symposium ICOM 2021, Prague Congress Center, Prague, Czech Republic, 25–27 August 2021; Mendelova Universita v Brne: Brno, Czech Republic, 2021; pp. 129–135. [Google Scholar]
- Martin-Lobera, C.; Aranda, F.; Lozano-Martinez, P.; Caballero, I.; Blanco, C.A. Bread as a Valuable Raw Material in Craft Ale Beer Brewing. Foods 2022, 11, 3013. [Google Scholar] [CrossRef]
- Almeida, J.; Thomas, J.; Murphy, K.; Griffiths, R.; Bengtsson, J. Circular Brew: Life cycle assessment of waste bread-based beer. In Proceedings of the 11th International Conference on Life Cycle Assessment of Food in conjuction with the 6th LCA AgriFood Asia and the 7th International Conference on Green and Sustainable Innovation, Bangkok, Thailand, 16–20 October 2018. [Google Scholar]
- Brancoli, P.; Bolton, K.; Erikkson, M. Environmental impacts of waste management and valorisation pathways for surplus bread in Sweden. Waste Manag. 2020, 117, 136–145. [Google Scholar] [CrossRef]
- Stanca, A.; Gianinetti, A.; Rizza, F.; Terzi, V. Barley: An Overview of a Versatile Cereal Grain with Many Food and Feed Uses. In Encyclopedia of Food Grains: The World of Food Grains, 2nd ed.; Wrigley, C., Corke, H., Seetharaman, K., Faubion, J., Eds.; Academic Press: Cambridge, MA, USA, 2016; Volume 1, pp. 147–152. [Google Scholar]
- Zhang, C.; Yang, Y.; Feng, Z.; Xiao, C.; Lang, T.; Du, W.; Liu, Y. Risk of Global External Cereals Supply under the Background of the COVID-19 Pandemic: Based on the Perspective of Trade Network. Foods 2021, 10, 1168. [Google Scholar] [CrossRef] [PubMed]
- Hassen, T.B.; Bilali, H.E. Impacts of the Russia-Ukraine War on Global Food Security: Towards More Sustainable and Resilient Food Systems? Foods 2022, 11, 2301. [Google Scholar] [CrossRef] [PubMed]
- Dymchenko, A.; Geršl, M.; Gregor, T. Trends in bread waste utilisation. Trends Food Sci. Technol. 2023, 132, 93–102. [Google Scholar] [CrossRef]
- Toast Ale. Homebrew Recipe Pale Ale; Toast Ale: London, UK, 2021. [Google Scholar]
- Palmer, J.; Kaminski, C. Water: A Comprehensive Guide for Brewers, 1st ed.; Brewers Publications: Boulders, CO, USA, 2013. [Google Scholar]
- Eumann, M. Water in Brewing. In Brewing: New Technologies, 1st ed.; Bamforth, C.W., Ed.; Woodhead Publishing: Cambridge, UK, 2006. [Google Scholar]
- Durello, R.S.; Silva, L.M.; Bogusz, S. Química do Lúpulo. Química Nova 2019, 42, 900–919. [Google Scholar] [CrossRef]
- Thesseling, F.A.; Bircham, P.W.; Mertens, S.; Voordeckers, K.; Verstrepen, K.J. A hands-on guide to brewing and analyzing beer in the laboratory. Curr. Protoc. Microbiol. 2019, 54, e91. [Google Scholar] [CrossRef] [PubMed]
- Parra, G.; Echevarria, M.; Perez, F. Beer Volatile Analysis: Optimization of HS/SPME Coupled to GC/MS/FID. J. Food Sci. 2011, 76, 205–211. [Google Scholar]
- Silva, G.; Augusto, F.; Poppi, R. Exploratory analysis of the volatile profile of beer by HS-SPME-GC. Food Chem. 2008, 111, 1057–1063. [Google Scholar] [CrossRef]
- Das, A.; Khawas, P.; Miyaji, T.; Deka, S. HPLC and GC-MS analyses of organic acids, carbohydrates, amino acids and volatile aromatic compounds in some varieties of rice beer from northeast India. J. Inst. Brew. 2014, 120, 244–252. [Google Scholar] [CrossRef]
- Castellari, M.; Sartini, E.; Spinabelli, U.; Riponi, C.; Galassi, S. Determination of Carboxylic Acids, Carbohydrates, Glycerol, Ethanol, and 5-HMF in Beer by High-Performance Liquid Chromatography and UV-Refractive Index Double Detection. J. Chromatogr. Sci. 2001, 39, 235–238. [Google Scholar] [CrossRef]
- Carvalho, N.; Minim, L.; Nascimento, M.; Ferreira, G.; Minim, V. Characterization of the consumer market and motivations for the consumption of craft beer. Br. Food J. 2018, 120, 378–391. [Google Scholar] [CrossRef]
- Kemp, S.E.; Hollowood, T.; Hort, J. Sensory Evaluation: A Practical Handbook; Wiley-Blackwell: Hoboken, NJ, USA, 2009. [Google Scholar]
- Barnes, Z.C. Brewing Process Control. In Handbook of Brewing, 2nd ed.; Priest, F.G., Stewart, G.G., Eds.; Taylor & Francis: Oxfordshire, UK, 2006; pp. 448–449. [Google Scholar]
- Betancur, M.I.; Motoki, K.; Spence, C.; Velasco, C. Factors influencing the choice of beer: A review. Food Res. Int. 2020, 137, 109367. [Google Scholar] [CrossRef] [PubMed]
- Viader, R.P.; Yde, M.S.H.; Hartvig, J.W.; Pagenstecher, M.; Carlsen, J.B.; Christensen, T.B.; Andersen, M.L. Optimization of Beer Brewing by Monitoring α-Amylase and β-Amylase Activities during Mashing. Beverages 2021, 7, 13. [Google Scholar] [CrossRef]
- Willaert, R. The Beer Brewing Process: Wort Production and Beer Fermentation. In Handbook of Food Products Manufacturing: Principles, Bakery, Beverages, Cereals, Cheese, Confectionary, Fats, Fruits, and Functional Foods, 1st ed.; Hui, Y.H., Ed.; Jon Wiley & Sons, Inc: New York, NY, USA, 2007. [Google Scholar]
- Langenaeken, N.A.; Schepper, C.F.; Schutter, D.P.; Courtin, C.M. Carbohydrate content and structure during malting and brewing: A mass balance study. J. Inst. Brew. 2020, 126, 253–262. [Google Scholar] [CrossRef]
- Choi, J.; Kang, J.; Rahman, A.; Lee, S. Increasing fermentable sugar yields by high- pressure treatment during beer mashing. Inst. Brew. Distill. 2016, 122, 143–146. [Google Scholar] [CrossRef]
- Denault, L.J.; Glenister, P.R.; Chau, S. Enzymology of the Mashing Step during Beer Production. J. Am. Soc. Brew. Chem. Sci. Beer 2018, 39, 46–52. [Google Scholar] [CrossRef]
- Guyot-Declerck, C.; François, N.; Ritter, C.; Govaerts, B.; Collin, S. Influence of pH and ageing on beer organoleptic properties. A sensory analysis based on AEDA data. Food Qual. Prefer. 2005, 16, 157–162. [Google Scholar] [CrossRef]
- Rodamn, A.D.; Gerogiorgis, D.I. Multi-objective process optimisation of beer fermentation via dynamic simulation. Food Bioprod. Process. 2016, 100, 255–274. [Google Scholar] [CrossRef]
- Angelino, S. Volatiles in Beer. In Volatiles Compounds in Food and Beverages, 1st ed.; Maarse, H., Ed.; Marcel Dekker, Inc.: New York, NY, USA, 1991. [Google Scholar]
- Olaniran, A.; Hiralal, L.; Mokoena, M.; Pillay, B. Flavour-active volatile compounds in beer: Production, regulation and control. J. Inst. Brew. 2017, 123, 13–23. [Google Scholar] [CrossRef]
- Kobayashi, M.; Shimizu, H.; Shioya, S. Beer Volatile Compounds and Their Application to Low-Malt Beer Fermentation. J. Biosci. Bioeng. 2008, 106, 317–323. [Google Scholar] [CrossRef]
- Johnson, J.A.; Linko, Y. Analysis of bread flour constituents. Qual. Plant. Et Mater. Veg. 1964, 11, 265–268. [Google Scholar] [CrossRef]
- Hansen, Å.; Hansen, B. Flavour of sourdough wheat bread crumb. Z. Für Lebensm. Unters. Und Forsch. 1996, 202, 244–249. [Google Scholar] [CrossRef]
- Gee, D.; Ramirez, W. A Flavour Model for Beer Fermentation. J. Inst. Brew. 1994, 100, 321–329. [Google Scholar] [CrossRef]
- Sammartino, M. Enzymes in Brewing; Master Brewers Association of the Americas Technical Quarterly: Chicago, IL, USA, 2015; Volume 52, pp. 156–164. [Google Scholar]
- Kong, X.; Zhou, H.; Qian, H. Enzymatic hydrolysis of wheat gluten by proteases and properties of the resulting hydrolysates. Food Chem. 2007, 102, 759–763. [Google Scholar] [CrossRef]
- Karnowski, M. Homebrew Beyond the Basics, 1st ed.; Sterling Publishing Co., Inc.: New York, NY, USA, 2018. [Google Scholar]
- Gomaa, A. Application of Enzymes in Brewing. J. Nutr. Food Sci. Forecast. 2018, 1, 1002. [Google Scholar]
- Ward, O.P. Proteases. Compr. Biotechnol. 2011, 1, 604–615. [Google Scholar]
- Benešová, K.; Běláková, S.; Mikulíková, R.; Svoboda, Z. Activity of Proteolytic Enzymes During Malting and Brewing. Kvas. Prum. 2017, 63, 2–7. [Google Scholar] [CrossRef]
- O’Rourke, T. The function of enzymes in brewing. BREWER Int. 2002, 9, 14–18. [Google Scholar]
- Alves, S.L.; Herberts, R.A.; Hollatz, C.; Trichez, D.; Miletti, L.C.; Araujo, P.S.; Stambuk, B.U. Molecular Analysis of Maltotriose Active Transport and Fermentation by Saccharomyces cerivisiae Reveals a Determinant Role for the AGT1 Permease. Appl. Environ. Microbiol. 2008, 74, 1494–1501. [Google Scholar] [CrossRef]
- Zhao, X.; Procopio, S.; Becker, T. Flavor impacts of glycerol in the processing of yeast fermented beverages: A review. J. Food Sci. Technol. 2015, 52, 7588–7598. [Google Scholar] [CrossRef]
- Monošík, R.; Magdolen, P.; Stredanský, M.; Šturdík, E. Monitoring of monosaccharides, oligosaccharides, ethanol and glycerol during wort fermentation by biosensors, HPLC and spectrophotometry. Food Chem. 2013, 138, 220–226. [Google Scholar] [CrossRef]
- Carley, S.; Yahng, L. Willingness-to-pay for sustainable beer. PLoS ONE 2018, 13, e0204917. [Google Scholar] [CrossRef] [PubMed]
- Rivaroli, S.; Kozák, V.; Spadoni, R. What motivates Czech and international “millennial-aged” university students to consume craft beers? Int. J. Wine Bus. Res. 2019, 31, 441–455. [Google Scholar] [CrossRef]
- Dymchenko, A.; Gerš, M.; Gregor, T. Brewing beer using bakery leftovers as a substitute for malt. Brew. Sci. 2023, 76, 1–10. [Google Scholar]
- Garavaglia, C.; Swinnen, J. The Craft Beer Revolution: An International Perspective. Choices 2017, 32, 1–8. [Google Scholar]
- Galanakis, C.M. The Future of Food. Foods 2024, 13, 506. [Google Scholar] [CrossRef] [PubMed]
- Hussain, M.A.; Bekhit, A. Innovative Foods: The Future of Food Supply, Nutrition and Health. Foods 2023, 12, 1359. [Google Scholar] [CrossRef] [PubMed]
- Ness, B. Beyond the Pale (Ale): An Exploration of the Sustainability Priorities and Innovative Measures in the Craft Beer Sector. Sustainability 2018, 10, 4108. [Google Scholar] [CrossRef]
- Breda, C.; Barros, A.I.; Gouvinhas, I. Characterization of bioactive compounds and antioxidant capacity of Portuguese craft beers. Int. J. Gastron. Food Sci. 2022, 27, 100473. [Google Scholar] [CrossRef]
- Silva, A.P.; Jager, G.; Zyl, H.V.; Voss, H.; Pintado, M.; Hogg, T.; Graaf, C.D. Cheers, proost, saúde: Cultural, contextual and psychological factors of wine and beer consumption in Portugal and in the Netherlands. Crit. Rev. Food Sci. Nutr. 2017, 57, 1340–1349. [Google Scholar] [CrossRef]
- Costa, P.; Franco, M. The role of cooperation networks in the craft beer business in Portugal: A collaborative entrepreneurship perspective. J. Gen. Manag. 2022, 1, 03063070221117923. [Google Scholar]
- Goode, D.L.; Halbert, C.; Arendt, E.K. Optimization of Mashing Conditions When Mashing with Unmalted Sorghum and Commercial Enzymes. J. Am. Soc. Brew. Chem. Sci. Beer 2003, 61, 69–78. [Google Scholar] [CrossRef]
- Economia e Transição Digital e Agricultura. Portaria 91/2022 de 9 de Fevereiro. Diário República 2022, 28, 4–7. [Google Scholar]
- Ekin, H.N.; Didem, D.O. Kvass: A Fermented Traditional Beverage. In Fermented Food Products, 1st ed.; Sankaranarayanan, A., Amaresan, N., Dhanasekaran, D., Eds.; CRC Press: Boca Raton, FL, USA, 2020. [Google Scholar]


| Addition Time Window during BOILING | Hops (α-Acids Content) | ||
|---|---|---|---|
| Bramling Cross (6.80%) | Cascade (5.75%) | Centennial (9%) | |
| 0 min | 0 g | 20 g | 5 g |
| 15 min | 20 g | 0 g | 5 g |
| 30 min | 15 g | 10 g | 0 g |
| 45 min | 0 g | 10 g | 5 g |
| 60 min | 10 g | 5 g | 0 g |
| Compounds | Boiling | Fermentation | Ageing |
|---|---|---|---|
| CO2 | n.d. | 14.01 | 49.76 |
| Acetaldehyde | n.d. | 63.63 | n.d. |
| 2,3-Butanediol | n.d. | 0.66 | n.d. |
| Acetic acid | n.d. | 1.77 | n.d. |
| Oxime-, methoxy-phenyl | n.d. | 0.2 | n.d. |
| Linalool | 1.39 | 1.11 | 3.56 |
| 2-Phenylethanol | n.d. | 1.02 | 0.02 |
| Octanoic acid, ethyl ester | n.d. | 0.12 | 5.57 |
| β-citronelool | n.d. | 0.24 | 0.84 |
| 2-Phenyl ethyl acetate | n.d. | 0.22 | 0.25 |
| Nerol | 0.4 | 0.22 | 0.33 |
| α-Humelene | 0.08 | 0.05 | 0.02 |
| 2-Tert-butyl-4-Isopropyl-5-Methylphenol | 0.15 | 0.7 | 0.99 |
| Caryophyllene oxide | 0.03 | 0.58 | 0.42 |
| 1-Heptadecanol | 0.09 | 0.23 | 0.12 |
| Eicosane | n.d. | 0.19 | 0.11 |
| Octadecanoic acid, ethyl ester | n.d. | 0.03 | n.d. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Coelho, P.; Prista, C.; Sousa, I. Brewing Mainly from Stale Bread: A Pale Ale Case Study. Beverages 2024, 10, 23. https://doi.org/10.3390/beverages10020023
Coelho P, Prista C, Sousa I. Brewing Mainly from Stale Bread: A Pale Ale Case Study. Beverages. 2024; 10(2):23. https://doi.org/10.3390/beverages10020023
Chicago/Turabian StyleCoelho, Pedro, Catarina Prista, and Isabel Sousa. 2024. "Brewing Mainly from Stale Bread: A Pale Ale Case Study" Beverages 10, no. 2: 23. https://doi.org/10.3390/beverages10020023
APA StyleCoelho, P., Prista, C., & Sousa, I. (2024). Brewing Mainly from Stale Bread: A Pale Ale Case Study. Beverages, 10(2), 23. https://doi.org/10.3390/beverages10020023