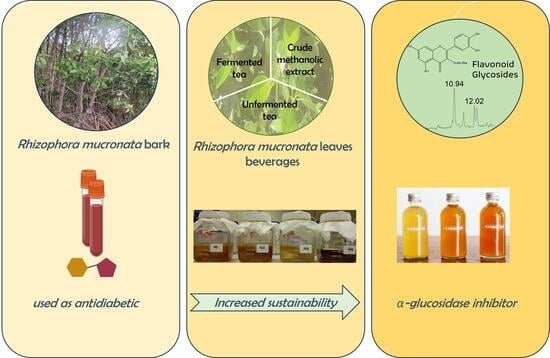
α-Glucosidase Inhibitory Activity of Tea and Kombucha from Rhizophora mucronata Leaves
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals
2.2. Plant Material
2.3. Preparation of Unfermented Tea Powder
2.4. Preparation of Kombucha Tea
2.5. Preparation of the Methanolic Extract
2.6. UPLC-HRMS Analysis
2.7. α-Glucosidase Inhibition Assay
2.8. Heavy Metal Analysis
3. Results
3.1. Identification of Phytochemicals by UPLC-HRMS
3.2. Effect of Kombucha Tea on α-Glucosidase Activity
3.3. Heavy Metal Analysis
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Kathiresan, K. Studies on tea from mangrove leaves. Environ. Ecol. 1995, 13, 321–323. [Google Scholar]
- Miranti, D.I.; Ichiura, H.; Ohtani, Y. The Bioactive Compounds and Antioxidant Activity of Food Products of Rhizophora stylosa Fruit (Coffee and Tea Mangrove). Int. J. For. Res. 2018, 2018, 2315329. [Google Scholar] [CrossRef]
- Harisman, E.K.; Puspitasari, Y.E. The kombucha from Rhizophora mucronata Lam herbal tea: Characteristics and the potential as an antidiabetic beverage. J. Pharm. Pharm. Res. 2020, 8, 410–421. [Google Scholar]
- Arulkumar, A.; Kumar, K.S.; Paramasivam, S. Antibacterial and invitro antioxidant potential of Indian mangroves. Biocatal. Agric. Biotechnol. 2020, 23, 101491. [Google Scholar] [CrossRef]
- Bibi, S.N.; Fawzi, M.M.; Gokhan, Z.; Rajesh, J.; Nadeem, N.; Rengasamy Kannan, R.R.; Albuquerque, R.D.D.G.; Pandian, S.K. Ethnopharmacology, Phytochemistry, and Global Distribution of Mangroves-A Comprehensive Review. Mar. Drugs 2019, 17, 231. [Google Scholar] [CrossRef] [PubMed]
- Ghosh, A.; Misra, S.; Dutta, A.K.; Choudhury, A. Pentacyclic triterpenoids and sterols from seven species of mangrove. Phytochemistry 1985, 24, 1725–1727. [Google Scholar] [CrossRef]
- Gurudeeban, S.; Ramanathan, T.; Satyavani, K. Antimicrobial and Radical Scavenging Effects of Alkaloid Extracts from Rhizophora mucronata. Pharm. Chem. J. 2015, 49, 34–37. [Google Scholar] [CrossRef]
- Taniguchi, K.; Funasaki, M.; Kishida, A.; Sadhu, S.K.; Ahmed, F.; Ishibashi, M.; Ohsaki, A. Two new coumarins and a new xanthone from the leaves of Rhizophora mucronata. Bioorg. Med. Chem. Lett. 2018, 28, 1063–1066. [Google Scholar] [CrossRef] [PubMed]
- Sachithanandam, V.; Lalitha, P.; Parthiban, A.; Muthukumaran, J.; Jain, M.; Misra, R.; Mageswaran, T.; Sridhar, R.; Purvaja, R.; Ramesh, R. A comprehensive in silico and in vitro studies on quinizarin: A promising phytochemical derived from Rhizophora mucronata Lam. J. Biomol. Struct. Dyn. 2022, 40, 7218–7229. [Google Scholar] [CrossRef] [PubMed]
- Kapp, J.M.; Sumner, W. Kombucha: A systematic review of the empirical evidence of human health benefit. Ann. Epidemiol. 2019, 30, 66–70. [Google Scholar] [CrossRef]
- Kim, J.; Adhikari, K. Current trends in kombucha: Marketing perspectives and the need for improved sensory research. Beverages 2020, 6, 15. [Google Scholar] [CrossRef]
- Dufresne, C.; Farnworth, E. Tea, Kombucha, and health: A review. Food Res. Int. 2000, 33, 409–421. [Google Scholar] [CrossRef]
- Reiss, J. Influence of different sugars on the metabolism of the tea fungus. Z. Lebensm. Unters. Forsch. 1994, 198, 258–261. [Google Scholar] [CrossRef]
- Kallel, L.; Desseaux, V.; Hamdi, M.; Stocker, P.; Ajandouz, E.H. Insights into the fermentation biochemistry of Kombucha teas and potential impacts of Kombucha drinking on starch digestion. Food Res. Int. 2012, 49, 226–232. [Google Scholar] [CrossRef]
- Koh, L.W.; Wong, L.L.; Loo, Y.Y.; Kasapis, S.; Huang, D. Evaluation of different teas against starch digestibility by mammalian glycosidases. J. Agric. Food Chem. 2010, 58, 148–154. [Google Scholar] [CrossRef]
- Aloulou, A.; Hamden, K.; Elloumi, D.; Ali, M.B.; Hargafi, K.; Jaouadi, B.; Ayadi, F.; Elfeki, A.; Ammar, E. Hypoglycemic and antilipidemic properties of kombucha tea in alloxan-induced diabetic rats. BMC Complement. Altern. Med. 2012, 12, 63. [Google Scholar] [CrossRef]
- Bhattacharya, S.; Gachhui, R.; Sil, P.C. Effect of Kombucha, a fermented black tea in attenuating oxidative stress mediated tissue damage in alloxan induced diabetic rats. Food Chem. Toxicol. 2013, 60, 328–340. [Google Scholar] [CrossRef]
- Hosseini, S.A.; Gorjian, M.; Rasouli, L.; Shirali, S. A comparison between the effect of green tea and Kombucha prepared from green tea on the weight of diabetic rats. Biosci. Biotechnol. Res. Asia 2015, 12, 141–146. [Google Scholar] [CrossRef]
- Watawana, M.I.; Jayawardena, N.; Gunawardhana, C.B.; Waisundara, V.Y. Enhancement of the antioxidant and starch hydrolase inhibitory activities of king coconut water (Cocos nucifera var. aurantiaca) by fermentation with kombucha “tea fungus”. Int. J. Food Sci. Technol. 2016, 51, 490–498. [Google Scholar] [CrossRef]
- Gamboa-Gómez, C.I.; Simental-Mendía, L.E.; González-Laredo, R.F.; Alcantar-Orozco, E.J.; Monserrat-Juarez, V.H.; Ramírez-España, J.C.; Gallegos-Infante, J.A.; Moreno-Jiménez, M.R.; Rocha-Guzmán, N.E. In vitro and in vivo assessment of anti-hyperglycemic and antioxidant effects of Oak leaves (Quercus convallata and Quercus arizonica) infusions and fermented beverages. Food Res. Int. 2017, 102, 690–699. [Google Scholar] [CrossRef]
- Zubaidah, E.; Afgani, C.A.; Kalsum, U.; Srianta, I.; Blanc, P.J. Comparison of in vivo antidiabetes activity of snake fruit Kombucha, black tea Kombucha and metformin. Biocatal. Agric. Biotechnol. 2019, 17, 465–469. [Google Scholar] [CrossRef]
- DiNicolantonio, J.J.; Bhutani, J.; O’Keefe, J.H. Acarbose: Safe and effective for lowering postprandial hyperglycaemia and improving cardiovascular outcomes. Open Heart 2015, 2, e000327. [Google Scholar] [CrossRef]
- Priebe, M.G.; Eelderink, C.; Wachters-Hagedoorn, R.E.; Vonk, R.J. Starch Digestion and Applications of Slowly Available Starch. In Starch in Food: Structure, Function and Applications, 2nd ed.; Sjoo, M., Nilsson, L., Eds.; Woodhead Publishing: Cambridge, UK, 2018; pp. 805–826. Available online: https://www.elsevier.com/books/starch-in-food/sjoo/978-0-08-100868-3 (accessed on 24 March 2020).
- Zubaidah, E.; Apriyadi, T.E.; Kalsum, U.; Widyastuti, E.; Estiasih, T.; Srianta, I.; Blanc, P.J. In vivo evaluation of snake fruit Kombucha as hyperglycemia therapeutic agent. Int. Food Res. J. 2018, 25, 453–457. [Google Scholar]
- Adhikari, A.; Ray, M.; Kumar, T.; Sharmistha, S. Anti-diabetic activity of Rhizophora mucronata leaves in streptozotocin-nicotinamide induced animal model. J. Middle East N. Afr. Sci. 2018, 4, 4–10. [Google Scholar]
- Pandey, A.K.; Gupta, P.P.; Lal, V.K. Hypoglycemic effect of Rhizophora mucronata in streptozotocin induced diabetic rats. J. Complement. Integr. Med. 2014, 11, 179–183. [Google Scholar] [CrossRef] [PubMed]
- Ray, M.; Adhikari, A.; Sur, T.K.; Mondal, C.; Pathak, A.; Das, A.K. Pharmacognostic and anti-hyperglycemic evaluation of the leaves of Sunderban mangrove, Rhizophora mucronata L. Rev. Mex. Fis. 2014, 60, 2289–2294. [Google Scholar]
- Lawag, I.L.; Aguinaldo, A.M.; Naheed, S.; Mosihuzzaman, M. α-Glucosidase inhibitory activity of selected Philippine plants. J. Ethnopharmacol. 2012, 144, 217–219. [Google Scholar] [CrossRef] [PubMed]
- Trinh, B.T.D.; Staerk, D.; Jager, A.K. Screening for potential α-glucosidase and α-amylase inhibitory constituents from selected Vietnames plants used to treat type 2 diabetes. J. Ethnopharmacol. 2016, 186, 189–195. [Google Scholar] [CrossRef]
- Paz-Alberto, A.M.; Celestino, A.B.; Sigua, G.C. Phytoremediation of Pb in the sediment of a mangrove ecosystem. J. Soils Sediments 2014, 14, 251–258. [Google Scholar] [CrossRef]
- World Health Organization. WHO Guidelines for Assessing Quality of Herbal Medicines with Reference to Contaminants and Residues; World Health Organization: Geneva, Switzerland, 2007. [Google Scholar]
- Pattanaik, C.; Reddy, C.S.; Dhal, N.K.; Das, R. Utilisation of mangrove forests in Bhitarkanika wildlife sanctuary, Orissa. Indian J. Tradit. Knowl. 2008, 7, 598–603. [Google Scholar]
- Adhikari, A.; Ray, M.; Das, A.; Sur, T. Antidiabetic and antioxidant activity of Rhizophora mucronata leaves (Indian sundarban mangrove): An in vitro and in vivo study. AYU (Int. Q. J. Res. Ayurveda) 2016, 37, 76. [Google Scholar] [CrossRef]
- Anjaneyulu, A.S.R.; Anjaneyulu, V.; Rao, V.L. New beyerane and isopimarane diterpenoids from Rhizophora mucronata. J. Asian Nat. Prod. Res. 2002, 4, 53–60. [Google Scholar] [CrossRef] [PubMed]
- Gurib-Fakim, A.; Subratty, H.; Narod, F.; Govinden-Soulange, J.; Mahomoodally, F. Biological activity from indigenous medicinal plants of Mauritius. Pure Appl. Chem. 2005, 77, 41–51. [Google Scholar] [CrossRef][Green Version]
- Ilman, M.; Dargusch, P.; Dart, P. Onrizal A historical analysis of the drivers of loss and degradation of Indonesia’s mangroves. Land Use Policy 2016, 54, 448–459. [Google Scholar] [CrossRef]
- Gill, A.M.; Tomlinson, P.B. Studies on the Growth of Red Mangrove (Rhizophora mangle L.) 3. Phenology of the Shoot. Biotropica 1971, 3, 109. [Google Scholar] [CrossRef]
- Biswas, S.R.; Mallik, A.U.; Choudhury, J.K.; Nishat, A. A unified framework for the restoration of Southeast Asian mangroves-bridging ecology, society and economics. Wetl. Ecol. Manag. 2009, 17, 365–383. [Google Scholar] [CrossRef]
- Gurudeeban, S.; Kaliamurthi, S.; Sheik, H.S.; Thiruganasambandam, R. Molecular docking, isolation and biological evaluation of Rhizophora mucronata flavonoids as anti-nociceptive agents. Biomed. Prev. Nutr. 2014, 4, 555–560. [Google Scholar] [CrossRef]
- Vukics, V.; Guttman, A. Structural Characterization of Flavonoid Glycosides by Multi-Stages Mass Spectrometry. Mass Spectrom. Rev. 2010, 29, 1–16. [Google Scholar] [CrossRef]
- Tourino, S.; Fuguet, E.; Jauregui, O.; Saura-Calixto, F.; Cascante, M.; Torres, J.L. High-resolution liquid chromatography/electrospray ionization time-of-flight mass spectrometry combined with liquid chromatography/electrospray ionization tandem mass spectrometry to identify polyphenols from grape antioxidant dietary fiber. Rapid Commun. Mass Spectrom. 2008, 22, 3489–3500. [Google Scholar] [CrossRef]
- Hanhineva, K.; Rogachev, I.; Kokko, H.; Mintz-Oron, S.; Venger, I.; Kärenlampi, S.; Aharoni, A. Non-targeted analysis of spatial metabolite composition in strawberry (Fragaria × ananassa) flowers. Phytochemistry 2008, 69, 2463–2481. [Google Scholar] [CrossRef]
- Lin, L.-Z.; Chen, P.; Ozcan, M.; Harnly, J.M. Chromatographic Profiles and Identification of New Phenolic Components of Ginkgo biloba Leaves and Selected Products. J. Agric. Food Chem. 2008, 56, 6671–6679. [Google Scholar] [CrossRef]
- European Pharmacopeia Herbal Drugs. Monograph 1433. Pharmaeuropa 2008, 20, 302–303. [Google Scholar]
- Hajeb, P.; Sloth, J.J.; Shakibazadeh, S.; Mahyudin, N.A.; Afsah-Hejri, L. Toxic elements in food: Occurrence, binding, and reduction approaches. Compr. Rev. Food Sci. Food Saf. 2014, 13, 457–472. [Google Scholar] [CrossRef] [PubMed]
- Jayabalan, R.; Marimuthu, S.; Swaminathan, K. Changes in content of organic acids and tea polyphenols during kombucha tea fermentation. Food Chem. 2007, 102, 392–398. [Google Scholar] [CrossRef]
- Satyavani, K.; Gurudeeban, S.; Ramanathan, T.; Muthusankar, A. Influence of Rhizophora apiculata Flavonoids on Chemical and Thermal Induced Nociceptive Models. Br. J. Pharm. Res. 2015, 7, 102–109. [Google Scholar] [CrossRef]
- Chiavaroli, A.; Sinan, K.I.; Zengin, G.; Mahomoodally, M.F.; Sadeer, N.B.; Etienne, O.K.; Cziáky, Z.; Jekő, J.; Glamocilja, J.; Sokovic, M.; et al. Identification of Chemical Profiles and Biological Properties of Rhizophora racemosa g. Mey. Extracts Obtained by Different Methods and Solvents. Antioxidants 2020, 9, 533. [Google Scholar] [CrossRef] [PubMed]
- Cruz, S.M.; Marroquín, N.; Alvarez, L.E.; Chang, D.E.; Cáceres, A. Evaluation of Mangrove (Rhizophora mangle L.) products as coloring, antimicrobial and antioxidant agents. Int. J. Phytocosmetics Nat. Ingred. 2015, 2, 12. [Google Scholar] [CrossRef]
- Vittaya, L.; Charoendat, U.; Janyong, S.; Ui-eng, J.; Leesakul, N. Comparative analyses of saponin, phenolic, and flavonoid contents in various parts of Rhizophora mucronata and Rhizophora apiculata and their growth inhibition of aquatic pathogenic bacteria. J. Appl. Pharm. Sci. 2022, 12, 111–121. [Google Scholar] [CrossRef]
- Al-Qudah, M.A.; Otoom, N.K.; Al-Jaber, H.I.; Saleh, A.M.; Abu Zarga, M.H.; Afifi, F.U.; Abu Orabi, S.T. New flavonol glycoside from Scabiosa prolifera L. aerial parts with in vitro antioxidant and cytotoxic activities. Nat. Prod. Res. 2017, 31, 2865–2874. [Google Scholar] [CrossRef]
- Hashim, A.N.; Swilam, N.F.; Moustafa, E.S.; Bakry, S.M.; Labib, R.M.; Barakat, H.H.; Singab, A.B.; Linscheid, M.W.; Nawwar, M.A. A cytotoxic flavonol glycoside from Melaleuca leucadendra leaves extract with immunostimulant activity. Pharmazie 2018, 73, 61–64. [Google Scholar] [CrossRef]
- Sharma, S.; Joshi, R.; Kumar, D. Quantitative analysis of flavonols, flavonol glycoside and homoisoflavonoids in Polygonatum verticillatum using UHPLC-DAD-QTOF-IMS and evaluation of their antioxidant potential. Phytochem. Anal. 2020, 31, 333–339. [Google Scholar] [CrossRef]
- Sun, Q.; Pan, G.; Xu, W.; Lu, X.; Bai, C.; Liu, M.; Chen, Y. Isolation and structure elucidation of a new flavonol glycoside from Sabia Parviflora. Nat. Prod. Res. 2021, 35, 2408–2413. [Google Scholar] [CrossRef]
- Ramil, R.J.D.; Ramil, M.D.I.; Konno, T.; Murata, T.; Kobayashi, K.; Buyankhishig, B.; Agrupis, S.C.; Sasaki, K. A new hexenoic acid glycoside with cytotoxic activity from the leaves of Psychotria luzoniensis. Nat. Prod. Res. 2021, 35, 5036–5041. [Google Scholar] [CrossRef] [PubMed]
- Proenca, C.; Freitas, M.; Ribeiro, D.; Oliveira, E.F.T.; Sousa, J.L.C.; Tom, S.M.; Ramos, M.J.; Silva, A.M.S.; Fernandes, P.A.; Fernandes, E. α-Glucosidase inhibition by flavonoids: An in vitro and in silico structure—Activity relationship study. J. Enzym. Inhib. Med. Chem. 2017, 32, 1216–1228. [Google Scholar] [CrossRef] [PubMed]
- Tadera, K.; Minami, Y.; Takamatsu, K.; Matsuoka, T. Inhibition of α-glucosidase and α-amylase by flavonoids. J. Nutr. Sci. Vitaminol. 2006, 52, 149–153. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.Q.; Zhou, F.C.; Gao, F.; Bian, J.S.; Shan, F. Comparative evaluation of quercetin, isoquercetin and rutin as inhibitors of α-glucosidase. J. Agric. Food Chem. 2009, 57, 11463–11468. [Google Scholar] [CrossRef] [PubMed]
- Şöhretoğlu, D.; Sari, S. Flavonoids as alpha-glucosidase inhibitors: Mechanistic approaches merged with enzyme kinetics and molecular modelling. Phytochem. Rev. 2020, 19, 1081–1092. [Google Scholar] [CrossRef]
- Li, S.; Zhang, W.; Wang, R.; Li, C.; Lin, X.; Wang, L. Screening and identification of natural α -glucosidase and α -amylase inhibitors from partridge tea (Mallotus furetianus Muell-Arg) and in silico analysis. Food Chem. 2022, 388, 133004. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Xu, L.; Ye, Y.H.; Zhu, M.F.; Li, J.; Tu, Z.C.; Yang, S.H.; Liao, H. Phytochemical profiles and screening of α-glucosidase inhibitors of four Acer species leaves with ultra-filtration combined with UPLC-QTOF-MS/MS. Ind. Crops Prod. 2019, 129, 156–168. [Google Scholar] [CrossRef]
- Maldonado-Román, M.; Jiménez-Collazo, J.; Malavé-Llamas, K.; Musa-Wasil, J.C. Mangroves and Their Response to a Heavy Metal Polluted Wetland in The North Coast of Puerto Rico. J. Trop. Life Sci. 2012, 6, 210–218. [Google Scholar] [CrossRef]
- Gasser, U.; Klier, B.; Kühn, A.V.; Steinhoff, B. Current findings on the heavy metal content in herbal drugs. Pharmeur. Sci. Notes 2009, 49, 37–50. [Google Scholar]
- Commission Regulation Commission Regulation (EC) No 1881/2006 of 19 December 2006 setting maximum levels for certain contaminants in foodstuffs. Off. J. Eur. Union 2006, 362, 5–24.

| RT (min) | Name | Measured m/z | Calculated m/z | Error (ppm) | Molecular Formula |
|---|---|---|---|---|---|
| 10.94 | Rutin (Quercetin-3-O-rutinoside) | 609.1433 | 609.1477 | 3.5 | C27H29O16 |
| 11.45 | Tetrahydroxyflavone-O-hexoside | 447.0918 | 447.0927 | −2.5 | C21H19O11 |
| 12.04 | Tetrahydroxyflavone-O-deoxyhexose-O-hexoside | 593.1494 | 593.1506 | −2.0 | C27H29O15 |
| 12.11 | Tetrahydroxy-methoxyflavone-O-deoxyhexose-O-hexoside | 623.1584 | 623.1612 | −4.5 | C28H31O16 |
| Kombucha Tea | Tea Powder | 80% MeOH Extract | Acarbose | |||
|---|---|---|---|---|---|---|
| 0 Days | 7 Days | 14 Days | ||||
| ST1 | 0.13 ± 0.01 | 0.09 ± 0.04 | 0.116 ± 0.006 | |||
| ST2 | 0.10 ± 0.01 | 0.09 ± 0.02 | 0.112 ± 0.006 | |||
| NC | 0.124 ± 0.003 | 0.081 ± 0.009 | 0.127± 0.007 | |||
| 0.12 ± 0.02 | 0.0435 ± 0.0007 | 2.4 ± 0.2 | ||||
| Element | Leaves Level (ppm) | Tea Powder Level (ppm) | Limit Level (ppm) | References |
|---|---|---|---|---|
| Cd | 0.0117 ± 0.0006 | 0.0026 ± 0.0001 | 0.3 | [31] |
| Pb | 0.1112 ± 0.0036 | 0.0063 ± 0.0031 | 10.0 | [31] |
| Hg | 0.0006 ± 0.0001 | ND * | 0.1 | [44] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Puspitasari, Y.E.; Tuenter, E.; Breynaert, A.; Foubert, K.; Herawati, H.; Hariati, A.M.; Aulanni’am, A.; De Bruyne, T.; Hermans, N. α-Glucosidase Inhibitory Activity of Tea and Kombucha from Rhizophora mucronata Leaves. Beverages 2024, 10, 22. https://doi.org/10.3390/beverages10010022
Puspitasari YE, Tuenter E, Breynaert A, Foubert K, Herawati H, Hariati AM, Aulanni’am A, De Bruyne T, Hermans N. α-Glucosidase Inhibitory Activity of Tea and Kombucha from Rhizophora mucronata Leaves. Beverages. 2024; 10(1):22. https://doi.org/10.3390/beverages10010022
Chicago/Turabian StylePuspitasari, Yunita Eka, Emmy Tuenter, Annelies Breynaert, Kenn Foubert, Herawati Herawati, Anik Martinah Hariati, Aulanni’am Aulanni’am, Tess De Bruyne, and Nina Hermans. 2024. "α-Glucosidase Inhibitory Activity of Tea and Kombucha from Rhizophora mucronata Leaves" Beverages 10, no. 1: 22. https://doi.org/10.3390/beverages10010022
APA StylePuspitasari, Y. E., Tuenter, E., Breynaert, A., Foubert, K., Herawati, H., Hariati, A. M., Aulanni’am, A., De Bruyne, T., & Hermans, N. (2024). α-Glucosidase Inhibitory Activity of Tea and Kombucha from Rhizophora mucronata Leaves. Beverages, 10(1), 22. https://doi.org/10.3390/beverages10010022