Computer-Aided Design and 3D Printing of Hemipelvic Endoprosthesis for Personalized Limb-Salvage Reconstruction after Periacetabular Tumor Resection
Abstract
1. Introduction
2. Patients and Methods
2.1. Patients
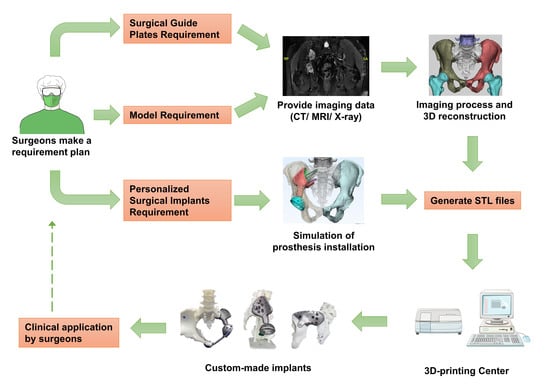
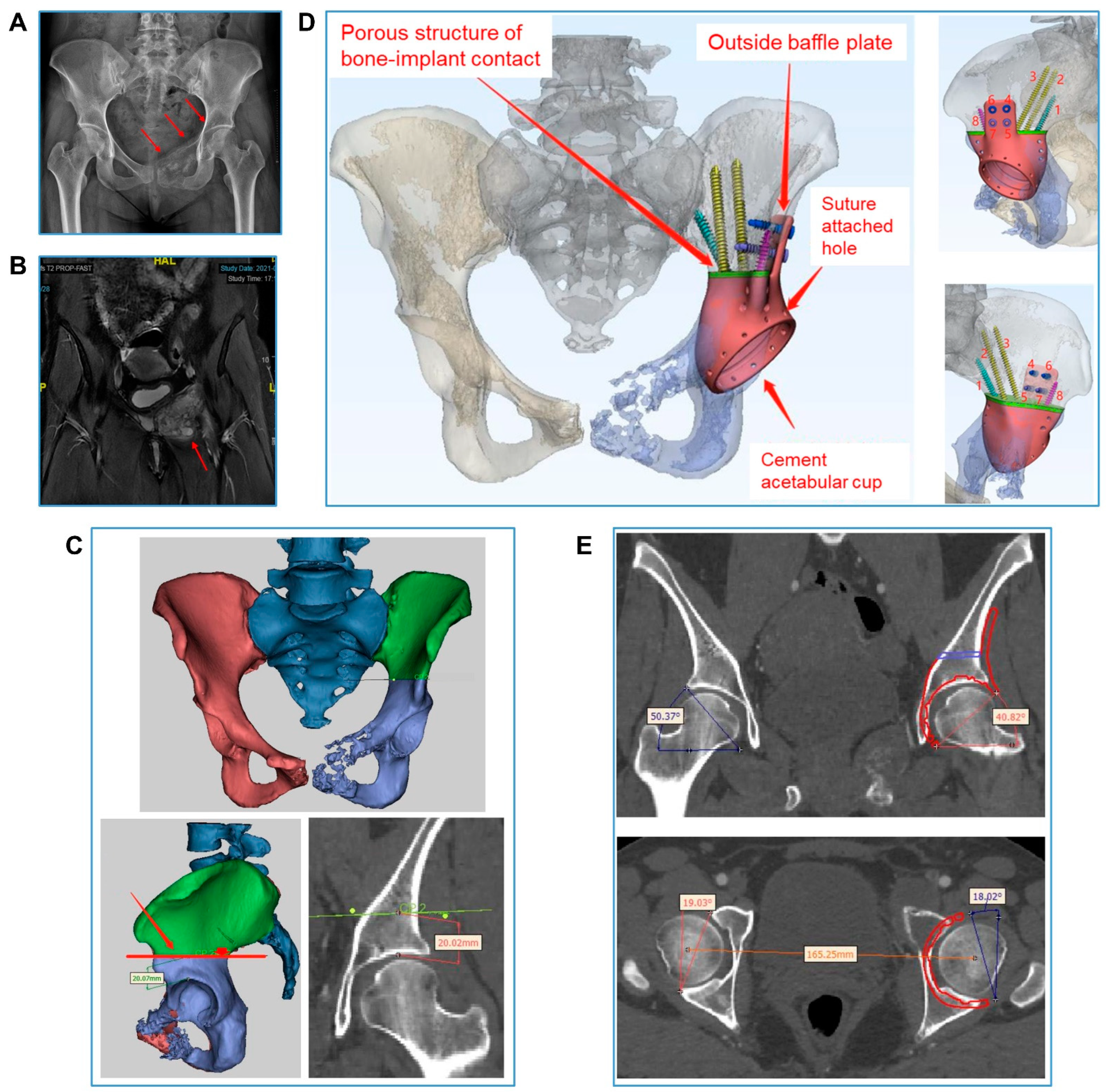
2.2. Hemipelvic Endoprosthesis Design and Manufacture
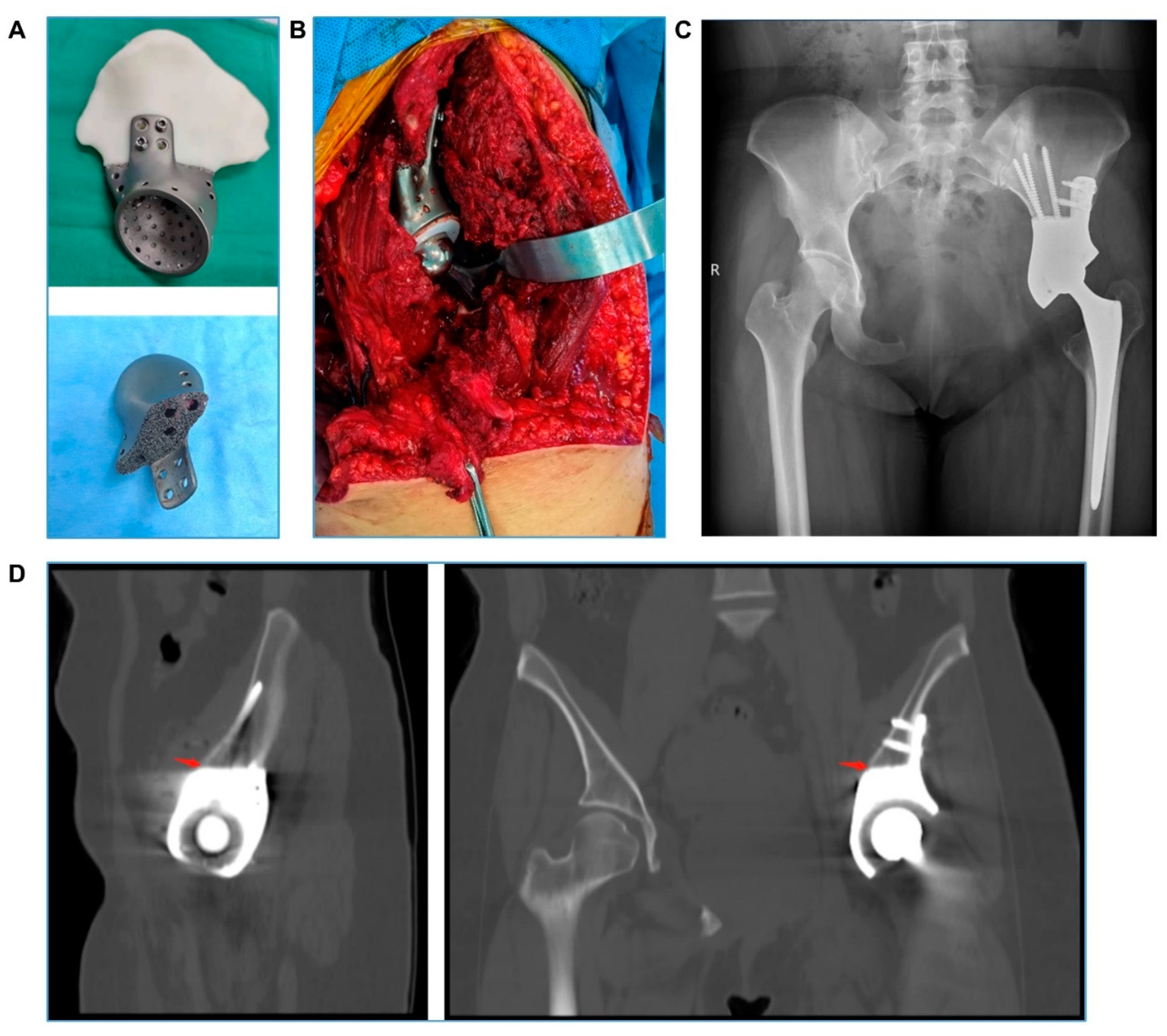
2.3. Surgery and Follow-Up
2.4. Statistical Analysis
3. Results
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Hu, X.; Deng, K.; Ye, H.; Sun, Z.; Huang, W.; Sun, Y.; Yan, W. Trends in Tumor Site-Specific Survival of Bone Sarcomas from 1980 to 2018: A Surveillance, Epidemiology and End Results-Based Study. Cancers 2021, 13, 5381. [Google Scholar] [CrossRef] [PubMed]
- Lavignac, P.; Prieur, J.; Fabre, T.; Descamps, J.; Niglis, L.; Carlier, C.; Bouthors, C.; Baron-Trocellier, T.; Sailhan, F.; Bonnevialle, P.; et al. Surgical treatment of peri-acetabular metastatic disease: Retrospective, multicentre study of 91 THA cases. Orthop. Traumatol. Surg. Res. 2020, 106, 1025–1032. [Google Scholar] [CrossRef] [PubMed]
- Enneking, W.F.; Dunham, W.K. Resection and reconstruction for primary neoplasms involving the innominate bone. J. Bone Jt. Surg. Am. 1978, 60, 731–746. [Google Scholar] [CrossRef]
- Fujiwara, T.; Ogura, K.; Christ, A.; Bartelstein, M.; Kenan, S.; Fabbri, N.; Healey, J. Periacetabular reconstruction following limb-salvage surgery for pelvic sarcomas. J. Bone Oncol. 2021, 31, 100396. [Google Scholar] [CrossRef]
- Spinelli, M.S.; Ziranu, A.; Piccioli, A.; Maccauro, G. Surgical treatment of acetabular metastasis. Eur. Rev. Med. Pharmacol. Sci. 2016, 20, 3005–3010. [Google Scholar]
- Guo, W.; Sun, X.; Zang, J.; Qu, H. Intralesional excision versus wide resection for giant cell tumor involving the acetabulum: Which is better? Clin. Orthop. Relat. Res. 2012, 470, 1213–1220. [Google Scholar] [CrossRef][Green Version]
- Tsuda, Y.; Evans, S.; Stevenson, J.D.; Parry, M.; Fujiwara, T.; Laitinen, M.; Outani, H.; Jeys, L. Is the Width of a Surgical Margin Associated with the Outcome of Disease in Patients with Peripheral Chondrosarcoma of the Pelvis? A Multicenter Study. Clin. Orthop. Relat. Res. 2019, 477, 2432–2440. [Google Scholar] [CrossRef]
- Abdel, M.P.; von Roth, P.; Perry, K.I.; Rose, P.S.; Lewallen, D.G.; Sim, F.H. Early Results of Acetabular Reconstruction after Wide Periacetabular Oncologic Resection. J. Bone Jt. Surg. Am. 2017, 99, e9. [Google Scholar] [CrossRef]
- Houdek, M.T.; Wunder, J.S.; Abdel, M.P.; Griffin, A.M.; Hevesi, M.; Rose, P.S.; Ferguson, P.C.; Lewallen, D.G. Comparison of reconstructive techniques after acetabular resection for pelvic chondrosarcoma. Bone Jt. J. 2021, 103-B, 391–397. [Google Scholar] [CrossRef]
- Kostakos, T.A.; Nayar, S.K.; Alcock, H.; Savvidou, O.; Vlasis, K.; Papagelopoulos, P.J. Acetabular reconstruction in oncological surgery: A systematic review and meta-analysis of implant survivorship and patient outcomes. Surg. Oncol. 2021, 38, 101635. [Google Scholar] [CrossRef]
- Fang, C.; Cai, H.; Kuong, E.; Chui, E.; Siu, Y.C.; Ji, T.; Drstvenšek, I. Surgical applications of three-dimensional printing in the pelvis and acetabulum: From models and tools to implants. Unfallchirurg 2019, 122, 278–285. [Google Scholar] [CrossRef] [PubMed]
- Dhawan, A.; Kennedy, P.M.; Rizk, E.B.; Ozbolat, I.T. Three-dimensional Bioprinting for Bone and Cartilage Restoration in Orthopaedic Surgery. J. Am. Acad. Orthop. Surg. 2019, 27, e215–e226. [Google Scholar] [CrossRef] [PubMed]
- Wixted, C.M.; Peterson, J.R.; Kadakia, R.J.; Adams, S.B. Three-dimensional Printing in Orthopaedic Surgery: Current Applications and Future Developments. J. Am. Acad. Orthop. Surg. Glob. Res. Rev. 2021, 5, e20.00230–11. [Google Scholar] [CrossRef] [PubMed]
- Javaid, M.; Haleem, A. Current status and challenges of Additive manufacturing in orthopaedics: An overview. J. Clin. Orthop. Trauma. 2019, 10, 380–386. [Google Scholar] [CrossRef]
- Sung, Y.T.; Wu, J.S. The Visual Analogue Scale for Rating, Ranking and Paired-Comparison (VAS-RRP): A new technique for psychological measurement. Behav. Res. Methods 2018, 50, 1694–1715. [Google Scholar] [CrossRef]
- Enneking, W.F.; Dunham, W.; Gebhardt, M.C.; Malawar, M.; Pritchard, D.J. A system for the functional evaluation of reconstructive procedures after surgical treatment of tumors of the musculoskeletal system. Clin. Orthop. Relat. Res. 1993, 286, 241–246. [Google Scholar] [CrossRef]
- Woo, S.H.; Sung, M.J.; Park, K.S.; Yoon, T.R. Three-dimensional-printing Technology in Hip and Pelvic Surgery: Current Landscape. Hip. Pelvis. 2020, 32, 1–10. [Google Scholar] [CrossRef]
- Bockhorn, L.; Gardner, S.S.; Dong, D.; Karmonik, C.; Elias, S.; Gwathmey, F.W.; Harris, J.D. Application of three-dimensional printing for pre-operative planning in hip preservation surgery. J. Hip. Preserv. Surg. 2019, 6, 164–169. [Google Scholar] [CrossRef]
- Liu, X.; Liu, Y.; Lu, W.; Liao, S.; Du, Q.; Deng, Z.; Lu, W. Combined Application of Modified Three-Dimensional Printed Anatomic Templates and Customized Cutting Blocks in Pelvic Reconstruction after Pelvic Tumor Resection. J. Arthroplast. 2019, 34, 338–345.e1. [Google Scholar] [CrossRef]
- Liang, H.; Ji, T.; Zhang, Y.; Wang, Y.; Guo, W. Reconstruction with 3D-printed pelvic endoprostheses after resection of a pelvic tumour. Bone Jt. J. 2017, 99-B, 267–275. [Google Scholar] [CrossRef]
- Shelton, T.J.; Monazzam, S.; Calafi, A.; Leshikar, H.B.; Haus, B.M. Preoperative 3D Modeling and Printing for Guiding Periacetabular Osteotomy. J. Pediatr. Orthop. 2021, 41, 149–158. [Google Scholar] [CrossRef] [PubMed]
- Baraza, N.; Chapman, C.; Zakani, S.; Mulpuri, K. 3D—Printed Patient Specific Instrumentation in Corrective Osteotomy of the Femur and Pelvis: A Review of the Literature. 3D Print. Med. 2020, 6, 34. [Google Scholar] [CrossRef] [PubMed]
- Xu, L.; Qin, H.; Tan, J.; Cheng, Z.; Luo, X.; Tan, H.; Huang, W. Clinical study of 3D printed personalized prosthesis in the treatment of bone defect after pelvic tumor resection. J. Orthop. Translat. 2021, 29, 163–169. [Google Scholar] [CrossRef]
- Ji, T.; Yang, Y.; Tang, X.; Liang, H.; Yan, T.; Yang, R.; Guo, W. 3D-Printed Modular Hemipelvic Endoprosthetic Reconstruction Following Periacetabular Tumor Resection: Early Results of 80 Consecutive Cases. J. Bone Jt. Surg. Am. 2020, 102, 1530–1541. [Google Scholar] [CrossRef]
- Wu, J.; Xie, K.; Luo, D.; Wang, L.; Wu, W.; Yan, M.; Ai, S.; Dai, K.; Hao, Y. Three-dimensional printing-based personalized limb salvage and reconstruction treatment of pelvic tumors. J. Surg. Oncol. 2021, 124, 420–430. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Min, L.; Lu, M.; Zhang, Y.; Wang, Y.; Luo, Y.; Zhou, Y.; Duan, H.; Tu, C. What are the Complications of Three-dimensionally Printed, Custom-made, Integrative Hemipelvic Endoprostheses in Patients with Primary Malignancies Involving the Acetabulum, and What is the Function of These Patients? Clin. Orthop. Relat. Res. 2020, 478, 2487–2501. [Google Scholar] [CrossRef]
- Wang, B.; Hao, Y.; Pu, F.; Jiang, W.; Shao, Z. Computer-aided designed, three dimensional-printed hemipelvic prosthesis for peri-acetabular malignant bone tumour. Int. Orthop. 2018, 42, 687–694. [Google Scholar] [CrossRef]
- Biranje, S.S.; Sun, J.; Cheng, L.; Cheng, Y.; Shi, Y.; Yu, S.; Jiao, H.; Zhang, M.; Lu, X.; Han, W.; et al. Development of Cellulose Nanofibril/Casein-Based 3D Composite Hemostasis Scaffold for Potential Wound-Healing Application. ACS Appl. Mater. Interfaces 2022, 14, 3792–3808. [Google Scholar] [CrossRef]
- Tripathi, D.; Sharma, A.; Tyagi, P.; Beniwal, C.S.; Mittal, G.; Jamini, A.; Singh, H.; Tyagi, A. Fabrication of Three-Dimensional Bioactive Composite Scaffolds for Hemostasis and Wound Healing. AAPS PharmSciTech 2021, 22, 138. [Google Scholar] [CrossRef]
- Zhang, L.; Yang, G.; Johnson, B.N.; Jia, X. Three-dimensional (3D) printed scaffold and material selection for bone repair. Acta Biomater. 2019, 84, 16–33. [Google Scholar] [CrossRef]
- Yang, Y.; Chu, L.; Yang, S.; Zhang, H.; Qin, L.; Guillaume, O.; Eglin, D.; Richards, R.G.; Tang, T. Dual-functional 3D-printed composite scaffold for inhibiting bacterial infection and promoting bone regeneration in infected bone defect models. Acta Biomater. 2018, 79, 265–275. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Li, T.; Ma, H.; Zhai, D.; Deng, C.; Wang, J.; Zhuo, S.; Chang, J.; Wu, C. 3D-printed scaffolds with bioactive elements-induced photothermal effect for bone tumor therapy. Acta Biomater. 2018, 73, 531–546. [Google Scholar] [CrossRef]
- Ma, H.; Feng, C.; Chang, J.; Wu, C. 3D-printed bioceramic scaffolds: From bone tissue engineering to tumor therapy. Acta Biomater. 2018, 79, 37–59. [Google Scholar] [CrossRef] [PubMed]
- Hao, Y.; Luo, D.; Wu, J.; Wang, L.; Xie, K.; Yan, M.; Dai, K.; Hao, Y. A novel revision system for complex pelvic defects utilizing 3D-printed custom prosthesis. J. Orthop. Translat. 2021, 31, 102–109. [Google Scholar] [CrossRef] [PubMed]
- De Paolis, M.; Sambri, A.; Zucchini, R.; Frisoni, T.; Spazzoli, B.; Taddei, F.; Donati, D.M. Custom-made 3D-Printed Prosthesis in Periacetabular Resections Through a Novel Ileo-adductor Approach. Orthopedics 2022, 45, e110–e114. [Google Scholar] [CrossRef]
- Zhu, D.; Fu, J.; Wang, L.; Guo, Z.; Wang, Z.; Fan, H. Reconstruction with customized, 3D-printed prosthesis after resection of periacetabular Ewing’s sarcoma in children using “triradiate cartilage-based” surgical strategy: A technical note. J. Orthop. Translat. 2021, 28, 108–117. [Google Scholar] [CrossRef]


| Case | Age (Years) | Sex | BMI (kg/m2) | Initial Symptoms and Signs | VAS at Admission | Disease Course (Months) | Tumor Characteristics | Surgical History | Neoadjuvant Oncological Therapy | |||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Diagnosis | Side | Zone | Stage * | |||||||||
| 1 | 32 | F | 21.6 | Hip pain, limitation of motion | 6 | 20 | Recurrent fibrosarcoma | L | II | IIB | Piecemeal resection | Chemotherapy |
| 2 | 67 | M | 24.8 | Hip pain | 7 | 48 | Chondrosarcoma | R | I–II | IIB | / | / |
| 3 | 31 | F | 26.2 | Hip pain | 5 | 3 | Chondrosarcoma | L | II–III | IIB | / | / |
| 4 | 41 | M | 20.5 | Hip pain | 6 | 15 | Chondrosarcoma | R | I–II | IIB | / | / |
| 5 | 32 | M | 28.7 | Hip pain, limitation of motion | 6 | 7 | Tendon sheaths giant cell tumor | L | II–III | 3 | / | Denosumab |
| 6 | 44 | F | 21.5 | Hip pain | 5 | 6 | Epithelioid Hemangioendothelioma | R | II–III | IB | / | / |
| 7 | 19 | M | 15.9 | Hip pain | 7 | 2 | Osteosarcoma | R | I–II | IIIB | / | Chemotherapy |
| 8 | 38 | F | 22.9 | Hip pain | 4 | 3 | Epithelioid Hemangioendothelioma | L | I–II | IB | / | Radiotherapy |
| 9 | 58 | F | 22.3 | Hip pain | 7 | 5 | Chondrosarcoma | L | II–III | IIB | / | / |
| 10 | 22 | M | 27.4 | Hip pain, limitation of motion | 8 | 10 | Ewing sarcoma | R | I–II | IIIB | / | Chemotherapy, Radiotherapy |
| 11 | 23 | F | 33.3 | Hip pain | 7 | 7 | Ewing sarcoma | L | I–II, IV | IIIB | / | Chemotherapy |
| 12 | 47 | F | 23.7 | Hip pain, limitation of motion | 8 | 5 | Metastatic lung adenocarcinoma | R | II–III | T2N0M1 | Primary tumor surgery | Chemotherapy |
| 13 | 60 | M | 27.4 | Hip pain, limitation of motion | 8 | 6 | Metastatic renal clear cell cancer | L | II–III | T1N0M1 | Primary tumor surgery | Targeted therapy, Immunotherapy, Radiotherapy |
| 14 | 57 | M | 25.7 | Hip pain | 9 | 7 | Metastatic renal clear cell cancer | L | II–III | T1N0M1 | Primary tumor surgery | Targeted therapy |
| 15 | 60 | F | 21.3 | Hip pain | 5 | 2 | Metastatic cyst-adenocarcinoma of the submandibular gland | R | II | T1N0M1 | Primary tumor surgery | Chemotherapy |
| 16 | 53 | M | 23.9 | Hip pain | 5 | 1 | Metastatic hepatocellular cancer | L | II | T1N0M1 | Primary tumor surgery | Targeted therapy, Immunotherapy |
| Case | Resection Type * | Surgery Time (min) | Blood Loss (mL) | Surgical Margin | Perioperative Complication | VAS at 7th Post-Surgery Day | Post-Discharge Follow-Up | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Distance (mm) | Pathology | Time (Month) | Revision Surgery | Adjuvant Therapy | Oncological Outcome | VAS | MSTS Score (%) | ||||||
| 1 | II | 205 | 400 | 33 | Negative | / | 0 | 9 | / | / | No evidence of disease | 0 | 27 (90%) |
| 2 | I–II | 325 | 700 | 25 | Negative | Pneumonia | 0 | 13 | / | / | No evidence of disease | 0 | 27 (90%) |
| 3 | II–III | 365 | 800 | 20 | Negative | / | 0 | 13 | / | / | No evidence of disease | 0 | 29 (96.7%) |
| 4 | I–II | 305 | 1100 | 20 | Negative | / | 1 | 39 | Done for hip dislocation caused by a traffic accident at 17th month | / | No evidence of disease | 0 | 30 (100%) |
| 5 | II–III | 320 | 3600 | 25 | Negative | DVT | 3 | 14 | / | / | No evidence of disease | 0 | 8 (26.7%) |
| 6 | II–III | 330 | 3000 | 26 | Negative | / | 0 | 10 | / | / | No evidence of disease | 0 | 27 (90%) |
| 7 | I–II | 215 | 500 | 14 | Negative | / | 2 | 18 | / | Chemotherapy | Alive with disease | 0 | 26 (86.7%) |
| 8 | I–II | 340 | 1900 | 21 | Negative | DVT, deep infection | 0 | 17 | / | / | No evidence of disease | 0 | 28 (93.3%) |
| 9 | II–III | 350 | 3200 | 25 | Negative | / | 1 | 10 | / | / | No evidence of disease | 0 | 27 (90%) |
| 10 | I–II | 230 | 2000 | 20 | Negative | Superficial infection | 2 | 9 | / | Chemotherapy | Alive with disease | 0 | 26 (86.7%) |
| 11 | I–II, IV | 260 | 1300 | 10 | Negative | / | 1 | 38 | / | Chemotherapy | Alive with disease | 0 | 27 (90%) |
| 12 | II–III | 210 | 900 | 20 | Negative | / | 1 | 6 | / | Chemotherapy, Bisphosphonates | No evidence of disease | 0 | 27 (90%) |
| 13 | II–III | 380 | 1600 | 18 | Negative | / | 2 | 7 | Done for hip dislocation at 3rd month | Targeted therapy, Immunotherapy, Denosumab | No evidence of disease | 0 | 23 (76.7%) |
| 14 | II–III | 390 | 2600 | 16 | Negative | Pneumonia | 1 | 12 | / | Targeted therapy, Bisphosphonates | No evidence of disease | 0 | 27 (90%) |
| 15 | II | 200 | 600 | 20 | Negative | / | 0 | 46 | / | Chemotherapy, Bisphosphonates | Alive with disease | 0 | 28 (93.3%) |
| 16 | II | 210 | 800 | 17 | Negative | / | 1 | 23 | / | Targeted therapy, Denosumab | No evidence of disease | 0 | 25 (83.3%) |
| Reference, Publication Year and Journal | Institution | Study Period | Patient Number | Age (Years) | Sex (Male /Female) | Resection Type | Surgical Duration (min) | Blood Loss (mL) | Surgical Margin | Complication | Follow-Up Months | MSTS (%) |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Ji et al. [24], 2020, J Bone Joint Surg Am | People’s Hospital, Peking University | 2015~2017 | 80 | 41.9 (11~78) | 42/38 | II (n = 23) II + III (n = 57) | 276 (150~570) | 1898.5 (300 to 6000) | R0 for 61 of 64 primary tumors; R1 for 16 metastatic tumors | Wound dehiscence (n = 8) Deep infection (n = 5) Hip dislocation (n = 2) Hematoma (n = 2) Acute arterial thrombosis (n = 1) Screw breakage (n = 1) | 32.5 (9~52) | 83.9% (43~100%) |
| Wu et al. [25], 2021, J Surg Oncol | Shanghai Ninth People’s Hospital, Shanghai Jiao Tong University School of Medicine | 2014~2019 | 28 # | 48.1 ± 11.6 | 15/13 | I + II (n = 10) I + II + III (n = 6) II + III (n = 4) II (n = 4) I (n = 4) | 393 (220~600) | 4404 (600~11,000) | Wide for 26; Marginal for 2 | Superficial infection (n = 6) Hip dislocation (n = 3) | 32.3 (3~75) | 23.2 (17~29) |
| Wang et al. [26], 2020, Clin Orthop Relat Res | West China Hospital, Sichuan University | 2016~2017 | 13 | 46 (31~66) | 6/7 | I + II (n = 3) I + II + III (n = 10) | 260 (170~540) | 2600 (900~8200) | Wide for all | Delayed wound healing (n = 2) | 27 (24~31) | 23 (15~27) |
| Wang et al. [27], 2018, Int Orthop | Union Hospital, Tongji Medical College, Huazhong University of Science and Technology | 2015~2016 | 11 | 47 (21~63) | 5/6 | Not specified | 271 ± 45.5 | 3236 ±1665 | Wide for 9; Marginal for 2 | Delayed wound healing (n = 1) Hip dislocation (n = 2) | 15.5 (6~24) | 19.2 (13~25) |
| Current study | Fudan University Shanghai Cancer Center | 2018~2021 | 16 | 42.8 (19~67) | 8/8 | I + II (n = 5) II + III (n = 7) II (n = 3) I + II + IV (n = 1) | 289.7 (200~390) | 1563 (400 to 3600) | Wide for all | Deep venous thrombosis (n = 2) Pneumonia (n =2) Would infection (n =2) Hip dislocation (n = 2) | 17.75 (6~46) | 85.8% (26.7~100%) or 25.8 (8~30) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hu, X.; Chen, Y.; Cai, W.; Cheng, M.; Yan, W.; Huang, W. Computer-Aided Design and 3D Printing of Hemipelvic Endoprosthesis for Personalized Limb-Salvage Reconstruction after Periacetabular Tumor Resection. Bioengineering 2022, 9, 400. https://doi.org/10.3390/bioengineering9080400
Hu X, Chen Y, Cai W, Cheng M, Yan W, Huang W. Computer-Aided Design and 3D Printing of Hemipelvic Endoprosthesis for Personalized Limb-Salvage Reconstruction after Periacetabular Tumor Resection. Bioengineering. 2022; 9(8):400. https://doi.org/10.3390/bioengineering9080400
Chicago/Turabian StyleHu, Xianglin, Yong Chen, Weiluo Cai, Mo Cheng, Wangjun Yan, and Wending Huang. 2022. "Computer-Aided Design and 3D Printing of Hemipelvic Endoprosthesis for Personalized Limb-Salvage Reconstruction after Periacetabular Tumor Resection" Bioengineering 9, no. 8: 400. https://doi.org/10.3390/bioengineering9080400
APA StyleHu, X., Chen, Y., Cai, W., Cheng, M., Yan, W., & Huang, W. (2022). Computer-Aided Design and 3D Printing of Hemipelvic Endoprosthesis for Personalized Limb-Salvage Reconstruction after Periacetabular Tumor Resection. Bioengineering, 9(8), 400. https://doi.org/10.3390/bioengineering9080400