Co-Graft of Acellular Dermal Matrix and Split Thickness Skin Graft—A New Reconstructive Surgical Method in the Treatment of Hidradenitis Suppurativa
Abstract
:1. Introduction
2. Materials and Methods
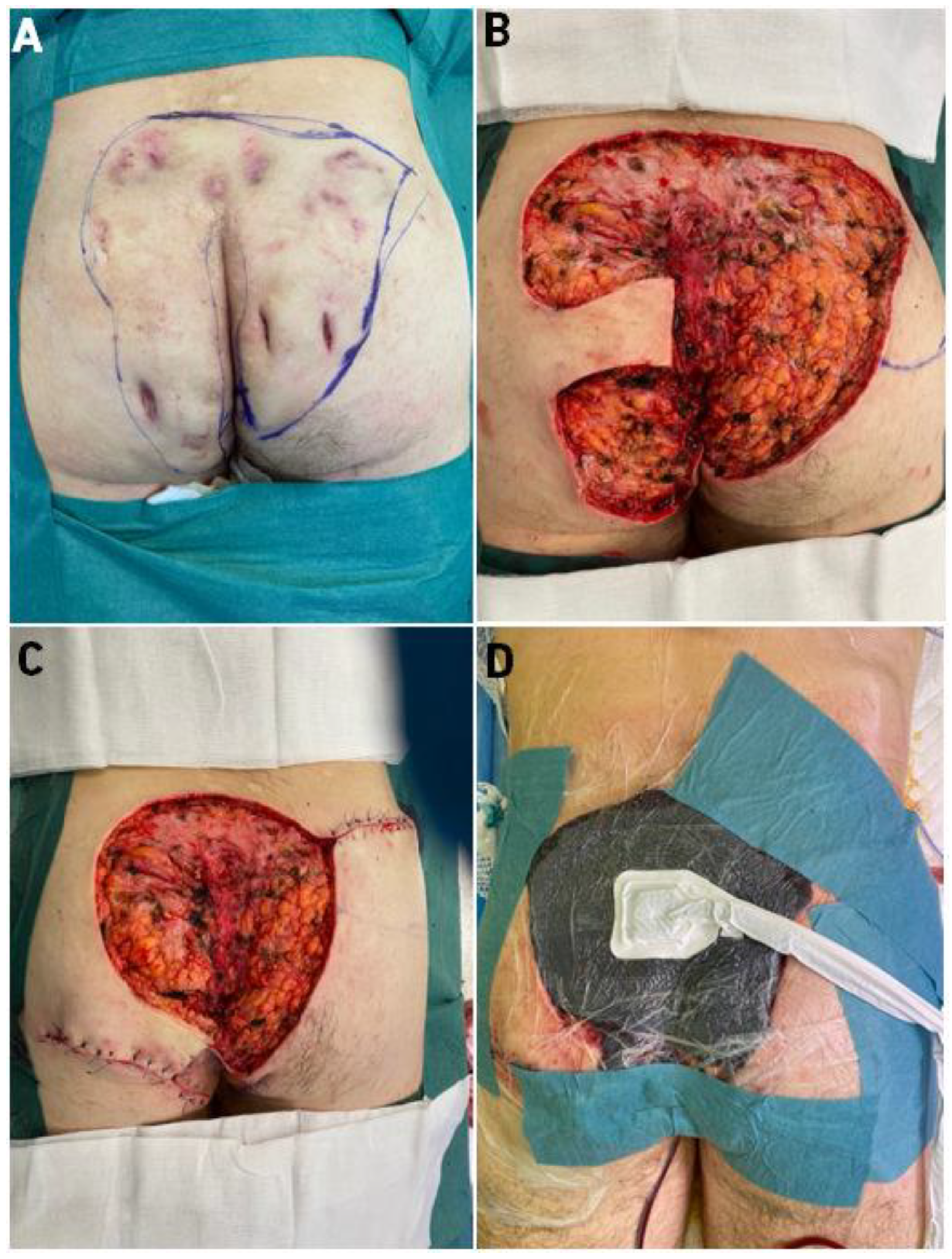
2.1. First Case
2.2. Second Case
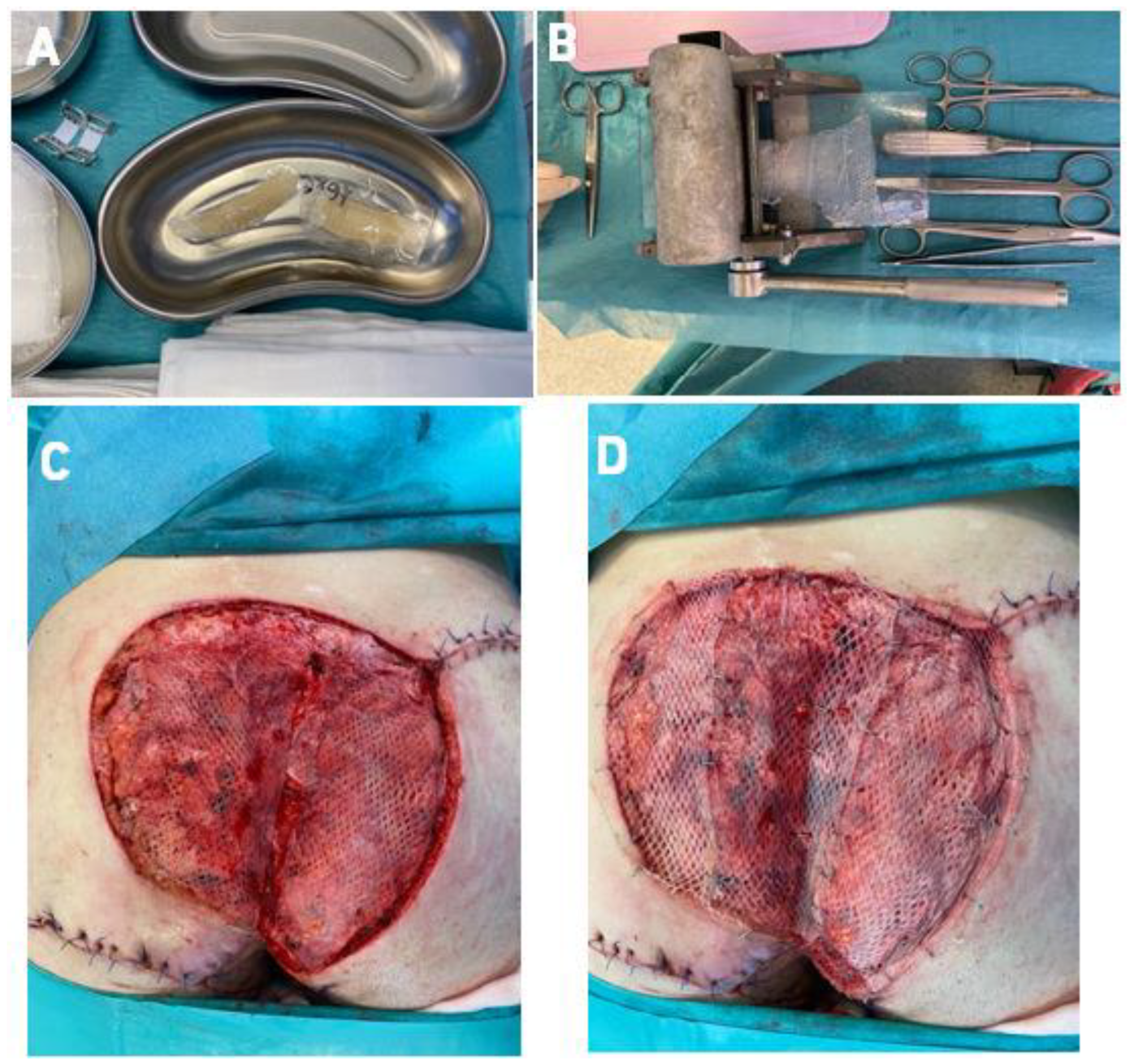
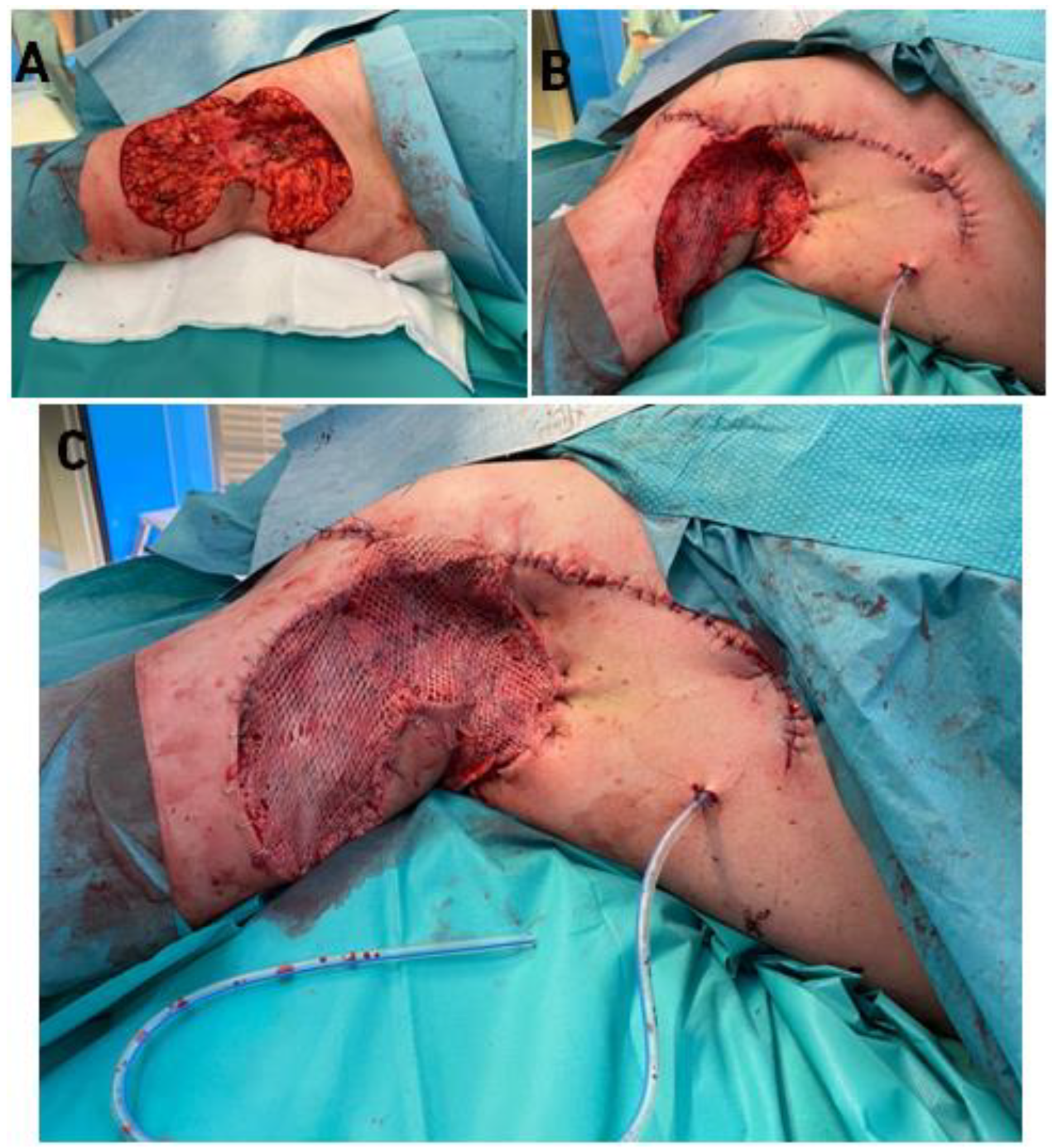
2.3. Surgical Technique-Co-Graft of ADM and STSG
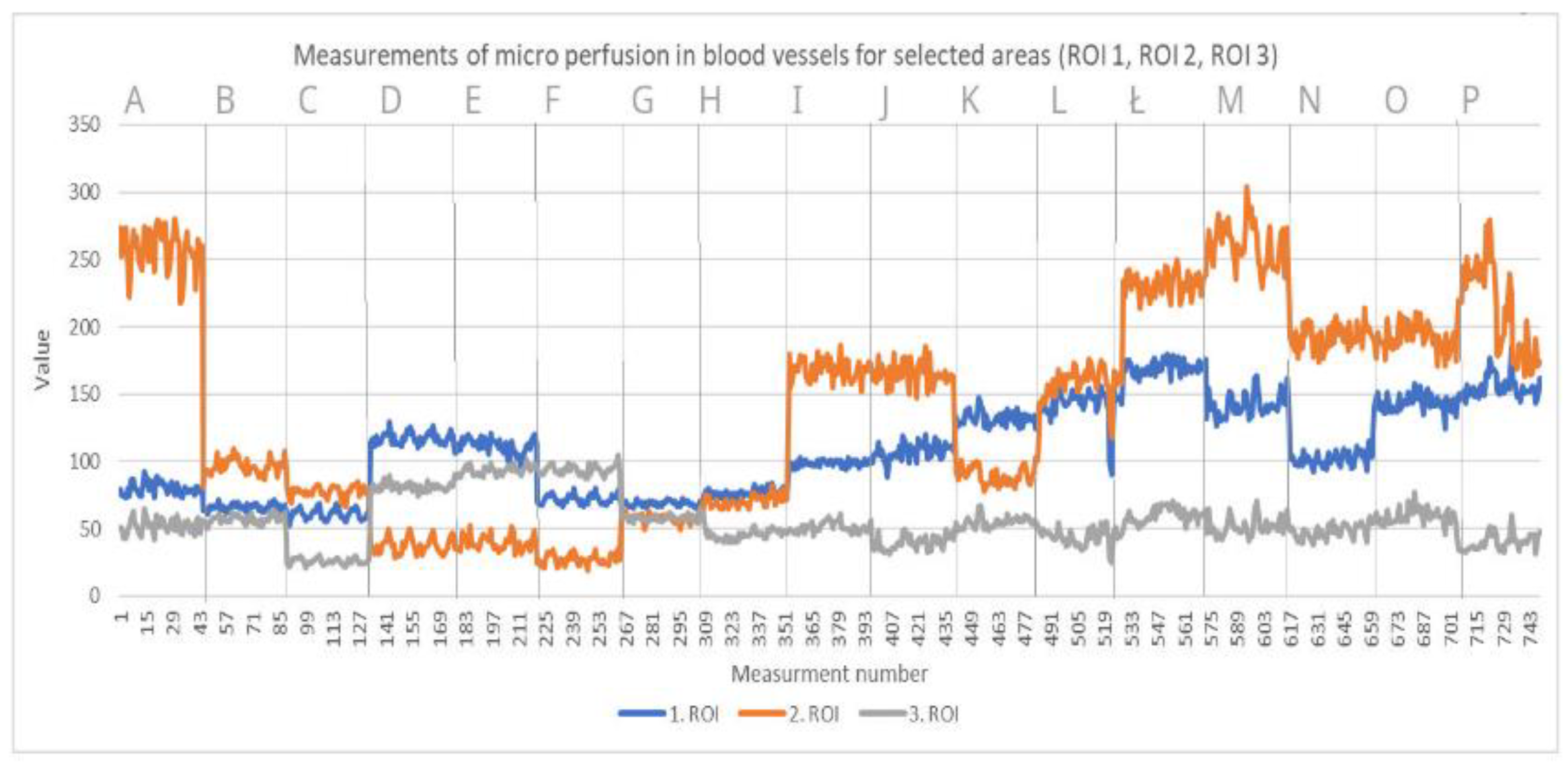
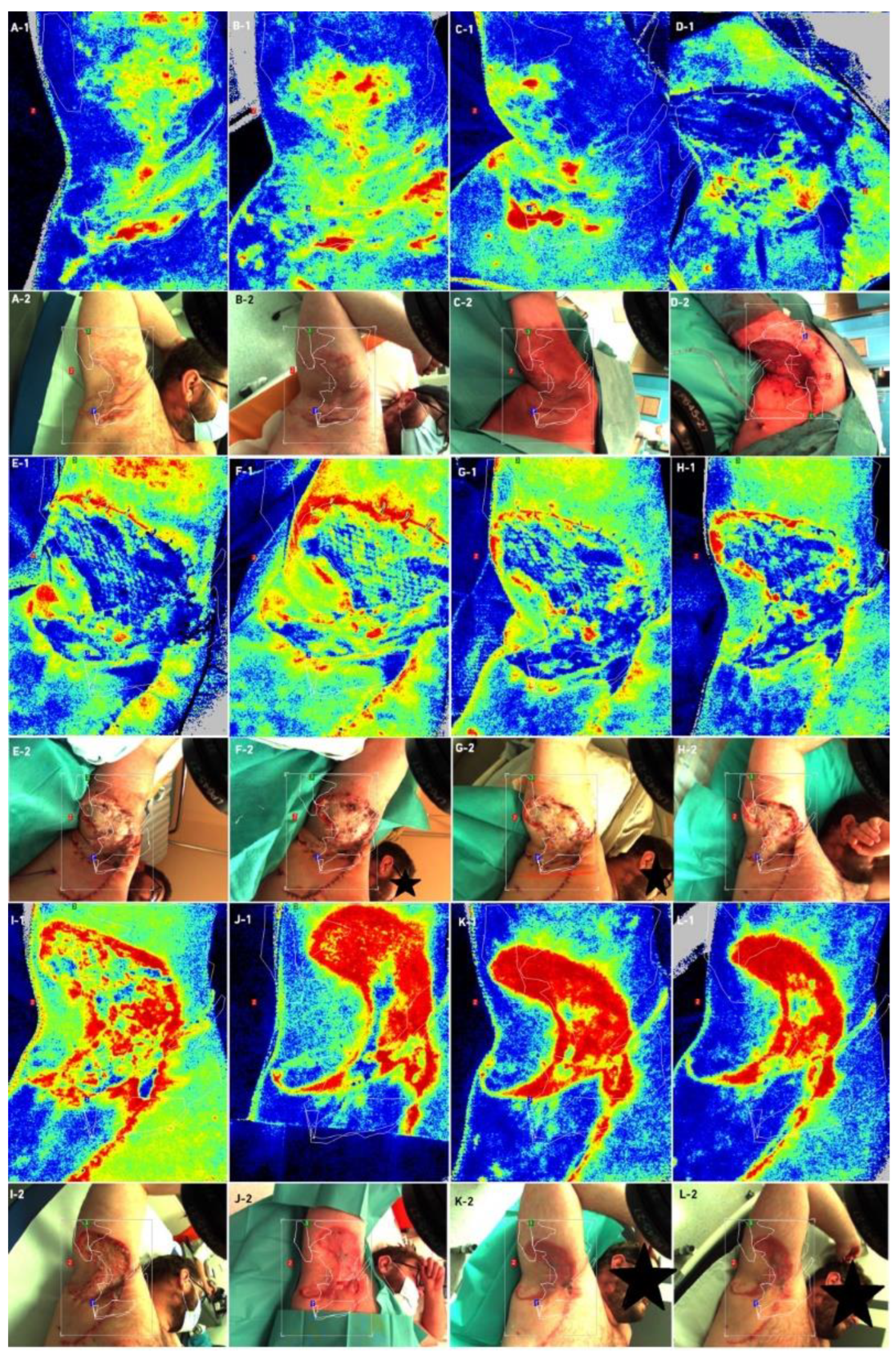
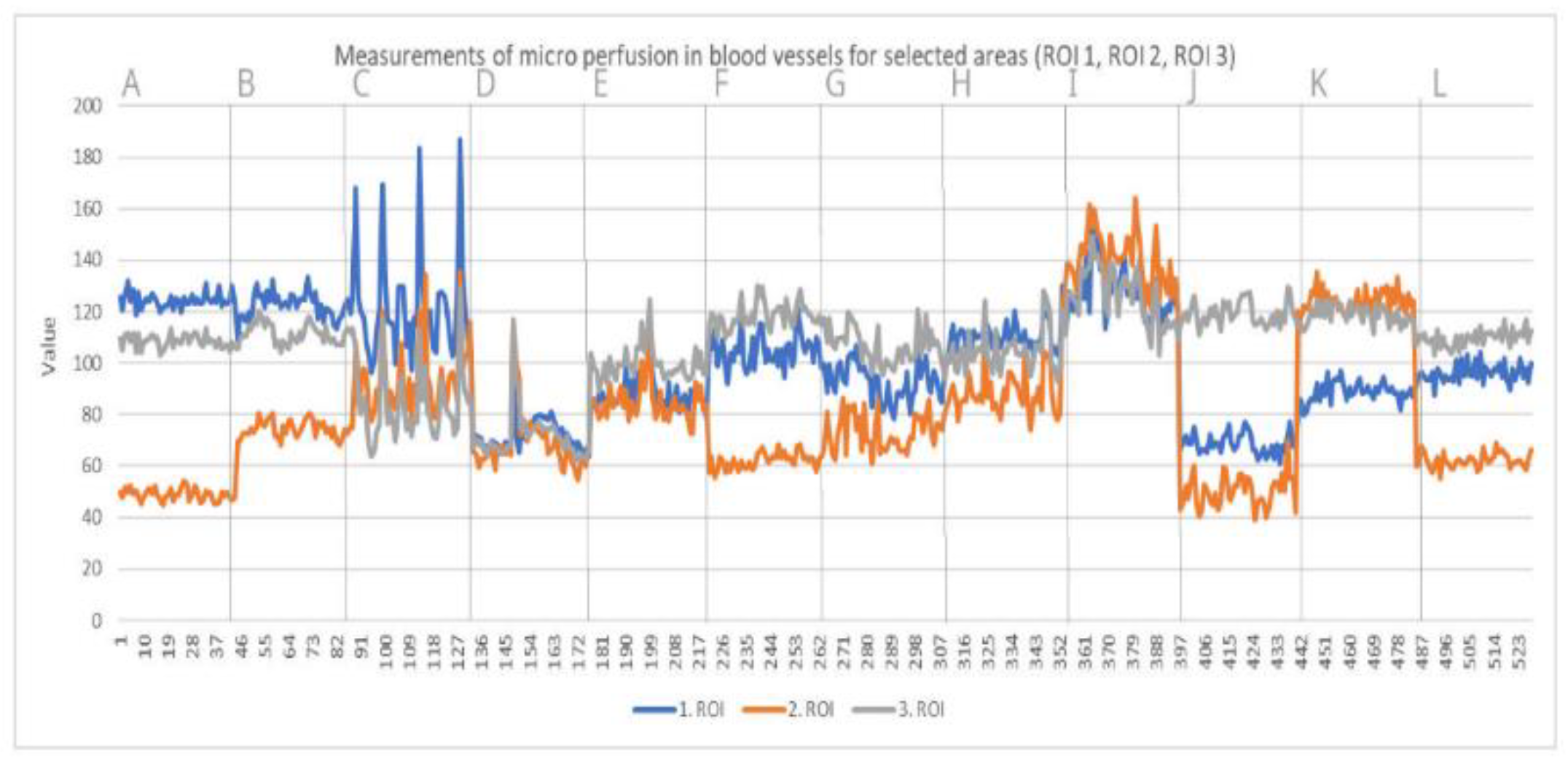
2.4. Examinations
- Laser Speckle Contrast Analysis (LASCA)
- Cutometer® dual MPA 580, Courage + Khazaka electronic GmbH, Mathias-Bruggen 50829
- Temperature measurements
- Corneometer measurments
- Mexameter measurments
- Tewameter measurments
- Cutometer measurments
- Skin Ultrasound DUB SkinScanner v5.31 Dubai Silicon Oasis, Dubai UAE
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Zouboulis, C.C.; Del Marmol, V.; Mrowietz, U.; Prens, E.P.; Tzellos, T.; Jemec, G.B. Hidradenitis suppurativa/acne inversa: Criteria for diagnosis, severity assessment, classification and disease evaluation. Dermatology 2015, 2, 184–190. [Google Scholar] [CrossRef] [PubMed]
- Lipsker, D.; Severac, F.; Freysz, M. The ABC of hidradenitis suppurativa: A validated glossary on how to name lesions. Dermatology 2016, 2, 137–142. [Google Scholar] [CrossRef]
- Chen, W.; Plewig, G. Should hidradenitis suppurativa/acne inversa best be renamed as “dissecting terminal hair folliculitis”? Exp. Dermatol. 2017, 6, 544–547. [Google Scholar] [CrossRef] [PubMed]
- Gierek, M.; Kitala, D.; Łabuś, W.; Szyluk, K.; Niemiec, P.; Ochała-Gierek, G. Impact of Hidradenitis Suppurativa Surgical Treatment on Health-Related Life Quality. J. Clin. Med. 2022, 11, 4327. [Google Scholar] [CrossRef]
- Nguyen, T.V.; Damiani, G.; Orenstein, L.; Hamzavi, I.; Jemec, G.B. Hidradenitis suppurativa: An update on epidemiology, phenotypes, diagnosis, pathogenesis, comorbidities and quality of life. J. Eur. Acad. Dermatol. Venereol. 2021, 35, 50–61. [Google Scholar] [CrossRef]
- Matusiak, Ł.; Kaszuba, A.; Krasowska, D.; Placek, W.; Szepietowski, J. Epidemiology of hidradenitis suppurativa in Poland in relation to international data. Derm. Rev./Przegl. Derm. 2017, 104, 377–384. [Google Scholar] [CrossRef]
- Collier, E.K.; Parvataneni, R.K.; Lowes, M.A.; Naik, H.B.; Okun, M.; Shi, V.; Hsiao, J.L. Diagnosis and management of hidradenitis suppurativa in women. Am. J. Obstet. Gynecol. 2020, 224, 54–61. [Google Scholar] [CrossRef]
- López-Llunell, C.; Romaní, J.; Garbayo-Salmons, P.; Agut-Busquet, E. Vulvar hidradenitis suppurativa: Clinical cross-sectional study of 25 patients. J. Dermatol. 2021, 48, 457–463. [Google Scholar] [CrossRef]
- Scuderi, N.; Monfrecola, A.; Dessy, L.A.; Fabbrocini, G.; Megna, M.; Monfrecola, G. Medical and Surgical Treatment of Hidradenitis Suppurativa: A Review. Ski. Appendage Disord. 2017, 3, 95–110. [Google Scholar] [CrossRef]
- Jemec, G.B.; Kimball, A.B. Hidradenitis suppurativa: Epidemiology and scope of the problem. J. Am. Acad. Dermatol. 2015, 73 (Suppl. 1), S4–S7. [Google Scholar] [CrossRef]
- Gierek, M.; Ochała-Gierek, G.; Kitala, D.; Łabuś, W.; Bergler-Czop, B. Surgical management of hidradenitis suppurativa. Adv. Dermatol. Allergol. 2022, 15, 35–41. [Google Scholar] [CrossRef]
- Fosnot, J.; Kovach, S.J.; Serletti, J.M. Acellular Dermal Matrix: General Principles for the Plastic Surgeon. Aesthetic Surg. J. 2011, 31 (Suppl. 7), 5S–12S. [Google Scholar] [CrossRef]
- Carlsson, A.H.; Gronet, E.M.; Rose, L.F.; Chan, R. Clinical Applications of Acellular Dermal Matrices in Reconstructive Surgery. Ski. Tissue Eng. Regen. Med. 2016, 6, 109–124. [Google Scholar] [CrossRef]
- Yu, P.; Qi, Z. Prospective Randomized Comparison of Scar Appearances between Cograft of Acellular Dermal Matrix with Autologous Split-Thickness Skin and Autologous Split-Thickness Skin Graft Alone for Full-Thickness Skin Defects of the Extremities. Plast. Reconstr. Surg. 2016, 137, 906e. [Google Scholar] [CrossRef] [PubMed]
- Del Molino del Barrio, I.; Kirby, J.; Ali, S. The Role of Chemokine and Glycosaminoglycan Interaction in Chemokine-Mediated Migration In Vitro and In Vivo. Methods Enzymol. 2016, 570, 309–333. [Google Scholar] [CrossRef] [PubMed]
- Barkan, D.; Green, J.E.; Chambers, A.F. Extracellular matrix: A gatekeeper in the transition from dormancy to metastatic growth. Eur. J. Cancer 2010, 46, 1181–1188. [Google Scholar] [CrossRef] [PubMed]
- Busuioc, C.J.; Popescu, F.C.; Mogoşanu, G.D.; Pârvănescu, H.; Streba, L.; Mogoantă, L. Histological and immunohistochemical study of cutaneous angiogenesis process in experimental third-degree skin burns treated with allograft. Rom. J. Morphol. Embryol. (Rev. Roum. Morphol. Embryol.) 2012, 53, 1061–1067. [Google Scholar]
- Kitala, D.; Kawecki, M.; Klama-Baryła, A.; Łabuś, W.; Kraut, M.; Glik, J.; Ryszkiel, I.; Kawecki, M.P.; Nowak, M. Allogeneic vs. Autologous Skin Grafts in the Therapy of Patients with Burn Injuries: A Restrospective, Open-label Clinical Study with Pair Matching. Adv. Clin. Exp. Med. 2016, 25, 923–929. [Google Scholar] [CrossRef] [PubMed]
- Glik, J.; Kawecki, M.; Kitala, D.; Klama-Baryła, A.; Łabuś, W.; Grabowski, M.; Durdzińska, A.; Nowak, M.; Misiuga, M.; Kasperczyk, A. A new option for definitive burn wound closure—Pair matching type of retrospective case-control study of hand burns in the hospitalised patients group in the Dr Stanislaw Sakiel Centre for Burn Treatment between 2009 and 2015. Int. Wound J. 2017, 14, 849–855. [Google Scholar] [CrossRef] [PubMed]
- Prim, P.M.; Kim, H.S.; Shapiro, L.E.; Lee, J.S.; Kaan, J.H.; Jackson, J.D.; Yoo, J.J.; Atala, A.; Lee, S.J. In vitro skin expansion: Wound healing assessment. Wound Repair Regen. 2017, 25, 398–407. [Google Scholar] [CrossRef]
- Grossova, I.; Zajicek, R.; Kubok, R.; Smula, M.C. The treatment of palmar contact burns in children: A five-year review. Ann. Burn. Fire Disasters 2017, 30, 5–8. [Google Scholar]
- Łabuś, W.; Glik, J.; Klama-Baryła, A.; Kitala, D.; Kraut, M.; Maj, M.; Nowak, M.; Misiuga, M.; Marcinkowski, A.; Trzebicka, B.; et al. Atomic force microscopy in the production of a biovital skin graft based on human acellular dermal matrix produced in-house and in vitro cultured human fibroblasts. J. Biomed. Mater. Res. Part B Appl. Biomater. 2018, 106, 726–733. [Google Scholar] [CrossRef] [PubMed]
- Kawecki, M.; Łabuś, W.; Klama-Baryla, A.; Kitala, D.; Kraut, M.; Glik, J.; Misiuga, M.; Nowak, M.; Bielecki, T.; Kasperczyk, A. A review of decellurization methods caused by an urgent need for quality control of cell-free extracellular matrix’ scaffolds and their role in regenerative medicine. J. Biomed. Mater. Res. Part B Appl. Biomater. 2018, 106, 909–923. [Google Scholar] [CrossRef] [PubMed]
- Guo, R.; Teng, J.; Xu, S.; Ma, L.; Huang, A.; Gao, C. Comparison studies of the in vivo treatment of full-thickness excisional wounds and burns by an artificial bilayer dermal equivalent and J-1 acellular dermal matrix. Wound Repair Regen. 2014, 22, 390–398. [Google Scholar] [CrossRef]
- Garcia, O., Jr.; Scott, J.R. Analysis of acellular dermal matrix integration and revascularization following tissue expander breast reconstruction in a clinically relevant large-animal model. Plast. Reconstr. Surg. 2013, 131, 741e–751e. [Google Scholar] [CrossRef]
- Butterfield, J.L. 440 Consecutive immediate, implant-based, single-surgeon breast reconstructions in 281 patients: A comparison of early outcomes and costs between SurgiMend fetal bovine and AlloDerm human cadaveric acellular dermal matrices. Plast. Reconstr. Surg. 2013, 131, 940–951. [Google Scholar] [CrossRef] [PubMed]
- Ott, H.C.; Matthiesen, T.S.; Goh, S.K.; Black, L.D.; Kren, S.M.; Netoff, T.I.; Taylor, D.A. Perfusion-decellularized matrix: Using nature’s platform to engineer a bioartificial heart. Nat. Med. 2008, 14, 213–221. [Google Scholar] [CrossRef]
- Evans, A.K.; Kon, N.D. Extracellular matrix for repair of type IV laryngotracheo-esophageal cleft. Int. J. Pediatric Otorhinolaryngol. 2015, 79, 2484–2486. [Google Scholar] [CrossRef] [PubMed]
- Valerio, I.L.; Campbell, P.; Sabino, J.; Dearth, C.L.; Fleming, M. The use of urinary bladder matrix in the treatment of trauma and combat casualty wound care. Regen. Med. 2015, 10, 611–622. [Google Scholar] [CrossRef]
- Sandby-Møller, J.; Poulsen, T.; Wulf, H.C. Epidermal thickness at different body sites: Relationship to age, gender, pigmentation, blood content, skin type and smoking habits. Acta Derm.-Venereol. 2003, 83, 410–413. [Google Scholar] [CrossRef]
- Yudovsky, D.; Pilon, L. Rapid and accurate estimation of blood saturation, melanin content, and epidermis thickness from spectral diffuse reflectance. Appl. Opt. 2010, 49, 1707–1719. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.; Hwang, K. Skin thickness of Korean adults. Surg. Radiol. Anat. (SRA) 2002, 24, 183–189. [Google Scholar] [CrossRef] [PubMed]
- Gschwantler-Kaulich, D.; Schrenk, P.; Bjelic-Radisic, V.; Unterrieder, K.; Leser, C.; Fink-Retter, A.; Salama, M.; Singer, C. Mesh versus acellular dermal matrix in immediate implant-based breast reconstruction—A prospective randomized trial. Eur. J. Surg. Oncol. 2016, 42, 665–671. [Google Scholar] [CrossRef] [PubMed]
- Hallberg, H.; Rafnsdottir, S.; Selvaggi, G.; Strandell, A.; Samuelsson, O.; Stadig, I.; Svanberg, T.; Hansson, E.; Lewin, R. Benefits and risks with acellular dermal matrix (ADM) and mesh support in immediate breast reconstruction: A systematic review and meta-analysis. J. Plast. Surg. Hand Surg. 2018, 52, 130–147. [Google Scholar] [CrossRef] [PubMed]
- Ibrahim, A.M.; Koolen, P.G.; Ganor, O.; Markarian, M.K.; Tobias, A.M.; Lee, B.T.; Lin, S.J.; Mureau, M.A. Does acellular dermal matrix really improve aesthetic outcome in tissue expander/implant-based breast reconstruction? Aesthetic Plast. Surg. 2015, 39, 359–368. [Google Scholar] [CrossRef]
- Madsen, R.J., Jr.; Chim, J.; Ang, B.; Fisher, O.; Hansen, J. Variance in the origin of the pectoralis major muscle: Implications for implant-based breast reconstruction. Ann. Plast. Surg. 2015, 74, 111–113. [Google Scholar] [CrossRef] [PubMed]
- Mendenhall, S.D.; Anderson, L.A.; Ying, J.; Boucher, K.M.; Liu, T.; Neumayer, L.A.; Agarwal, J.P. The BREASTrial: Stage I. Outcomes from the time of tissue expander and acellular dermal matrix placement to definitive reconstruction. Plast. Reconstr. Surg. 2015, 135, 29e–42e. [Google Scholar] [CrossRef]
- Haney, N.M.; Huang, M.M.; Liu, J.L.; Hawksworth, D.J.; Burnett, A.L. Acellular Dermal Matrix Tissues in Genitourinary Reconstructive Surgery: A Review of the Literature and Case Discussions. Sex. Med. Rev. 2021, 9, 488–497. [Google Scholar] [CrossRef]
- Walter, R.J.; Matsuda, T.; Reyes, H.M.; Walter, J.M.; Hanumadass, M. Characterization of acellular dermal matrices (ADMs) prepared by two different methods. Burn. J. Int. Soc. Burn Inj. 1998, 24, 104–113. [Google Scholar] [CrossRef]
- Rössner, E.; Smith, M.D.; Petschke, B.; Schmidt, K.; Vitacolonna, M.; Syring, C.; von Versen, R.; Hohenberger, P. Epiflex(®) a new decellularised human skin tissue transplant: Manufacture and properties. Cell Tissue Bank. 2011, 12, 209–217. [Google Scholar] [CrossRef]
- Ge, L.; Zheng, S.; Wei, H. Comparison of histological structure and biocompatibility between human acellular dermal matrix (ADM) and porcine ADM. Burn. J. Int. Soc. Burn Inj. 2009, 35, 46–50. [Google Scholar] [CrossRef]
- Gáspár, K.; Erdei, I.; Péter, Z.; Dezsö, B.; Hunyadi, J.; Juhász, I. Role of acellular dermal matrix allograft in minimal invasive coverage of deep burn wound with bone exposed--case report and histological evaluation. Int. Wound J. 2006, 3, 51–58. [Google Scholar] [CrossRef] [PubMed]
- Karakol, P.; Bozkurt, M. Recent strategic approach in postburn extremity scars and contractures. J. Plast. Surg. Hand Surg. 2021, 55, 153–161. [Google Scholar] [CrossRef] [PubMed]
- Shang, F.; Hou, Q. Effects of allogenic acellular dermal matrix combined with autologous razor-thin graft on hand appearance and function of patients with extensive burn combined with deep hand burn. Int. Wound J. 2021, 18, 279–286. [Google Scholar] [CrossRef]
- Chen, X.; Feng, X.; Xie, J.; Ruan, S.; Lin, Y.; Lin, Z.; Shen, R.; Zhang, F. Application of acellular dermal xenografts in full-thickness skin burns. Exp. Ther. Med. 2013, 6, 194–198. [Google Scholar] [CrossRef]
- Hicks, K.E.; Huynh, M.N.; Jeschke, M.; Malic, C. Dermal regenerative matrix use in burn patients: A systematic review. J. Plast. Reconstr. Aesthetic Surg. 2019, 72, 1741–1751. [Google Scholar] [CrossRef]
- Lee, Y.J.; Park, M.C.; Park, D.H.; Hahn, H.M.; Kim, S.M.; Lee, I.J. Effectiveness of Acellular Dermal Matrix on Autologous Split-Thickness Skin Graft in Treatment of Deep Tissue Defect: Esthetic Subjective and Objective Evaluation. Aesthetic Plast. Surg. 2017, 41, 1049–1057. [Google Scholar] [CrossRef] [PubMed]
- Chaffin, A.E.; Buckley, M.C. Extracellular matrix graft for the surgical management of Hurley stage III hidradenitis suppurativa: A pilot case series. J. Wound Care 2020, 29, 624–630. [Google Scholar] [CrossRef]



Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gierek, M.; Łabuś, W.; Słaboń, A.; Ziółkowska, K.; Ochała-Gierek, G.; Kitala, D.; Szyluk, K.; Niemiec, P. Co-Graft of Acellular Dermal Matrix and Split Thickness Skin Graft—A New Reconstructive Surgical Method in the Treatment of Hidradenitis Suppurativa. Bioengineering 2022, 9, 389. https://doi.org/10.3390/bioengineering9080389
Gierek M, Łabuś W, Słaboń A, Ziółkowska K, Ochała-Gierek G, Kitala D, Szyluk K, Niemiec P. Co-Graft of Acellular Dermal Matrix and Split Thickness Skin Graft—A New Reconstructive Surgical Method in the Treatment of Hidradenitis Suppurativa. Bioengineering. 2022; 9(8):389. https://doi.org/10.3390/bioengineering9080389
Chicago/Turabian StyleGierek, Marcin, Wojciech Łabuś, Anna Słaboń, Karolina Ziółkowska, Gabriela Ochała-Gierek, Diana Kitala, Karol Szyluk, and Paweł Niemiec. 2022. "Co-Graft of Acellular Dermal Matrix and Split Thickness Skin Graft—A New Reconstructive Surgical Method in the Treatment of Hidradenitis Suppurativa" Bioengineering 9, no. 8: 389. https://doi.org/10.3390/bioengineering9080389
APA StyleGierek, M., Łabuś, W., Słaboń, A., Ziółkowska, K., Ochała-Gierek, G., Kitala, D., Szyluk, K., & Niemiec, P. (2022). Co-Graft of Acellular Dermal Matrix and Split Thickness Skin Graft—A New Reconstructive Surgical Method in the Treatment of Hidradenitis Suppurativa. Bioengineering, 9(8), 389. https://doi.org/10.3390/bioengineering9080389







