Long-Term Dose Optimization of Adalimumab via Dose Spacing in Patients with Psoriasis
Abstract
:1. Introduction
2. Methods
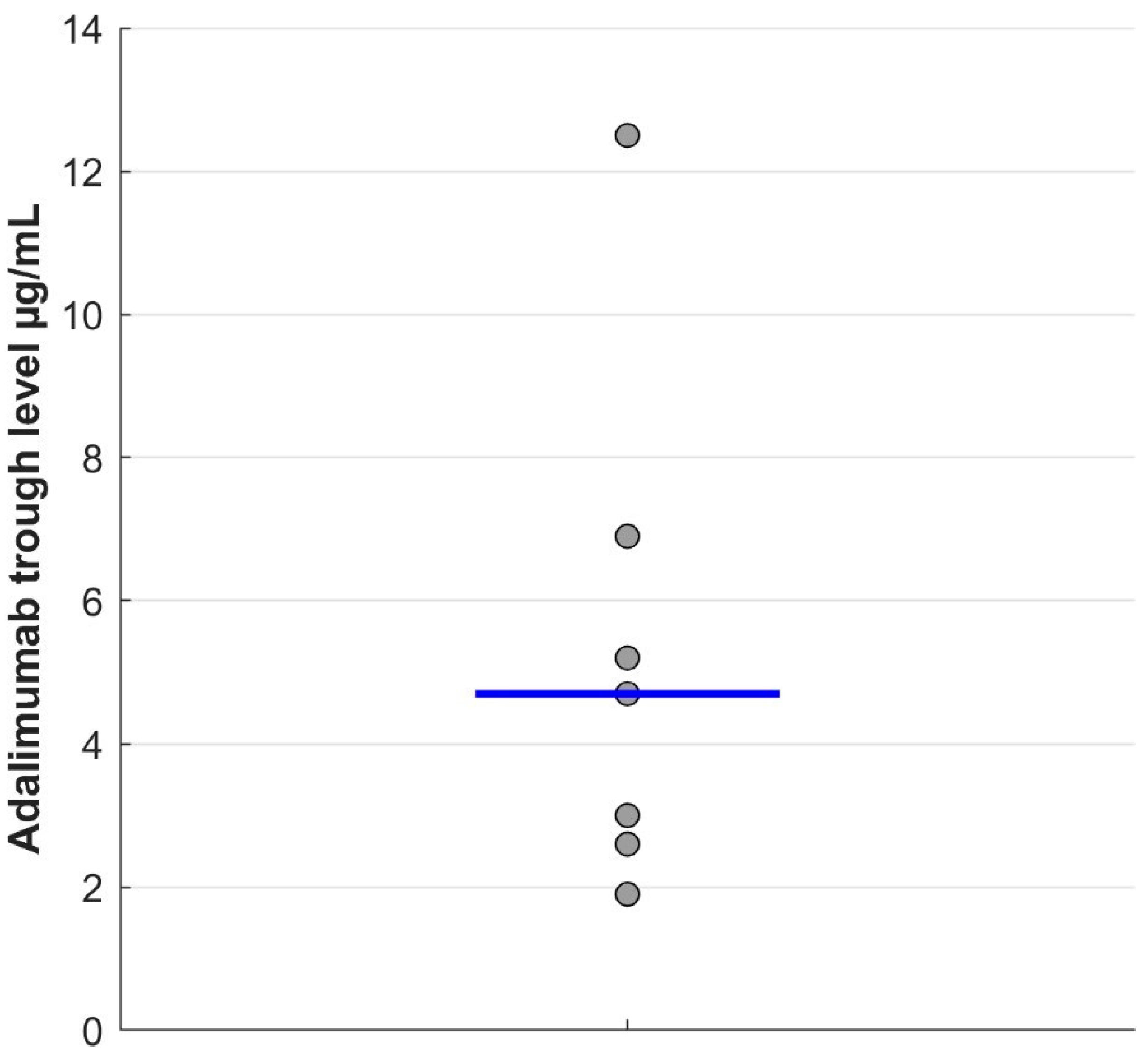
3. Results
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Esposito, M.; Gisondi, P.; Conti, A.; Giunta, A.; Del Giglio, M.; Di Mercurio, M.; Veneziano, L.; Ferrucci, G.; Bianchi, L.; Chimenti, S.; et al. Dose adjustment of biologic therapies for psoriasis in dermatological practice: A retrospective study. J. Eur. Acad. Dermatol. Venereol. 2017, 31, 863–869. [Google Scholar] [CrossRef]
- van Muijen, M.E.; van der Schoot, L.S.; van den Reek, J.M.P.A.; de Jong, E.M.G.J. Attitudes and behaviour regarding dose reduction of biologics for psoriasis: A survey among dermatologists worldwide. Arch. Dermatol. Res. 2021, 314, 687–695. [Google Scholar] [CrossRef]
- Atalay, S.; van den Reek, J.M.; den Broeder, A.A.; van Vugt, L.J.; Otero, M.E.; Njoo, M.D.; Mommers, J.M.; Ossenkoppele, P.M.; Koetsier, M.I.; Berends, M.A.; et al. Comparison of tightly controlled dose reduction of biologics with usual care for patients with psoriasis: A randomized clinical trial. JAMA Dermatol. 2020, 156, 393–400. [Google Scholar] [CrossRef]
- Michielsens, C.A.J.; van Muijen, M.E.; Verhoef, L.M.; van den Reek, J.M.P.A.; de Jong, E.M.G.J. Dose Tapering of Biologics in Patients with Psoriasis: A Scoping Review. Drugs 2021, 81, 349–366. [Google Scholar] [CrossRef] [PubMed]
- Llamas-Velasco, M.; Daudén, E. Reduced doses of biological therapies in psoriasis may increase efficiency without decreasing drug survival. Dermatol. Ther. 2020, 33, e14134. [Google Scholar] [CrossRef] [PubMed]
- Barclani, F.; Loi, C.; Prignano, F.; Ricceri, F.; Giordano, F.; Patrizi, A.; Magnano, M. Down-titration of infliximab: The real-life use in psoriatic patients. J. Drugs Dermatol. 2016, 15, 1584–1586. [Google Scholar]
- Baniandres, O.; Rodriguez-Soria, V.J.; Romero-Jimenez, R.M.; Suarez, R. Dose modification in biologic therapy for moderate to severe psoriasis: A descriptive analysis in a clinical practice setting. Actas Dermo-Sifiliográficas 2015, 106, 569–577. [Google Scholar] [CrossRef] [PubMed]
- Michielsens, C.A.J.; den Broeder, N.; Mulder, M.L.M.; van den Hoogen, F.H.J.; Verhoef, L.M.; den Broeder, A.A. Tumour necrosis factor inhibitor dose adaptation in psoriatic arthritis and axial spondyloarthritis (TAPAS): A retrospective cohort study. Rheumatology 2022, 61, 2307–2315. [Google Scholar] [CrossRef] [PubMed]
- Atalay, S.; van den Reek, J.M.; Otero, M.E.; Njoo, M.D.; Mommers, J.M.; Ossenkoppele, P.M.; Koetsier, M.I.; Berends, M.A.M.; van de Kerkhof, P.C.; Groenewoud, H.; et al. Health Economic Consequences of a Tightly Controlled Dose Reduction Strategy for Adalimumab, Etanercept and Ustekinumab Compared with Standard Psoriasis Care: A Cost-utility Analysis of the CONDOR Study. Acta Derm. Venereol. 2020, 100, adv00340. [Google Scholar] [CrossRef] [PubMed]
- Lecluse, L.L.; Driessen, R.J.; Spuls, P.I.; de Jong, E.M.; Stapel, S.O.; van Doorn, M.B.; Bos, J.D.; Wolbink, G.J. Extent and clinical consequences of antibody formation against adalimumab in patients with plaque psoriasis. Arch. Dermatol. 2010, 146, 127–132. [Google Scholar] [CrossRef] [PubMed]
- Menting, S.P.; van Lümig, P.P.; de Vries, A.C.Q.; van den Reek, J.M.; van der Kleij, D.; de Jong, E.M.; Spuls, P.I.; Lecluse, L.L. Extent and consequences of antibody formation against adalimumab in patients with psoriasis: One-year follow-up. JAMA Dermatol. 2014, 150, 130–136. [Google Scholar] [CrossRef] [PubMed]
- Menting, S.P.; Coussens, E.; Pouw, M.F.; van den Reek, J.M.; Temmerman, L.; Boonen, H.; de Jong, E.M.; Spuls, P.I.; Lambert, J. Developing a Therapeutic Range of Adalimumab Serum Concentrations in Management of Psoriasis: A Step Toward Personalized Treatment. JAMA Dermatol. 2015, 151, 616–622. [Google Scholar] [CrossRef] [PubMed]
- Wilkinson, N.; Tsakok, T.; Dand, N.; Bloem, K.; Duckworth, M.; Baudry, D.; Pushpa-Rajah, A.; Griffiths, C.E.; Reynolds, N.J.; Barker, J.; et al. Defining the Therapeutic Range for Adalimumab and Predicting Response in Psoriasis: A Multicenter Prospective Observational Cohort Study. J. Investig. Dermatol. 2019, 139, 115–123. [Google Scholar] [CrossRef] [PubMed]
- Arican, O.; Aral, M.; Sasmaz, S.; Ciragil, P. Serum levels of TNF-alpha, IFN-gamma, IL-6, IL-8, IL-12, IL-17, and IL-18 in patients with active psoriasis and correlation with disease severity. Mediat. Inflamm. 2005, 2005, 273–279. [Google Scholar] [CrossRef] [PubMed]
| N (%) | Median (Range) | ||
|---|---|---|---|
| Sex | Male | 6 (85.7%) | |
| Female | 1 (14.3%) | ||
| Age (years) | 61 (41–73) | ||
| Duration of Psoriasis Until Dose Spacing (Months) | 35 (6–52) | ||
| BMI (kg/m2) | 26.1 (22.2–40.1) | ||
| Previous Systemic Treatments * | Acitretin | 6 (85.7%) | |
| Methotrexate | 6 (85.7%) | ||
| Efalizumab | 2 (28.6%) | ||
| Alefacept | 1 (14.3%) | ||
| Presence of Psoriatic Arthritis ** | Yes | 3 (42.9%) | |
| No | 4 (57.1%) | ||
| Use of Biosimilars ** | Yes | 2 (28.6%) | |
| No | 5 (71.4%) | ||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Benzaquen, M.; Munshi, M.; Bossart, S.; Feldmeyer, L.; Emelianov, V.; Yawalkar, N.; Cazzaniga, S.; Heidemeyer, K. Long-Term Dose Optimization of Adalimumab via Dose Spacing in Patients with Psoriasis. Bioengineering 2022, 9, 387. https://doi.org/10.3390/bioengineering9080387
Benzaquen M, Munshi M, Bossart S, Feldmeyer L, Emelianov V, Yawalkar N, Cazzaniga S, Heidemeyer K. Long-Term Dose Optimization of Adalimumab via Dose Spacing in Patients with Psoriasis. Bioengineering. 2022; 9(8):387. https://doi.org/10.3390/bioengineering9080387
Chicago/Turabian StyleBenzaquen, Michael, Mohammad Munshi, Simon Bossart, Laurence Feldmeyer, Vladimir Emelianov, Nikhil Yawalkar, Simone Cazzaniga, and Kristine Heidemeyer. 2022. "Long-Term Dose Optimization of Adalimumab via Dose Spacing in Patients with Psoriasis" Bioengineering 9, no. 8: 387. https://doi.org/10.3390/bioengineering9080387
APA StyleBenzaquen, M., Munshi, M., Bossart, S., Feldmeyer, L., Emelianov, V., Yawalkar, N., Cazzaniga, S., & Heidemeyer, K. (2022). Long-Term Dose Optimization of Adalimumab via Dose Spacing in Patients with Psoriasis. Bioengineering, 9(8), 387. https://doi.org/10.3390/bioengineering9080387







