Concept, Design, and Early Prototyping of a Low-Cost, Minimally Invasive, Fully Implantable Left Ventricular Assist Device
Abstract
:1. Introduction
2. Materials and Methods
2.1. Concept Development
2.2. Mathematical Modeling
- -
- Using ΔP = 1.33 × 104 N/m2 and blood density ρ, we calculated the specific work “y”.
- -
- For different impeller diameters D (11–15 mm) and flow rates V (1–10 L/min), the specific diameter δ was calculated.
- -
- For each calculated specific diameter (δ), on the Cordier diagram, we graphically estimated [39] the specific speed (σ) at the intersection of δ with the optimal efficiency interval for axial pumps. After that, the rotation speed of the pump (n) was derived:
2.3. Preliminary Pump Design
2.4. Computational Fluid Dynamics
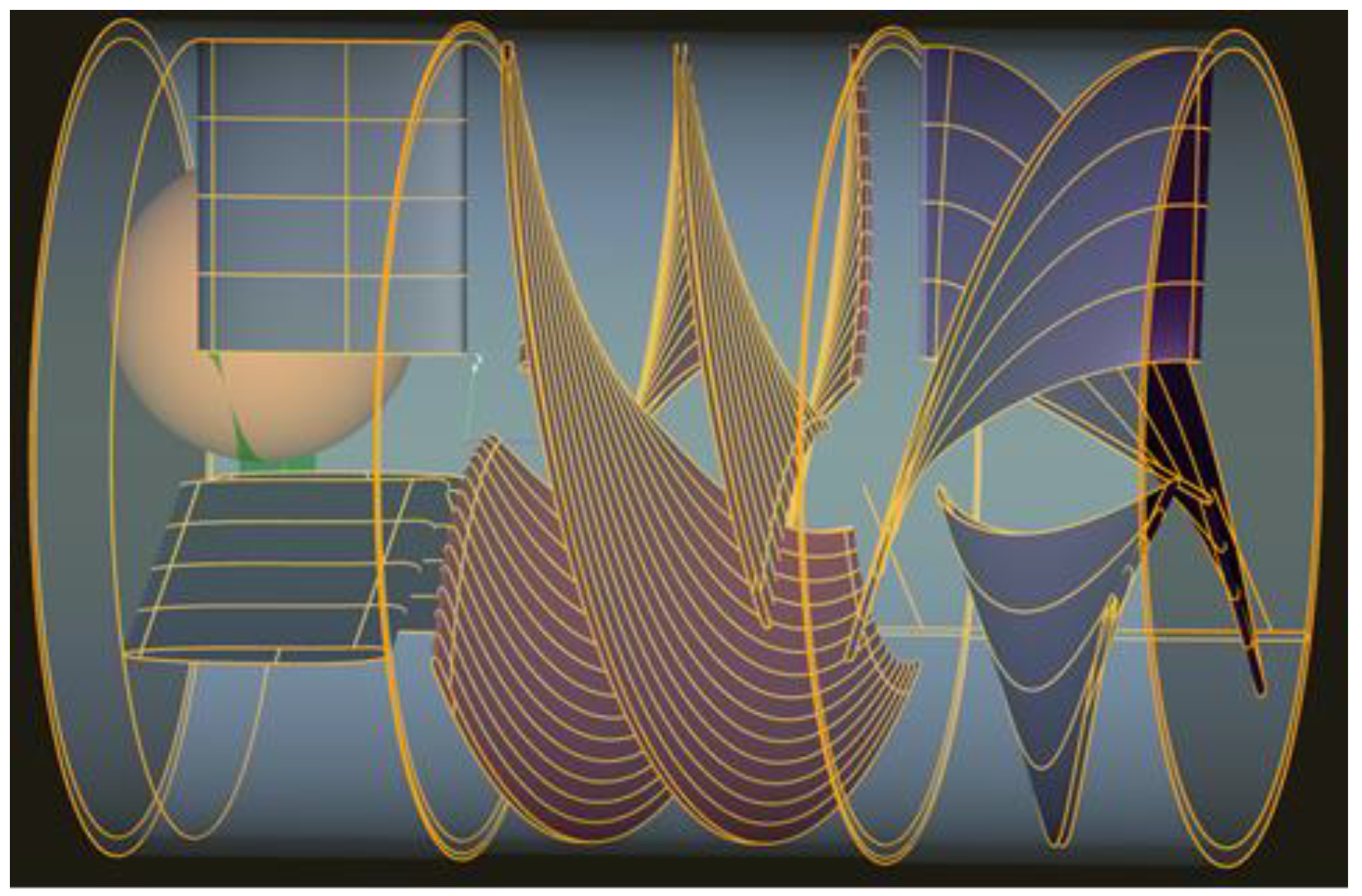
2.5. Computer-Assisted Design
2.6. Early Prototyping
3. Results
3.1. Concept and Design
3.2. Mathematical Modeling
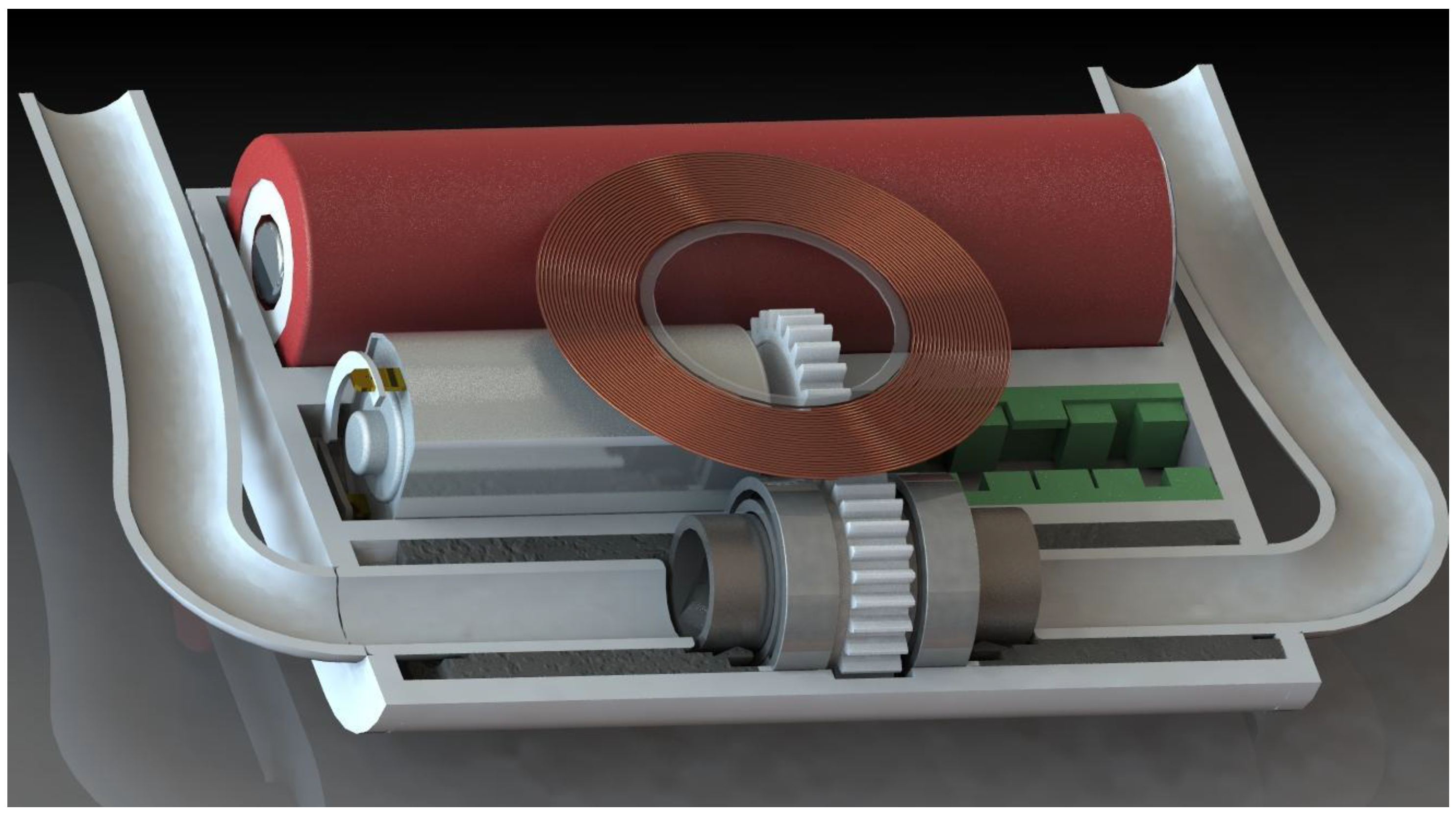
3.3. The Preliminary Design of the Pump
3.4. Computed Fluid Dynamics
3.5. Computer-Assisted Design
3.6. Early Prototyping
4. Discussion
5. Conclusions
6. Patents
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- McDonagh, T.A.; Metra, M.; Adamo, M.; Gardner, R.S.; Baumbach, A.; Böhm, M.; Burri, H.; Butler, J.; Čelutkienė, J.; Chioncel, O.; et al. 2021 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure: Developed by the Task Force for the diagnosis and treatment of acute and chronic heart failure of the European Society of Cardiology (ESC) With the special contribution of the Heart Failure Association (HFA) of the ESC. Eur. Heart J. 2021, 42, 3599–3726. [Google Scholar] [PubMed]
- Russell, S.D.; Miller, L.W.; Pagani, F.D. Advanced heart failure: A call to action. Congest. Heart Fail. 2008, 14, 316–321. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hunt, S.A.; Abraham, W.T.; Chin, M.H.; Feldman, A.M.; Francis, G.S.; Ganiats, T.G.; Jessup, M.; Konstam, M.A.; Mancini, D.M.; Michl, K.; et al. 2009 Focused Update Incorporated Into the ACC/AHA 2005 Guidelines for the Diagnosis and Management of Heart Failure in Adults: A Report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines Developed in Collaboration with the International Society for Heart and Lung Transplantation. J. Am. Coll. Cardiol. 2009, 53, e1–e90. [Google Scholar] [PubMed]
- Ponikowski, P.; Voors, A.A.; Anker, S.D.; Bueno, H.; Cleland, J.G.F.; Coats, A.J.S.; Falk, V.; González-Juanatey, J.R.; Harjola, V.-P.; Jankowska, E.A.; et al. 2016 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure: The Task Force for the diagnosis and treatment of acute and chronic heart failure of the European Society of Cardiology (ESC) Developed with the special contribution of the Heart Failure Association (HFA) of the ESC. Eur. Heart J. 2016, 37, 2129–2200. [Google Scholar]
- Crespo-Leiro, M.G.; Metra, M.; Lund, L.H.; Milicic, D.; Costanzo, M.R.; Filippatos, G.; Gustafsson, F.; Tsui, S.; Barge-Caballero, E.; de Jonge, N.; et al. Advanced heart failure: A position statement of the Heart Failure Association of the European Society of Cardiology. Eur. J. Heart Fail. 2018, 20, 1505–1535. [Google Scholar] [CrossRef]
- Chaudhry, S.P.; Stewart, G.C. Advanced Heart Failure: Prevalence, Natural History, and Prognosis. Heart Fail. Clin. 2016, 12, 323–333. [Google Scholar] [CrossRef]
- Costanzo, M.R.; Mills, R.M.; Wynne, J. Characteristics of “Stage D” heart failure: Insights from the Acute Decompensated Heart Failure National Registry Longitudinal Module (ADHERE LM). Am. Heart J. 2008, 155, 339–347. [Google Scholar] [CrossRef]
- Abouezzeddine, O.F.; Redfield, M.M. Who has advanced heart failure? Definition and epidemiology. Congest. Heart Fail. 2011, 17, 160–168. [Google Scholar] [CrossRef]
- Lippi, G.; Sanchis-Gomar, F. Global epidemiology and future trends of heart failure. AME Med. J. 2020, 5, 15. [Google Scholar] [CrossRef]
- Savarese, G.; Lund, L.H. Global Public Health Burden of Heart Failure. Card. Fail. Rev. 2017, 3, 7–11. [Google Scholar] [CrossRef]
- Heidenreich, P.A.; Albert, N.M.; Allen, L.A.; Bluemke, D.A.; Butler, J.; Fonarow, G.C.; Ikonomidis, J.S.; Khavjou, O.; Konstam, M.A.; Maddox, T.M.; et al. Forecasting the impact of heart failure in the United States. A policy statement from the American heart association. Circ. Heart Fail. 2013, 6, 606–619. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Benjamin, E.; Muntner, P.; Alonso, A.; Bittencourt, M.S.; Callaway, C.W.; Carson, A.P.; American Heart Association Council on Epidemiology and Prevention Statistics Committee and Stroke Statistics Subcommitee. Heart Disease and Stroke Statistics—2019 Update: A report from the American Heart Association. Circulation 2019, 139, e56–e528. [Google Scholar] [CrossRef] [PubMed]
- Roth, G.A.; Mensah, G.A.; Johnson, C.O.; Addolorato, G.; Ammirati, E.; Baddour, L.M.; Barengo, N.C.; Beaton, A.Z.; Benjamin, E.J.; Benziger, C.P.; et al. Global Burden of Cardiovascular Diseases and Risk Factors, 1990–2019: Update from the GBD 2019 Study. J. Am. Coll. Cardiol. 2020, 76, 2982–3021. [Google Scholar] [CrossRef] [PubMed]
- Friedrich, E.B.; Böhm, M. Management of end stage heart failure. Heart 2007, 93, 626–631. [Google Scholar] [CrossRef] [Green Version]
- Khush, K.K.; Cherikh, W.S.; Chambers, D.C.; Harhay, M.O.; Hayes, D.; Hsich, E.; Meiser, B.; Potena, L.; Robinson, A.; Rossano, J.W.; et al. The International Thoracic Organ Transplant Registry of the International Society for Heart and Lung Transplantation: Thirty-sixth adult heart transplantation report—2019; focus theme: Donor and recipient size match. J. Heart Lung Transplant. 2019, 38, 1056–1066. [Google Scholar] [CrossRef]
- Truby, L.K.; Rogers, J.G. Advanced Heart Failure: Epidemiology, Diagnosis, and Therapeutic Approaches. JACC Heart Fail. 2020, 8, 523–536. [Google Scholar] [CrossRef]
- Kirklin, J.K.; Naftel, D.C.; Stevenson, L.W.; Kormos, R.L.; Pagani, F.D.; Miller, M.A.; Ulisney, K.; Young, J.B. INTERMACS Database for Durable Devices for Circulatory Support: First Annual Report. J. Heart Lung Transplant. 2008, 27, 1065–1072. [Google Scholar] [CrossRef]
- Han, J.; Trumble, D.R. Cardiac assist devices: Early concepts, current technologies, and future innovations. Bioengineering 2019, 6, 18. [Google Scholar] [CrossRef] [Green Version]
- Sidhu, K.; Lam, P.H.; Mehra, M.R. Evolving trends in mechanical circulatory support: Clinical development of a fully magnetically levitated durable ventricular assist device. Trends Cardiovasc. Med. 2020, 30, 223–229. [Google Scholar] [CrossRef]
- Graefe, R.; Groß-Hardt, S. Second-generation ventricular assist devices. In Mechanical Circulatory and Respiratory Support; Gregory, S.D., Stevens, M.C., Fraser, J.F., Eds.; Academic Press: Cambridge, MA, USA, 2018; pp. 117–150. ISBN 9780128104910. [Google Scholar]
- Vieira, J.L.; Ventura, H.O.; Mehra, M.R. Mechanical circulatory support devices in advanced heart failure: 2020 and beyond. Prog. Cardiovasc. Dis. 2020, 63, 630–639. [Google Scholar] [CrossRef]
- Rose, E.A.; Moskowitz, A.J.; Packer, M.; Sollano, J.A.; Williams, D.L.; Tierney, A.R.; Heitjan, D.F.; Meier, P.; Ascheim, D.D.; Levitan, R.G.; et al. The REMATCH Trial: Rationale, Design, and End Points. Ann. Thorac. Surg. 1999, 67, 723–730. [Google Scholar] [CrossRef]
- Slaughter, M.S.; Rogers, J.G.; Milano, C.A.; Russell, S.D.; Conte, J.V.; Feldman, D.; Sun, B.; Tatooles, A.J.; Delgado, R.M., III; Long, J.W.; et al. Advanced Heart Failure Treated with Continuous-Flow Left Ventricular Assist Device. N. Engl. J. Med. 2009, 361, 2241–2251. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rogers, J.G.; Pagani, F.D.; Tatooles, A.J.; Bhat, G.; Slaughter, M.S.; Birks, E.J.; Boyce, S.W.; Najjar, S.S.; Jeevanandam, V.; Anderson, A.S.; et al. Intrapericardial Left Ventricular Assist Device for Advanced Heart Failure. N. Engl. J. Med. 2017, 376, 451–460. [Google Scholar] [CrossRef] [PubMed]
- Milano, C.A.; Rogers, J.G.; Tatooles, A.J.; Bhat, G.; Slaughter, M.S.; Birks, E.J.; Mokadam, N.A.; Mahr, C.; Miller, J.S.; Markham, D.W.; et al. HVAD: The ENDURANCE Supplemental Trial. JACC Heart Fail. 2018, 6, 792–802. [Google Scholar] [CrossRef]
- Mehra, M.R.; Uriel, N.; Naka, Y.; Cleveland, J.C., Jr.; Yuzefpolskaya, M.; Salerno, C.T.; Walsh, M.N.; Milano, C.A.; Patel, C.B.; Hutchins, S.W.; et al. A Fully Magnetically Levitated Left Ventricular Assist Device—Final Report. N. Engl. J. Med. 2019, 380, 1618–1627. [Google Scholar] [CrossRef]
- Jorde, U.P.; Kushwaha, S.S.; Tatooles, A.J.; Naka, Y.; Bhat, G.; Long, J.W.; Horstmanshof, D.A.; Kormos, R.L.; Teuteberg, J.J.; Slaughter, M.S.; et al. Results of the destination therapy post-food and drug administration approval study with a continuous flow left ventricular assist device: A prospective study using the INTERMACS registry (interagency registry for mechanically assisted circulatory support. J. Am. Coll. Cardiol. 2014, 63, 1751–1757. [Google Scholar] [CrossRef] [Green Version]
- Kormos, R.L.; Cowger, J.; Pagani, F.D.; Teuteberg, J.J.; Goldstein, D.J.; Jacobs, J.P.; Higgins, R.S.; Stevenson, L.W.; Stehlik, J.; Atluri, P.; et al. The Society of Thoracic Surgeons Intermacs database annual report: Evolving indications, outcomes, and scientific partnerships. J. Heart Lung Transplant. 2019, 38, 114–126. [Google Scholar] [CrossRef]
- Molina, E.J.; Shah, P.; Kiernan, M.S.; Cornwell, W.K.; Copeland, H.; Takeda, K.; Fernandez, F.G.; Badhwar, V.; Habib, R.H.; Jacobs, J.P.; et al. The Society of Thoracic Surgeons Intermacs 2020 Annual Report. Ann. Thorac. Surg. 2021, 111, 778–792. [Google Scholar] [CrossRef]
- Cascino, T.M.; Aaronson, K.D.; Stewart, G.C. Identifying Stage D Heart Failure: Data from the Most Recent Registries. Curr. Heart Fail. Rep. 2019, 16, 130–139. [Google Scholar] [CrossRef]
- Ali, J.M.; Abu-Omar, Y. Complications associated with mechanical circulatory support. Ann. Transl. Med. 2020, 8, 835. [Google Scholar] [CrossRef]
- Marasco, S.F.; Summerhayes, R.; Quayle, M.; McGiffin, D.; Luthe, M. Cost comparison of heart transplant vs. Left ventricular assist device therapy at one year. Clin. Transplant. 2016, 30, 598–605. [Google Scholar] [CrossRef] [PubMed]
- Miller, L.W.; Guglin, M.; Rogers, J. Cost of ventricular assist devices can we afford the progress? Circulation 2013, 127, 743–748. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tadmouri, A.; Blomkvist, J.; Landais, C.; Seymour, J.; Azmoun, A. Cost-effectiveness of left ventricular assist devices for patients with end-stage heart failure: Analysis of the French hospital discharge database. ESC Heart Fail. 2018, 5, 75–86. [Google Scholar] [CrossRef] [PubMed]
- Baras Shreibati, J.; Goldhaber-Fiebert, J.D.; Banerjee, D.; Owens, D.K.; Hlatky, M.A. Cost-Effectiveness of Left Ventricular Assist Devices in Ambulatory Patients with Advanced Heart Failure. JACC Heart Fail. 2017, 5, 110–119. [Google Scholar] [CrossRef] [PubMed]
- Di Nora, C.; Guidetti, F.; Livi, U.; Antonini-Canterin, F. Role of Cardiac Rehabilitation After Ventricular Assist Device Implantation. Heart Fail. Clin. 2021, 17, 273–278. [Google Scholar] [CrossRef] [PubMed]
- Scardulla, F.; Pasta, S.; D’Acquisto, L.; Sciacca, S.; Agnese, V.; Vergara, C.; Quarteroni, A.M.; Clemenza, F.; Bellavia, D.; Pilato, M. Shear stress alterations in the celiac trunk of patients with a continuous-flow left ventricular assist device as shown by in-silico and in-vitro flow analyses. J. Heart Lung Transplant. 2017, 36, 906–913. [Google Scholar] [CrossRef]
- Scardulla, F.; Bellavia, D.; D’Acquisto, L.; Raffa, G.M.; Pasta, S. Particle image velocimetry study of the celiac trunk hemodynamic induced by continuous-flow left ventricular assist device. Med. Eng. Phys. 2017, 47, 47–54. [Google Scholar] [CrossRef]
- Rosarius, N.; Sieß, T.; Reul, H.; Rau, G. Concept, realization, and first in vitro testing of an intraarterial microaxial blood pump with an integrated drive unit. Artif. Organs 1994, 18, 512–516. [Google Scholar] [CrossRef]
- Reul, H. Technical requirements and limitations of miniaturized axial flow pumps for circulatory support. Cardiology 1994, 84, 187–193. [Google Scholar] [CrossRef]
- Mohite, P.N.; Sabashnikov, A.; Simon, A.R.; Weymann, A.; Patil, N.P.; Unsoeld, B.; Bireta, C.; Popov, A.F. Does CircuLite Synergy assist device as partial ventricular support have a place in modern management of advanced heart failure? Expert Rev. Med. Devices 2015, 12, 49–60. [Google Scholar] [CrossRef]
- Lim, H.S.; Shaw, S.; Carter, A.W.; Jayawardana, S.; Mossialos, E.; Mehra, M.R. A clinical and cost-effectiveness analysis of the HeartMate 3 left ventricular assist device for transplant-ineligible patients: A United Kingdom perspective. J. Heart Lung Transplant. 2022, 41, 174–186. [Google Scholar] [CrossRef] [PubMed]
- Schmitto, J.D.; Hanke, J.S.; Rojas, S.V.; Avsar, M.; Haverich, A. First implantation in man of a new magnetically levitated left ventricular assist device (HeartMate III). J. Heart Lung Transplant. 2015, 34, 858–860. [Google Scholar] [CrossRef] [PubMed]
- Subramanian, S.; Kodavatiganti, R.; Plummer, S.; High, K. Perioperative management of a patient with an axial-flow rotary ventricular assist device for laparoscopic ileo-colectomy. J. Anaesthesiol. Clin. Pharmacol. 2012, 28, 101–105. [Google Scholar]
- Patel, C.B.; Blue, L.; Cagliostro, B.; Bailey, S.H.; Entwistle, J.W.; John, R.; Thohan, V.; Cleveland, J.C., Jr.; Goldstein, D.J.; Uriel, N.; et al. Left ventricular assist systems and infection-related outcomes: A comprehensive analysis of the MOMENTUM 3 trial. J. Heart Lung Transplant. 2020, 39, 774–781. [Google Scholar] [CrossRef] [Green Version]
- Juraszek, A.; Smólski, M.; Kołsut, P.; Szymański, J.; Litwiński, P.; Kuśmierski, K.; Zakrzewska-Koperska, J.; Sterliński, M.; Dziodzio, T.; Kuśmierczyk, M. Prevalence and management of driveline infections in mechanical circulatory support—A single center analysis. J. Cardiothorac. Surg. 2021, 16, 216. [Google Scholar] [CrossRef]
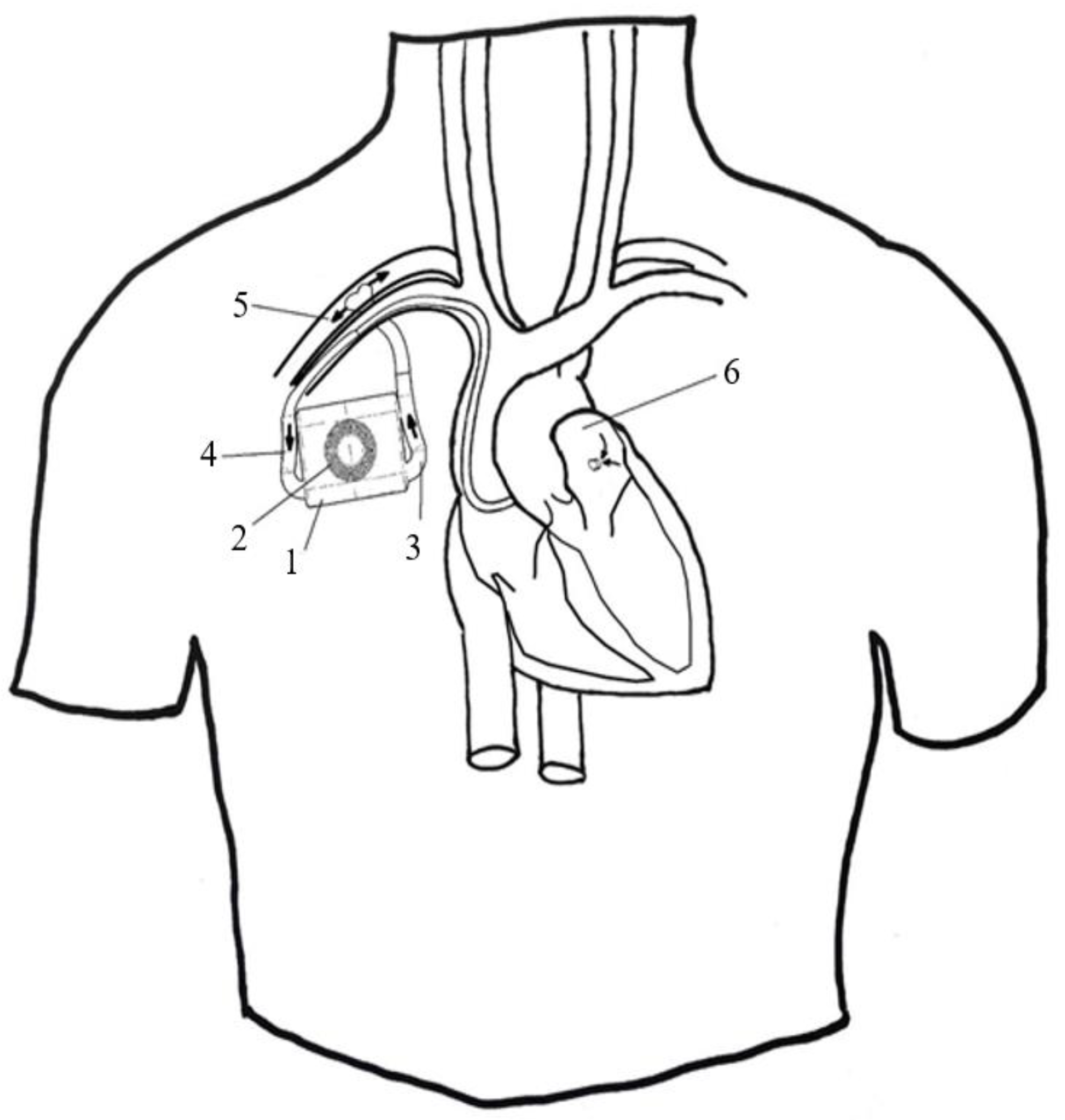




| Diameter (mm) | Rotation per Minute (Maximum Efficiency) | Corresponding Flow (Q) (L) |
|---|---|---|
| 11 | 15,956 | 9.9 |
| 12 | 14,615 | 11.8 |
| 13 | 13,515 | 13.8 |
| 14 | 12,551 | 16 |
| 15 | 11,672 | 18.5 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pleșoianu, F.A.; Pleșoianu, C.E.; Bararu Bojan, I.; Bojan, A.; Țăruș, A.; Tinică, G. Concept, Design, and Early Prototyping of a Low-Cost, Minimally Invasive, Fully Implantable Left Ventricular Assist Device. Bioengineering 2022, 9, 201. https://doi.org/10.3390/bioengineering9050201
Pleșoianu FA, Pleșoianu CE, Bararu Bojan I, Bojan A, Țăruș A, Tinică G. Concept, Design, and Early Prototyping of a Low-Cost, Minimally Invasive, Fully Implantable Left Ventricular Assist Device. Bioengineering. 2022; 9(5):201. https://doi.org/10.3390/bioengineering9050201
Chicago/Turabian StylePleșoianu, Florin Alexandru, Carmen Elena Pleșoianu, Iris Bararu Bojan, Andrei Bojan, Andrei Țăruș, and Grigore Tinică. 2022. "Concept, Design, and Early Prototyping of a Low-Cost, Minimally Invasive, Fully Implantable Left Ventricular Assist Device" Bioengineering 9, no. 5: 201. https://doi.org/10.3390/bioengineering9050201
APA StylePleșoianu, F. A., Pleșoianu, C. E., Bararu Bojan, I., Bojan, A., Țăruș, A., & Tinică, G. (2022). Concept, Design, and Early Prototyping of a Low-Cost, Minimally Invasive, Fully Implantable Left Ventricular Assist Device. Bioengineering, 9(5), 201. https://doi.org/10.3390/bioengineering9050201








