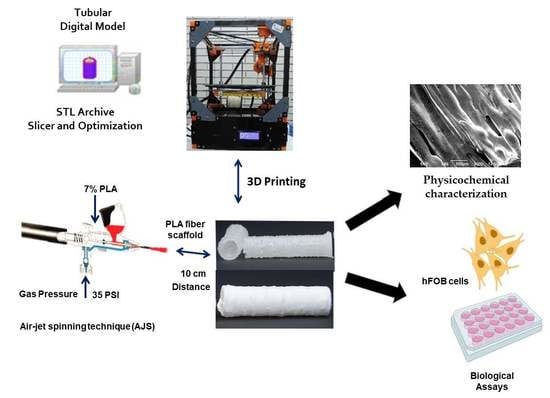
3D-Printed Tubular Scaffolds Decorated with Air-Jet-Spun Fibers for Bone Tissue Applications
Abstract
:1. Introduction
2. Materials and Methods
2.1. Fabrication
2.2. Physicochemical Characterization
2.3. In Vitro Studies
2.4. Statistical Analysis
3. Results and Discussion
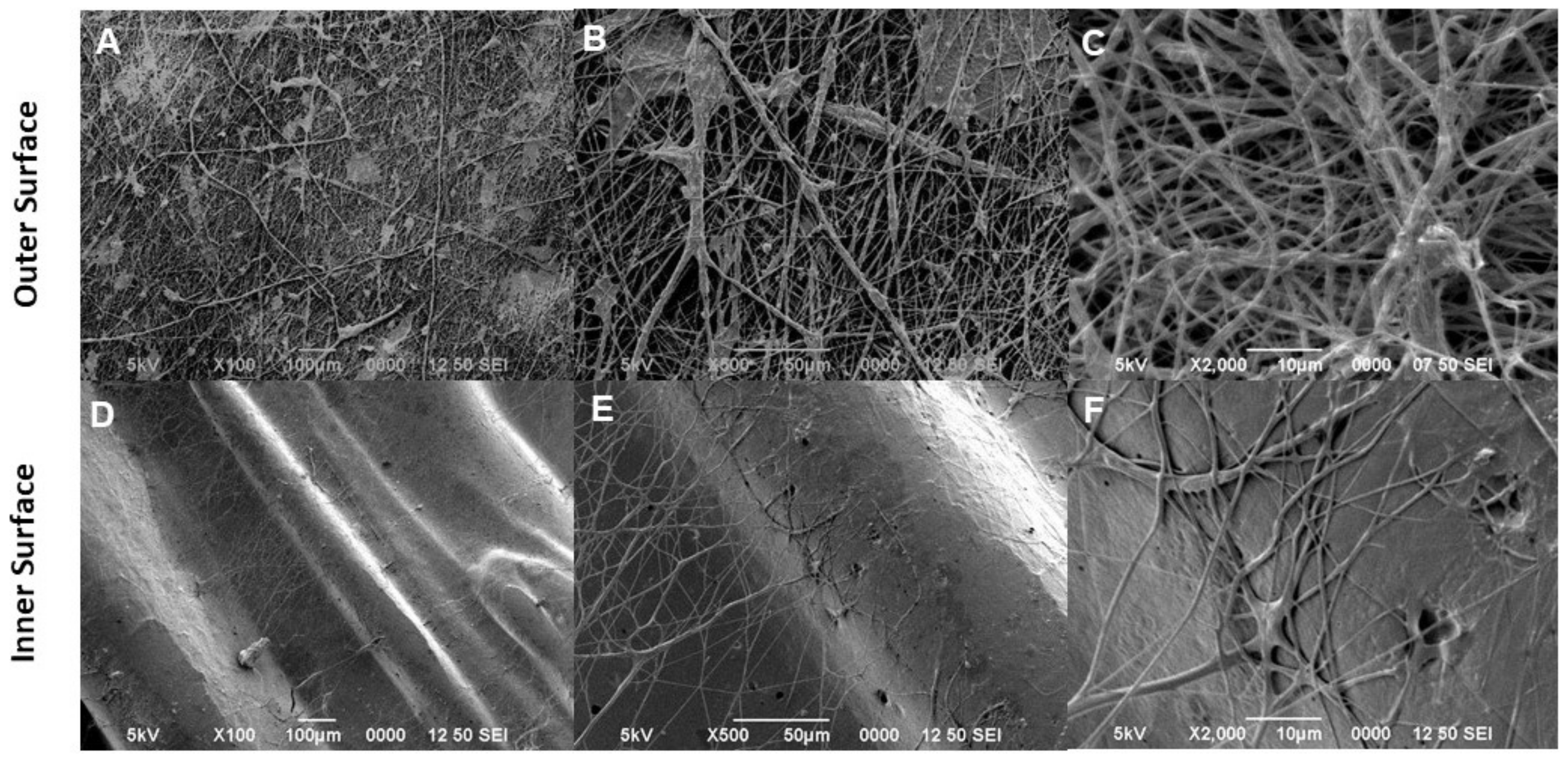
3.1. Morphological Characterization
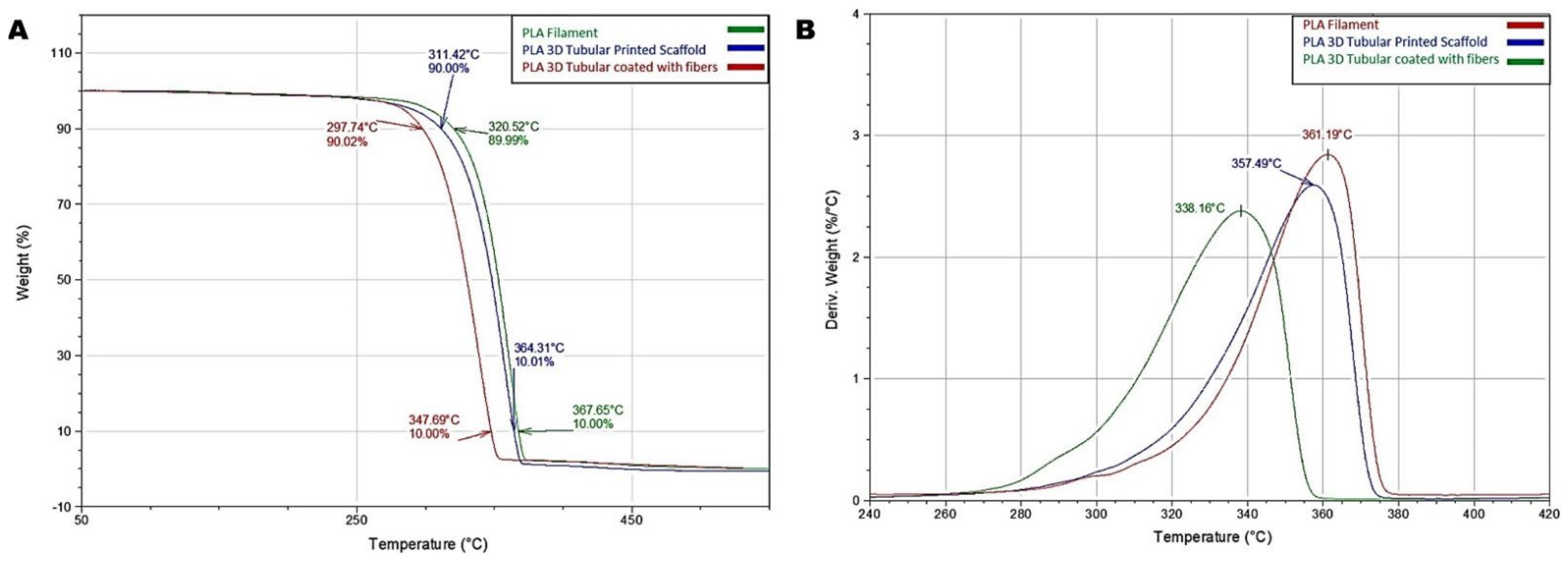
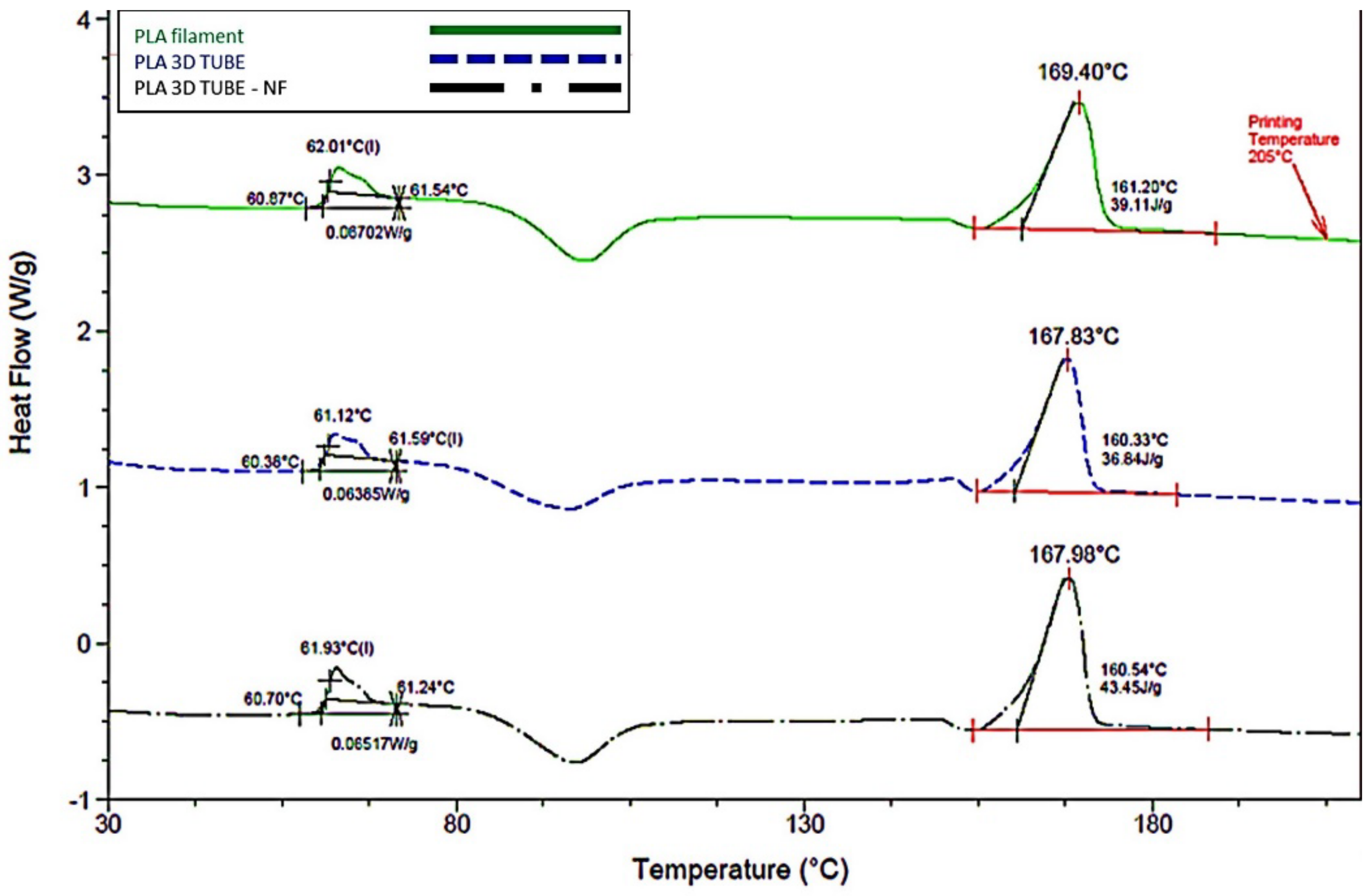
3.2. Thermal Response
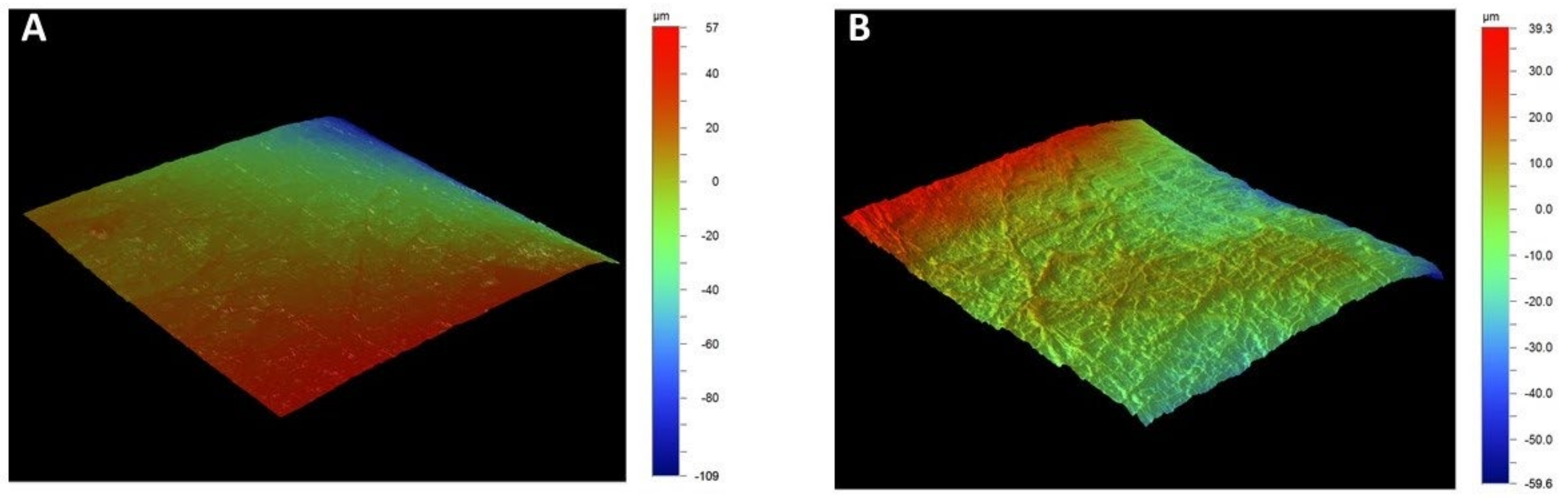
3.3. Profilometry
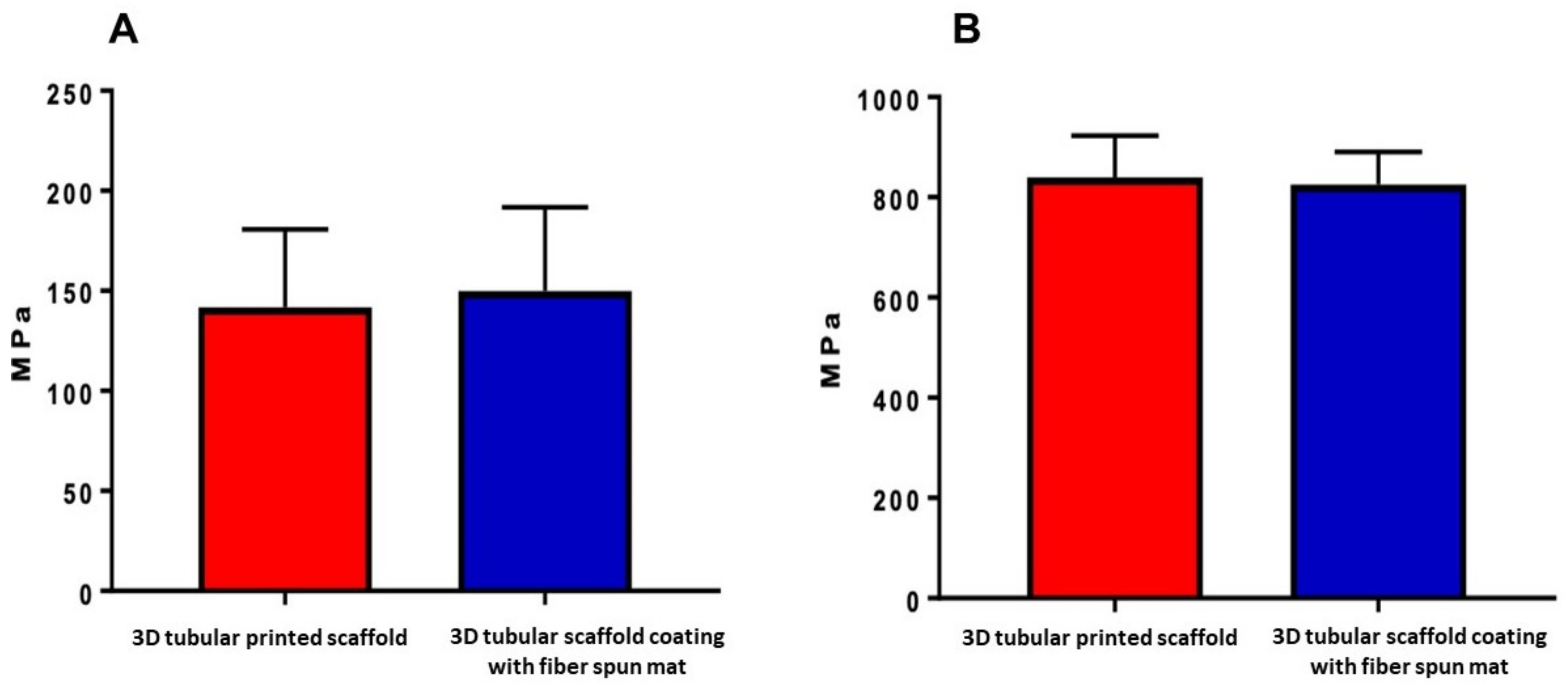
3.4. Mechanical Characterization
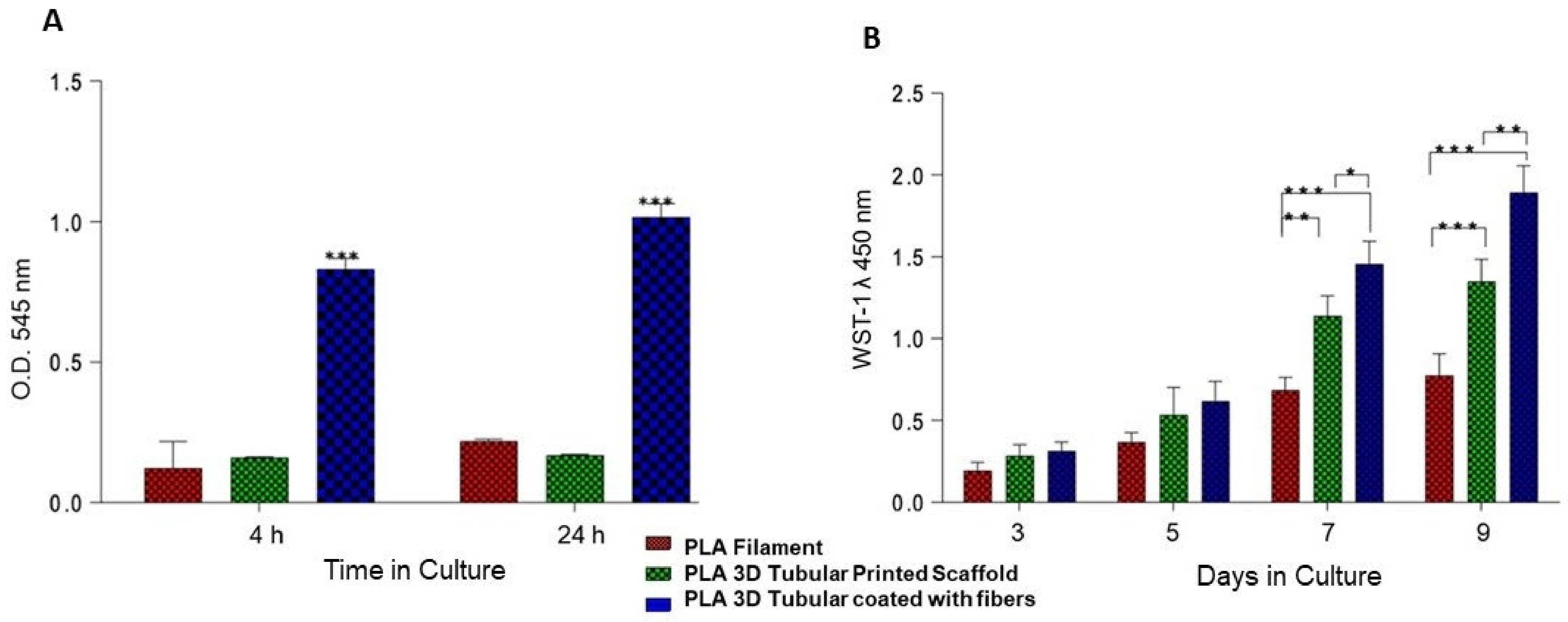
3.5. Cellular Adhesion and Proliferation
3.6. Cellular Morphology
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Guarino, V.; Gloria, A.; Raucci, M.G.; De Santis, R.; Ambrosio, L. Bio-inspired composite and cell instructive platforms for bone regeneration. Int. Mater. Rev. 2012, 57, 256–275. [Google Scholar] [CrossRef]
- Ambrosio, L.; Guarino, V.; Sanginario, V.; Torricelli, P.; Fini, M.; Ginebra, M.-P.; A Planell, J.; Giardino, R. Injectable calcium-phosphate-based composites for skeletal bone treatments. Biomed. Mater. 2012, 7, 024113. [Google Scholar] [CrossRef] [PubMed]
- Bahraminasab, M. Challenges on optimization of 3D-printed bone scaffolds. Biomed. Eng. Online 2020, 19, 69. [Google Scholar] [CrossRef] [PubMed]
- Bhattacharjee, P.; Kundu, B.; Naskar, D.; Kim, H.-W.; Maiti, T.K.; Bhattacharya, D.; Kundu, S.C. Silk scaffolds in bone tissue engineering: An overview. Acta Biomater. 2017, 63, 1–17. [Google Scholar] [CrossRef]
- Vyas, C.; Ates, G.; Aslan, E.; Hart, J.; Huang, B.; Bartolo, P. Three-Dimensional Printing and Electrospinning Dual-Scale Polycaprolactone Scaffolds with Low-Density and Oriented Fibers to Promote Cell Alignment. 3D Print. Add. Manuf. 2020, 7, 105–113. [Google Scholar] [CrossRef]
- Guarino, V.; Cirillo, V.; Ambrosio, L. Bicomponent electrospun scaffolds to design extracellular matrix tissue analogs. Expert Rev. Med. Devices 2016, 13, 83–102. [Google Scholar] [CrossRef]
- Alvarez Perez, M.A.; Guarino, V.; Cirillo, V.; Ambrosio, L. In vitro mineralization and bone osteogenesis in poly(ε-caprolactone)/gelatin nanofibers. J. Biomed. Mater. Res. Part A 2012, 100, 3008–3019. [Google Scholar] [CrossRef]
- Longoni, A.; Li, J.; Lindberg, G.C.; Rnjak-Kovacina, J.; Wise, L.M.; Hooper, G.J.; Woodfield, T.B.; Kieser, D.C.; Lim, K.S. Strategies for inclusion of growth factors into 3D printed bone grafts. Essays Biochem. 2021, 65, 569–585. [Google Scholar] [CrossRef]
- De Witte, T.-M.; Fratila-Apachitei, L.E.; Zadpoor, A.A.; Peppas, N.A. Bone tissue engineering via growth factor delivery: From scaffolds to complex matrices. Regen. Biomater. 2018, 5, 197–211. [Google Scholar] [CrossRef] [Green Version]
- Manferdini, C.; Guarino, V.; Zini, N.; Raucci, M.G.; Ferrari, A.; Grassi, F.; Gabusi, E.; Squarzoni, S.; Facchini, A.; Ambrosio, L.; et al. Mineralization behavior with mesenchymal stromal cells in a biomimetic hyaluronic acid-based scaffold. Biomaterials 2010, 31, 3986–3996. [Google Scholar] [CrossRef]
- Kowalczewski, C.J.; Saul, J.M. Biomaterials for the Delivery of Growth Factors and Other Therapeutic Agents in Tissue Engineering Approaches to Bone Regeneration. Front. Pharmacol. 2018, 9, 513. [Google Scholar] [CrossRef] [Green Version]
- Guarino, V.; Altobelli, R.; Cirillo, V.; Cummaro, A.; Ambrosio, L. Additive electrospraying: A route to process electrospun scaffolds for controlled molecular release. Polym. Adv. Technol. 2015, 26, 1359–1369. [Google Scholar] [CrossRef]
- Guarino, V.; Lewandowska, M.; Bil, M.; Polak, B.; Ambrosio, L. Morphology and degradation properties of PCL/HYAFF11® composite scaffolds with multi-scale degradation rate. Compos. Sci. Technol. 2010, 70, 1826–1837. [Google Scholar] [CrossRef]
- Khodir, W.W.A.; Guarino, V.; Alvarez-Perez, M.; Cafiero, C.; Ambrosio, L. Trapping tetracycline-loaded nanoparticles into polycaprolactone fiber networks for periodontal regeneration therapy. J. Bioact. Compat. Polym. 2013, 28, 258–273. [Google Scholar] [CrossRef]
- Guarino, V.; Gloria, A.; Raucci, M.G.; Ambrosio, L. Hydrogel-Based Platforms for the Regeneration of Osteochondral Tissue and Intervertebral Disc. Polymers 2012, 4, 1590–1612. [Google Scholar] [CrossRef] [Green Version]
- Guduric, V.; Metz, C.; Siadous, R.; Bareille, R.; Levato, R.; Engel, E.; Fricain, J.-C.; Devillard, R.; Luzanin, O.; Catros, S. Layer-by-layer bioassembly of cellularized polylactic acid porous membranes for bone tissue engineering. J. Mater. Sci. Mater. Electron. 2017, 28, 78. [Google Scholar] [CrossRef] [Green Version]
- Chen, H.; Han, Q.; Wang, C.; Liu, Y.; Chen, B.; Wang, J. Porous Scaffold Design for Additive Manufacturing in Orthopedics: A Review. Front. Bioeng. Biotechnol. 2020, 8, 609. [Google Scholar] [CrossRef]
- Guarino, V.; Causa, F.; Salerno, A.; Ambrosio, L.; Netti, P.A. Design and manufacture of microporous polymeric materials with hierarchal complex structure for biomedical application. Mater. Sci. Technol. 2008, 24, 1111–1117. [Google Scholar] [CrossRef]
- Santos-Rosales, V.; Magariños, B.; Starbird, R.; Suárez-González, J.; Fariña, J.B.; Alvarez-Lorenzo, C.; García-González, C.A. Supercritical CO2 technology for one-pot foaming and sterilization of polymeric scaffolds for bone regeneration. Int. J. Pharm. 2021, 605, 120801. [Google Scholar] [CrossRef]
- Serrano-Bello, J.; Cruz-Maya, I.; Suaste-Olmos, F.; González-Alva, P.; Altobelli, R.; Ambrosio, L.; Medina, L.A.; Guarino, V.; Alvarez-Perez, M.A. In vivo Regeneration of Mineralized Bone Tissue in Anisotropic Biomimetic Sponges. Front. Bioeng. Biotechnol. 2020, 8, 587. [Google Scholar] [CrossRef]
- Yuan, B.; Zhou, S.-Y.; Chen, X.-S. Rapid prototyping technology and its application in bone tissue engineering. J. Zhejiang Univ. Sci. B 2017, 18, 303–315. [Google Scholar] [CrossRef] [Green Version]
- Miszuk, J.; Liang, Z.; Hu, J.; Sanyour, H.; Hong, Z.; Fong, H.; Sun, H. Elastic Mineralized 3D Electrospun PCL Nanofibrous Scaffold for Drug Release and Bone Tissue Engineering. ACS Appl. Bio Mater. 2021, 4, 3639–3648. [Google Scholar] [CrossRef]
- Narayanan, G.; Vernekar, V.N.; Kuyinu, E.; Laurencin, C.T. Poly (lactic acid)-based biomaterials for orthopaedic regenerative engineering. Adv. Drug Deliv. Rev. 2016, 107, 247–276. [Google Scholar] [CrossRef]
- Wang, C.; Huang, W.; Zhoue, Y.; He, L.; He, Z.; Chen, Z.; He, X.; Tian, S.; Liao, J.; Lu, B.; et al. 3D printing of bone tissue engineering scaffolds. Bioact. Mater. 2020, 5, 82–91. [Google Scholar] [CrossRef]
- Pobloth, A.; Schell, H.; Petersen, A.; Beierlein, K.; Kleber, C.; Schmidt-Bleek, K.; Duda, G.N. Tubular open-porous β-tricalcium phosphate polycaprolactone scaffolds as guiding structure for segmental bone defect regeneration in a novel sheep model. J. Tissue Eng. Regen. Med. 2017, 12, 897–911. [Google Scholar] [CrossRef]
- Sun, Y.-X.; Zhang, J.-F.; Li, D.-J.; Wu, X.-M.; Xu, L.-L.; Pan, X.-H.; Li, G. Comparing the osteoconductive potential between tubular and cylindrical beta-tricalcium phosphate scaffolds: An experimental study in rats. J. Biomed. Mater. Res. Part B Appl. Biomater. 2017, 106, 1934–1940. [Google Scholar] [CrossRef]
- Zhang, B.; Wang, L.; Song, P.; Pei, X.; Sun, H.; Wu, L.; Zhou, C.; Wang, K.; Fan, Y.; Zhang, Y. 3D printed bone tissue regenerative PLA/HA scaffolds with comprehensive performance optimizations. Mater. Des. 2021, 201, 109490. [Google Scholar] [CrossRef]
- Arai, K.; Murata, D.; Verissimo, A.R.; Mukae, Y.; Itoh, M.; Nakamura, A.; Morita, S.; Nakayama, K. Fabrication of scaffold-free tubular cardiac constructs using a Bio-3D printer. PLoS ONE 2018, 13, e0209162. [Google Scholar] [CrossRef] [Green Version]
- Savoji, H.; Huyer, L.D.; Mohammadi, M.H.; Lai, B.F.L.; Rafatian, N.; Bannerman, D.; Shoaib, M.; Bobicki, E.R.; Ramachandran, A.; Radisic, M. 3D Printing of Vascular Tubes Using Bioelastomer Prepolymers by Freeform Reversible Embedding. ACS Biomater. Sci. Eng. 2020, 6, 1333–1343. [Google Scholar] [CrossRef]
- Versteegden, L.R.; van Kampen, K.A.; Janke, H.P.; Tiemessen, D.M.; Hoogenkamp, H.R.; Hafmans, T.G.; Roozen, E.A.; Lomme, R.M.; van Goor, H.; Oosterwijk, E.; et al. Tubular collagen scaffolds with radial elasticity for hollow organ regeneration. Acta Biomater. 2017, 52, 1–8. [Google Scholar] [CrossRef]
- Ramakrishna, H.; Li, T.; He, T.; Temple, J.; King, M.W.; Spagnoli, A. Tissue engineering a tendon-bone junction with biodegradable braided scaffolds. Biomater. Res. 2019, 23, 11. [Google Scholar] [CrossRef] [PubMed]
- Cirillo, V.; Guarino, V.; Ambrosio, L. Design of bioactive electrospun scaffolds for bone tissue engineering. J. Appl. Biomater. Funct. Mater. 2012, 10, 223–228. [Google Scholar] [CrossRef] [PubMed]
- Cirillo, V.; Clements, B.A.; Guarino, V.; Bushman, J.; Kohn, J.; Ambrosio, L. A comparison of the performance of mono- and bi-component electrospun conduits in a rat sciatic model. Biomaterials 2014, 35, 8970–8982. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, X.-D.; Chen, X.; Xu, M.-L.; Wang, C.-N.; Zhang, L.-Z.; Zhao, Y.-H.; Zhu, C.-L.; Chen, Y.; Wu, J.; Yang, Y.-M. A partition-type tubular scaffold loaded with PDGF-releasing microspheres for spinal cord repair facilitates the directional migration and growth of cells. Neural Regen. Res. 2018, 13, 1231–1240. [Google Scholar] [CrossRef]
- Wang, W.; Nune, K.; Tan, L.; Zhang, N.; Dong, J.; Yan, J.; Misra, R.; Yang, K. Bone regeneration of hollow tubular magnesium-strontium scaffolds in critical-size segmental defects: Effect of surface coatings. Mater. Sci. Eng. C 2019, 100, 297–307. [Google Scholar] [CrossRef]
- Vazquez-Vazquez, F.C.; Chanes-Cuevas, O.A.; Masuoka, D.; Alatorre, J.A.; Chavarria-Bolaños, D.; Vega-Baudrit, J.R.; Serrano-Bello, J.; Alvarez-Perez, M.A. Biocompatibility of Developing 3D-Printed Tubular Scaffold Coated with Nanofibers for Bone Applications. J. Nanomater. 2019, 2019, 6105818. [Google Scholar] [CrossRef] [Green Version]
- Mohammadian, F.; Eatemadi, A. Drug loading and delivery using nanofibers scaffolds. Artif. Cells Nanomed. Biotechnol. 2016, 45, 881–888. [Google Scholar] [CrossRef]
- Granados-Hernández, M.V.; Serrano-Bello, J.; Montesinos, J.J.; Alvarez-Gayosso, C.; Medina-Velázquez, L.A.; Alvarez-Fregoso, O.; Alvarez-Perez, M.A. In vitro and in vivo biological characterization of poly(lactic acid) fiber scaffolds synthesized by air jet spinning. J. Biomed. Mater. Res. Part B Appl. Biomater. 2017, 106, 2435–2446. [Google Scholar] [CrossRef]
- Scaglione, S.; Guarino, V.; Sandri, M.; Tampieri, A.; Ambrosio, L.; Quarto, R. In vivo lamellar bone formation in fibre coated MgCHA–PCL-composite scaffolds. J. Mater. Sci. Mater. Med. 2012, 23, 117–128. [Google Scholar] [CrossRef]
- Perez, A.R.T.; Roberson, D.A.; Wicker, R.B. Fracture Surface Analysis of 3D-Printed Tensile Specimens of Novel ABS-Based Materials. J. Fail. Anal. Prev. 2014, 14, 343–353. [Google Scholar] [CrossRef]
- Engler, A.J.; Sen, S.; Sweeney, H.L.; Discher, D.E. Matrix Elasticity Directs Stem Cell Lineage Specification. Cell 2006, 126, 677–689. [Google Scholar] [CrossRef] [Green Version]
- Zafar, M.; Najeeb, S.; Khurshid, Z.; Vazirzadeh, M.; Zohaib, S.; Najeeb, B.; Sefat, F. Potential of Electrospun Nanofibers for Biomedical and Dental Applications. Materials 2016, 9, 73. [Google Scholar] [CrossRef]
- Rehfeldt, F.; Engler, A.J.; Eckhardt, A.; Ahmed, F.; Discher, D.E. Cell responses to the mechanochemical microenvironment—Implications for regenerative medicine and drug delivery. Adv. Drug Deliv. Rev. 2007, 59, 1329–1339. [Google Scholar] [CrossRef] [Green Version]
- Kareem, M.M.; Hodgkinson, T.; Sanchez, M.S.; Dalby, M.J.; Tanner, K.E. Hybrid core–shell scaffolds for bone tissue engineering. Biomed. Mater. 2019, 14, 025008. [Google Scholar] [CrossRef]
- Fasolino, I.; Guarino, V.; Cirillo, V.; Ambrosio, L. 5-Azacytidine-mediated hMSC behavior on electrospun scaffolds for skeletal muscle regeneration. J. Biomed. Mater. Res. Part A 2017, 105, 2551–2561. [Google Scholar] [CrossRef]
- Frenot, A.; Chronakis, I.S. Polymer nanofibers assembled by electrospinning. Curr. Opin. Colloid Interface Sci. 2003, 8, 64–75. [Google Scholar] [CrossRef]
- Curcio, E.; Macchiarini, P.; De Bartolo, L. Oxygen mass transfer in a human tissue-engineered trachea. Biomaterials 2010, 31, 5131–5136. [Google Scholar] [CrossRef]
- Curcio, E.; Piscioneri, A.; Morelli, S.; Salerno, S.; Macchiarini, P.; De Bartolo, L. Kinetics of oxygen uptake by cells potentially used in a tissue engineered trachea. Biomaterials 2014, 35, 6829–6837. [Google Scholar] [CrossRef]
- Li, X.; Wang, X.; Yao, D.; Jiang, J.; Guo, X.; Gao, Y.; Li, Q.; Shen, C. Effects of aligned and random fibers with different diameter on cell behaviors. Colloids Surf. B Biointerfaces 2018, 171, 461–467. [Google Scholar] [CrossRef]



Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vazquez-Vazquez, F.C.; Chavarria-Bolaños, D.; Ortiz-Magdaleno, M.; Guarino, V.; Alvarez-Perez, M.A. 3D-Printed Tubular Scaffolds Decorated with Air-Jet-Spun Fibers for Bone Tissue Applications. Bioengineering 2022, 9, 189. https://doi.org/10.3390/bioengineering9050189
Vazquez-Vazquez FC, Chavarria-Bolaños D, Ortiz-Magdaleno M, Guarino V, Alvarez-Perez MA. 3D-Printed Tubular Scaffolds Decorated with Air-Jet-Spun Fibers for Bone Tissue Applications. Bioengineering. 2022; 9(5):189. https://doi.org/10.3390/bioengineering9050189
Chicago/Turabian StyleVazquez-Vazquez, Febe Carolina, Daniel Chavarria-Bolaños, Marine Ortiz-Magdaleno, Vincenzo Guarino, and Marco Antonio Alvarez-Perez. 2022. "3D-Printed Tubular Scaffolds Decorated with Air-Jet-Spun Fibers for Bone Tissue Applications" Bioengineering 9, no. 5: 189. https://doi.org/10.3390/bioengineering9050189
APA StyleVazquez-Vazquez, F. C., Chavarria-Bolaños, D., Ortiz-Magdaleno, M., Guarino, V., & Alvarez-Perez, M. A. (2022). 3D-Printed Tubular Scaffolds Decorated with Air-Jet-Spun Fibers for Bone Tissue Applications. Bioengineering, 9(5), 189. https://doi.org/10.3390/bioengineering9050189