Applications of Ultrasound-Mediated Gene Delivery in Regenerative Medicine
Abstract
1. Introduction
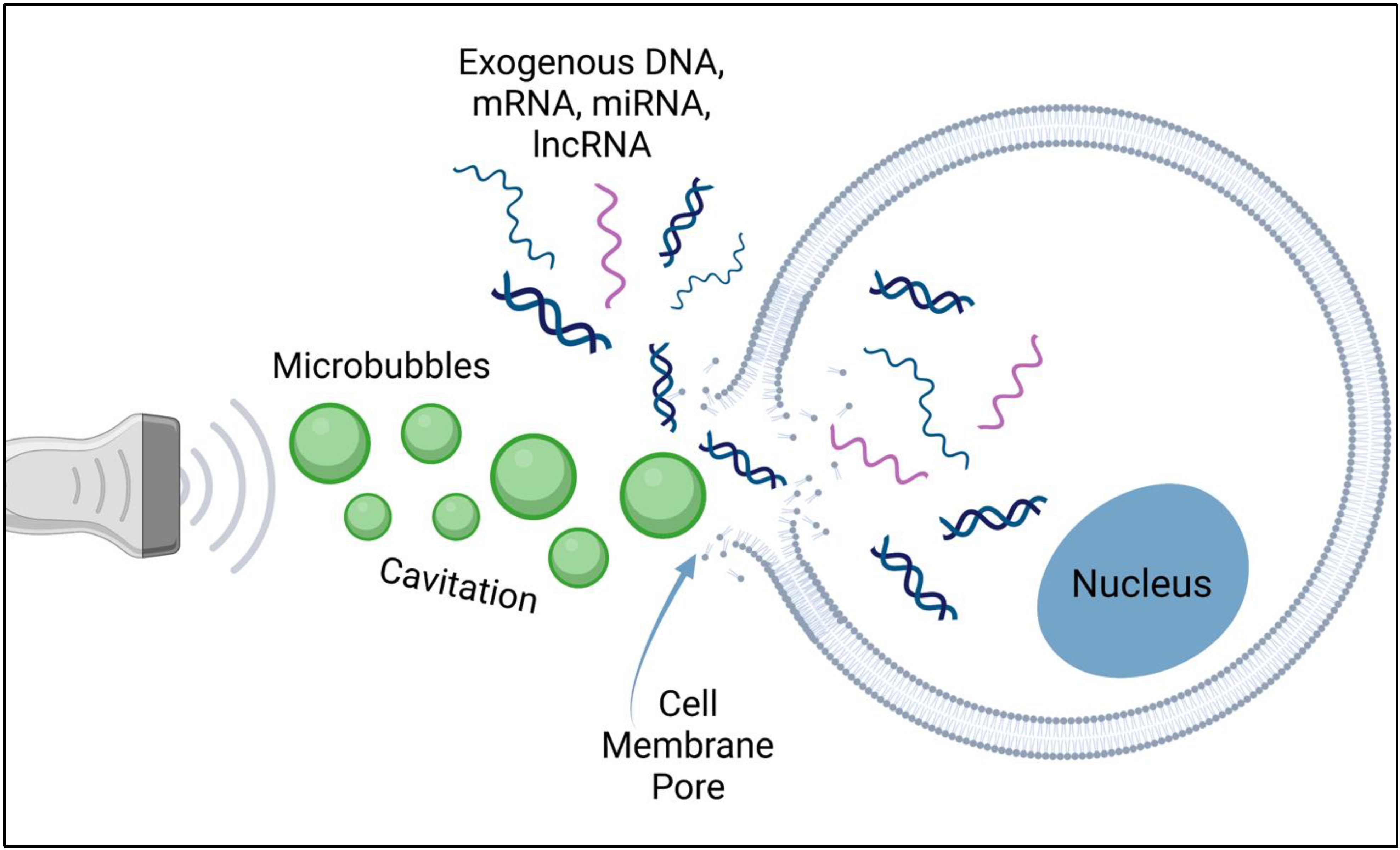
2. Sonoporation-Based Gene Delivery
3. Applications of Sonoporation for Tissue Regeneration
3.1. Sonoporation for Skeletal Tissue Regeneration
3.2. Treatment of Myocardial Ischemia with Sonoporation
3.3. Treatment of Peripheral Ischemia with Sonoporation
3.4. Sonoporation for Pancreatic Islet Regeneration
3.5. Other Applications of Sonoporation for Tissue Regeneration
4. Considerations for Clinical Translation
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Jacques, E.; Suuronen, E.J. The Progression of Regenerative Medicine and its Impact on Therapy Translation. Clin. Transl. Sci. 2020, 13, 440–450. [Google Scholar] [CrossRef] [PubMed]
- Jessop, Z.M.; Al-Sabah, A.; Francis, W.R.; Whitaker, I.S. Transforming healthcare through regenerative medicine. BMC Med. 2016, 14, 115. [Google Scholar] [CrossRef] [PubMed]
- Fang, Y.L.; Chen, X.G.; Godbey, W.T. Gene delivery in tissue engineering and regenerative medicine. J. Biomed. Mater. Res. Part B Appl. Biomater. 2015, 103, 1679–1699. [Google Scholar] [CrossRef] [PubMed]
- Ruiz, M.M.; Regueiro, J.R. New Tools in Regenerative Medicine: Gene Therapy. Adv. Exp. Med. Biol. 2012, 741, 254–275. [Google Scholar] [CrossRef]
- Bleiziffer, O.; Eriksson, E.; Yao, F.; Horch, R.E.; Kneser, U. Gene transfer strategies in tissue engineering. J. Cell. Mol. Med. 2007, 11, 206–223. [Google Scholar] [CrossRef]
- Sung, Y.K.; Kim, S.W. Recent advances in the development of gene delivery systems. Biomater. Res. 2019, 23, 8. [Google Scholar] [CrossRef]
- Waehler, R.; Russell, S.J.; Curiel, D.T. Engineering targeted viral vectors for gene therapy. Nat. Rev. Genet. 2007, 8, 573–587. [Google Scholar] [CrossRef]
- Bouard, D.; Alazard-Dany, N.; Cosset, F.-L. Viral vectors: From virology to transgene expression. Br. J. Pharmacol. 2009, 157, 153–165. [Google Scholar] [CrossRef]
- Liu, Y.; Wang, D.-A. Viral vector-mediated transgenic cell therapy in regenerative medicine: Safety of the process. Expert Opin. Biol. Ther. 2014, 15, 559–567. [Google Scholar] [CrossRef]
- Venkatesan, J.K.; Rey-Rico, A.; Cucchiarini, M. Current Trends in Viral Gene Therapy for Human Orthopaedic Regenerative Medicine. Tissue Eng. Regen. Med. 2019, 16, 345–355. [Google Scholar] [CrossRef]
- Alaee, F.; Sugiyama, O.; Virk, M.S.; Tang, H.; Drissi, H.; Lichtler, A.C.; Lieberman, J.R. Suicide gene approach using a dual-expression lentiviral vector to enhance the safety of ex vivo gene therapy for bone repair. Gene Ther. 2013, 21, 139–147. [Google Scholar] [CrossRef] [PubMed]
- Nayerossadat, N.; Ali, P.A.; Maedeh, T. Viral and nonviral delivery systems for gene delivery. Adv. Biomed. Res. 2012, 1, 27. [Google Scholar] [CrossRef] [PubMed]
- Wolff, J.A.; Budker, V. The Mechanism of Naked DNA Uptake and Expression. Adv. Genet. 2005, 54, 3–20. [Google Scholar] [CrossRef]
- Schillinger, U.; Wexel, G.; Hacker, C.; Kullmer, M.; Koch, C.; Gerg, M.; Vogt, S.; Ueblacker, P.; Tischer, T.; Hensler, D.; et al. A Fibrin Glue Composition as Carrier for Nucleic Acid Vectors. Pharm. Res. 2008, 25, 2946–2962. [Google Scholar] [CrossRef] [PubMed]
- Kaipel, M.; Schützenberger, S.; Hofmann, A.T.; Ferguson, J.; Nau, T.; Redl, H.; Feichtinger, G.A. Evaluation of fibrin-based gene-activated matrices for BMP2/7 plasmid codelivery in a rat nonunion model. Int. Orthop. 2014, 38, 2607–2613. [Google Scholar] [CrossRef] [PubMed]
- Plank, C.; Schillinger, U.; Scherer, F.; Bergemann, C.; Remy, J.-S.; Krötz, F.; Anton, M.; Lausier, J.; Rosenecker, J. The Magnetofection Method: Using Magnetic Force to Enhance Gene Delivery. Biol. Chem. 2003, 384, 737–747. [Google Scholar] [CrossRef]
- Ramamoorth, M.; Narvekar, A. Non viral vectors in gene therapy—An overview. J. Clin. Diagn. Res. 2015, 9, GE01–GE06. [Google Scholar] [CrossRef]
- Zu, H.; Gao, D. Non-viral Vectors in Gene Therapy: Recent Development, Challenges, and Prospects. AAPS J. 2021, 23, 78. [Google Scholar] [CrossRef]
- Lu, Q.L.; Liang, H.-D.; Partridge, T.; Blomley, M.J.K. Microbubble ultrasound improves the efficiency of gene transduction in skeletal muscle in vivo with reduced tissue damage. Gene Ther. 2003, 10, 396–405. [Google Scholar] [CrossRef]
- D’Mello, S.; Atluri, K.; Geary, S.M.; Hong, L.; Elangovan, S.; Salem, A.K. Bone Regeneration Using Gene-Activated Matrices. AAPS J. 2017, 19, 43–53. [Google Scholar] [CrossRef]
- Gantenbein, B.; Tang, S.; Guerrero, J.; Higuita-Castro, N.; Salazar-Puerta, A.I.; Croft, A.S.; Gazdhar, A.; Purmessur, D. Non-viral Gene Delivery Methods for Bone and Joints. Front. Bioeng. Biotechnol. 2020, 8, 598466. [Google Scholar] [CrossRef] [PubMed]
- Sheyn, D.; Kimelman-Bleich, N.; Pelled, G.; Zilberman, Y.; Gazit, D.; Gazit, Z. Ultrasound-based nonviral gene delivery induces bone formation in vivo. Gene Ther. 2007, 15, 257–266. [Google Scholar] [CrossRef] [PubMed]
- Tomizawa, M.; Shinozaki, F.; Motoyoshi, Y.; Sugiyama, T.; Yamamoto, S.; Sueishi, M. Sonoporation: Gene transfer using ultrasound. World J. Methodol. 2013, 3, 39–44. [Google Scholar] [CrossRef] [PubMed]
- Belling, J.N.; Heidenreich, L.K.; Tian, Z.; Mendoza, A.M.; Chiou, T.-T.; Gong, Y.; Chen, N.Y.; Young, T.D.; Wattanatorn, N.; Park, J.H.; et al. Acoustofluidic sonoporation for gene delivery to human hematopoietic stem and progenitor cells. Proc. Natl. Acad. Sci. USA 2020, 117, 10976–10982. [Google Scholar] [CrossRef]
- Rychak, J.J.; Klibanov, A.L. Nucleic acid delivery with microbubbles and ultrasound. Adv. Drug Deliv. Rev. 2014, 72, 82–93. [Google Scholar] [CrossRef]
- Fechheimer, M.; Boylan, J.F.; Parker, S.; Sisken, J.E.; Patel, G.L.; Zimmer, S.G. Transfection of mammalian cells with plasmid DNA by scrape loading and sonication loading. Proc. Natl. Acad. Sci. USA 1987, 84, 8463–8467. [Google Scholar] [CrossRef]
- Tachibana, K.; Uchida, T.; Ogawa, K.; Yamashita, N.; Tamura, K. Induction of cell-membrane porosity by ultrasound. Lancet 1999, 353, 1409. [Google Scholar] [CrossRef]
- Klibanov, A.L. Microbubble contrast agents: Targeted ultrasound imaging and ultrasound-assisted drug-delivery applications. Investig. Radiol. 2006, 41, 354–362. [Google Scholar] [CrossRef]
- Muskula, P.R.; Main, M.L. Safety with Echocardiographic Contrast Agents. Circ. Cardiovasc. Imaging 2017, 10, e005459. [Google Scholar] [CrossRef]
- Kaur, H.; Uludag, H.; El-Bialy, T. Effect of Nonviral Plasmid Delivered Basic Fibroblast Growth Factor and Low Intensity Pulsed Ultrasound on Mandibular Condylar Growth: A Preliminary Study. BioMed Res. Int. 2014, 2014, 426710. [Google Scholar] [CrossRef]
- Nomikou, N.; Feichtinger, G.; Saha, S.; Nuernberger, S.; Heimel, P.; Redl, H.; McHale, A. Ultrasound-responsive gene-activated matrices for osteogenic gene therapy using matrix-assisted sonoporation. J. Tissue Eng. Regen. Med. 2018, 12, e250–e260. [Google Scholar] [CrossRef] [PubMed]
- Nomikou, N.; Feichtinger, G.A.; Redl, H.; McHale, A.P. Ultrasound-mediated gene transfer (sonoporation) in fibrin-based matrices: Potential for use in tissue regeneration. J. Tissue Eng. Regen. Med. 2016, 10, 29–39. [Google Scholar] [CrossRef] [PubMed]
- Lentacker, I.; De Cock, I.; Deckers, R.; De Smedt, S.; Moonen, C. Understanding ultrasound induced sonoporation: Definitions and underlying mechanisms. Adv. Drug Deliv. Rev. 2014, 72, 49–64. [Google Scholar] [CrossRef] [PubMed]
- Qin, P.; Xu, L.; Han, T.; Du, L.; Yu, A.C. Effect of non-acoustic parameters on heterogeneous sonoporation mediated by single-pulse ultrasound and microbubbles. Ultrason. Sonochem. 2016, 31, 107–115. [Google Scholar] [CrossRef] [PubMed]
- Feichtinger, G.A.; Hofmann, A.T.; Slezak, P.; Schützenberger, S.; Kaipel, M.; Schwartz, E.; Neef, A.; Nomikou, N.; Nau, T.; van Griensven, M.; et al. Sonoporation Increases Therapeutic Efficacy of Inducible and Constitutive BMP2/7 In Vivo Gene Delivery. Hum. Gene Ther. Methods 2014, 25, 57–71. [Google Scholar] [CrossRef]
- Nomikou, N.; Tiwari, P.; Trehan, T.; Gulati, K.; McHale, A.P. Studies on neutral, cationic and biotinylated cationic microbubbles in enhancing ultrasound-mediated gene delivery in vitro and in vivo. Acta Biomater. 2012, 8, 1273–1280. [Google Scholar] [CrossRef]
- Li, Y.S.; Davidson, E.; Reid, C.N.; McHale, A.P. Optimising ultrasound-mediated gene transfer (sonoporation) in vitro and prolonged expression of a transgene in vivo: Potential applications for gene therapy of cancer. Cancer Lett. 2009, 273, 62–69. [Google Scholar] [CrossRef]
- Nishida, K.; Doita, M.; Takada, T.; Kakutani, K.-I.; Miyamoto, H.; Shimomura, T.; Maeno, K.; Kurosaka, M. Sustained Transgene Expression in Intervertebral Disc Cells In Vivo Mediated by Microbubble-Enhanced Ultrasound Gene Therapy. Spine 2006, 31, 1415–1419. [Google Scholar] [CrossRef]
- Bez, M.; Sheyn, D.; Tawackoli, W.; Avalos, P.; Shapiro, G.; Giaconi, J.C.; Da, X.; Ben David, S.; Gavrity, J.; Awad, H.A.; et al. In situ bone tissue engineering via ultrasound-mediated gene delivery to endogenous progenitor cells in mini-pigs. Sci. Transl. Med. 2017, 9, eaal3128. [Google Scholar] [CrossRef]
- Bez, M.; Kremen, T.; Tawackoli, W.; Avalos, P.; Sheyn, D.; Shapiro, G.; Giaconi, J.C.; Ben David, S.; Snedeker, J.; Gazit, Z.; et al. Ultrasound-Mediated Gene Delivery Enhances Tendon Allograft Integration in Mini-Pig Ligament Reconstruction. Mol. Ther. 2018, 26, 1746–1755. [Google Scholar] [CrossRef]
- Li, Y.S.; Reid, C.N.; McHale, A.P. Enhancing ultrasound-mediated cell membrane permeabilisation (sonoporation) using a high frequency pulse regime and implications for ultrasound-aided cancer chemotherapy. Cancer Lett. 2008, 266, 156–162. [Google Scholar] [CrossRef] [PubMed]
- Perez, R.; Won, J.-E.; Knowles, J.C.; Kim, H.-W. Naturally and synthetic smart composite biomaterials for tissue regeneration. Adv. Drug Deliv. Rev. 2013, 65, 471–496. [Google Scholar] [CrossRef] [PubMed]
- Osawa, K.; Okubo, Y.; Nakao, K.; Koyama, N.; Bessho, K. Osteoinduction by microbubble-enhanced transcutaneous sonoporation of human bone morphogenetic protein-2. J. Gene Med. 2009, 11, 633–641. [Google Scholar] [CrossRef] [PubMed]
- Fujii, H.; Sun, Z.; Li, S.-H.; Wu, J.; Fazel, S.; Weisel, R.D.; Rakowski, H.; Lindner, J.; Li, R.-K. Ultrasound-Targeted Gene Delivery Induces Angiogenesis After a Myocardial Infarction in Mice. JACC Cardiovasc. Imaging 2009, 2, 869–879. [Google Scholar] [CrossRef]
- Kwekkeboom, R.F.; Sluijter, J.; van Middelaar, B.J.; Metz, C.H.; Brans, M.A.; Kamp, O.; Paulus, W.J.; Musters, R.J. Increased local delivery of antagomir therapeutics to the rodent myocardium using ultrasound and microbubbles. J. Control. Release 2016, 222, 18–31. [Google Scholar] [CrossRef]
- Fujii, H.; Li, S.-H.; Wu, J.; Miyagi, Y.; Yau, T.M.; Rakowski, H.; Egashira, K.; Guo, J.; Weisel, R.D.; Li, R.-K. Repeated and targeted transfer of angiogenic plasmids into the infarcted rat heart via ultrasound targeted microbubble destruction enhances cardiac repair. Eur. Heart J. 2011, 32, 2075–2084. [Google Scholar] [CrossRef]
- Sun, L.; Huang, C.-W.; Wu, J.; Chen, K.-J.; Li, S.-H.; Weisel, R.D.; Rakowski, H.; Sung, H.-W.; Li, R.-K. The use of cationic microbubbles to improve ultrasound-targeted gene delivery to the ischemic myocardium. Biomaterials 2013, 34, 2107–2116. [Google Scholar] [CrossRef]
- Taniyama, Y.; Tachibana, K.; Hiraoka, K.; Aoki, M.; Yamamoto, S.; Matsumoto, K.; Nakamura, T.; Ogihara, T.; Kaneda, Y.; Morishita, R. Development of safe and efficient novel nonviral gene transfer using ultrasound: Enhancement of transfection efficiency of naked plasmid DNA in skeletal muscle. Gene Ther. 2002, 9, 372–380. [Google Scholar] [CrossRef]
- Leong-Poi, H.; Kuliszewski, M.A.; Lekas, M.; Sibbald, M.; Teichert-Kuliszewska, K.; Klibanov, A.L.; Stewart, D.J.; Lindner, J.R. Therapeutic Arteriogenesis by Ultrasound-Mediated VEGF165 Plasmid Gene Delivery to Chronically Ischemic Skeletal Muscle. Circ. Res. 2007, 101, 295–303. [Google Scholar] [CrossRef]
- Kobulnik, J.; Kuliszewski, M.A.; Stewart, D.J.; Lindner, J.R.; Leong-Poi, H. Comparison of Gene Delivery Techniques for Therapeutic Angiogenesis: Ultrasound-Mediated Destruction of Carrier Microbubbles Versus Direct Intramuscular Injection. J. Am. Coll. Cardiol. 2009, 54, 1735–1742. [Google Scholar] [CrossRef]
- Smith, A.H.; Kuliszewski, M.A.; Liao, C.; Rudenko, D.; Stewart, D.J.; Leong-Poi, H. Sustained Improvement in Perfusion and Flow Reserve After Temporally Separated Delivery of Vascular Endothelial Growth Factor and Angiopoietin-1 Plasmid Deoxyribonucleic Acid. J. Am. Coll. Cardiol. 2012, 59, 1320–1328. [Google Scholar] [CrossRef] [PubMed]
- Cao, W.J.; Rosenblat, J.D.; Roth, N.C.; Kuliszewski, M.A.; Matkar, P.N.; Rudenko, D.; Liao, C.; Lee, P.J.; Leong-Poi, H. Therapeutic Angiogenesis by Ultrasound-Mediated MicroRNA-126-3p Delivery. Arter. Thromb. Vasc. Biol. 2015, 35, 2401–2411. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.; Shimoda, M.; Wang, M.-Y.; Ding, J.; Noguchi, H.; Matsumoto, S.; Grayburn, P.A. Regeneration of pancreatic islets in vivo by ultrasound-targeted gene therapy. Gene Ther. 2010, 17, 1411–1420. [Google Scholar] [CrossRef][Green Version]
- Chen, S.; Shimoda, M.; Chen, J.; Matsumoto, S.; Grayburn, P.A. Ectopic transgenic expression of NKX2.2 induces differentiation of adult pancreatic progenitors and mediates islet regeneration. Cell Cycle 2012, 11, 1544–1553. [Google Scholar] [CrossRef][Green Version]
- Chen, S.; Shimoda, M.; Chen, J.; Matsumoto, S.; Grayburn, P.A. Transient overexpression of cyclin D2/CDK4/GLP1 genes induces proliferation and differentiation of adult pancreatic progenitors and mediates islet regeneration. Cell Cycle 2012, 11, 695–705. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Chen, S.; Huang, P.; Meng, X.-L.; Clayton, S.; Shen, J.-S.; Grayburn, P.A. In vivo targeted delivery of ANGPTL8 gene for beta cell regeneration in rats. Diabetologia 2015, 58, 1036–1044. [Google Scholar] [CrossRef][Green Version]
- Henkel, J.; Woodruff, M.; Epari, D.; Steck, R.; Glatt, V.; Dickinson, I.C.; Choong, P.; Schuetz, M.A.; Hutmacher, D.W. Bone Regeneration Based on Tissue Engineering Conceptions—A 21st Century Perspective. Bone Res. 2013, 1, 216–248. [Google Scholar] [CrossRef]
- Kimelman-Bleich, N.; Pelled, G.; Zilberman, Y.; Kallai, I.; Mizrahi, O.; Tawackoli, W.; Gazit, Z.; Gazit, D. Targeted Gene-and-host Progenitor Cell Therapy for Nonunion Bone Fracture Repair. Mol. Ther. 2011, 19, 53–59. [Google Scholar] [CrossRef]
- Aihara, H.; Miyazaki, J.-I. Gene transfer into muscle by electroporation in vivo. Nat. Biotechnol. 1998, 16, 867–870. [Google Scholar] [CrossRef]
- Nakashima, M.; Mizunuma, K.; Murakami, T.; Akamine, A. Induction of dental pulp stem cell differentiation into odontoblasts by electroporation-mediated gene delivery of growth/differentiation factor 11 (Gdf11). Gene Ther. 2002, 9, 814–818. [Google Scholar] [CrossRef]
- Liao, Z.-K.; Tsai, K.-C.; Wang, H.-T.; Tseng, S.-H.; Deng, W.-P.; Chen, W.-S.; Hwang, L.-H. Sonoporation-mediated anti-angiogenic gene transfer into muscle effectively regresses distant orthotopic tumors. Cancer Gene Ther. 2011, 19, 171–180. [Google Scholar] [CrossRef] [PubMed][Green Version]
- McMahon, J.M.; Wells, K.E.; Bamfo, J.E.; Cartwright, M.A.; Wells, D.J. Inflammatory responses following direct injection of plasmid DNA into skeletal muscle. Gene Ther. 1998, 5, 1283–1290. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Betz, O.B.; Betz, V.M.; Nazarian, A.; Egermann, M.; Gerstenfeld, L.C.; Einhorn, T.; Vrahas, M.S.; Bouxsein, M.L.; Evans, C.H. Delayed administration of adenoviral BMP-2 vector improves the formation of bone in osseous defects. Gene Ther. 2007, 14, 1039–1044. [Google Scholar] [CrossRef] [PubMed]
- Delalande, A.; Bureau, M.-F.; Midoux, P.; Bouakaz, A.; Pichon, C. Ultrasound-assisted microbubbles gene transfer in tendons for gene therapy. Ultrasonics 2010, 50, 269–272. [Google Scholar] [CrossRef] [PubMed]
- Shapiro, G.; Wong, A.W.; Bez, M.; Yang, F.; Tam, S.; Even, L.; Sheyn, D.; Ben-David, S.; Tawackoli, W.; Pelled, G.; et al. Multiparameter evaluation of in vivo gene delivery using ultrasound-guided, microbubble-enhanced sonoporation. J. Control. Release 2016, 223, 157–164. [Google Scholar] [CrossRef]
- Frantz, S.; Bauersachs, J.; Ertl, G. Post-infarct remodelling: Contribution of wound healing and inflammation. Cardiovasc. Res. 2008, 81, 474–481. [Google Scholar] [CrossRef]
- Chen, S.; Shimoda, M.; Chen, J.; Grayburn, P.A. Stimulation of adult resident cardiac progenitor cells by durable myocardial expression of thymosin beta 4 with ultrasound-targeted microbubble delivery. Gene Ther. 2012, 20, 225–233. [Google Scholar] [CrossRef]
- Kopechek, J.A.; McTiernan, C.F.; Chen, X.; Zhu, J.; Mburu, M.; Feroze, R.; Whitehurst, D.A.; Lavery, L.; Cyriac, J.; Villanueva, F.S. Ultrasound and Microbubble-targeted Delivery of a microRNA Inhibitor to the Heart Suppresses Cardiac Hypertrophy and Preserves Cardiac Function. Theranostics 2019, 9, 7088–7098. [Google Scholar] [CrossRef]
- Liu, Y.; Li, L.; Su, Q.; Liu, T.; Ma, Z.; Yang, H. Ultrasound-Targeted Microbubble Destruction Enhances Gene Expression of microRNA-21 in Swine Heart via Intracoronary Delivery. Echocardiography 2015, 32, 1407–1416. [Google Scholar] [CrossRef]
- Wang, W.; Tayier, B.; Guan, L.; Yan, F.; Mu, Y. Pre-transplantation of Bone Marrow Mesenchymal Stem Cells Amplifies the Therapeutic Effect of Ultrasound-Targeted Microbubble Destruction–Mediated Localized Combined Gene Therapy in Post-Myocardial Infarction Heart Failure Rats. Ultrasound Med. Biol. 2022, 48, 830–845. [Google Scholar] [CrossRef]
- Bastarrachea, R.A.; Chen, J.; Kent, J.W., Jr.; Nava-Gonzalez, E.J.; Rodriguez-Ayala, E.; Daadi, M.M.; Jorge, B.; Laviada-Molina, H.; Comuzzie, A.G.; Chen, S.; et al. Engineering brown fat into skeletal muscle using ultrasound-targeted microbubble destruction gene delivery in obese Zucker rats: Proof of concept design. IUBMB Life 2017, 69, 745–755. [Google Scholar] [CrossRef] [PubMed]
- Nakashima, M.; Tachibana, K.; Iohara, K.; Ito, M.; Ishikawa, M.; Akamine, A. Induction of Reparative Dentin Formation by Ultrasound-Mediated Gene Delivery of Growth/Differentiation Factor 11. Hum. Gene Ther. 2003, 14, 591–597. [Google Scholar] [CrossRef] [PubMed]
- Nakashima, M. Induction of Dentin Formation on Canine Amputated Pulp by Recombinant Human Bone Morphogenetic Proteins (BMP)-2 and -4. J. Dent. Res. 1994, 73, 1515–1522. [Google Scholar] [CrossRef]
- Sugano, M.; Negishi, Y.; Endo-Takahashi, Y.; Hamano, N.; Usui, M.; Suzuki, R.; Maruyama, K.; Aramaki, Y.; Yamamoto, M. Gene delivery to periodontal tissue using Bubble liposomes and ultrasound. J. Periodontal Res. 2013, 49, 398–404. [Google Scholar] [CrossRef] [PubMed]
- Liao, A.-H.; Huang, Y.-J.; Chuang, H.-C.; Wang, C.-H.; Shih, C.-P.; Chiang, C.-P. Minoxidil-Coated Lysozyme-Shelled Microbubbes Combined with Ultrasound for the Enhancement of Hair Follicle Growth: Efficacy In Vitro and In Vivo. Front. Pharmacol. 2021, 12, 668754. [Google Scholar] [CrossRef] [PubMed]
- Ryu, J.-Y.; Won, E.-J.; Lee, H.A.R.; Kim, J.H.; Hui, E.; Kim, H.P.; Yoon, T.-J. Ultrasound-activated particles as CRISPR/Cas9 delivery system for androgenic alopecia therapy. Biomaterials 2020, 232, 119736. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Z.-Z.; Xia, G.-Y.; Zhang, Y.; Dong, L.; He, B.-Z.; Sun, J.-G. Attenuation of hepatic fibrosis through ultrasound-microbubble-mediated HGF gene transfer in rats. Clin. Imaging 2013, 37, 104–110. [Google Scholar] [CrossRef] [PubMed]
- Yang, D.; Gao, Y.-H.; Tan, K.-B.; Zuo, Z.-X.; Yang, W.-X.; Hua, X.; Li, P.-J.; Zhang, Y.; Wang, G. Inhibition of hepatic fibrosis with artificial microRNA using ultrasound and cationic liposome-bearing microbubbles. Gene Ther. 2013, 20, 1140–1148. [Google Scholar] [CrossRef]
- Snipstad, S.; Vikedal, K.; Maardalen, M.; Kurbatskaya, A.; Sulheim, E.; Davies, C.D.L. Ultrasound and microbubbles to beat barriers in tumors: Improving delivery of nanomedicine. Adv. Drug Deliv. Rev. 2021, 177, 113847. [Google Scholar] [CrossRef]
- Idbaih, A.; Canney, M.; Belin, L.; Desseaux, C.; Vignot, A.; Bouchoux, G.; Asquier, N.; Law-Ye, B.; Leclercq, D.; Bissery, A.; et al. Safety and Feasibility of Repeated and Transient Blood-Brain Barrier Disruption by Pulsed Ultrasound in Patients with Recurrent Glioblastoma. Clin. Cancer Res. 2019, 25, 3793–3801. [Google Scholar] [CrossRef]
- Yang, L.; Yan, F.; Ma, J.; Zhang, J.; Liu, L.; Guan, L.; Zheng, H.; Li, T.-S.; Liang, D.; Mu, Y. Ultrasound-Targeted Microbubble Destruction-Mediated Co-Delivery of Cxcl12 (Sdf-1alpha) and Bmp2 Genes for Myocardial Repair. J. Biomed. Nanotechnol. 2019, 15, 1299–1312. [Google Scholar] [CrossRef] [PubMed]
- Be, P.P.; Alam, M.; Li, S.; Chow, S.K.H.; Zheng, Y. Low-Intensity Pulsed Ultrasound Stimulation for Bone Fractures Healing: A Review. J. Ultrasound Med. 2021, 41, 547–563. [Google Scholar] [CrossRef]
- Yuana, Y.; Jiang, L.; Lammertink, B.H.A.; Vader, P.; Deckers, R.; Bos, C.; Schiffelers, R.M.; Moonen, C.T. Microbubbles-Assisted Ultrasound Triggers the Release of Extracellular Vesicles. Int. J. Mol. Sci. 2017, 18, 1610. [Google Scholar] [CrossRef] [PubMed]
- Bazan-Peregrino, M.; Rifai, B.; Carlisle, R.C.; Choi, J.; Arvanitis, C.D.; Seymour, L.W.; Coussios, C.C. Cavitation-enhanced delivery of a replicating oncolytic adenovirus to tumors using focused ultrasound. J. Control. Release 2013, 169, 40–47. [Google Scholar] [CrossRef] [PubMed]
- Filippi, M.; Dasen, B.; Scherberich, A. Rapid Magneto-Sonoporation of Adipose-Derived Cells. Materials 2021, 14, 4877. [Google Scholar] [CrossRef]
| Delivery Method | Mechanism | Advantages | Limitations | References |
|---|---|---|---|---|
| Naked DNA Injection | Direct injection of DNA at targeted site | Simplest and least expensive delivery method, localized DNA uptake | Poor and variable expression levels, damage to tissue surrounding injection site | [19] |
| Gene-Activated Matrix | Scaffolds implanted for extended release of DNA at targeted site | Directed and sustained gene expression, both in vivo and ex vivo approaches available, 3D template for tissue regeneration | May require other viral or non-viral vectors to increase expression, possible DNA damage during scaffold formation | [20] |
| Magnetofection | Magnetic particles complexed with DNA and an external magnetic field | Fast delivery of nucleic acids, high transduction efficiency, low-dose requirements | Localization can be difficult in vivo, particle size impacts cell entry, cytotoxicity | [6] |
| Electroporation | High voltage electric pulses to increase membrane permeability | High throughput, low cost, more efficient than naked DNA injection or sonoporation | Variable transfection efficiency, limited cell viability, non-homogenous tissue regeneration, potential tissue damage | [21,22] |
| Sonoporation | Ultrasound waves create pores in cell membrane due to cavitation | Noninvasive, less tissue damage compared to electroporation, ultrasound is highly accepted in the clinical setting, more efficient than naked DNA injection, systemic injection is possible | Low transfection efficiency, cell membrane damage is possible, low reproducibility | [23] |
| Regeneration Model | Animal Model | References |
|---|---|---|
| Bone Regeneration | Mouse | [22,31,35,43] |
| Pig | [39] | |
| Soft Tissue-Bone Integration | Pig | [40] |
| Myocardial Angiogenesis | Mouse | [44,45] |
| Rat | [46,47] | |
| Peripheral Angiogenesis | Rabbit | [48] |
| Rat | [49,50,51,52] | |
| Pancreatic Islet Regeneration | Rat | [53,54,55,56] |
| Model | Animal | Ultrasound | Frequency (MHz) | Conclusion | References |
|---|---|---|---|---|---|
| Ectopic | Mouse | Rich-Mar Sonitron 2000 | 1 | Sonoporation applied with intramuscular injection of rhBMP-9 plasmid and lipid-stabilized microbubbles resulted in ectopic bone formation | [22] |
| Rich-Mar Sonitron 2000 | Repeated sonoporation with BMP-2 plasmid significantly increased osteoinduction compared to one treatment session | [43] | |||
| Sonidel SP100 | Using 4 W/cm2 sonoporation and constitutive BMP2/7 co-expression plasmid significantly increased ectopic bone formation, but with variable morphology and irregular shape | [35] | |||
| Sonidel SP100 | Use of a GAM and BMP2/7 co-expression plasmid significantly enhanced ectopic bone formation compared to standard sonoporation | [31] | |||
| Femur Defect | Rat | Sonidel SP100 | 1 | Use of a BMP2/7 co-expression plasmid resulted in fracture union in 33% of rats, compared to the 0% union rate in the control group, although this result was not statistically significant | [35] |
| Tibia Defect | Pig | Philips Sonos 5500; S3 transducer | 1.3 | Using a collagen scaffold and hBMP-6 plasmid led to complete radiographic and functional healing, similar to that shown with autograft implantation | [39] |
| ACL Reconstruction | Pig | Philips Sonos 5500; S3 transducer | 1.3 | Collagen scaffold and BMP-6 plasmid injection significantly enhanced osteointegration and tissue continuity, with no ectopic bone formation | [40] |
| Model | Animal | Ultrasound | Frequency (MHz) | Conclusion | References |
|---|---|---|---|---|---|
| Ischemia/reperfusion (I/R) Injury | Mouse | Siemens Acuson Sequoia C256; 15L8 transducer | 8 | Injection of either VEGF or SCF plasmids resulted in greater capillary and arteriolar density, myocardial perfusion, and enhanced cardiac function compared to the control group | [44] |
| Philips Sonos 5500; S12 transducer | 7 | Myocardial perfusion and ventricular function improved progressively with the number of treatments of stem cell factor (SCF) and stromal cell-derived factor-1α (SDF-1α) plasmids | [45] | ||
| Rat | Siemens Acuson Sequoia C256; 15L8 transducer | 8 | Cationic microbubble delivery of the AKT gene produced the greatest increase in ventricular function and myocardial perfusion, resulting in decreased infarct size and reducing apoptosis | [46] | |
| GE Healthcare Vivid 7; M3S transducer | 1.6 | Antagomir delivery to the myocardium is dependent on ultrasound frequency and mode, and delivery primarily occurred at the anterior wall of the heart | [47] |
| Model | Animal | Ultrasound | Frequency (MHz) | Conclusion | References |
|---|---|---|---|---|---|
| Hindlimb ischemia | Rabbit | Not specified | 1 | Angiographic score and capillary density of animals treated with ultrasound and HGF plasmid was significantly greater than the control, resulting in a significant increase in blood flow and blood pressure ratio | [48] |
| Rat | Philips Sonos 5500; S3 transducer | 1.3 | Infusion of VEGF-165 plasmid resulted in significant improvement in microvascular blood flow and increased vessel density, with transfection localized predominantly to the vascular endothelium of arterioles | [49] | |
| Both IM and IV delivery of VEGF-165 plasmid produced significant increases in microvascular blood volume and blood flow, but microvascular blood flow was greater in IV-treated animals | [50] | ||||
| Temporally separated VEGF and Ang-1 plasmid delivery resulted in increased blood flow, vessel density, and sustained an increase in flow reserve | [51] | ||||
| Treatment with miR-126-3p resulted in significant improvements in microvascular perfusion, and repeated treatment exhibited an even greater angiogenic response | [52] |
| Model | Animal | Ultrasound | Frequency (MHz) | Conclusion | References |
|---|---|---|---|---|---|
| STZ-induced diabetes | Rat | Philips Sonos 5500; S3 transducer | 1.3 | RIP3.1-NeuroD1 plasmid promoted islet regeneration from surviving beta-cells, with normalization of glucose, insulin, and C-peptide levels up to 30 days, but pretreating with SP600125 could extend the duration of islet regeneration and normoglycemia to 90 days | [53] |
| Injection of the Nkx2.2 gene induced robust proliferation and differentiation of adult pancreatic progenitors, curing STZ-induced diabetes for 3 months | [54] | ||||
| A single sonoporation treatment with cyclin D2/CDK4/GLP-1 plasmids induced β-cell regeneration with reversal of diabetes for 6 months without evidence of toxicity or activation of oncogenes | [55] | ||||
| ANGPTL8 gene targeted to the pancreas significantly alleviated but did not totally reverse STZ-induced diabetes, but treatment did promote the proliferation of adult and aged beta cells, expanding the beta-cell mass and improving glucose tolerance | [56] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Krut, Z.; Gazit, D.; Gazit, Z.; Pelled, G. Applications of Ultrasound-Mediated Gene Delivery in Regenerative Medicine. Bioengineering 2022, 9, 190. https://doi.org/10.3390/bioengineering9050190
Krut Z, Gazit D, Gazit Z, Pelled G. Applications of Ultrasound-Mediated Gene Delivery in Regenerative Medicine. Bioengineering. 2022; 9(5):190. https://doi.org/10.3390/bioengineering9050190
Chicago/Turabian StyleKrut, Zoe, Dan Gazit, Zulma Gazit, and Gadi Pelled. 2022. "Applications of Ultrasound-Mediated Gene Delivery in Regenerative Medicine" Bioengineering 9, no. 5: 190. https://doi.org/10.3390/bioengineering9050190
APA StyleKrut, Z., Gazit, D., Gazit, Z., & Pelled, G. (2022). Applications of Ultrasound-Mediated Gene Delivery in Regenerative Medicine. Bioengineering, 9(5), 190. https://doi.org/10.3390/bioengineering9050190






