Bioengineering Approaches for Delivering Growth Factors: A Focus on Bone and Cartilage Regeneration
Abstract
1. Introduction
| Growth Factors | Source | Effector Cells | Function | Pathways |
|---|---|---|---|---|
| TGFβ [6,7,8,9,10] | Platelets | MSCs Fibroblasts? (Collagen) | Osteogenesis Chondrogenesis Collagen Type 2 synthesis Proteoglycan synthesis | MAPK ERK SAPK/JNK |
| BMPs [11,12,13,14,15,16] | Platelets | MSCs Fibroblasts? (Collagen) Endothelial cells | Osteogenesis Chondrogenesis Collagen Type 2 synthesis Proteoglycan synthesis Angiogenesis | MAPK ERK SAPK/JNK |
| VEGF [17,18] | Osteoblasts Pre-osteogenic cells Chondrocytes | Endothelial cells | Neovascularization Osteogenic cells recruitment | RAS-raf-ERK/1/2 PI3K/AKT |
| PDGF [19,20,21,22] | Platelets | MSCs Chondrocytes Inflammatory Cells | Mitosis Chemotaxis Extracellular Matrix Formation Cartilage formation Osteogenesis | ERK1/2 PI3K/AKT |
| IGF [23,24,25,26] | Osteoblasts Chondrocyte (Hepatocytes) | MSCs Myeloid Precursor Cells Osteoclasts | Osteogenesis Chondrogenesis Osteoclast differentiation Osteoblast chemotaxis Osteoclast function Type 1 collagen release | Mtorc2/AKT ERK1/2 PI3K/AKT |
| FGF [27,28,29,30] | MSCs Osteoblasts Chondrocytes (Inflammatory) Endothelial cells Macrophages | MSCs Osteoblasts Endothelial cells | Chondrogenesis Osteoblast proliferation Angiogenesis Inflammation MSC proliferation Bone formation | PLC3-K/AKT Ras/MAPK PLC PKC STAT1/p21 |
2. Growth Factors Helping Bone and Cartilage Regeneration
2.1. Transforming Growth Factor-Beta
2.2. Bone Morphogenetic Proteins
2.3. Vascular Endothelial Growth Factor
2.4. Platelet-Derived Growth Factor
2.5. Insulin-like Growth Factors
2.6. Fibroblast Growth Factor
3. Platelet-Rich Plasma
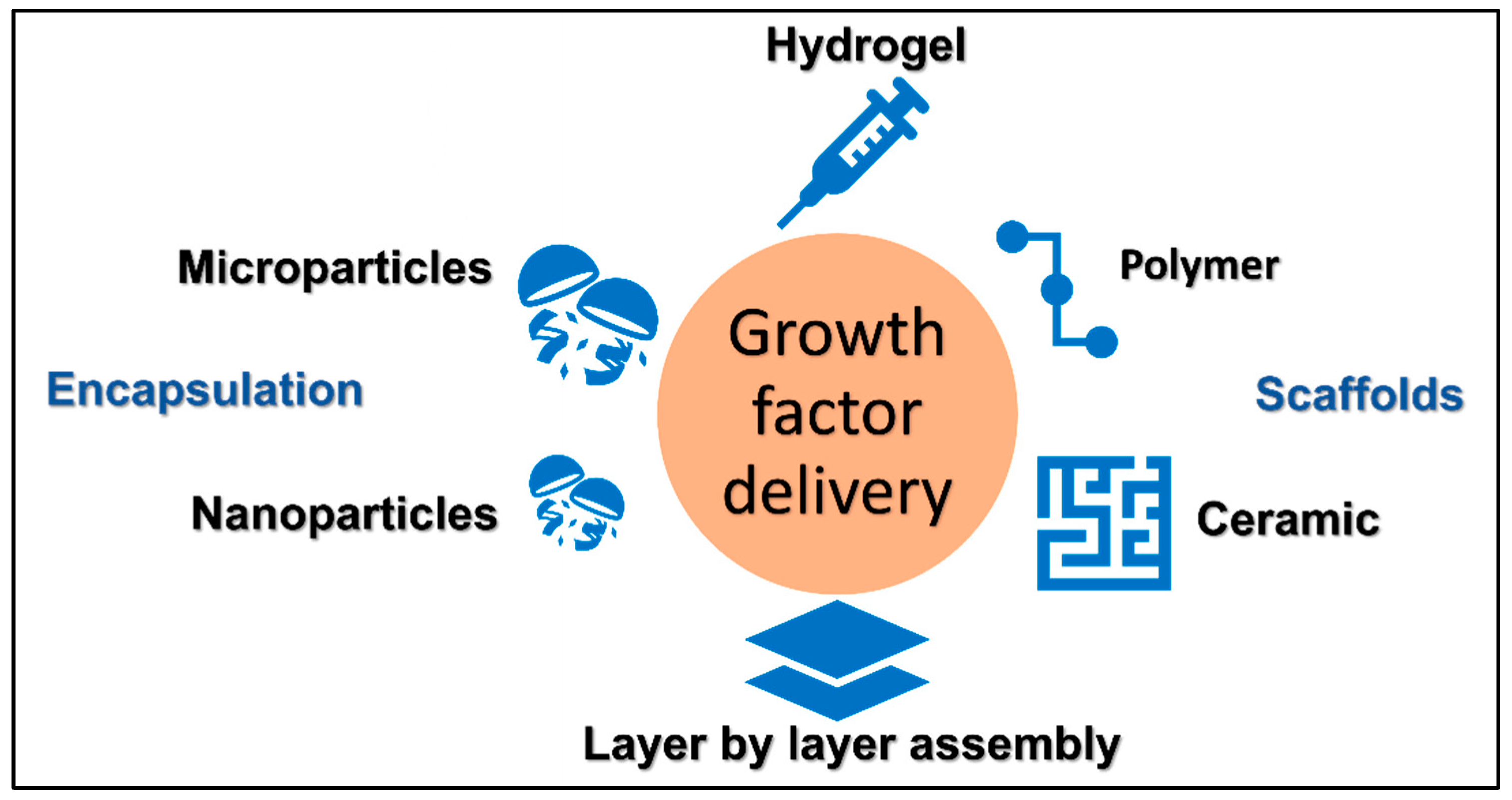
4. Delivery of Growth Factors via Scaffolds
4.1. Metal-Based Scaffolds
4.2. Ceramics
4.3. Polymers
5. Encapsulation Technology for Growth Factor Delivery
5.1. Physical Encapsulation
5.2. Microparticles
5.3. Nanoparticles
6. Layer by Layer Assembly Technology for Growth Factor Delivery
7. Hydrogel Technology for Growth Factor Delivery
8. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- El-Jawhari, J.J.; Jones, E.; Giannoudis, P.V. The roles of immune cells in bone healing; what we know, do not know and future perspectives. Injury 2016, 47, 2399–2406. [Google Scholar] [CrossRef]
- Bahney, C.S.; Zondervan, R.L.; Allison, P.; Theologis, A.; Ashley, J.W.; Ahn, J.; Miclau, T.; Marcucio, R.S.; Hankenson, K.D. Cellular biology of fracture healing. J. Orthop. Res. 2019, 37, 35–50. [Google Scholar] [CrossRef]
- Stewart, S.K. Fracture Non-Union: A Review of Clinical Challenges and Future Research Needs. Malays. Orthop. J. 2019, 13, 1–10. [Google Scholar] [CrossRef]
- El-Jawhari, J.J.; Ganguly, P.; Jones, E.; Giannoudis, P.V. Bone Marrow Multipotent Mesenchymal Stromal Cells as Autologous Therapy for Osteonecrosis: Effects of Age and Underlying Causes. Bioengineering 2021, 8, 69. [Google Scholar] [CrossRef]
- Vo, T.N.; Kasper, F.K.; Mikos, A.G. Strategies for Controlled Delivery of Growth Factors and Cells for Bone Regeneration. Adv. Drug Deliv. Rev. 2012, 64, 1292–1309. [Google Scholar] [CrossRef]
- Ramoshebi, L.N.; Matsaba, T.N.; Teare, J.; Renton, L.; Patton, J.; Ripamonti, U. Tissue engineering: TGF-beta superfamily members and delivery systems in bone regeneration. Expert Rev. Mol. Med. 2002, 4, 1–11. [Google Scholar] [CrossRef]
- Derynck, R.; Zhang, Y.E. Smad-dependent and Smad-independent pathways in TGF-beta family signalling. Nature 2003, 425, 577–584. [Google Scholar] [CrossRef]
- Madry, H.; Rey-Rico, A.; Venkatesan, J.K.; Johnstone, B.; Cucchiarini, M. Transforming growth factor Beta-releasing scaffolds for cartilage tissue engineering. Tissue Eng. Part B Rev. 2014, 20, 106–125. [Google Scholar] [CrossRef]
- Coricor, G.; Serra, R. TGF-beta regulates phosphorylation and stabilization of Sox9 protein in chondrocytes through p38 and Smad dependent mechanisms. Sci. Rep. 2016, 6, 638616. [Google Scholar] [CrossRef]
- Dimitriou, R.; Giannoudis, P.V. Discovery and development of BMPs. Injury 2005, 36 (Suppl. S3), S28–S33. [Google Scholar] [CrossRef]
- Scarfi, S. Use of bone morphogenetic proteins in mesenchymal stem cell stimulation of cartilage and bone repair. World J. Stem. Cells 2016, 8, 1–12. [Google Scholar] [CrossRef]
- Gomez-Puerto, M.C.; Iyengar, P.V.; Garcia de Vinuesa, A.; Ten Dijke, P.; Sanchez-Duffhues, G. Bone morphogenetic protein receptor signal transduction in human disease. J. Pathol. 2019, 247, 9–20. [Google Scholar] [CrossRef]
- Lienemann, P.S.; Lutolf, M.P.; Ehrbar, M. Biomimetic hydrogels for controlled biomolecule delivery to augment bone regeneration. Adv. Drug Deliv. Rev. 2012, 64, 1078–1089. [Google Scholar] [CrossRef]
- Tsiridis, E.; Gamie, Z.; Conaghan, P.G.; Giannoudis, P.V. Biological options to enhance periprosthetic bone mass. Injury 2007, 38, 704–713. [Google Scholar] [CrossRef]
- Williams, J.C.; Maitra, S.; Anderson, M.J.; Christiansen, B.A.; Reddi, A.H.; Lee, M.A. BMP-7 and Bone Regeneration: Evaluation of Dose-Response in a Rodent Segmental Defect Model. J. Orthop. Trauma 2015, 29, e336–e341. [Google Scholar] [CrossRef]
- Kaito, T.; Morimoto, T.; Mori, Y.; Kanayama, S.; Makino, T.; Takenaka, S.; Sakai, Y.; Otsuru, S.; Yoshioka, Y.; Yoshikawa, H. BMP-2/7 heterodimer strongly induces bone regeneration in the absence of increased soft tissue inflammation. Spine J. 2018, 18, 139–146. [Google Scholar] [CrossRef]
- Street, J.; Bao, M.; de Guzman, L.; Bunting, S.; Peale, F.V., Jr.; Ferrara, N.; Steinmetz, H.; Hoeffel, J.; Cleland, J.L.; Daugherty, A.; et al. Vascular endothelial growth factor stimulates bone repair by promoting angiogenesis and bone turnover. Proc. Natl. Acad. Sci. USA 2002, 99, 9656–9661. [Google Scholar] [CrossRef]
- Simons, M.; Gordon, E.; Claesson-Welsh, L. Mechanisms and regulation of endothelial VEGF receptor signalling. Nat. Rev. Mol. Cell Biol. 2016, 17, 611–625. [Google Scholar] [CrossRef]
- Hankenson, K.D.; Dishowitz, M.; Gray, C.; Schenker, M. Angiogenesis in bone regeneration. Injury 2011, 42, 556–561. [Google Scholar] [CrossRef]
- Hulth, A.; Johnell, O.; Miyazono, K.; Lindberg, L.; Heinegard, D.; Heldin, C.H. Effect of transforming growth factor-beta and platelet-derived growth factor-BB on articular cartilage in rats. J. Orthop. Res. 1996, 14, 547–553. [Google Scholar] [CrossRef]
- Alderton, F.; Rakhit, S.; Kong, K.C.; Palmer, T.; Sambi, B.; Pyne, S.; Pyne, N.J. Tethering of the platelet-derived growth factor beta receptor to G-protein-coupled receptors. A novel platform for integrative signaling by these receptor classes in mammalian cells. J. Biol. Chem. 2001, 276, 28578–28585. [Google Scholar] [CrossRef]
- Ng, F.; Boucher, S.; Koh, S.; Sastry, K.S.; Chase, L.; Lakshmipathy, U.; Choong, C.; Yang, Z.; Vemuri, M.C.; Rao, M.S.; et al. PDGF, TGF-beta, and FGF signaling is important for differentiation and growth of mesenchymal stem cells (MSCs): Transcriptional profiling can identify markers and signaling pathways important in differentiation of MSCs into adipogenic, chondrogenic, and osteogenic lineages. Blood 2008, 112, 295–307. [Google Scholar] [CrossRef]
- Guntur, A.R.; Rosen, C.J. IGF-1 regulation of key signaling pathways in bone. Bonekey Rep. 2013, 2, 2437. [Google Scholar] [CrossRef]
- Shi, Y.; Chen, J.; Karner, C.M.; Long, F. Hedgehog signaling activates a positive feedback mechanism involving insulin-like growth factors to induce osteoblast differentiation. Proc. Natl. Acad. Sci. USA 2015, 112, 4678–4683. [Google Scholar] [CrossRef]
- Crane, J.L.; Cao, X. Function of matrix IGF-1 in coupling bone resorption and formation. J. Mol. Med. 2014, 92, 107–115. [Google Scholar] [CrossRef]
- Wu, S.; Morrison, A.; Sun, H.; De Luca, F. Nuclear factor-kappaB (NF-kappaB) p65 interacts with Stat5b in growth plate chondrocytes and mediates the effects of growth hormone on chondrogenesis and on the expression of insulin-like growth factor-1 and bone morphogenetic protein-2. J. Biol. Chem. 2011, 286, 24726–24734. [Google Scholar] [CrossRef]
- Chen, L.; Liu, J.; Guan, M.; Zhou, T.; Duan, X.; Xiang, Z. Growth Factor and Its Polymer Scaffold-Based Delivery System for Cartilage Tissue Engineering. Int. J. Nanomed. 2020, 15, 6097–6111. [Google Scholar] [CrossRef]
- Ornitz, D.M. FGF signaling in the developing endochondral skeleton. Cytokine Growth Factor Rev. 2005, 16, 205–213. [Google Scholar] [CrossRef]
- Hildner, F.; Peterbauer, A.; Wolbank, S.; Nurnberger, S.; Marlovits, S.; Redl, H.; van Griensven, M.; Gabriel, C. FGF-2 abolishes the chondrogenic effect of combined BMP-6 and TGF-beta in human adipose derived stem cells. J. Biomed. Mater. Res. A 2010, 94, 978–987. [Google Scholar] [CrossRef]
- Ito, T.; Sawada, R.; Fujiwara, Y.; Tsuchiya, T. FGF-2 increases osteogenic and chondrogenic differentiation potentials of human mesenchymal stem cells by inactivation of TGF-beta signaling. Cytotechnology 2008, 56, 1–7. [Google Scholar] [CrossRef]
- Zhang, Y.; Xing, F.; Luo, R.; Duan, X. Platelet-Rich Plasma for Bone Fracture Treatment: A Systematic Review of Current Evidence in Preclinical and Clinical Studies. Front. Med. 2021, 8676033. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez, I.A.; Growney Kalaf, E.A.; Bowlin, G.L.; Sell, S.A. Platelet-rich plasma in bone regeneration: Engineering the delivery for improved clinical efficacy. Biomed. Res. Int. 2014, 2014, 392398. [Google Scholar] [CrossRef] [PubMed]
- Kaur, P.; Maria, A. Efficacy of platelet rich plasma and hydroxyapatite crystals in bone regeneration after surgical removal of mandibular third molars. J. Maxillofac. Oral. Surg. 2013, 12, 51–59. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Liu, X.; Wang, P.; Chen, W.; Weir, M.D.; Bao, C.; Xu, H.H. Human embryonic stem cells and macroporous calcium phosphate construct for bone regeneration in cranial defects in rats. Acta Biomater. 2014, 10, 4484–4493. [Google Scholar] [CrossRef]
- Qiu, G.; Shi, Z.; Xu, H.H.K.; Yang, B.; Weir, M.D.; Li, G.; Song, Y.; Wang, J.; Hu, K.; Wang, P.; et al. Bone regeneration in minipigs via calcium phosphate cement scaffold delivering autologous bone marrow mesenchymal stem cells and platelet-rich plasma. J. Tissue Eng. Regen. Med. 2018, 12, e937–e948. [Google Scholar] [CrossRef]
- Hede, K.; Christensen, B.B.; Jensen, J.; Foldager, C.B.; Lind, M. Combined Bone Marrow Aspirate and Platelet-Rich Plasma for Cartilage Repair: Two-Year Clinical Results. Cartilage 2021, 13 (Suppl. S1), 937S–947S. [Google Scholar] [CrossRef]
- Mitchell, A.C.; Briquez, P.S.; Hubbell, J.A.; Cochran, J.R. Engineering growth factors for regenerative medicine applications. Acta Biomater. 2016, 30, 1–12. [Google Scholar] [CrossRef]
- De Witte, T.M.; Fratila-Apachitei, L.E.; Zadpoor, A.A.; Peppas, N.A. Bone tissue engineering via growth factor delivery: From scaffolds to complex matrices. Regen. Biomater. 2018, 5, 197–211. [Google Scholar] [CrossRef]
- Turnbull, G.; Clarke, J.; Picard, F.; Riches, P.; Jia, L.; Han, F.; Li, B.; Shu, W. 3D bioactive composite scaffolds for bone tissue engineering. Bioact. Mater. 2018, 3, 278–314. [Google Scholar] [CrossRef]
- Janbaz, S.; Noordzij, N.; Widyaratih, D.S.; Hagen, C.W.; Fratila-Apachitei, L.E.; Zadpoor, A.A. Origami lattices with free-form surface ornaments. Sci. Adv. 2017, 3, eaao1595. [Google Scholar] [CrossRef]
- Polo-Corrales, L.; Latorre-Esteves, M.; Ramirez-Vick, J.E. Scaffold design for bone regeneration. J. Nanosci. Nanotechnol. 2014, 14, 15–56. [Google Scholar] [CrossRef] [PubMed]
- Lin, Z.; Wu, J.; Qiao, W.; Zhao, Y.; Wong, K.H.M.; Chu, P.K.; Bian, L.; Wu, S.; Zheng, Y.; Cheung, K.M.C.; et al. Precisely controlled delivery of magnesium ions thru sponge-like monodisperse PLGA/nano-MgO-alginate core-shell microsphere device to enable in-situ bone regeneration. Biomaterials 2018, 174, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Jeong, J.; Kim, J.H.; Shim, J.H.; Hwang, N.S.; Heo, C.Y. Bioactive calcium phosphate materials and applications in bone regeneration. Biomater. Res. 2019, 23, 4. [Google Scholar] [CrossRef]
- Lim, H.K.; Kwon, Y.J.; Hong, S.J.; Choi, H.G.; Chung, S.M.; Yang, B.E.; Lee, J.H.; Byun, S.H. Bone regeneration in ceramic scaffolds with variable concentrations of PDRN and rhBMP-2. Sci. Rep. 2021, 11, 11470. [Google Scholar] [CrossRef] [PubMed]
- Seong, Y.J.; Song, E.H.; Park, C.; Lee, H.; Kang, I.G.; Kim, H.E.; Jeong, S.H. Porous calcium phosphate-collagen composite microspheres for effective growth factor delivery and bone tissue regeneration. Mater. Sci. Eng. C Mater. Biol. Appl. 2020, 109, 110480. [Google Scholar] [CrossRef] [PubMed]
- El-Jawhari, J.J.; Sanjurjo-Rodriguez, C.; Jones, E.; Giannoudis, P.V. Collagen-containing scaffolds enhance attachment and proliferation of non-cultured bone marrow multipotential stromal cells. J. Orthop. Res. 2016, 34, 597–606. [Google Scholar] [CrossRef] [PubMed]
- El-Jawhari, J.J.; Moisley, K.; Jones, E.; Giannoudis, P.V. A crosslinked collagen membrane versus a non-crosslinked bilayer collagen membrane for supporting osteogenic functions of human bone marrow-multipotent stromal cells. Eur. Cell Mater. 2019, 37292–37309. [Google Scholar] [CrossRef]
- Calabrese, G.; Giuffrida, R.; Fabbi, C.; Figallo, E.; Lo Furno, D.; Gulino, R.; Colarossi, C.; Fullone, F.; Giuffrida, R.; Parenti, R.; et al. Collagen-Hydroxyapatite Scaffolds Induce Human Adipose Derived Stem Cells Osteogenic Differentiation In Vitro. PLoS ONE 2016, 11, e0151181. [Google Scholar] [CrossRef]
- Rao, S.H.; Harini, B.; Shadamarshan, R.P.K.; Balagangadharan, K.; Selvamurugan, N. Natural and synthetic polymers/bioceramics/bioactive compounds-mediated cell signalling in bone tissue engineering. Int. J. Biol. Macromol. 2018, 110, 88–96. [Google Scholar] [CrossRef]
- Fasolino, I.; Raucci, M.G.; Soriente, A.; Demitri, C.; Madaghiele, M.; Sannino, A.; Ambrosio, L. Osteoinductive and anti-inflammatory properties of chitosan-based scaffolds for bone regeneration. Mater. Sci. Eng. C Mater. Biol. Appl. 2019, 105, 110046. [Google Scholar] [CrossRef]
- Azevedo, A.S.; Sa, M.J.; Fook, M.V.; Neto, P.I.; Sousa, O.B.; Azevedo, S.S.; Teixeira, M.W.; Costa, F.S.; Araujo, A.L. Use of chitosan and beta-tricalcium phosphate, alone and in combination, for bone healing in rabbits. J. Mater. Sci. Mater. Med. 2014, 25, 481–486. [Google Scholar] [CrossRef] [PubMed]
- Hong, J.Y.; Kang, S.W.; Kim, J.W.; Suh, S.W.; Ko, Y.J.; Park, J.H. Optimal condition of heparin-conjugated fibrin with bone morphogenetic protein-2 for spinal fusion in a rabbit model. Cytotherapy 2014, 16, 1441–1448. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.; Zhang, Z.; Ma, X.; Duan, Z.; Hui, J.; Zhu, C.; Zhang, D.; Fan, D.; Shang, L.; Chen, F. Newly Designed Human-Like Collagen to Maximize Sensitive Release of BMP-2 for Remarkable Repairing of Bone Defects. Biomolecules 2019, 9, 450. [Google Scholar] [CrossRef] [PubMed]
- Donnaloja, F.; Jacchetti, E.; Soncini, M.; Raimondi, M.T. Natural and Synthetic Polymers for Bone Scaffolds Optimization. Polymers 2020, 12, 905. [Google Scholar] [CrossRef]
- Alba-Perez, A.; Jayawarna, V.; Childs, P.G.; Dalby, M.J.; Salmeron-Sanchez, M. Plasma zpolymerized nanoscale coatings of controlled thickness for efficient solid-phase presentation of growth factors. Mater. Sci. Eng. C 2020, 113, 110966. [Google Scholar] [CrossRef] [PubMed]
- Solovieva, A.; Miroshnichenko, S.; Kovalskii, A.; Permyakova, E.; Popov, Z.; Dvořáková, E.; Kiryukhantsev-Korneev, P.; Obrosov, A.; Polčak, J.; Zajíčková, L.; et al. Immobilization of platelet-rich plasma onto COOH plasma-coated PCL nanofibers boost viability and proliferation of human mesenchymal stem cells. Polymers 2017, 9, 736. [Google Scholar] [CrossRef]
- Solovieva, A.O.; Permyakova, E.S.; Ershov, K.I.; Bakhareva, K.I.; Miroshnichenko, S.M.; Kiryukhantsev-Korneev, P.V.; Konopatsky, A.S.; Polčak j Shtansky, D.V.; Manakhov, A.M. Plasma-coated PCL scaffolds with immobilized platelet-rich plasma enhance the wound healing in diabetics mice. Plasma Process. Polym. 2022, e2200032. [Google Scholar] [CrossRef]
- Zou, L.; Zhang, Y.; Liu, X.; Chen, J.; Zhang, Q. Biomimetic mineralization on natural and synthetic polymers to prepare hybrid scaffolds for bone tissue engineering. Colloids Surf. B Biointerfaces 2019, 178, 222–229. [Google Scholar] [CrossRef]
- Mehrasa, M.; Asadollahi, M.A.; Ghaedi, K.; Salehi, H.; Arpanaei, A. Electrospun aligned PLGA and PLGA/gelatin nanofibers embedded with silica nanoparticles for tissue engineering. Int. J. Biol. Macromol. 2015, 79, 687–695. [Google Scholar] [CrossRef]
- Ashwin, B.; Abinaya, B.; Prasith, T.P.; Chandran, S.V.; Yadav, L.R.; Vairamani, M.; Patil, S.; Selvamurugan, N. 3D-poly (lactic acid) scaffolds coated with gelatin and mucic acid for bone tissue engineering. Int. J. Biol. Macromol. 2020, 162, 523–532. [Google Scholar] [CrossRef]
- Patel, J.M.; Saleh, K.S.; Burdick, J.A.; Mauck, R.L. Bioactive factors for cartilage repair and regeneration: Improving delivery, retention, and activity. Acta Biomater. 2019, 93, 222–238. [Google Scholar] [CrossRef] [PubMed]
- Dang, M.; Saunders, L.; Niu, X.; Fan, Y.; Ma, P.X. Biomimetic delivery of signals for bone tissue engineering. Bone Res. 2018, 6, 25. [Google Scholar] [CrossRef] [PubMed]
- Cheng, R.; Liu, L.; Xiang, Y.; Lu, Y.; Deng, L.; Zhang, H.; Santos, H.A.; Cui, W. Advanced liposome-loaded scaffolds for therapeutic and tissue engineering applications. Biomaterials 2020, 232, 119706. [Google Scholar] [CrossRef]
- Willerth, S.M. How can microsphere-mediated delivery of small molecules serve as a novel tool for engineering tissues from stem cells? Ther. Deliv. 2019, 10, 671–674. [Google Scholar] [CrossRef]
- Kudva, A.K.; Dikina, A.D.; Luyten, F.P.; Alsberg, E.; Patterson, J. Gelatin microspheres releasing transforming growth factor drive in vitro chondrogenesis of human periosteum derived cells in micromass culture. Acta Biomater. 2019, 90, 287–299. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.; Jha, A.K.; Duncan, R.L.; Jia, X. Heparin-decorated, hyaluronic acid-based hydrogel particles for the controlled release of bone morphogenetic protein 2. Acta Biomater. 2011, 7, 3050–3059. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Kusada, K.; Kitagawa, H. Phase Control of Noble Monometallic and Alloy Nanomaterials by Chemical Reduction Methods. ChemPlusChem 2021, 86, 504–519. [Google Scholar] [CrossRef]
- Funda, G.; Taschieri, S.; Bruno, G.A.; Grecchi, E.; Paolo, S.; Girolamo, D.; Del Fabbro, M. Nanotechnology Scaffolds for Alveolar Bone Regeneration. Materials 2020, 13, 201. [Google Scholar] [CrossRef]
- Kim, H.J.; Han, M.A.; Shin, J.Y.; Jeon, J.H.; Lee, S.J.; Yoon, M.Y.; Kim, H.J.; Choi, E.J.; Do, S.H.; Yang, V.C.; et al. Intra-articular delivery of synovium-resident mesenchymal stem cells via BMP-7-loaded fibrous PLGA scaffolds for cartilage repair. J. Control Release 2019, 302, 169–180. [Google Scholar] [CrossRef]
- Quintos-Meneses, H.A.; Aranda-Lara, L.; Morales-Ávila, E.; Ocampo-García, B.; Contreras, I.; Ramírez-Nava, G.J.; Santos-Cuevas, C.L.; Estrada, J.A.; Luna-Gutiérrez, M.A.; Ferro-Flores, G.; et al. A Multimodal Theranostic System Prepared from High-Density Lipoprotein Carrier of Doxorubicin and 177 Lu. J. Biomed. Nanotechnol. 2021, 17, 2125–2141. [Google Scholar] [CrossRef]
- Mandapalli, P.K.; Labala, S.; Jose, A.; Bhatnagar, S.; Janupally, R.; Sriram, D.; Venuganti, V.V. Layer-by-Layer Thin Films for Co-Delivery of TGF-beta siRNA and Epidermal Growth Factor to Improve Excisional Wound Healing. AAPS PharmSciTech 2017, 18, 809–820. [Google Scholar] [CrossRef] [PubMed]
- Enriquez-Ochoa, D.; Robles-Ovalle, P.; Mayolo-Deloisa, K.; Brunck, M.E.G. Immobilization of Growth Factors for Cell Therapy Manufacturing. Front. Bioeng. Biotechnol. 2020, 8, 620. [Google Scholar] [CrossRef] [PubMed]
- Ansboro, S.; Hayes, J.S.; Barron, V.; Browne, S.; Howard, L.; Greiser, U.; Lalor, P.; Shannon, F.; Barry, F.P.; Pandit, A.; et al. A chondromimetic microsphere for in situ spatially controlled chondrogenic differentiation of human mesenchymal stem cells. J. Control Release 2014, 17, 942–951. [Google Scholar] [CrossRef] [PubMed]
- Shah, N.J.; Hyder, M.N.; Quadir, M.A.; Dorval Courchesne, N.M.; Seeherman, H.J.; Nevins, M.; Spector, M.; Hammond, P.T. Adaptive growth factor delivery from a polyelectrolyte coating promotes synergistic bone tissue repair and reconstruction. Proc. Natl. Acad. Sci. USA 2014, 111, 12847–12852. [Google Scholar] [CrossRef]
- Sreekumaran, S.; Radhakrishnan, A.; Rauf, A.A.; Kurup, G.M. Nanohydroxyapatite incorporated photocrosslinked gelatin methacryloyl/poly(ethylene glycol)diacrylate hydrogel for bone tissue engineering. Prog. Biomater. 2021, 10, 43–51. [Google Scholar] [CrossRef]
- Kadri, R.; Bacharouch, J.; Elkhoury, K.; Ben Messaoud, G.; Kahn, C.; Desobry, S.; Linder, M.; Tamayol, A.; Francius, G.; Mano, J.F.; et al. Role of active nanoliposomes in the surface and bulk mechanical properties of hybrid hydrogels. Mater. Today Bio 2020, 6, 100046. [Google Scholar] [CrossRef]
- Bhakta, G.; Rai, B.; Lim, Z.X.; Hui, J.H.; Stein, G.S.; van Wijnen, A.J.; Nurcombe, V.; Prestwich, G.D.; Cool, S.M. Hyaluronic acid-based hydrogels functionalized with heparin that support controlled release of bioactive BMP-2. Biomaterials 2012, 33, 6113–6122. [Google Scholar] [CrossRef]
- Wen, B.; Karl, M.; Pendrys, D.; Shafer, D.; Freilich, M.; Kuhn, L. An evaluation of BMP-2 delivery from scaffolds with miniaturized dental implants in a novel rat mandible model. J. Biomed. Mater. Res. B Appl. Biomater. 2011, 97, 315–326. [Google Scholar] [CrossRef]
- Elkhoury, K.; Morsink, M.; Sanchez-Gonzalez, L.; Kahn, C.; Tamayol, A.; Arab-Tehrany, E. Biofabrication of natural hydrogels for cardiac, neural, and bone Tissue engineering Applications. Bioact. Mater. 2021, 6, 3904–3923. [Google Scholar] [CrossRef]
- Kuroda, Y.; Kawai, T.; Goto, K.; Matsuda, S. Clinical application of injectable growth factor for bone regeneration: A systematic review. Inflamm. Regen. 2019, 39, 20. [Google Scholar] [CrossRef]
- Deng, Z.H.; Li, Y.S.; Gao, X.; Lei, G.H.; Huard, J. Bone morphogenetic proteins for articular cartilage regeneration. Osteoarthr. Cartil. 2018, 26, 1153–1161. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Z.; Song, W.; Zhang, G.; Zhan, S.; Cai, Z.; Yu, W.; He, Y. The recombinant human fibroblast growth factor-18 (sprifermin) Improves Tendon-to-Bone Healing by promoting chondrogenesis In a Rat Rotator Cuff Repair Model. J. Shoulder Elbow Surg. 2022. [Google Scholar] [CrossRef] [PubMed]
- Grabowski, G.; Cornett, C.A. Bone graft and bone graft substitutes in spine surgery: Current concepts and controversies. J. Am. Acad. Orthop. Surg. 2013, 21, 51–60. [Google Scholar] [CrossRef] [PubMed]
- Oliveira, E.R.; Nie, L.; Podstawczyk, D.; Allahbakhsh, A.; Ratnayake, J.; Brasil, D.L.; Shavandi, A. Advances in Growth Factor Delivery for Bone Tissue Engineering. Int. J. Mol. Sci. 2021, 22, 903. [Google Scholar] [CrossRef] [PubMed]
| Method | Examples | Advantages | Disadvantages | Ref. |
|---|---|---|---|---|
| Scaffolds | Polymers: synthetic, natural, mixed, plasma coated |
|
| [46,47,48,49,50,51,52,53,54,55,56,57,58,59,60,61,62,63,64,65,66,67,68,69,70] |
| Ceramic Bioinert: zirconia and alumina, Bioactive: Bioactive glass, Hydroxyapatite, glass |
|
| [43,44,45] | |
| Metal-based scaffolds Titanium, Zirconium, Platinum, stainless steel |
|
| [41,42] | |
| Encapsulation | Physical encapsulation: Phase emulsion, freeze-drying solvent casting, gas foaming |
|
| [62,63] |
| Microparticles Synthetic polymers MPs: PLGA |
|
| [64,65,66] | |
| Nanoparticles: BMP-7 loaded NPs |
|
| [67,68,69,70] | |
| Layer-by-layer Assembly | 3-D bioprinting |
|
| [71,72,73,74] |
| Hydrogel | HA hydrogel, Collagen, Chitosan, Alginate |
|
| [75,76,77,78,79] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shakoor, S.; Kibble, E.; El-Jawhari, J.J. Bioengineering Approaches for Delivering Growth Factors: A Focus on Bone and Cartilage Regeneration. Bioengineering 2022, 9, 223. https://doi.org/10.3390/bioengineering9050223
Shakoor S, Kibble E, El-Jawhari JJ. Bioengineering Approaches for Delivering Growth Factors: A Focus on Bone and Cartilage Regeneration. Bioengineering. 2022; 9(5):223. https://doi.org/10.3390/bioengineering9050223
Chicago/Turabian StyleShakoor, Sheeba, Eleyna Kibble, and Jehan J. El-Jawhari. 2022. "Bioengineering Approaches for Delivering Growth Factors: A Focus on Bone and Cartilage Regeneration" Bioengineering 9, no. 5: 223. https://doi.org/10.3390/bioengineering9050223
APA StyleShakoor, S., Kibble, E., & El-Jawhari, J. J. (2022). Bioengineering Approaches for Delivering Growth Factors: A Focus on Bone and Cartilage Regeneration. Bioengineering, 9(5), 223. https://doi.org/10.3390/bioengineering9050223







