A Biomimetic Electrospun Membrane Supports the Differentiation and Maturation of Kidney Epithelium from Human Stem Cells
Abstract
:1. Introduction
2. Materials and Methods
2.1. Preparation of Silk Fibroin Solution
2.2. Electrospinning
2.3. Human Induced Pluripotent Stem Cell Culture
2.4. Differentiation of hiPS Cells into Intermediate Mesoderm (IM) Cells
2.5. Differentiation of IM Cells into Podocytes on Electrospun Silk Fibroin
2.6. Differentiation of IM Cells into Podocytes on Tissue Culture Plates
2.7. Attenuated Total Reflectance Accessory–Fourier Transformed Infrared (ATR-FTIR) Spectroscopy
2.8. Profilometer
2.9. Scanning Electron Microscopy (SEM)
2.10. Immunostaining and Microscopy Analysis
2.11. Quantitative Real-Time PCR
2.12. Western Blot
2.13. CCK-8 Cell Viability Assay
2.14. Statistical Analysis
3. Results and Discussions
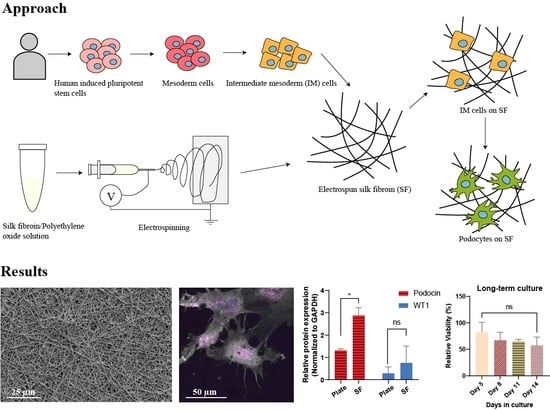
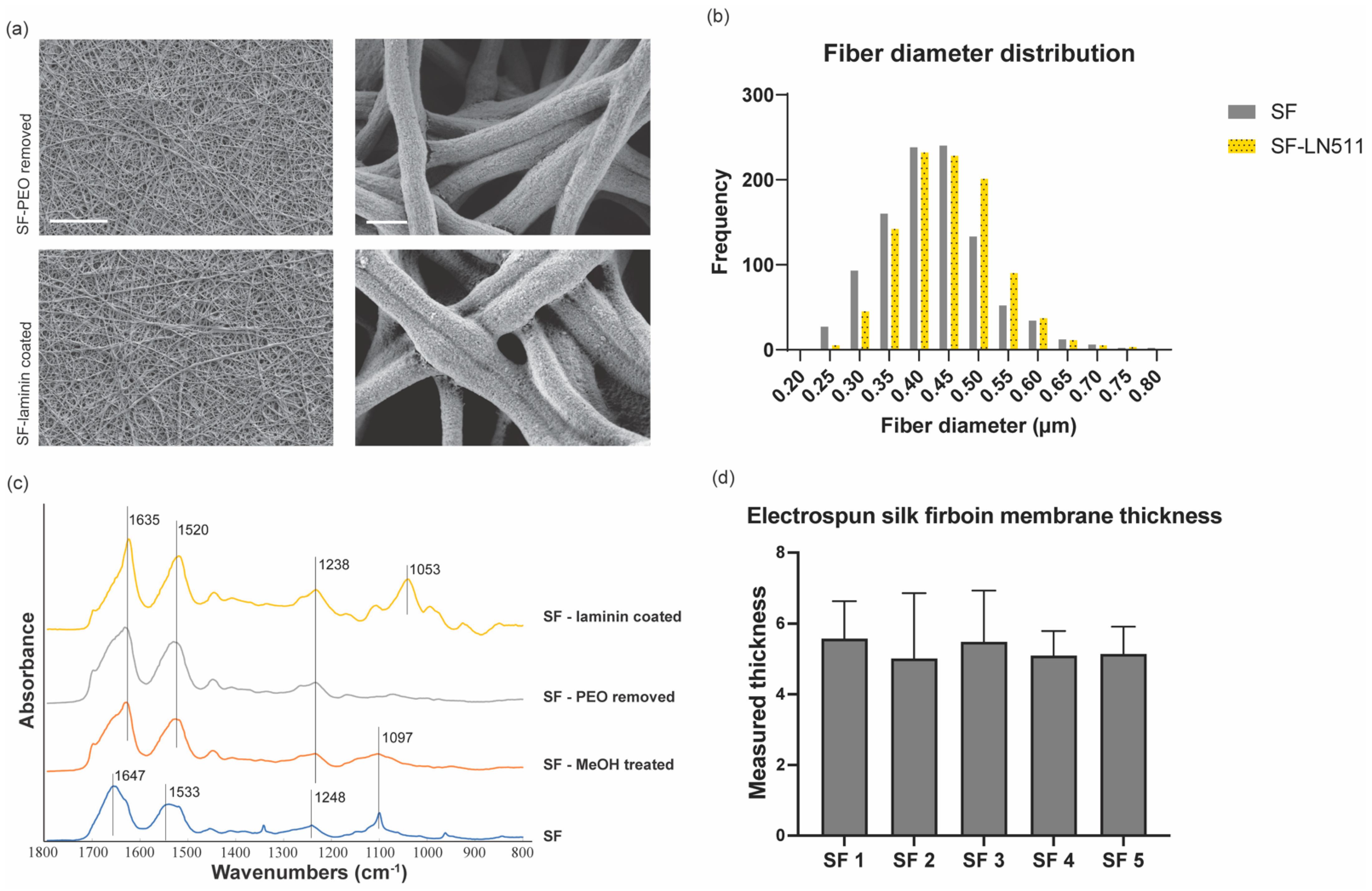
3.1. Fabrication and Characterization of Electrospun Silk Fibroin Membranes
3.2. Differentiation and Characterization of Human Stem Cell-Derived Podocytes on Electrospun Membranes
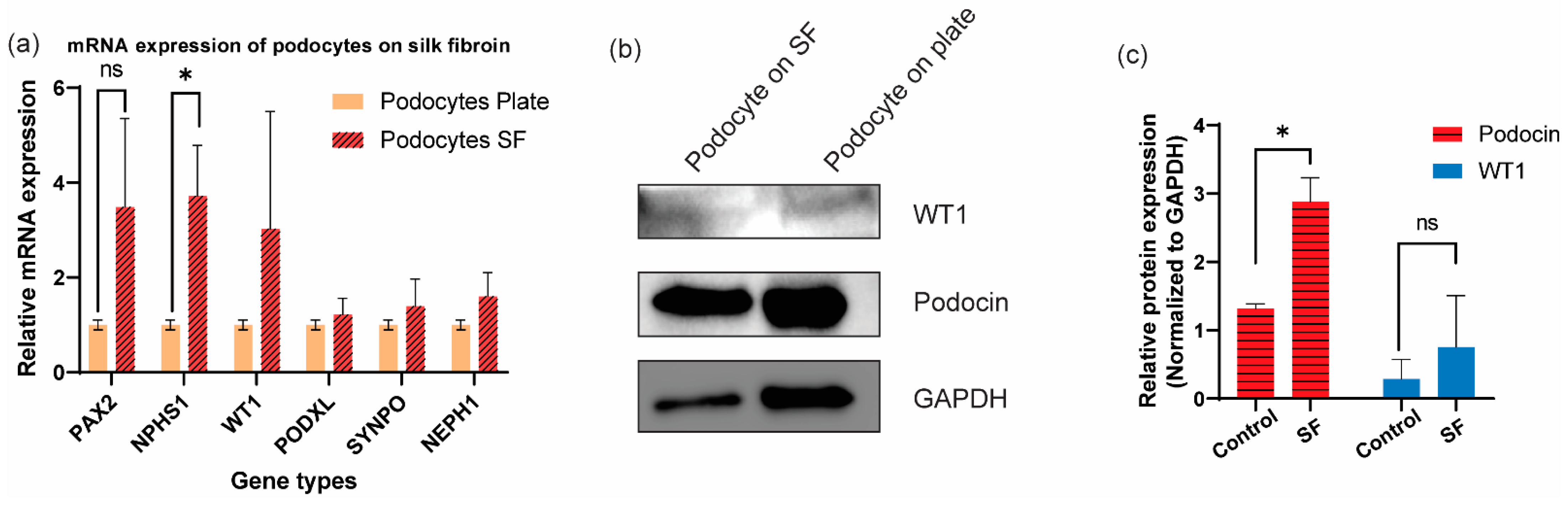
3.3. Additional Characterization of Differentiated Human Podocytes through Gene and Protein Level Quantifications
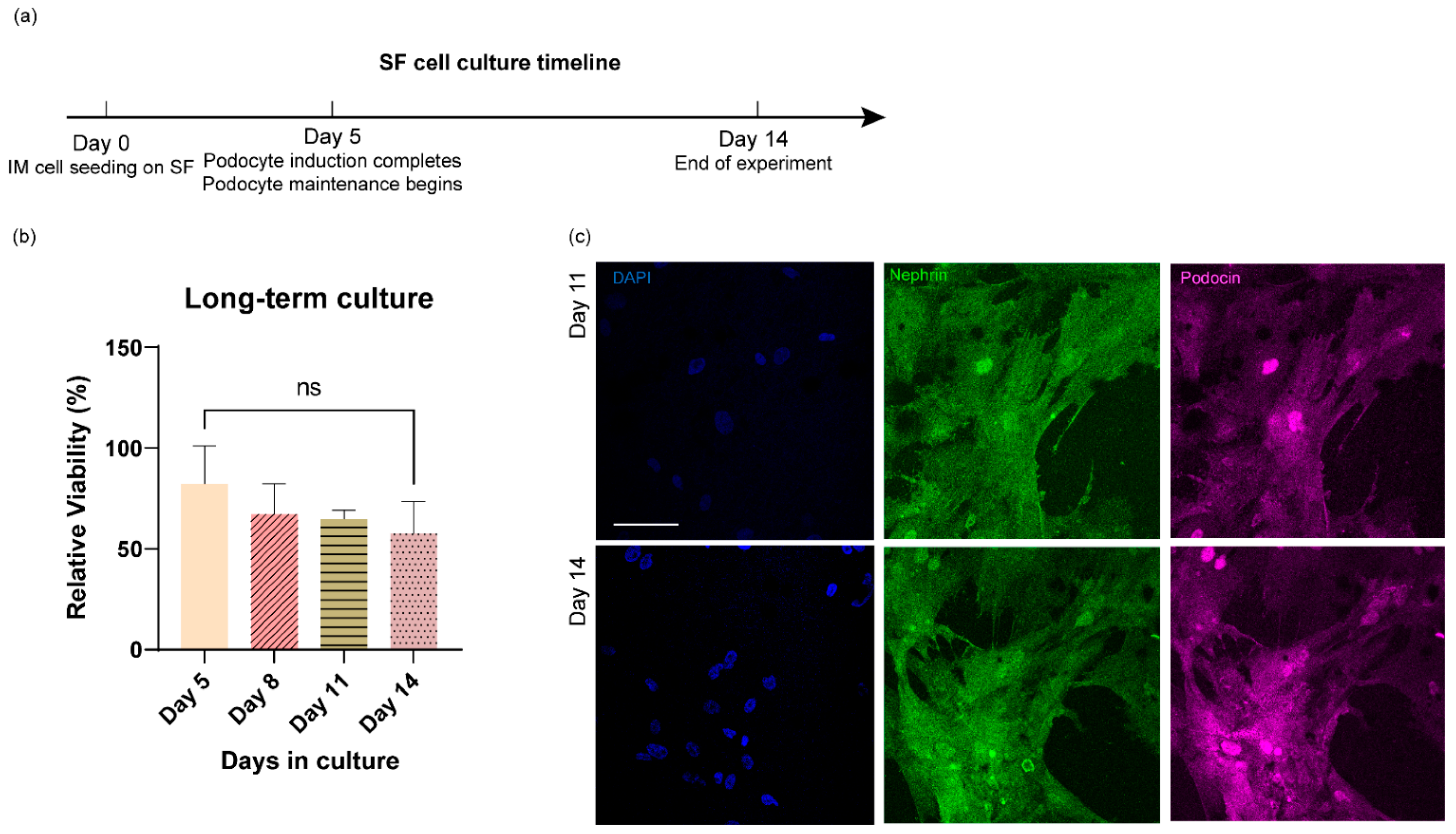
3.4. Long-Term Culture of Stem Cell-Derived Human Podocytes Differentiated on Electrospun Membranes
4. Summary
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Lennon, R.; Randles, M.J.; Humphries, M.J. The importance of podocyte adhesion for a healthy glomerulus. Front. Endocrinol. 2014, 5, 160. [Google Scholar] [CrossRef] [Green Version]
- Pavenstadt, H.; Kriz, W.; Kretzler, M. Cell biology of the glomerular podocyte. Physiol. Rev. 2003, 83, 253–307. [Google Scholar] [CrossRef] [Green Version]
- Jefferson, J.A.; Alpers, C.E.; Shankland, S.J. Podocyte biology for the bedside. Am. J. Kidney Dis. 2011, 58, 835–845. [Google Scholar] [CrossRef] [Green Version]
- Gödel, M.; Hartleben, B.; Herbach, N.; Liu, S.; Zschiedrich, S.; Lu, S.; Debreczeni-Mór, A.; Lindenmeyer, M.T.; Rastaldi, M.-P.; Hartleben, G. Role of mTOR in podocyte function and diabetic nephropathy in humans and mice. J. Clin. Investig. 2011, 121, 2197–2209. [Google Scholar] [CrossRef] [Green Version]
- Qi, W.; Keenan, H.A.; Li, Q.; Ishikado, A.; Kannt, A.; Sadowski, T.; Yorek, M.A.; Wu, I.-H.; Lockhart, S.; Coppey, L.J. Pyruvate kinase M2 activation may protect against the progression of diabetic glomerular pathology and mitochondrial dysfunction. Nat. Med. 2017, 23, 753. [Google Scholar] [CrossRef]
- Musah, S.; Dimitrakakis, N.; Camacho, D.M.; Church, G.M.; Ingber, D.E. Directed differentiation of human induced pluripotent stem cells into mature kidney podocytes and establishment of a Glomerulus Chip. Nat. Protoc. 2018, 13, 1662–1685. [Google Scholar] [CrossRef]
- Musah, S.; Mammoto, A.; Ferrante, T.C.; Jeanty, S.S.; Hirano-Kobayashi, M.; Mammoto, T.; Roberts, K.; Chung, S.; Novak, R.; Ingram, M. Mature induced-pluripotent-stem-cell-derived human podocytes reconstitute kidney glomerular-capillary-wall function on a chip. Nat. Biomed. Eng. 2017, 1, 0069. [Google Scholar] [CrossRef]
- Burt, M.; Bhattachaya, R.; Okafor, A.E.; Musah, S. Guided differentiation of mature kidney podocytes from human induced pluripotent stem cells under chemically defined conditions. JoVE (J. Vis. Exp.) 2020, e61299. Available online: https://www.jove.com/t/61299/guided-differentiation-mature-kidney-podocytes-from-human-induced?utm_source=joveglobal&utm_medium=twitter&utm_campaign=podocyte (accessed on 9 April 2022). [CrossRef]
- Liu, H.; Lin, J.; Roy, K. Effect of 3D scaffold and dynamic culture condition on the global gene expression profile of mouse embryonic stem cells. Biomaterials 2006, 27, 5978–5989. [Google Scholar] [CrossRef]
- Lim, J.Y.; Dreiss, A.D.; Zhou, Z.; Hansen, J.C.; Siedlecki, C.A.; Hengstebeck, R.W.; Cheng, J.; Winograd, N.; Donahue, H.J. The regulation of integrin-mediated osteoblast focal adhesion and focal adhesion kinase expression by nanoscale topography. Biomaterials 2007, 28, 1787–1797. [Google Scholar] [CrossRef]
- Satyam, A.; Tsokos, M.G.; Tresback, J.S.; Zeugolis, D.I.; Tsokos, G.C. Cell-Derived Extracellular Matrix-Rich Biomimetic Substrate Supports Podocyte Proliferation, Differentiation, and Maintenance of Native Phenotype. Adv. Funct. Mater. 2020, 30, 1908752. [Google Scholar] [CrossRef]
- Korolj, A.; Laschinger, C.; James, C.; Hu, E.; Velikonja, C.; Smith, N.; Gu, I.; Ahadian, S.; Willette, R.; Radisic, M. Curvature facilitates podocyte culture in a biomimetic platform. Lab A Chip 2018, 18, 3112–3128. [Google Scholar] [CrossRef]
- Xie, R.; Korolj, A.; Liu, C.; Song, X.; Lu, R.X.Z.; Zhang, B.; Ramachandran, A.; Liang, Q.; Radisic, M. h-FIBER: Microfluidic topographical hollow fiber for studies of glomerular filtration barrier. ACS Cent. Sci. 2020, 6, 903–912. [Google Scholar] [CrossRef]
- Zhang, Y.; Yang, F.; Liu, K.; Shen, H.; Zhu, Y.; Zhang, W.; Liu, W.; Wang, S.; Cao, Y.; Zhou, G. The impact of PLGA scaffold orientation on in vitro cartilage regeneration. Biomaterials 2012, 33, 2926–2935. [Google Scholar] [CrossRef]
- Uematsu, K.; Hattori, K.; Ishimoto, Y.; Yamauchi, J.; Habata, T.; Takakura, Y.; Ohgushi, H.; Fukuchi, T.; Sato, M. Cartilage regeneration using mesenchymal stem cells and a three-dimensional poly-lactic-glycolic acid (PLGA) scaffold. Biomaterials 2005, 26, 4273–4279. [Google Scholar] [CrossRef]
- Palamà, I.E.; Arcadio, V.; D’Amone, S.; Biasiucci, M.; Gigli, G.; Cortese, B. Therapeutic PCL scaffold for reparation of resected osteosarcoma defect. Sci. Rep. 2017, 7, 12672. [Google Scholar] [CrossRef] [Green Version]
- Wang, Y.; Gunasekara, D.B.; Reed, M.I.; DiSalvo, M.; Bultman, S.J.; Sims, C.E.; Magness, S.T.; Allbritton, N.L. A microengineered collagen scaffold for generating a polarized crypt-villus architecture of human small intestinal epithelium. Biomaterials 2017, 128, 44–55. [Google Scholar] [CrossRef]
- Seal, B.; Otero, T.; Panitch, A. Polymeric biomaterials for tissue and organ regeneration. Mater. Sci. Eng. R Rep. 2001, 34, 147–230. [Google Scholar] [CrossRef]
- Kundu, B.; Rajkhowa, R.; Kundu, S.C.; Wang, X. Silk fibroin biomaterials for tissue regenerations. Adv. Drug Deliv. Rev. 2013, 65, 457–470. [Google Scholar] [CrossRef]
- Brovold, M.; Almeida, J.I.; Pla-Palacín, I.; Sainz-Arnal, P.; Sánchez-Romero, N.; Rivas, J.J.; Almeida, H.; Dachary, P.R.; Serrano-Aulló, T.; Soker, S. Naturally-derived biomaterials for tissue engineering applications. Nov. Biomater. Regen. Med. 2018, 1077, 421–449. [Google Scholar]
- Murphy, A.R.; Kaplan, D.L. Biomedical applications of chemically-modified silk fibroin. J. Mater. Chem. 2009, 19, 6443–6450. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vepari, C.; Kaplan, D.L. Silk as a Biomaterial. Prog. Polym. Sci. 2007, 32, 991–1007. [Google Scholar] [CrossRef] [PubMed]
- Rockwood, D.N.; Preda, R.C.; Yücel, T.; Wang, X.; Lovett, M.L.; Kaplan, D.L. Materials fabrication from Bombyx mori silk fibroin. Nat. Protoc. 2011, 6, 1612. [Google Scholar] [CrossRef] [PubMed]
- Su, D.; Yao, M.; Liu, J.; Zhong, Y.; Chen, X.; Shao, Z. Enhancing mechanical properties of silk fibroin hydrogel through restricting the growth of β-sheet domains. ACS Appl. Mater. Interfaces 2017, 9, 17489–17498. [Google Scholar] [CrossRef] [PubMed]
- Su, D.; Jiang, L.; Chen, X.; Dong, J.; Shao, Z. Enhancing the gelation and bioactivity of injectable silk fibroin hydrogel with laponite nanoplatelets. ACS Appl. Mater. Interfaces 2016, 8, 9619–9628. [Google Scholar] [CrossRef] [PubMed]
- Jiang, C.; Wang, X.; Gunawidjaja, R.; Lin, Y.H.; Gupta, M.K.; Kaplan, D.L.; Naik, R.R.; Tsukruk, V.V. Mechanical properties of robust ultrathin silk fibroin films. Adv. Funct. Mater. 2007, 17, 2229–2237. [Google Scholar] [CrossRef]
- Zhang, Y.-Q.; Shen, W.-D.; Xiang, R.-L.; Zhuge, L.-J.; Gao, W.-J.; Wang, W.-B. Formation of silk fibroin nanoparticles in water-miscible organic solvent and their characterization. J. Nanoparticle Res. 2007, 9, 885–900. [Google Scholar] [CrossRef]
- Jin, H.-J.; Chen, J.; Karageorgiou, V.; Altman, G.H.; Kaplan, D.L. Human bone marrow stromal cell responses on electrospun silk fibroin mats. Biomaterials 2004, 25, 1039–1047. [Google Scholar] [CrossRef]
- Ko, E.; Lee, J.S.; Kim, H.; Yang, S.Y.; Yang, D.; Yang, K.; Lee, J.; Shin, J.; Yang, H.S.; Ryu, W. Electrospun silk fibroin nanofibrous scaffolds with two-stage hydroxyapatite functionalization for enhancing the osteogenic differentiation of human adipose-derived mesenchymal stem cells. ACS Appl. Mater. Interfaces 2017, 10, 7614–7625. [Google Scholar] [CrossRef]
- Luo, X.; Guo, Z.; He, P.; Chen, T.; Li, L.; Ding, S.; Li, H. Study on structure, mechanical property and cell cytocompatibility of electrospun collagen nanofibers crosslinked by common agents. Int. J. Biol. Macromol. 2018, 113, 476–486. [Google Scholar] [CrossRef]
- Catto, V.; Farè, S.; Cattaneo, I.; Figliuzzi, M.; Alessandrino, A.; Freddi, G.; Remuzzi, A.; Tanzi, M.C. Small diameter electrospun silk fibroin vascular grafts: Mechanical properties, in vitro biodegradability, and in vivo biocompatibility. Mater. Sci. Eng. C 2015, 54, 101–111. [Google Scholar] [CrossRef] [PubMed]
- Miguel, S.P.; Simões, D.; Moreira, A.F.; Sequeira, R.S.; Correia, I.J. Production and characterization of electrospun silk fibroin based asymmetric membranes for wound dressing applications. Int. J. Biol. Macromol. 2019, 121, 524–535. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.; Wang, D.; Yao, X.; Wang, M.; Zhao, Y.; Lu, Y.; Wang, Z.; Guo, Y. Biomimetic hybrid scaffold of electrospun silk fibroin and pancreatic decellularized extracellular matrix for islet survival. J. Biomater. Sci. Polym. Ed. 2021, 32, 151–165. [Google Scholar] [CrossRef] [PubMed]
- Niu, Y.; Chen, X.; Yao, D.; Peng, G.; Liu, H.; Fan, Y. Enhancing neural differentiation of induced pluripotent stem cells by conductive graphene/silk fibroin films. J. Biomed. Mater. Res. Part A 2018, 106, 2973–2983. [Google Scholar] [CrossRef]
- Yi, B.; Zhang, H.; Yu, Z.; Yuan, H.; Wang, X.; Zhang, Y. Fabrication of high performance silk fibroin fibers via stable jet electrospinning for potential use in anisotropic tissue regeneration. J. Mater. Chem. B 2018, 6, 3934–3945. [Google Scholar] [CrossRef]
- Liang, Y.; Mitriashkin, A.; Lim, T.T.; Goh, J.C.-H. Conductive polypyrrole-encapsulated silk fibroin fibers for cardiac tissue engineering. Biomaterials 2021, 276, 121008. [Google Scholar] [CrossRef]
- Galloway, C.A.; Dalvi, S.; Shadforth, A.M.; Suzuki, S.; Wilson, M.; Kuai, D.; Hashim, A.; MacDonald, L.A.; Gamm, D.M.; Harkin, D.G. Characterization of human iPSC-RPE on a prosthetic Bruch’s membrane manufactured from silk fibroin. Investig. Ophthalmol. Vis. Sci. 2018, 59, 2792–2800. [Google Scholar] [CrossRef] [Green Version]
- Ball, M.P.; Thakuria, J.V.; Zaranek, A.W.; Clegg, T.; Rosenbaum, A.M.; Wu, X.; Angrist, M.; Bhak, J.; Bobe, J.; Callow, M.J. A public resource facilitating clinical use of genomes. Proc. Natl. Acad. Sci. USA 2012, 109, 11920–11927. [Google Scholar] [CrossRef] [Green Version]
- Naylor, R.W.; Morais, M.R.P.T.; Lennon, R. Complexities of the glomerular basement membrane. Nat. Rev. Nephrol. 2021, 17, 112–127. [Google Scholar] [CrossRef]
- Kreidberg, J.A. Podocyte differentiation and glomerulogenesis. J. Am. Soc. Nephrol. 2003, 14, 806–814. [Google Scholar] [CrossRef] [Green Version]
- Yamamura, Y.; Furuichi, K.; Murakawa, Y.; Hirabayashi, S.; Yoshihara, M.; Sako, K.; Kitajima, S.; Toyama, T.; Iwata, Y.; Sakai, N. Identification of candidate PAX2-regulated genes implicated in human kidney development. Sci. Rep. 2021, 11, 9123. [Google Scholar] [CrossRef] [PubMed]
- Ohtaka, A.; Ootaka, T.; Sato, H.; Soma, J.; Sato, T.; Saito, T.; Ito, S. Significance of early phenotypic change of glomerular podocytes detected by Pax2 in primary focal segmental glomerulosclerosis. Am. J. Kidney Dis. 2002, 39, 475–485. [Google Scholar] [CrossRef] [PubMed]
- Kawachi, H.; Miyauchi, N.; Suzuki, K.; Han, G.D.; Orikasa, M.; Shimizu, F. Role of podocyte slit diaphragm as a filtration barrier. Nephrology 2006, 11, 274–281. [Google Scholar] [CrossRef]
- Srichai, M.B.; Konieczkowski, M.; Padiyar, A.; Konieczkowski, D.J.; Mukherjee, A.; Hayden, P.S.; Kamat, S.; El-Meanawy, M.A.; Khan, S.; Mundel, P. A WT1 co-regulator controls podocyte phenotype by shuttling between adhesion structures and nucleus. J. Biol. Chem. 2004, 279, 14398–14408. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Guo, J.-K.; Menke, A.L.; Gubler, M.-C.; Clarke, A.R.; Harrison, D.; Hammes, A.; Hastie, N.D.; Schedl, A. WT1 is a key regulator of podocyte function: Reduced expression levels cause crescentic glomerulonephritis and mesangial sclerosis. Hum. Mol. Genet. 2002, 11, 651–659. [Google Scholar] [CrossRef]



Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mou, X.; Shah, J.; Bhattacharya, R.; Kalejaiye, T.D.; Sun, B.; Hsu, P.-C.; Musah, S. A Biomimetic Electrospun Membrane Supports the Differentiation and Maturation of Kidney Epithelium from Human Stem Cells. Bioengineering 2022, 9, 188. https://doi.org/10.3390/bioengineering9050188
Mou X, Shah J, Bhattacharya R, Kalejaiye TD, Sun B, Hsu P-C, Musah S. A Biomimetic Electrospun Membrane Supports the Differentiation and Maturation of Kidney Epithelium from Human Stem Cells. Bioengineering. 2022; 9(5):188. https://doi.org/10.3390/bioengineering9050188
Chicago/Turabian StyleMou, Xingrui, Jessica Shah, Rohan Bhattacharya, Titilola D. Kalejaiye, Bowen Sun, Po-Chun Hsu, and Samira Musah. 2022. "A Biomimetic Electrospun Membrane Supports the Differentiation and Maturation of Kidney Epithelium from Human Stem Cells" Bioengineering 9, no. 5: 188. https://doi.org/10.3390/bioengineering9050188
APA StyleMou, X., Shah, J., Bhattacharya, R., Kalejaiye, T. D., Sun, B., Hsu, P.-C., & Musah, S. (2022). A Biomimetic Electrospun Membrane Supports the Differentiation and Maturation of Kidney Epithelium from Human Stem Cells. Bioengineering, 9(5), 188. https://doi.org/10.3390/bioengineering9050188