Densification: Hyaluronan Aggregation in Different Human Organs
Abstract
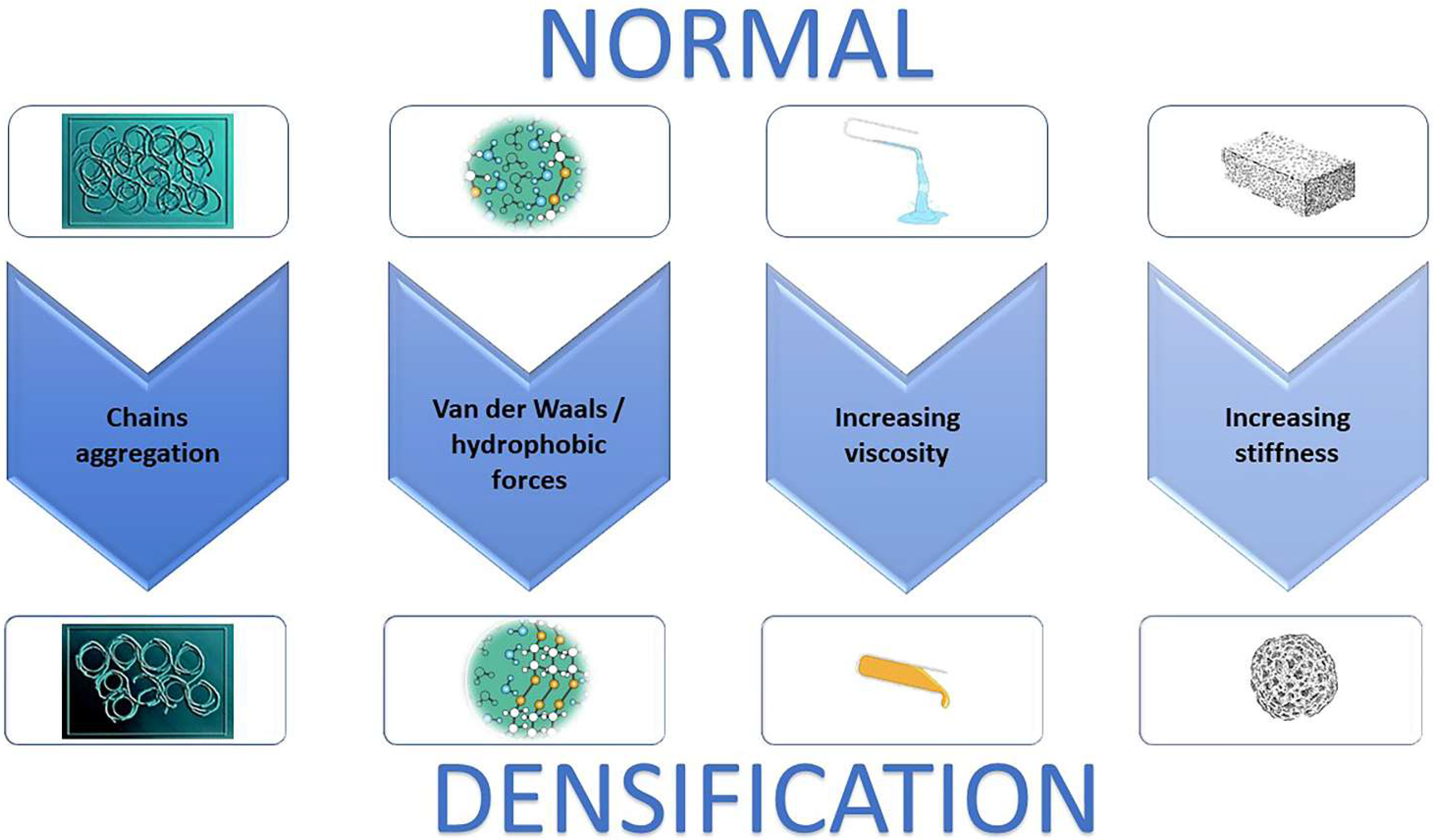
1. Introduction
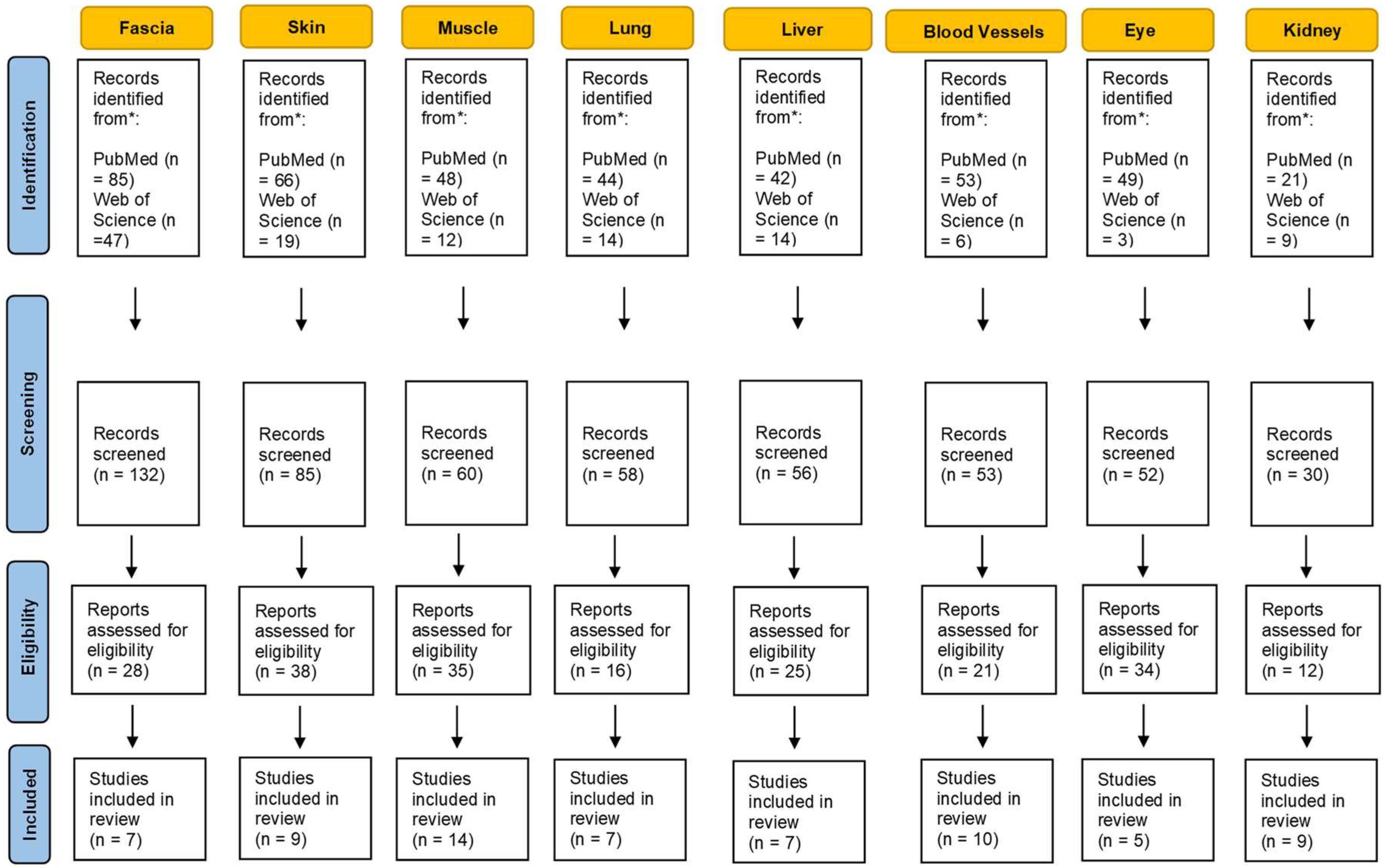
2. Materials and Methods
3. Results
3.1. Fascia
3.2. Skin
3.3. Muscle
3.4. Lung
3.5. Liver
3.6. Blood Vessels
3.7. Eyes
3.8. Kidney
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Jiang, D.; Liang, J.; Noble, P.W. Hyaluronan in tissue injury and repair. Annu. Rev. Cell Dev. Biol. 2007, 23, 435–461. [Google Scholar] [CrossRef] [PubMed]
- Hascall, V.C.; Majors, A.K.; De La Motte, C.A.; Evanko, S.P.; Wang, A.; Drazba, J.A.; Wight, T.N. Intracellular hyaluronan: A new frontier for inflammation? Biochim. Et Biophys. Acta (BBA)-Gen. Subj. 2004, 1673, 3–12. [Google Scholar] [CrossRef] [PubMed]
- Stridh, S.; Palm, F.; Hansell, P. Renal interstitial hyaluronan: Functional aspects during normal and pathological conditions. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2012, 302, R1235–R1249. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.; Spicer, A. Hyaluronan: A multifunctional, megaDalton, stealth molecule. Curr. Opin. Cell Biol. 2000, 12, 581. [Google Scholar] [CrossRef]
- Savani, R.C.; Delisser, H.M. Hyaluronan and its Receptors in Lung Health and Disease. In Proteoglycans in Lung Disease; CRC Press: Boca Raton, FL, USA, 2002; pp. 102–135. [Google Scholar]
- Zhang, L.; Bowen, T.; Grennan-Jones, F.; Paddon, C.; Giles, P.; Webber, J.; Ludgate, M. Thyrotropin receptor activation increases hyaluronan production in preadipocyte fibroblasts: Contributory role in hyaluronan accumulation in thyroid dysfunction. J. Biol. Chem. 2009, 284, 26447–26455. [Google Scholar] [CrossRef]
- Smith, T.J.; Wang, H.S.; Evans, C.H. Leukoregulin is a potent inducer of hyaluronan synthesis in cultured human orbital fibroblasts. Am. J. Physiol.-Cell Physiol. 1995, 268, C382–C388. [Google Scholar] [CrossRef]
- Marieb, E.A.; Zoltan-Jones, A.; Li, R.; Misra, S.; Ghatak, S.; Cao, J.; Toole, B.P. Emmprin promotes anchorage-independent growth in human mammary carcinoma cells by stimulating hyaluronan production. Cancer Res. 2004, 64, 1229–1232. [Google Scholar] [CrossRef]
- Gao, F.; Okunieff, P.; Han, Z.; Ding, I.; Wang, L.; Liu, W.; Zhang, L. Hypoxia-Induced Alterations in Hyaluronan and Hyaluronidase. In Oxygen Transport to Tissue XXVI; Springer: Boston, MA, USA, 2005; pp. 249–256. [Google Scholar]
- David-Raoudi, M.; Deschrevel, B.; Leclercq, S.; Galéra, P.; Boumediene, K.; Pujol, J.P. Chondroitin sulfate increases hyaluronan production by human synoviocytes through differential regulation of hyaluronan synthases: Role of p38 and Akt. Arthritis Rheum. Off. J. Am. Coll. Rheumatol. 2009, 60, 760–770. [Google Scholar] [CrossRef]
- Ohtani, T.; Memezawa, A.I.; Okuyama, R.; Sayo, T.; Sugiyama, Y.; Inoue, S.; Aiba, S. Increased hyaluronan production and decreased E-cadherin expression by cytokine-stimulated keratinocytes lead to spongiosis formation. J. Investig. Dermatol. 2009, 129, 1412–1420. [Google Scholar] [CrossRef]
- Toole, B.P.; Hascall, V.C. Hyaluronan and tumor growth. Am. J. Pathol. 2002, 161, 745. [Google Scholar] [CrossRef]
- Li, Y.; Heldin, P. Hyaluronan production increases the malignant properties of mesothelioma cells. Br. J. Cancer 2001, 85, 600–607. [Google Scholar] [CrossRef] [PubMed]
- Zoltan-Jones, A.; Huang, L.; Ghatak, S.; Toole, B.P. Elevated hyaluronan production induces mesenchymal and transformed properties in epithelial cells. J. Biol. Chem. 2003, 278, 45801–45810. [Google Scholar] [CrossRef] [PubMed]
- Abatangelo, G.; Vindigni, V.; Avruscio, G.; Pandis, L.; Brun, P. Hyaluronic Acid: Redefining Its Role. Cells 2020, 9, 1743. [Google Scholar] [CrossRef] [PubMed]
- Misra, S.; Ghatak, S.; Zoltan-Jones, A.; Toole, B.P. Regulation of multidrug resistance in cancer cells by hyaluronan. J. Biol. Chem. 2003, 278, 25285–25288. [Google Scholar] [CrossRef]
- Anderegg, U.; Simon, J.C.; Averbeck, M. More than just a filler—The role of hyaluronan for skin homeostasis. Exp. Dermatol. 2014, 23, 295–303. [Google Scholar] [CrossRef]
- Fraser, J.R.; Laurent, T.C.; Laurent, U.B. Hyaluronan: Its nature, distribution, functions and turnover. J. Intern. Med. 1997, 242, 27–33. [Google Scholar] [CrossRef]
- Tavianatou, A.G.; Caon, I.; Franchi, M.; Piperigkou, Z.; Galesso, D.; Karamanos, N.K. Hyaluronan: Molecular size-dependent signaling and biological functions in inflammation and cancer. FEBS J. 2019, 286, 2883–2908. [Google Scholar] [CrossRef]
- Tavianatou, A.G.; Piperigkou, Z.; Barbera, C.; Beninatto, R.; Masola, V.; Caon, I.; Onisto, M.; Franchi, M.; Galesso, D.; Karamanos, N.K. Molecular size-dependent specificity of hyaluronan on functional properties, morphology and matrix composition of mammary cancer cells. Matrix Biol. Plus 2019, 3, 100008. [Google Scholar] [CrossRef]
- Lee, D.H.; Oh, J.H.; Chung, J.H. Glycosaminoglycan and proteoglycan in skin aging. J. Dermatol. Sci. 2016, 83, 174–181. [Google Scholar] [CrossRef]
- Matteini, P.; Dei, L.; Carretti, E.; Volpi, N.; Goti, A.; Pini, R. Structural Behavior of Highly Concentrated Hyaluronan. Biomacromolecules 2009, 10, 1516–1522. [Google Scholar] [CrossRef]
- Pavan, P.G.; Stecco, A.; Stern, R.; Stecco, C. Painful connections: Densification versus fibrosis of fascia. Curr. Pain Headache Rep. 2014, 18, 441. [Google Scholar] [CrossRef] [PubMed]
- Amir, A.; Kim, S.; Stecco, A.; Jankowski, M.P.; Raghavan, P. Hyaluronan homeostasis and its role in pain and muscle stiffness. PM&R 2022. [Google Scholar] [CrossRef]
- Stecco, A.; Gesi, M.; Stecco, C.; Stern, R. Fascial components of the myofascial pain syndrome. Curr. Pain Headache Rep. 2013, 17, 352. [Google Scholar] [CrossRef]
- Stecco, C.; Stern, R.; Porzionato, A.; Macchi, V.; Masiero, S.; Stecco, A.; De Caro, R. Hyaluronan within fascia in the etiology of myofascial pain. Surg. Radiol. Anat. 2011, 33, 891–896. [Google Scholar] [CrossRef] [PubMed]
- Dintenfass, L. Lubrication in synovial joints: A theoretical analysis: A rheological approach to the problems of joint movements and joint lubrication. JBJS 1963, 45, 1241–1256. [Google Scholar] [CrossRef]
- Chytil, M.; Strand, S.; Christensen, B.E.; Pekař, M. Calorimetric and light scattering study of interactions and macromolecular properties of native and hydrophobically modified hyaluronan. Carbohydr. Polym. 2010, 81, 855–863. [Google Scholar] [CrossRef]
- Yucesoy, C.A.; Baan, G.C.; Huijing, P.A. Substantial inter-antagonistic epimuscular myofascial force transmission occurs in the rat between the deep flexor muscles and the muscles of the anterior crural and peroneal compartments. J. Electromyogr. Kinesiol. 2010, 20, 118–126. [Google Scholar] [CrossRef]
- Stecco, A.; Meneghini, A.; Stern, R.; Stecco, C.; Imamura, M. Ultrasonography in myofascial neck pain: Randomized clinical trial for diagnosis and follow-up. Surg. Radiol. Anat. 2014, 36, 243–253. [Google Scholar] [CrossRef]
- Menon, R.G.; Oswald, S.F.; Raghavan, P.; Regatte, R.R.; Stecco, A. T1ρ-Mapping for Musculoskeletal Pain Diagnosis: Case Series of Variation of Water Bound Glycosaminoglycans Quantification before and after Fascial Manipulation® in Subjects with Elbow Pain. Int. J. Environ. Res. Public Health 2020, 17, 708. [Google Scholar] [CrossRef]
- Luomala, T.; Pihlman, M.; Heiskanen, J.; Stecco, C. Case study: Could ultrasound and elastography visualized densified areas inside the deep fascia? J. Bodyw. Mov. Ther. 2014, 18, 462–468. [Google Scholar] [CrossRef]
- Stern, R.; Maibach, H.I. Hyaluronan in skin: Aspects of aging and its pharmacologic modulation. Clin. Dermatol. 2008, 26, 106–122. [Google Scholar] [CrossRef] [PubMed]
- Laurent, U.B.G.; Laurent, T.C. On the origin of hyaluronate in blood. Biochem. Int. 1981, 2, 195–199. [Google Scholar]
- Yoneda, M.; Suzuki, S.; Kimata, K. Hyaluronic acid associated with the surfaces of cultured fibroblasts is linked to a serum-derived 85-kDa protein. J. Biol. Chem. 1990, 265, 5247–5257. [Google Scholar] [CrossRef]
- Meyer, L.J.; Stern, R. Age-dependent changes of hyaluronan in human skin. J. Invest. Dermatol. 1994, 102, 385–389. [Google Scholar] [CrossRef] [PubMed]
- Piehl-Aulin, K.; Laurent, C.; Engstrom-Laurent, A.; Hellstrom, S.; Henriksson, J. Hyaluronan in human skeletal muscle of lower extremity: Concentration, distribution, and effect of exercise. J. Appl. Physiol. 1991, 71, 2493–2498. [Google Scholar] [CrossRef]
- Okita, M.; Yoshimura, T.; Nakano, J.; Motomura, M.; Eguchi, K. Effects of reduced joint mobility on sarcomere length, collagen fibril arrangement in the endomysium, and hyaluronan in rat soleus muscle. J. Muscle Res. Cell Motil. 2004, 25, 159–166. [Google Scholar] [CrossRef]
- Springer, J.; Schust, S.; Peske, K.; Tschirner, A.; Rex, A.; Engel, O.; Scherbakov, N.; Meisel, A.; von Haehling, S.; Boschmann, M.; et al. Catabolic signaling and muscle wasting after acute ischemic stroke in mice: Indication for a stroke-specific sarcopenia. Stroke 2014, 45, 3675–3683. [Google Scholar] [CrossRef]
- Raghavan, P.; Lu, Y.; Mirchandani, M.; Stecco, A. Human Recombinant Hyaluronidase Injections for Upper Limb Muscle Stiffness in Individuals with Cerebral Injury: A Case Series. EBioMedicine 2016, 9, 306–313. [Google Scholar] [CrossRef]
- Konrad, A.; Stafilidis, S.; Tilp, M. Effects of acute static, ballistic, and PNF stretching exercise on the muscle and tendon tissue properties. Scand. J. Med. Sci. Sports 2016, 27, 1070–1080. [Google Scholar] [CrossRef]
- Herda, T.J.; Cramer, J.T.; Ryan, E.D.; McHugh, M.P.; Stout, J.R. Acute effects of static versus dynamic stretching on isometric peak torque, electromyography, and mechanomyography of the biceps femoris muscle. J. Strength Cond. Res. 2008, 22, 809–817. [Google Scholar] [CrossRef]
- Nordez, A.; McNair, P.J.; Casari, P.; Cornu, C. Static and cyclic stretching: Their different effects on the passive torque-angle curve. J. Sci. Med. Sport. 2010, 13, 156–160. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Bishop, D.J. Warm up I: Potential mechanisms and the effects of passive warm up on exercise performance. Sports Med. 2003, 33, 439–454. [Google Scholar] [CrossRef] [PubMed]
- Buchthal, F.; Kaiser, E.; Knappeis, G.G. Elasticity, Viscosity and Plasticity in the Cross Striated Muscle Fibre. Acta Physiol. Scand. 2008, 8, 16–37. [Google Scholar] [CrossRef]
- Menon, R.G.; Raghavan, P.; Regatte, R.R. Quantifying muscle glycosaminoglycan levels in patients with post-stroke muscle stiffness using T1ρ MRI. Sci. Rep. 2019, 9, 14513. [Google Scholar]
- Lampi, M.C.; Reinhart-King, C.A. Targeting extracellular matrix stiffness to attenuate disease: From molecular mechanisms to clinical trials. Sci. Transl. Med. 2018, 10, eaao0475. [Google Scholar] [CrossRef]
- Lieber, R.L.; Steinman, S.; Barash, I.A.; Chambers, H. Structural and functional changes in spastic skeletal muscle. Muscle Nerve 2004, 29, 615–627. [Google Scholar] [CrossRef]
- Nettelbladt, O.; Hallgren, R. Hyaluronan (hyaluronic acid) in bronchoalveolar lavage fluid during the development of bleomycin-induced alveolitis in the rat. Am. Rev. Respir. Dis. 1989, 140, 1028–1032. [Google Scholar] [CrossRef]
- Juul, S.E.; Kinsella, M.G.; Jackson, J.C.; Truog, W.E.; Standaert, T.A.; Hodson, W.A. Changes in hyaluronan deposition during early respiratory distress syndrome in premature monkeys. Pediatr. Res. 1994, 35, 238–243. [Google Scholar] [CrossRef]
- Toole, B.P. Glycosaminoglycans in Morphogenesis. In Cellular Biology of Extracellular Matrix; Hay, E.D., Ed.; Plenum Publishing Corporation: New York, NY, USA, 1981; pp. 251–294. [Google Scholar]
- Bjermer, L.; Engström-Laurent, A.; Thunell, M.; Hällgren, R. Hyaluronic acid in bronchoalveolar lavage fluid in patients with sarcoidosis: Relationship to lavage mast cells. Thorax 1987, 42, 933–938. [Google Scholar] [CrossRef]
- Cui, Z.; Liao, J.; Cheong, N.; Longoria, C.; Cao, G.; DeLisser, H.M.; Savani, R.C. The Receptor for Hyaluronan-Mediated Motility (CD168) promotes inflammation and fibrosis after acute lung injury. Matrix Biol. 2019, 78–79, 255–271. [Google Scholar] [CrossRef]
- Collum, S.D.; Chen, N.Y.; Hernandez, A.M.; Hanmandlu, A.; Sweeney, H.; Mertens, T.C.J.; Weng, T.; Luo, F.; Molina, J.G.; Davies, J.; et al. Inhibition of hyaluronan synthesis attenuates pulmonary hypertension associated with lung fibrosis. Br. J. Pharmacol. 2017, 174, 3284–3301. [Google Scholar] [CrossRef] [PubMed]
- Arenson, B.M.; Bissell, D.M. Glycosaminoglycan, proteoglycan, and hepatic fibrosis. Gastroenterology 1987, 92, 536–538. [Google Scholar] [CrossRef]
- Wells, R.G. Cellular sources of extracellular matrix in hepatic fibrosis. Clin. Liver Dis. 2008, 12, 759–768. [Google Scholar] [CrossRef] [PubMed]
- Gressner, O.A.; Weiskirchen, R.; Gressner, A.M. Evolving concepts of liver fibrogenesis provide new diagnostic and therapeutic options. Comp. Hepatol. 2007, 6, 7. [Google Scholar] [CrossRef] [PubMed]
- Guha, I.N.; Parkes, J.; Roderick, P.; Chattopadhyay, D.; Cross, R.; Harris, S.; Kaye, P.; Burt, A.D.; Ryder, S.D.; Aithal, G.P.; et al. Noninvasive markers of fibrosis in nonalcoholic fatty liver disease: Validating the European liver fibrosis panel and exploring simple markers. Hepatology 2008, 47, 455–460. [Google Scholar] [CrossRef]
- Hansen, J.F.; Christiansen, K.M.; Staugaard, B.; Moessner, B.K.; Lillevang, S.; Krag, A.; Christensen, P.B. Combining liver stiffness with hyaluronic acid provides superior prognostic performance in chronic hepatitis C. PLoS ONE 2019, 14, e0212036. [Google Scholar] [CrossRef]
- Sakakibara, K.; Umeda, M.; Saito, S.; Nagase, S. Production of collagen and acidic glycosaminoglycans by an epithelial liver cell clone in culture. Exp. Cell Res. 1977, 110, 159–165. [Google Scholar] [CrossRef]
- Koizumi, T.; Nakamura, N.; Abe, H. Changes in acid mucopolysaccharide in the liver in hepatic fibrosis. Biochim. Et Biophys. Acta (BBA)-Gen. Subj. 1967, 148, 749–756. [Google Scholar] [CrossRef]
- Neuman, M.G.; Cohen, L.B.; Nanau, R.M. Hyaluronic acid as a non-invasive biomarker of liver fibrosis. Clin. Biochem. 2016, 49, 302–315. [Google Scholar] [CrossRef]
- Nobili, V.; Alisi, A.; Torre, G.; De Vito, R.; Pietrobattista, A.; Morino, G.; De Ville De Goyet, J.; Bedogni, G.; Pinzani, M. Hyaluronic acid predicts hepatic fibrosis in children with nonalcoholic fatty liver disease. Transl. Res. 2010, 156, 229–234. [Google Scholar] [CrossRef]
- Lydatakis, H.; Hager, I.P.; Kostadelou, E.; Mpousmpoulas, S.; Pappas, S.; Diamantis, I. Non-invasive markers to predict the liver fibrosis in nonalcoholic fatty liver disease. Liver Int. 2006, 26, 864–871. [Google Scholar] [CrossRef] [PubMed]
- Guechot, J.; Laudat, A.; Loria, A.; Serfaty, L.; Poupon, R.; Giboudeau, J. Diagnostic accuracy of hyaluronan and type III procollagen amino terminal peptide serum assays as markers of liver fibrosis in chronic viral hepatitis C evaluated by ROC curve analysis. Clin. Chem. 1996, 42, 558–563. [Google Scholar] [CrossRef] [PubMed]
- Kaneda, H.; Hashimoto, E.; Yatsuji, S.; Tokushige, K.; Shiratori, K. Hyaluronic acid levels can predict severe fibrosis and platelet counts can predict cirrhosis in patients with nonalcoholic fatty liver disease. J. Gastroenterol. Hepatol. 2006, 21, 1459–1465. [Google Scholar] [CrossRef] [PubMed]
- Caon, I.; Bartolini, B.; Moretto, P.; Parnigoni, A.; Caravà, E.; Vitale, D.L.; Alaniz, L.; Viola, M.; Karousou, E.; De Luca, G.; et al. Sirtuin 1 reduces hyaluronan synthase 2 expression by inhibiting nuclear translocation of NF-κB and expression of the long-noncoding RNA HAS2-AS1. J. Biol. Chem. 2020, 295, 3485–3496. [Google Scholar] [CrossRef] [PubMed]
- Lennon, F.E.; Singleton, P.A. Hyaluronan regulation of vascular integrity. Am. J. Cardiovasc. Dis. 2011, 1, 200. [Google Scholar]
- Bot, P.; Hoefer, I.; Piek, J.; Pasterkamp, G. Hyaluronic acid: Targeting immune modulatory components of the extracellular matrix in atherosclerosis. Curr. Med. Chem. 2008, 15, 786. [Google Scholar] [CrossRef]
- Papakonstantinou, E.; Roth, M.; Block, L.; Mirtsou-Fidani, V.; Argiriadis, P.; Karakiulakis, G. The differential distribution of hyaluronic acid in the layers of human atheromatic aortas is associated with vascular smooth muscle cell proliferation and migration. Atherosclerosis 1998, 138, 79. [Google Scholar] [CrossRef]
- Evanko, S.; Angello, J.; Wight, T. Formation of hyaluronan-and versican-rich pericellular matrix is required for proliferation and migration of vascular smooth muscle cells. Arterioscler. Thromb. Vasc. Biol. 1999, 19, 1004. [Google Scholar] [CrossRef]
- Wilkinson, T.; Bressler, S.; Evanko, S.; Braun, K.R.; Wight, T.N. Overexpression of hyaluronan synthases alters vascular smooth muscle cell phenotype and promotes monocyte adhesion. J. Cell. Physiol. 2006, 206, 378. [Google Scholar] [CrossRef]
- de la Motte, C.A.; Hascall, V.; Drazba, J.; Bandyopadhyay, S.K.; Strong, S.A. Mononuclear leukocytes bind to specific hyaluronan structures on colon mucosal smooth muscle cells treated with polyinosinic acid: Polycytidylic Acid. Am. J. Pathol. 2003, 163, 121. [Google Scholar] [CrossRef]
- Evanko, S.P.; Johnson, P.Y.; Braun, K.R.; Underhill, C.B.; Dudhia, J.; Wight, T.N. Platelet-derived growth factor stimulates the formation of versican-hyaluronan aggregates and pericellular matrix expansion in arterial smooth muscle cells. Arch. Biochem. Biophys. 2001, 394, 29–38. [Google Scholar] [CrossRef] [PubMed]
- Riessen, R.; Wight, T.; Pastore, C.; Henley, C.; Isner, J.M. Distribution of hyaluronan during extracellular matrix remodeling in human restenotic arteries and balloon-injured rat carotid arteries. Circulation 1996, 93, 1141. [Google Scholar] [CrossRef] [PubMed]
- Chajara, A.; Delpech, B.; Courel, M.; Leroy, M.; Basuyau, J.P.; Lévesque, H. Effect of aging on neointima formation and hyaluronan, hyaluronidase, and hyaluronectin production in injured rat aorta. Atherosclerosis 1998, 138, 53. [Google Scholar] [CrossRef]
- Sadowitz, B.; Seymour, K.; Gahtan, V.; Maier, K.G. The role of hyaluronic acid in atherosclerosis and intimal hyperplasia. J. Surg. Res. 2012, 173, e63–e72. [Google Scholar] [CrossRef]
- Lorentzen, K.A.; Chai, S.; Chen, H.; Danielsen, C.C.; Simonsen, U.; Wogensen, L. Mechanisms involved in extracellular matrix remodeling and arterial stiffness induced by hyaluronan accumulation. Atherosclerosis 2016, 244, 195–203. [Google Scholar] [CrossRef]
- Peng, Y.; Yu, Y.; Lin, L.; Liu, X.; Zhang, X.; Wang, P.; Hoffman, P.; Kim, S.Y.; Zhang, F.; Linhardt, R.J. Glycosaminoglycans from bovine eye vitreous humour and interaction with collagen type II. Glycoconj. J. 2018, 35, 119–128. [Google Scholar] [CrossRef]
- Chakrabarti, B.; Park, J.W. Glycosaminoglycans: Structure and interaction. CRC Crit. Rev. Biochem. 1980, 8, 225–313. [Google Scholar] [CrossRef]
- Stepp, M.A.; Menko, A.S. Immune responses to injury and their links to eye disease. Transl. Res. 2021, 236, 52–71. [Google Scholar] [CrossRef]
- Theocharis, D.A.; Skandalis, S.S.; Noulas, A.V.; Papageorgakopoulou, N.; Theocharis, A.D.; Karamanos, N.K. Hyaluronan and chondroitin sulfate proteoglycans in the supramolecular organization of the mammalian vitreous body. Connect. Tissue Res. 2008, 49, 124–128. [Google Scholar] [CrossRef]
- Narayanan, R.; Kuppermann, B.D. Hyaluronidase for pharmacologic vitreolysis. Dev. Ophthalmol. 2009, 44, 20–25. [Google Scholar]
- Comper, W.D.; Laurent, T.C. Physiological function of connective tissue polysaccharides. Physiol. Rev. 1978, 58, 255–315. [Google Scholar] [CrossRef] [PubMed]
- Oh, J.H.; Kim, Y.K.; Jung, J.Y.; Shin, J.E.; Kim, K.H.; Cho, K.H.; Eun, H.C.; Chung, J.H. Intrinsic aging- and photoaging dependent level changes of glycosaminoglycans and their correlation with water content in human skin. J. Dermatol. Sci. 2011, 62, 192–201. [Google Scholar] [CrossRef] [PubMed]
- Kaul, A.; Singampalli, K.L.; Parikh, U.M.; Yu, L.; Keswani, S.G.; Wang, X. Hyaluronan, a double-edged sword in kidney diseases. Pediatr. Nephrol. 2021, 37, 735–744. [Google Scholar] [CrossRef] [PubMed]
- Eddy, A.A. Molecular basis of renal fibrosis. Pediatr. Nephrol. 2000, 15, 290–301. [Google Scholar] [CrossRef] [PubMed]
- Selbi, W.; de la Motte, C.A.; Hascall, V.C.; Day, A.J.; Bowen, T.; Phillips, A.O. Characterization of hyaluronan cable structure and function in renal proximal tubular epithelial cells. Kidney Int. 2006, 70, 1287–1295. [Google Scholar] [CrossRef]
- Yang, J.; Liu, Y. Dissection of key events in tubular epithelial to myofibroblast transition and its implications in renal interstitial fibrosis. Am. J. Pathol. 2001, 159, 1465–1475. [Google Scholar] [CrossRef]
- Johnsson, C.; Hällgren, R.; Wahlberg, J.; Tufveson, G. Renal accumulation and distribution of hyaluronan after ureteral obstruction. Scand. J. Urol. Nephrol. 1997, 31, 327–331. [Google Scholar] [CrossRef]
- Morse, R.M.; Resnick, M.I. A new approach to the study of urinary macromolecules as a participant in calcium oxalate crystallization. J. Urol. 1988, 139, 869–873. [Google Scholar] [CrossRef]
- Han, D.H.; Song, H.K.; Lee, S.Y.; Song, J.H.; Piao, S.G.; Yoon, H.E.; Ghee, J.Y.; Yoon, H.J.; Kim, J.; Yang, C.W. Upregulation of hyaluronan and its binding receptors in an experimental model of chronic cyclosporine nephropathy. Nephrology 2010, 15, 216–224. [Google Scholar] [CrossRef]
- Hansell, P.; Göransson, V.; Odlind, C.; Gerdin, B.; Hällgren, R. Hyaluronan content in the kidney in different states of body hydration. Kidney Int. 2000, 58, 2061–2068. [Google Scholar] [CrossRef]
- Heatley, F.; Scott, J.E. A water molecule participates in the secondary structure of hyaluronan. Biochem. J. 1988, 254, 489–493. [Google Scholar] [CrossRef] [PubMed]
- Tømmeraas, K.; Melander, C. Kinetics of Hyaluronan Hydrolysis in Acidic Solution at Various pH Values. Biomacromolecules 2008, 9, 1535–1540. [Google Scholar] [CrossRef] [PubMed]
- Gatej, I.; Popa, M.; Rinaudo, M. Role of the pH on Hyaluronan Behavior in Aqueous Solution. Biomacromolecules 2005, 6, 61–67. [Google Scholar] [CrossRef] [PubMed]
- Juel, C.; Klarskov, C.; Nielsen, J.J.; Krustrup, P.; Mohr, M.; Bangsbo, J. Effect of high-intensity intermittent training on lactate and H+ release from human skeletal muscle. Am. J. Physiol. Endocrinol. Metab. 2004, 286, E245–E251. [Google Scholar] [CrossRef]
- Day, A.J.; Prestwich, G.D. Hyaluronan-binding proteins: Tying up the giant. J. Biol. Chem. 2002, 277, 4585–4588. [Google Scholar] [CrossRef]
- Wight, T.N. Versican: A versatile extracellular matrix proteoglycan in cell biology. Curr. Opin. Cell Biol. 2002, 14, 617–623. [Google Scholar] [CrossRef]
- Handorf, A.M.; Zhou, Y.; Halanski, M.A.; Li, W.J. Tissue stiffness dictates development, homeostasis, and disease progression. Organogenesis 2015, 11, 1–15. [Google Scholar] [CrossRef]
- Bae, Y.H.; Mui, K.L.; Hsu, B.Y.; Liu, S.L.; Cretu, A.; Razinia, Z.; Xu, T.; Puré, E.; Assoian, R.K. A FAK-Cas-Rac-lamellipodin signaling module transduces extracellular matrix stiffness into mechanosensitive cell cycling. Sci. Signal. 2014, 7, ra57. [Google Scholar] [CrossRef]
- Genovese, F.; Manresa, A.A.; Leeming, D.J.; Karsdal, M.A.; Boor, P. The extracellular matrix in the kidney: A source of novel non-invasive biomarkers of kidney fibrosis? Fibrogenesis Tissue Repair 2014, 7, 4. [Google Scholar] [CrossRef]
- Ebelt, N.D.; Zamloot, V.; Manuel, E.R. Targeting desmoplasia in pancreatic cancer as an essential first step to effective therapy. Oncotarget 2020, 11, 3486–3488. [Google Scholar] [CrossRef]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Stecco, A.; Cowman, M.; Pirri, N.; Raghavan, P.; Pirri, C. Densification: Hyaluronan Aggregation in Different Human Organs. Bioengineering 2022, 9, 159. https://doi.org/10.3390/bioengineering9040159
Stecco A, Cowman M, Pirri N, Raghavan P, Pirri C. Densification: Hyaluronan Aggregation in Different Human Organs. Bioengineering. 2022; 9(4):159. https://doi.org/10.3390/bioengineering9040159
Chicago/Turabian StyleStecco, Antonio, Mary Cowman, Nina Pirri, Preeti Raghavan, and Carmelo Pirri. 2022. "Densification: Hyaluronan Aggregation in Different Human Organs" Bioengineering 9, no. 4: 159. https://doi.org/10.3390/bioengineering9040159
APA StyleStecco, A., Cowman, M., Pirri, N., Raghavan, P., & Pirri, C. (2022). Densification: Hyaluronan Aggregation in Different Human Organs. Bioengineering, 9(4), 159. https://doi.org/10.3390/bioengineering9040159







