Impact of Microenvironmental Changes during Degeneration on Intervertebral Disc Progenitor Cells: A Comparison with Mesenchymal Stem Cells
Abstract
1. Introduction
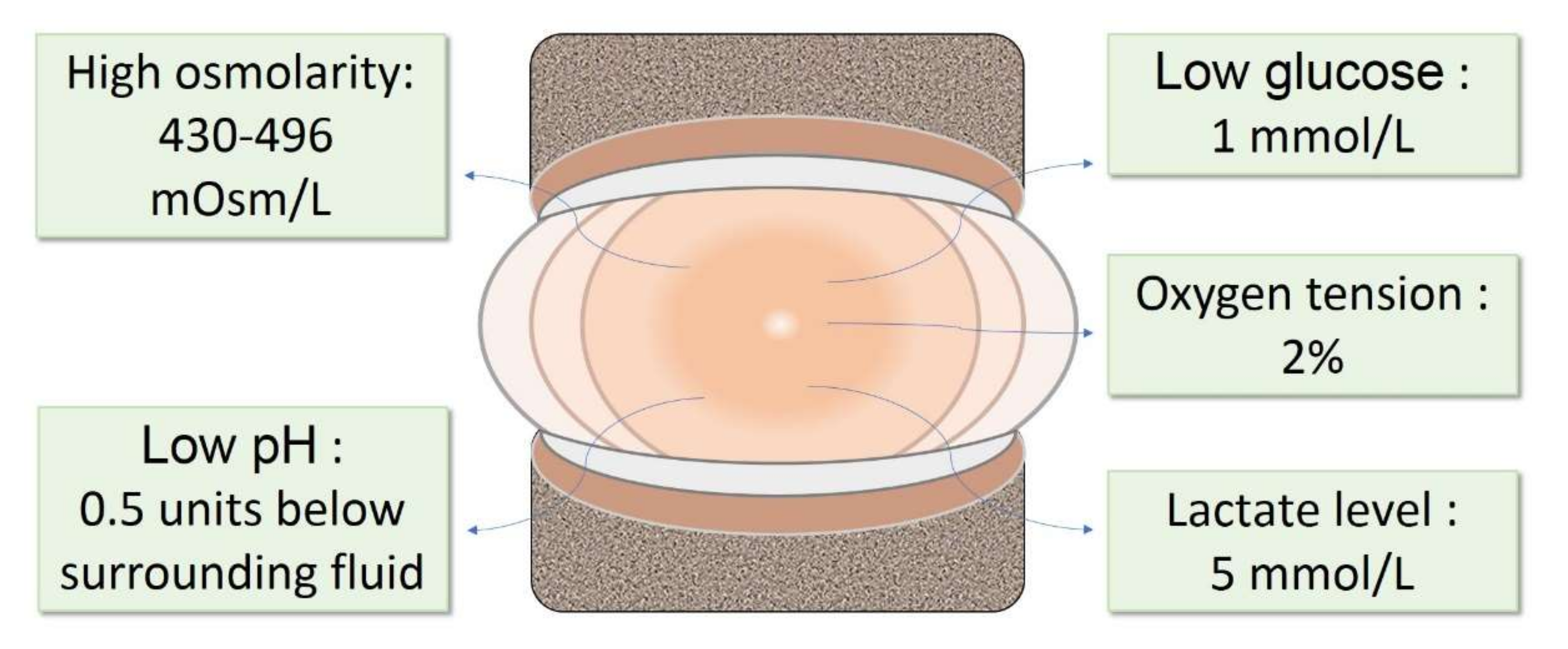
2. The IVD Microenvironment
3. Changes in IVD Microenvironment during Degeneration
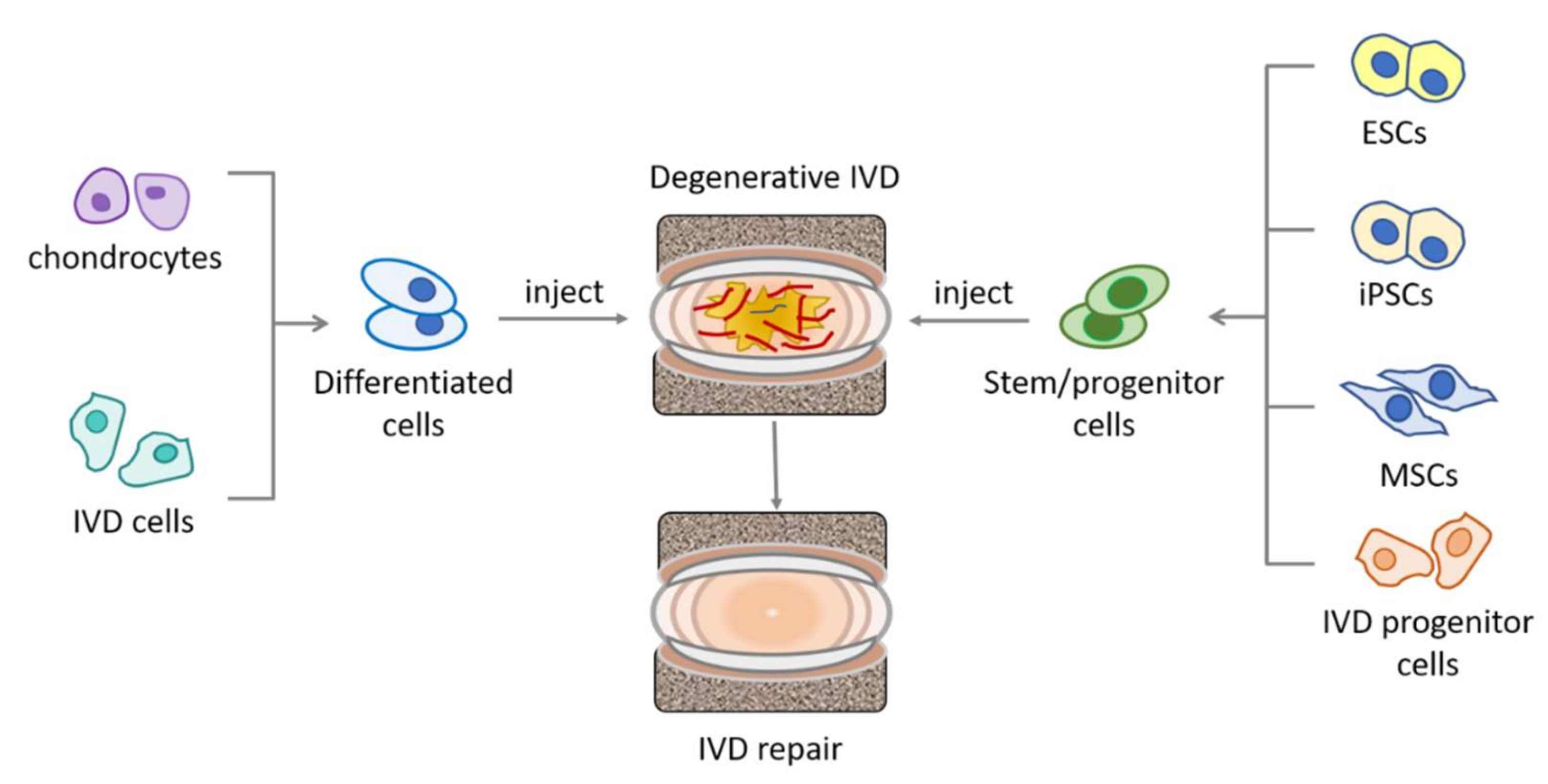
4. Stem Cells Exploited for IVD Repair
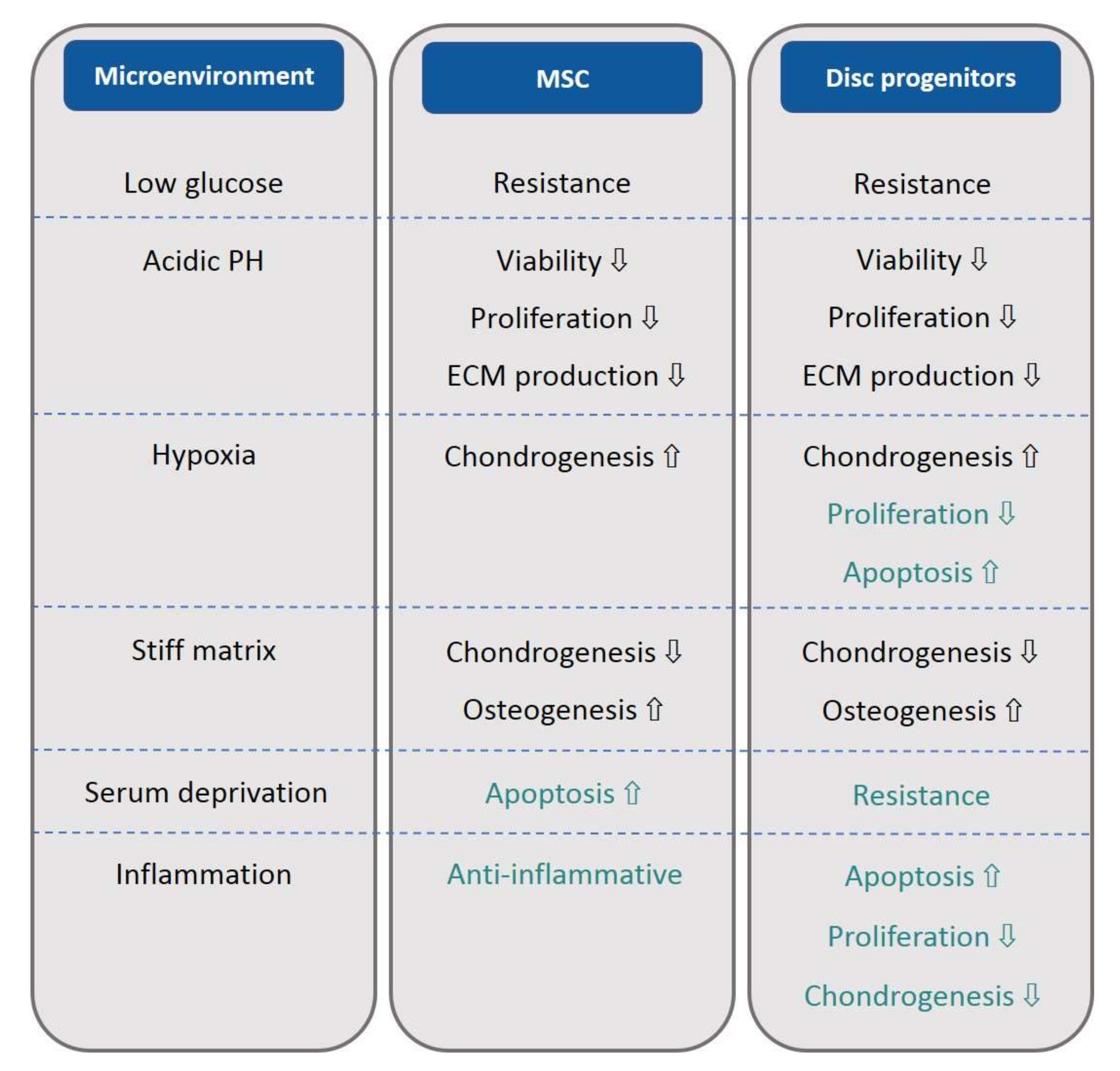
5. The Impact of the Disc Microenvironment on MSCs and Disc Progenitor Cells
6. Low Glucose and Serum Deficiency
6.1. MSCs
6.2. IVD Progenitor Cells
7. Acidity
7.1. MSCs
7.2. IVD Progenitor Cells
8. Lactic Acid Accumulation
9. Hypoxia
9.1. MSCs
9.2. IVD Progenitor Cells
10. Matrix Stiffness and Elasticity
10.1. MSCs
10.2. IVD Progenitor Cells
11. Osmolarity
MSCs
12. Inflammatory Factors
12.1. MSCs
12.2. IVD Progenitor Cells
13. Discussion
Funding
Conflicts of Interest
References
- Vos, T.; Flaxman, A.D.; Naghavi, M.; Lozano, R.; Michaud, C.; Ezzati, M.; Shibuya, K.; Salomon, J.A.; Abdalla, S.; Aboyans, V.; et al. Years lived with disability (YLDs) for 1160 sequelae of 289 diseases and injuries 1990–2010: A systematic analysis for the Global Burden of Disease Study 2010. Lancet 2012, 380, 2163–2196. [Google Scholar] [CrossRef]
- Foster, N.E.; Anema, J.R.; Cherkin, D.; Chou, R.; Cohen, S.P.; Gross, D.P.; Ferreira, P.H.; Fritz, J.M.; Koes, B.W.; Peul, W.; et al. Prevention and treatment of low back pain: Evidence, challenges, and promising directions. Lancet 2018, 391, 2368–2383. [Google Scholar] [CrossRef]
- Maher, C.; Underwood, M.; Buchbinder, R. Non-specific low back pain. Lancet 2017, 389, 736–747. [Google Scholar] [CrossRef]
- Nassr, A.; Lee, J.Y.; Bashir, R.S.; Rihn, J.A.; Eck, J.C.; Kang, J.D.; Lim, M.R. Does incorrect level needle localization during anterior cervical discectomy and fusion lead to accelerated disc degeneration? Spine 2009, 34, 189–192. [Google Scholar] [CrossRef] [PubMed]
- Lyu, F.-J.; Cheung, K.M.; Zheng, Z.; Wang, H.; Sakai, D.; Leung, V.Y. IVD progenitor cells: A new horizon for understanding disc homeostasis and repair. Nat. Rev. Rheumatol. 2019, 15, 102–112. [Google Scholar] [CrossRef]
- Huang, Y.-C.; Li, Z.; Li, J.; Lyu, F.-J. Interaction between stem cells and the microenvironment for musculoskeletal repair. Stem Cells Int. 2020, 2020, 7587428. [Google Scholar] [CrossRef]
- Brodin, H. Paths of nutrition in articular cartilage and intervertebral discs. Acta Orthop. Scand. 1954, 24, 177–183. [Google Scholar] [CrossRef]
- Holm, S.; Maroudas, A.; Urban, J.P.G.; Selstam, G.; Nachemson, A. Nutrition of the intervertebral disc: Solute transport and metabolism. Connect. Tissue Res. 1981, 8, 101–119. [Google Scholar] [CrossRef]
- Soukane, D.M.; Shirazi-Adl, A.; Urban, J.P.G. Investigation of solute concentrations in a 3D model of intervertebral disc. Eur. Spine J. 2008, 18, 254–262. [Google Scholar] [CrossRef]
- Bartels, E.M.; Fairbank, J.C.; Winlove, C.P.; Urban, J.P. Oxygen and lactate concentrations measured in vivo in the intervertebral discs of patients with scoliosis and back pain. Spine 1998, 23, 1–7; discussion 8. [Google Scholar] [CrossRef]
- Ichimura, K.; Tsuji, H.; Matsui, H.; Makiyama, N. Cell culture of the intervertebral disc of rats: Factors influencing culture, proteoglycan, collagen, and deoxyribonucleic acid synthesis. J. Spinal Disord. 1991, 4, 428–436. [Google Scholar] [CrossRef] [PubMed]
- Urban, J.P.G. The role of the physicochemical environment in determining disc cell behaviour. Biochem. Soc. Trans. 2002, 30, 858–863. [Google Scholar] [CrossRef] [PubMed]
- Ishihara, H.; Warensjo, K.; Roberts, S.; Urban, J.P. Proteoglycan synthesis in the intervertebral disk nucleus: The role of extracellular osmolality. Am. J. Physiol. Content 1997, 272, C1499–C1506. [Google Scholar] [CrossRef] [PubMed]
- Urban, J.P.; Roberts, S. Degeneration of the intervertebral disc. Arthritis Res. Ther. 2003, 5, 120–130. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Jackson, A.R.; Huang, C.-Y.; Gu, W.Y. Effect of endplate calcification and mechanical deformation on the distribution of glucose in intervertebral disc: A 3D finite element study. Comput. Methods Biomech. Biomed. Eng. 2011, 14, 195–204. [Google Scholar] [CrossRef] [PubMed]
- Kitano, T.; Zerwekh, J.E.; Usui, Y.; Edwards, M.L.; Flicker, P.L.; Mooney, V. Biochemical changes associated with the symptomatic human intervertebral disk. Clin. Orthop. Relat. Res. 1993, 1993, 372–377. [Google Scholar] [CrossRef]
- Diamant, B.; Karlsson, J.; Nachemson, A. Correlation between lactate levels and pH in discs of patients with lumbar rhizopathies. Experientia 1968, 24, 1195–1196. [Google Scholar] [CrossRef]
- Peng, Y.; Lv, F.-J. Symptomatic versus asymptomatic intervertebral disc degeneration: Is inflammation the key? Crit. Rev. Eukaryot. Gene Expr. 2015, 25, 13–21. [Google Scholar] [CrossRef]
- Richardson, S.M.; Doyle, P.; Minogue, B.M.; Gnanalingham, K.; Hoyland, J.A. Increased expression of matrix metalloproteinase-10, nerve growth factor and substance P in the painful degenerate intervertebral disc. Arthritis Res. Ther. 2009, 11, R126. [Google Scholar] [CrossRef]
- Lv, F.-J.; Peng, Y.; Lim, F.; Sun, Y.; Lv, M.; Zhou, L.; Wang, H.; Zheng, Z.; Cheung, K.; Leung, V. Matrix metalloproteinase 12 is an indicator of intervertebral disc degeneration co-expressed with fibrotic markers. Osteoarthr. Cartil. 2016, 24, 1826–1836. [Google Scholar] [CrossRef]
- Liu, C.; Liang, G.; Deng, Z.; Tan, J.; Zheng, Q.; Lyu, F.-J. The upregulation of COX2 in human degenerated nucleus pulposus: The association of inflammation with intervertebral disc degeneration. Mediat. Inflamm. 2021, 2021, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Igarashi, T.; Kikuchi, S.; Shubayev, V.; Myers, R.R. 2000 Volvo Award winner in basic science studies: Exogenous tumor necrosis factor-alpha mimics nucleus pulposus-induced neuropathology. Molecular, histologic, and behavioral comparisons in rats. Spine 2000, 25, 2975–2980. [Google Scholar] [CrossRef]
- Ahn, S.-H.; Cho, Y.-W.; Ahn, M.-W.; Jang, S.-H.; Sohn, Y.-K.; Kim, H.-S. mRNA expression of cytokines and chemokines in herniated lumbar intervertebral discs. Spine 2002, 27, 911–917. [Google Scholar] [CrossRef] [PubMed]
- O’donnell, J.L.; O’donnell, A.L. Prostaglandin E2 content in herniated lumbar disc disease. Spine 1996, 21, 1653–1655; discussion 1655–1656. [Google Scholar] [CrossRef] [PubMed]
- Shamji, M.F.; Setton, L.A.; Jarvis, W.; So, S.; Chen, J.; Jing, L.; Bullock, R.; Isaacs, R.E.; Brown, C.; Richardson, W.J. Proinflammatory cytokine expression profile in degenerated and herniated human intervertebral disc tissues. Arthritis Rheum. 2010, 62, 1974–1982. [Google Scholar]
- Lyu, F.-J.; Cui, H.; Pan, H.; MC Cheung, K.; Cao, X.; Iatridis, J.C.; Zheng, Z. Painful intervertebral disc degeneration and inflammation: From laboratory evidence to clinical interventions. Bone Res. 2021, 9, 1653–1655; discussion 1655–1656. [Google Scholar] [CrossRef]
- Phillips, K.; Cullen, K.; Chiverton, N.; Michael, A.; Cole, A.; Breakwell, L.; Haddock, G.; Bunning, R.; Cross, A.; Le Maitre, C. Potential roles of cytokines and chemokines in human intervertebral disc degeneration: Interleukin-1 is a master regulator of catabolic processes. Osteoarthr. Cartil. 2015, 23, 1165–1177. [Google Scholar] [CrossRef]
- Hoyland, J.A.; Le Maitre, C.; Freemont, A.J. Investigation of the role of IL-1 and TNF in matrix degradation in the intervertebral disc. Rheumatology 2008, 47, 809–814. [Google Scholar] [CrossRef]
- Kang, J.D.; Stefanovic-Racic, M.; McIntyre, L.A.; Georgescu, H.I.; Evans, C.H. Toward a biochemical understanding of human intervertebral disc degeneration and herniation. Spine 1997, 22, 1065–1073. [Google Scholar] [CrossRef]
- Walter, B.A.; Mageswaran, P.; Mo, X.; Boulter, D.J.; Mashaly, H.; Nguyen, X.V.; Prevedello, L.M.; Thoman, W.; Raterman, B.D.; Kalra, P.; et al. MR Elastography–derived Stiffness: A biomarker for intervertebral disc degeneration. Radiology 2017, 285, 167–175. [Google Scholar] [CrossRef]
- Leung, V.Y.; Aladin, D.M.; Lv, F.; Tam, V.; Sun, Y.; Lau, R.Y.; Hung, S.-C.; Ngan, A.H.; Tang, B.; Lim, C.T.; et al. Mesenchymal stem cells reduce intervertebral disc fibrosis and facilitate repair. Stem Cells 2014, 32, 2164–2177. [Google Scholar] [CrossRef] [PubMed]
- Antoniou, J.; Steffen, T.; Nelson, F.; Winterbottom, N.; Hollander, A.P.; Poole, R.A.; Aebi, M.; Alini, M. The human lumbar intervertebral disc: Evidence for changes in the biosynthesis and denaturation of the extracellular matrix with growth, maturation, ageing, and degeneration. J. Clin. Investig. 1996, 98, 996–1003. [Google Scholar] [CrossRef] [PubMed]
- Melrose, J.; Smith, S.M.; Fuller, E.S.; Young, A.A.; Roughley, P.J.; Dart, A.; Little, C.B. Biglycan and fibromodulin fragmentation correlates with temporal and spatial annular remodelling in experimentally injured ovine intervertebral discs. Eur. Spine J. 2007, 16, 2193–2205. [Google Scholar] [CrossRef] [PubMed]
- Patel, K.P.; Sandy, J.D.; Akeda, K.; Miyamoto, K.; Chujo, T.; An, H.S.; Masuda, K. Aggrecanases and Aggrecanase-generated fragments in the human intervertebral disc at early and advanced stages of disc degeneration. Spine 2007, 32, 2596–2603. [Google Scholar] [CrossRef]
- Anderson, D.G.; Li, X.; Balian, G. A fibronectin fragment alters the metabolism by rabbit intervertebral disc cells in vitro. Spine 2005, 30, 1242–1246. [Google Scholar] [CrossRef]
- Wuertz, K.; Urban, J.P.G.; Klasen, J.; Ignatius, A.; Wilke, H.J.; Claes, L.; Neidlinger-Wilke, C. Influence of extracellular osmolarity and mechanical stimulation on gene expression of intervertebral disc cells. J. Orthop. Res. 2007, 25, 1513–1522. [Google Scholar] [CrossRef]
- Gorensek, M.; Joksimovic, C.; Kregar-Velikonja, N.; Gorensek, M.; Knezevic, M.; Jeras, M.; Pavlovčič, V.; CÖR, A. Nucleus pulposus repair with cultured autologous elastic cartilage derived chondrocytes. Cell Mol. Biol. Lett. 2004, 9, 363–373. [Google Scholar]
- Iwashina, T.; Mochida, J.; Sakai, D.; Yamamoto, Y.; Miyazaki, T.; Ando, K.; Hotta, T. Feasibility of using a human nucleus pulposus cell line as a cell source in cell transplantation therapy for intervertebral disc degeneration. Spine 2006, 31, 1177–1186. [Google Scholar] [CrossRef]
- Okuma, M.; Mochida, J.; Nishimura, K.; Sakabe, K.; Seiki, K. Reinsertion of stimulated nucleus pulposus cells retards intervertebral disc degeneration: Anin vitro andin vivo experimental study. J. Orthop. Res. 2000, 18, 988–997. [Google Scholar] [CrossRef]
- Sheikh, H.; Zakharian, K.; De La Torre, R.P.; Facek, C.; Vasquez, A.; Chaudhry, G.R.; Svinarich, D.; Perez-Cruet, M.J. In vivo intervertebral disc regeneration using stem cell–derived chondroprogenitors. J. Neurosurg. Spine 2009, 10, 265–272. [Google Scholar] [CrossRef]
- Tang, R.; Jing, L.; Willard, V.P.; Wu, C.-L.; Guilak, F.; Chen, J.; Setton, L.A. Differentiation of human induced pluripotent stem cells into nucleus pulposus-like cells. Stem Cell Res. Ther. 2018, 9, 61. [Google Scholar] [CrossRef] [PubMed]
- Lv, F.-J.; Tuan, R.S.; Cheung, K.M.C.; Leung, V.Y.L. Concise review: The surface markers and identity of human mesenchymal stem cells. Stem Cells 2014, 32, 1408–1419. [Google Scholar] [CrossRef] [PubMed]
- Martin-Rendon, E.; Sweeney, D.; Lu, F.; Girdlestone, J.; Navarrete, C.; Watt, S.M. 5-Azacytidine-treated human mesenchymal stem/progenitor cells derived from umbilical cord, cord blood and bone marrow do not generate cardiomyocytesin vitroat high frequencies. Vox Sang. 2008, 95, 137–148. [Google Scholar] [CrossRef] [PubMed]
- Lv, F.; Lu, M.; MC Cheung, K.; Leung, V.Y.; Zhou, G. Intrinsic properties of Mesemchymal stem cells from human bone marrow, umbilical cord and umbilical cord blood comparing the different sources of MSC. Curr. Stem Cell Res. Ther. 2012, 7, 389–399. [Google Scholar] [CrossRef] [PubMed]
- Panepucci, R.A.; Siufi, J.L.; Silva, W.A.; Proto-Siquiera, R.; Neder, L.; Orellana, M.; Rocha, V.; Covas, D.T.; Zago, M.A. Comparison of gene expression of umbilical cord vein and bone marrow–derived mesenchymal stem cells. Stem Cells 2004, 22, 1263–1278. [Google Scholar] [CrossRef] [PubMed]
- Deng, Z.; Jin, J.; Wang, S.; Qi, F.; Chen, X.; Liu, C.; Li, Y.; Ma, Y.; Lyu, F.; Zheng, Q. Narrative review of the choices of stem cell sources and hydrogels for cartilage tissue engineering. Ann. Transl. Med. 2020, 8, 1598. [Google Scholar] [CrossRef]
- Qi, F.; Deng, Z.; Ma, Y.; Wang, S.; Liu, C.; Lyu, F.; Wang, T.; Zheng, Q. From the perspective of embryonic tendon development: Various cells applied to tendon tissue engineering. Ann. Transl. Med. 2020, 8, 131. [Google Scholar] [CrossRef]
- Liu, S.; Herault, Y.; Pavlovic, G.; Leask, A. Skin progenitor cells contribute to bleomycin-induced skin fibrosis. Arthritis Rheumatol. 2014, 66, 707–713. [Google Scholar] [CrossRef]
- Wang, K.; Liu, H.; Yang, J.; Ma, C.; Zhang, Z.; Zheng, D.; Guan, W. Liver epithelioid progenitor cells derived from fetal Luxi bovine alleviate liver fibrosis. Cytotechnology 2017, 70, 129–140. [Google Scholar] [CrossRef]
- Ning, Z.; Xiao-Ming, G.; Xun, M.; Li, Z.; Hui, Z.; Liang, S. Interleukin-1 beta affects the biological properties of rat nucleus pulposus-derived mesenchymal stem cells. Chin. J. Tissue Eng. Res. 2014, 18, 4437–4443. [Google Scholar]
- Matta, A.; Karim, M.Z.; Isenman, D.E.; Erwin, W.M. Molecular therapy for degenerative disc disease: Clues from secretome analysis of the notochordal cell-rich nucleus pulposus. Sci. Rep. 2017, 7, 45623. [Google Scholar] [CrossRef] [PubMed]
- Wuertz, K.; Godburn, K.; Neidlinger-Wilke, C.; Urban, J.; Iatridis, J.C. Behavior of mesenchymal stem cells in the chemical microenvironment of the intervertebral disc. Spine 2008, 33, 1843–1849. [Google Scholar] [CrossRef] [PubMed]
- Weil, B.R.; Abarbanell, A.M.; Herrmann, J.L.; Wang, Y.; Meldrum, D.R. High glucose concentration in cell culture medium does not acutely affect human mesenchymal stem cell growth factor production or proliferation. Am. J. Physiol. Integr. Comp. Physiol. 2009, 296, R1735–R1743. [Google Scholar] [CrossRef]
- Liang, C.; Li, H.; Tao, Y.; Zhou, X.; Li, F.; Chen, G.; Chen, Q. Responses of human adipose-derived mesenchymal stem cells to chemical microenvironment of the intervertebral disc. J. Transl. Med. 2012, 10, 49. [Google Scholar] [CrossRef] [PubMed]
- Stolzing, A.; Coleman, N.; Scutt, A. Glucose-Induced replicative senescence in mesenchymal stem cells. Rejuvenation Res. 2006, 9, 31–35. [Google Scholar] [CrossRef] [PubMed]
- Zhu, W.; Chen, J.; Cong, X.; Hu, S.; Chen, X. Hypoxia and serum deprivation-induced apoptosis in mesenchymal stem cells. Stem Cells 2006, 24, 416–425. [Google Scholar] [CrossRef]
- Fu, S.; Jin, D.; Liu, S.; Wang, L.; Wang, Z.; Mei, G.; Zou, Z.-L.; Wu, J.-Q.; Xu, Z.-Y. Protective effect of neuropeptide substance P on bone marrow mesenchymal stem cells against apoptosis induced by serum deprivation. Stem Cells Int. 2015, 2015, 270328. [Google Scholar] [CrossRef]
- He, Z.; Pu, L.; Yuan, C.; Jia, M.; Wang, J. Nutrition deficiency promotes apoptosis of cartilage endplate stem cells in a caspase-independent manner partially through upregulating BNIP3. Acta Biochim. Biophys. Sin. 2016, 49, 25–32. [Google Scholar] [CrossRef]
- Turner, S.A.; Wright, K.T.; Jones, P.N.; Balain, B.; Roberts, S. Temporal Analyses of the Response of Intervertebral Disc Cells and Mesenchymal Stem Cells to Nutrient Deprivation. Stem Cells Int. 2016, 2016, 5415901. [Google Scholar] [CrossRef]
- Wuertz, K.; Godburn, K.; Iatridis, J. MSC response to pH levels found in degenerating intervertebral discs. Biochem. Biophys. Res. Commun. 2009, 379, 824–829. [Google Scholar] [CrossRef]
- Li, H.; Liang, C.; Tao, Y.; Zhou, X.; Li, F.; Chen, G.; Chen, Q.-X. Acidic pH conditions mimicking degenerative intervertebral discs impair the survival and biological behavior of human adipose-derived mesenchymal stem cells. Exp. Biol. Med. (Maywood) 2012, 237, 845–852. [Google Scholar] [CrossRef] [PubMed]
- Han, B.; Wang, H.-C.; Li, H.; Tao, Y.-Q.; Liang, C.-Z.; Li, F.-C.; Chen, G.; Chen, Q.-X. Nucleus pulposus mesenchymal stem cells in acidic conditions mimicking degenerative intervertebral discs give better performance than adipose tissue-derived mesenchymal stem cells. Cells Tissues Organs 2014, 199, 342–352. [Google Scholar] [CrossRef] [PubMed]
- Massa, A.; Perut, F.; Chano, T.; Woloszyk, A.; Mitsiadis, T.; Avnet, S.; Baldini, N. The effect of extracellular acidosis on the behaviour of mesenchymal stem cells in vitro. Eur. Cells Mater. 2017, 33, 252–267. [Google Scholar] [CrossRef]
- Gao, W.; Zhang, H.; Chang, G.; Xie, Z.; Wang, H.; Ma, L.; Han, Z.C.; Li, Q.; Pang, T. Decreased intracellular pH induced by cariporide differentially contributes to human umbilical cord-derived mesenchymal stem cells differentiation. Cell. Physiol. Biochem. 2014, 33, 185–194. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Tao, H.; Wang, H.; Dong, F.; Zhang, R.; Li, J.; Ge, P.; Song, P.; Zhang, H.; Xu, P.; et al. Biological behavior of human nucleus pulposus mesenchymal stem cells in response to changes in the acidic environment during intervertebral disc degeneration. Stem Cells Dev. 2017, 26, 901–911. [Google Scholar] [CrossRef]
- Schneider, C.-C.; Ateschrang, A.; Königsrainer, I.; Glatzle, J.; Bühler, S.; Schäfer, R.; Northoff, H.; Königsrainer, A.; Zieker, D. Lactate influences the gene expression profile of human mesenchymal stem cells (hMSC) in a dose dependant manner. Cell. Physiol. Biochem. 2012, 30, 1547–1556. [Google Scholar] [CrossRef]
- Huang, S.; Leung, V.Y.; Long, D.; Chan, D.; Lu, W.W.; Cheung, K.M.; Zhou, G. Coupling of small leucine-rich proteoglycans to hypoxic survival of a progenitor cell-like subpopulation in Rhesus Macaque intervertebral disc. Biomaterials 2013, 34, 6548–6558. [Google Scholar] [CrossRef]
- Feng, Y.; Zhu, M.; Dangelmajer, S.; Lee, Y.M.; Wijesekera, O.; Castellanos, C.X.; Denduluri, A.; Chaichana, K.L.; Li, Q.; Zhang, H.; et al. Hypoxia-cultured human adipose-derived mesenchymal stem cells are non-oncogenic and have enhanced viability, motility, and tropism to brain cancer. Cell Death Dis. 2015, 6, e1797. [Google Scholar] [CrossRef]
- Safwani, W.K.Z.W.; Choi, J.R.; Yong, K.W.; Ting, I.; Adenan, N.A.M.; Pingguan-Murphy, B. Hypoxia enhances the viability, growth and chondrogenic potential of cryopreserved human adipose-derived stem cells. Cryobiology 2017, 75, 91–99. [Google Scholar] [CrossRef]
- Antebi, B.; Ii, L.A.R.; Walker, K.P.; Asher, A.M.; Kamucheka, R.M.; Alvarado, L.; Mohammadipoor, A.; Cancio, L.C. Short-term physiological hypoxia potentiates the therapeutic function of mesenchymal stem cells. Stem Cell Res. Ther. 2018, 9, 265. [Google Scholar] [CrossRef]
- Li, H.; Tao, Y.; Liang, C.; Han, B.; Li, F.; Chen, G.; Chen, Q. Influence of hypoxia in the intervertebral disc on the biological behaviors of rat adipose- and nucleus pulposus-derived mesenchymal stem cells. Cells Tissues Organs 2013, 198, 266–277. [Google Scholar] [CrossRef] [PubMed]
- Grayson, W.L.; Zhao, F.; Izadpanah, R.; Bunnell, B.; Ma, T. Effects of hypoxia on human mesenchymal stem cell expansion and plasticity in 3D constructs. J. Cell. Physiol. 2006, 207, 331–339. [Google Scholar] [CrossRef] [PubMed]
- Grayson, W.L.; Zhao, F.; Bunnell, B.; Ma, T. Hypoxia enhances proliferation and tissue formation of human mesenchymal stem cells. Biochem. Biophys. Res. Commun. 2007, 358, 948–953. [Google Scholar] [CrossRef] [PubMed]
- Choi, J.R.; Pingguan-Murphy, B.; Abas, W.A.B.W.; Azmi, M.A.N.; Omar, S.Z.; Chua, K.H.; Safwani, W.K.Z.W. Impact of low oxygen tension on stemness, proliferation and differentiation potential of human adipose-derived stem cells. Biochem. Biophys. Res. Commun. 2014, 448, 218–224. [Google Scholar] [CrossRef]
- Fotia, C.; Massa, A.; Boriani, F.; Baldini, N.; Granchi, D. Hypoxia enhances proliferation and stemness of human adipose-derived mesenchymal stem cells. Cytotechnology 2015, 67, 1073–1084. [Google Scholar] [CrossRef]
- Tsai, C.-C.; Chen, Y.-J.; Yew, T.-L.; Chen, L.-L.; Wang, J.-Y.; Chiu, C.-H.; Hung, S.-C. Hypoxia inhibits senescence and maintains mesenchymal stem cell properties through down-regulation of E2A-p21 by HIF-TWIST. Blood 2011, 117, 459–469. [Google Scholar] [CrossRef]
- Kanichai, M.; Ferguson, D.; Prendergast, P.J.; Campbell, V.A. Hypoxia promotes chondrogenesis in rat mesenchymal stem cells: A role for AKT and hypoxia-inducible factor (HIF)-1α. J. Cell. Physiol. 2008, 216, 708–715. [Google Scholar] [CrossRef]
- Meyer, E.G.; Buckley, C.T.; Thorpe, S.D.; Kelly, D.J. Low oxygen tension is a more potent promoter of chondrogenic differentiation than dynamic compression. J. Biomech. 2010, 43, 2516–2523. [Google Scholar] [CrossRef]
- Valorani, M.G.; Montelatici, E.; Germani, A.; Biddle, A.; D’Alessandro, D.; Strollo, R.; Patrizi, M.P.; Lazzari, L.; Nye, E.; Otto, W.R.; et al. Pre-culturing human adipose tissue mesenchymal stem cells under hypoxia increases their adipogenic and osteogenic differentiation potentials. Cell Prolif. 2012, 45, 225–238. [Google Scholar] [CrossRef]
- Yu, X.; Wan, Q.; Cheng, G.; Cheng, X.; Zhang, J.; Pathak, J.L.; Li, Z. CoCl2, a mimic of hypoxia, enhances bone marrow mesenchymal stem cells migration and osteogenic differentiation via STAT3 signaling pathway. Cell Biol. Int. 2018, 42, 1321–1329. [Google Scholar] [CrossRef]
- D’Ippolito, G.; Diabira, S.; Howard, G.A.; Roos, B.A.; Schiller, P.C. Low oxygen tension inhibits osteogenic differentiation and enhances stemness of human MIAMI cells. Bone 2006, 39, 513–522. [Google Scholar] [CrossRef] [PubMed]
- Potier, E.; Ferreira, E.; Andriamanalijaona, R.; Pujol, J.-P.; Oudina, K.; Logeart-Avramoglou, D.; Petite, H. Hypoxia affects mesenchymal stromal cell osteogenic differentiation and angiogenic factor expression. Bone 2007, 40, 1078–1087. [Google Scholar] [CrossRef] [PubMed]
- Merceron, C.; Vinatier, C.; Portron, S.; Masson, M.; Amiaud, J.; Guigand, L.; Chérel, Y.; Weiss, P.; Guicheux, J. Differential effects of hypoxia on osteochondrogenic potential of human adipose-derived stem cells. Am. J. Physiol. Cell Physiol. 2010, 298, C355–C364. [Google Scholar] [CrossRef] [PubMed]
- Safwani, W.K.Z.W.; Wong, C.W.; Yong, K.W.; Choi, J.R.; Adenan, N.A.M.; Omar, S.Z.; Abas, W.A.B.W.; Pingguan-Murphy, B. The effects of hypoxia and serum-free conditions on the stemness properties of human adipose-derived stem cells. Cytotechnology 2016, 68, 1859–1872. [Google Scholar] [CrossRef] [PubMed]
- Schiller, Z.A.; Schiele, N.R.; Sims, J.K.; Lee, K.; Kuo, C.K. Adipogenesis of adipose-derived stem cells may be regulated via the cytoskeleton at physiological oxygen levels in vitro. Stem Cell Res. Ther. 2013, 4, 79. [Google Scholar] [CrossRef] [PubMed]
- Yao, Y.; Deng, Q.; Sun, C.; Song, W.; Liu, H.; Zhou, Y. A genome-wide analysis of the gene expression profiles and alternative splicing events during the hypoxia-regulated osteogenic differentiation of human cartilage endplate-derived stem cells. Mol. Med. Rep. 2017, 16, 1991–2001. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Yao, Y.; Deng, Q.; Song, W.; Zhang, H.; Li, Y.; Yang, Y.; Fan, X.; Liu, M.; Shang, J.; Sun, C.; et al. MIF plays a key role in regulating tissue-specific chondro-osteogenic differentiation fate of human cartilage endplate stem cells under hypoxia. Stem Cell Rep. 2016, 7, 249–262. [Google Scholar] [CrossRef]
- Olivares-Navarrete, R.; Lee, E.M.; Smith, K.; Hyzy, S.L.; Doroudi, M.; Williams, J.K.; Gall, K.; Boyan, B.D.; Schwartz, Z. Substrate stiffness controls osteoblastic and chondrocytic differentiation of mesenchymal stem cells without exogenous stimuli. PLoS ONE 2017, 12, e0170312. [Google Scholar] [CrossRef]
- Park, J.S.; Chu, J.S.; Tsou, A.D.; Diop, R.; Tang, Z.; Wang, A.; Li, S. The effect of matrix stiffness on the differentiation of mesenchymal stem cells in response to TGF-beta. Biomaterials 2011, 32, 3921–3930. [Google Scholar] [CrossRef]
- Li, X.; Huang, Y.; Zheng, L.; Liu, H.; Niu, X.; Huang, J.; Zhao, F.; Fan, Y. Effect of substrate stiffness on the functions of rat bone marrow and adipose tissue derived mesenchymal stem cells in vitro. J. Biomed. Mater. Res. Part A 2014, 102, 1092–1101. [Google Scholar] [CrossRef]
- Lin, C.-H.; Su, J.J.-M.; Lee, S.-Y.; Lin, Y.-M. Stiffness modification of photopolymerizable gelatin-methacrylate hydrogels influences endothelial differentiation of human mesenchymal stem cells. J. Tissue Eng. Regen. Med. 2018, 12, 2099–2111. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Y.-C.; Jiao, H.-L.; Lee, M.-S.; Wang, T.; Turng, L.-S.; Li, Q.; Li, W.-J. Endogenous biological factors modulated by substrate stiffness regulate endothelial differentiation of mesenchymal stem cells. J. Biomed. Mater. Res. Part A 2018, 106, 1595–1603. [Google Scholar] [CrossRef] [PubMed]
- Navaro, Y.; Bleich-Kimelman, N.; Hazanov, L.; Mironi-Harpaz, I.; Shachaf, Y.; Garty, S.; Smith, Y.; Pelled, G.; Gazit, D.; Seliktar, D.; et al. Matrix stiffness determines the fate of nucleus pulposus–derived stem cells. Biomaterials 2015, 49, 68–76. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Zhu, C.; Li, J.; Zhou, P.; Chen, M.; Yang, H.; Li, B. The effect of the fibre orientation of electrospun scaffolds on the matrix production of rabbit annulus fibrosus-derived stem cells. Bone Res. 2015, 3, 15012. [Google Scholar] [CrossRef]
- Zhu, C.; Li, J.; Liu, C.; Zhou, P.; Yang, H.; Li, B. Modulation of the gene expression of annulus fibrosus-derived stem cells using poly(ether carbonate urethane)urea scaffolds of tunable elasticity. Acta Biomater. 2016, 29, 228–238. [Google Scholar] [CrossRef]
- Walker, P.A.; Jimenez, F.; Cox, C.S., Jr. Progenitor cell therapy for traumatic brain injury: Effect of serum osmolarity on cell viability and cytokine production. Regen. Med. 2010, 5, 65–71. [Google Scholar] [CrossRef]
- Chen, X.; Tang, L.; Han, Z.; Wang, W.; Meng, J. Coculture with bone marrow-derived mesenchymal stem cells attenuates inflammation and apoptosis in lipopolysaccharide-stimulated alveolar epithelial cells via enhanced secretion of keratinocyte growth factor and angiopoietin-1 modulating the Toll-like receptor-4 signal pathway. Mol. Med. Rep. 2019, 19, 1891–1902. [Google Scholar] [CrossRef]
- Pedrazza, L.; Cubillos-Rojas, M.; de Mesquita, F.C.; Luft, C.; Cunha, A.A.; Rosa, J.L.; De Oliveira, J.R. Mesenchymal stem cells decrease lung inflammation during sepsis, acting through inhibition of the MAPK pathway. Stem Cell Res. Ther. 2017, 8, 289. [Google Scholar] [CrossRef]
- Li, F.; Zhao, S.-Z. Control of cross talk between angiogenesis and inflammation by mesenchymal stem cells for the treatment of ocular surface diseases. Stem Cells Int. 2016, 2016, 7961816. [Google Scholar] [CrossRef]
- Takeda, K.; Webb, T.L.; Ning, F.; Shiraishi, Y.; Regan, D.P.; Chow, L.; Smith, M.J.; Ashino, S.; Guth, A.M.; Hopkins, S.; et al. Mesenchymal Stem Cells Recruit CCR2+ Monocytes To Suppress Allergic Airway Inflammation. J. Immunol. 2018, 200, 1261–1269. [Google Scholar] [CrossRef]
- Ohara, M.; Ohnishi, S.; Hosono, H.; Yamamoto, K.; Yuyama, K.; Nakamura, H.; Fu, Q.; Maehara, O.; Suda, G.; Sakamoto, N. Extracellular vesicles from amnion-derived mesenchymal stem cells ameliorate hepatic inflammation and fibrosis in rats. Stem Cells Int. 2018, 2018, 3212643. [Google Scholar] [CrossRef] [PubMed]
- Prockop, D.J. Inflammation, fibrosis, and modulation of the process by mesenchymal stem/stromal cells. Matrix Biol. 2016, 51, 7–13. [Google Scholar] [CrossRef] [PubMed]
- Navone, S.E.; Marfia, G.; Canzi, L.; Ciusani, E.; Canazza, A.; Visintini, S.; Campanella, R.; Parati, E.A. Expression of neural and neurotrophic markers in nucleus pulposus cells isolated from degenerated intervertebral disc. J. Orthop. Res. 2012, 30, 1470–1477. [Google Scholar] [CrossRef] [PubMed]
- Islam, A.; Mbimba, T.; Younesi, M.; Akkus, O. Effects of substrate stiffness on the tenoinduction of human mesenchymal stem cells. Acta Biomater. 2017, 58, 244–253. [Google Scholar] [CrossRef]
- Zhao, W.; Li, X.; Liu, X.; Zhang, N.; Wen, X. Effects of substrate stiffness on adipogenic and osteogenic differentiation of human mesenchymal stem cells. Mater. Sci. Eng. C 2014, 40, 316–323. [Google Scholar] [CrossRef]
- Xu, J.; Sun, M.; Tan, Y.; Wang, H.; Wang, H.; Li, P.; Xu, Z.; Xia, Y.; Li, L.; Li, Y. Effect of matrix stiffness on the proliferation and differentiation of umbilical cord mesenchymal stem cells. Differentiation 2017, 96, 30–39. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lyu, F.-J. Impact of Microenvironmental Changes during Degeneration on Intervertebral Disc Progenitor Cells: A Comparison with Mesenchymal Stem Cells. Bioengineering 2022, 9, 148. https://doi.org/10.3390/bioengineering9040148
Lyu F-J. Impact of Microenvironmental Changes during Degeneration on Intervertebral Disc Progenitor Cells: A Comparison with Mesenchymal Stem Cells. Bioengineering. 2022; 9(4):148. https://doi.org/10.3390/bioengineering9040148
Chicago/Turabian StyleLyu, Feng-Juan. 2022. "Impact of Microenvironmental Changes during Degeneration on Intervertebral Disc Progenitor Cells: A Comparison with Mesenchymal Stem Cells" Bioengineering 9, no. 4: 148. https://doi.org/10.3390/bioengineering9040148
APA StyleLyu, F.-J. (2022). Impact of Microenvironmental Changes during Degeneration on Intervertebral Disc Progenitor Cells: A Comparison with Mesenchymal Stem Cells. Bioengineering, 9(4), 148. https://doi.org/10.3390/bioengineering9040148




