Automation of Measurements for Personalized Medical Appliances by Means of CAD Software—Application in Robin Sequence Orthodontic Appliances
Abstract
1. Introduction
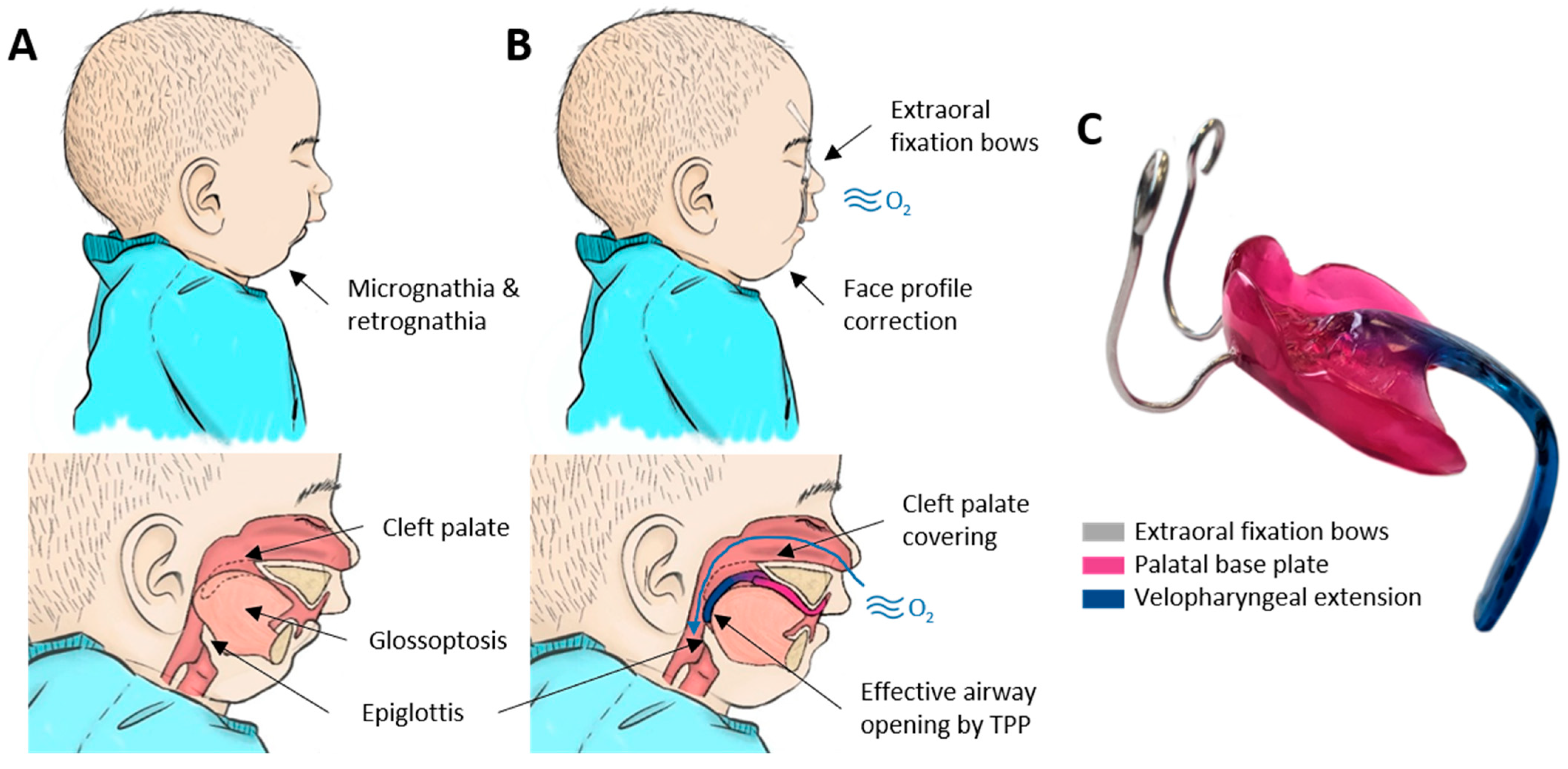
2. Materials and Methods
2.1. Sample Digitalization
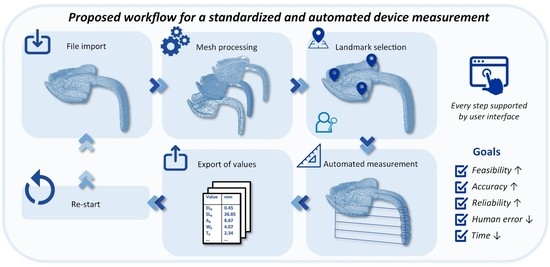
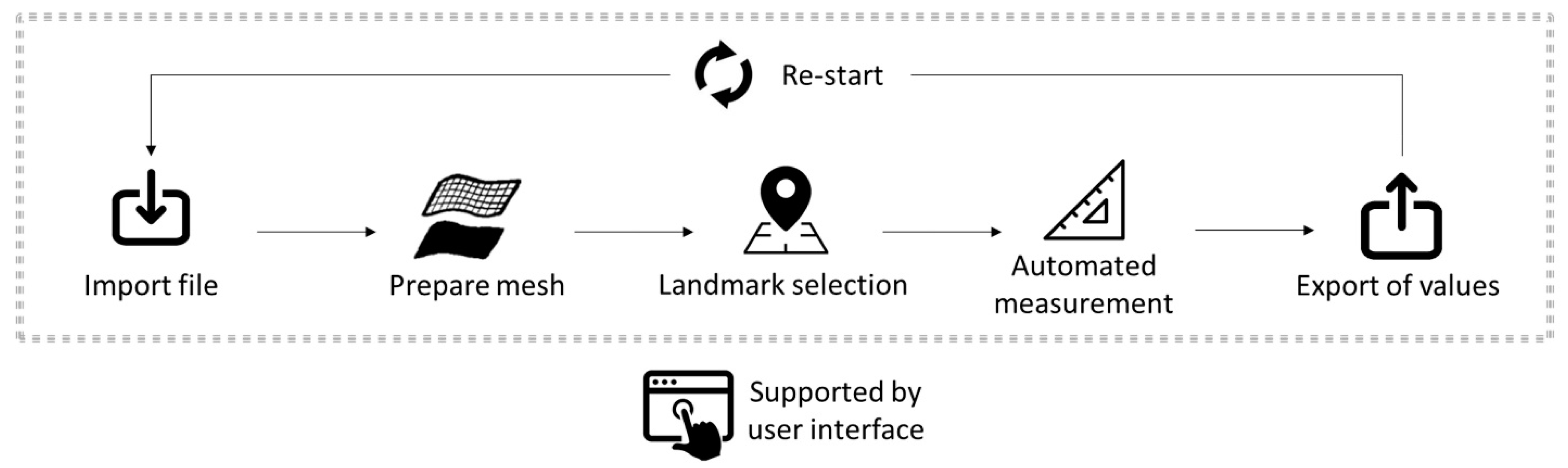
2.2. Automatization of the Measurement Protocol
- Human UI plugin (Version 0.8.1.3., year 2019, developed by A. Heumann, M. Syp, N. Holland, B. Ringley).
- Elefront (Version 5.0.0., year 2022, funded by Front Inc. and developed by Keyan Rahimzadeh, Ramon van der Heijden, and Alan Tai).
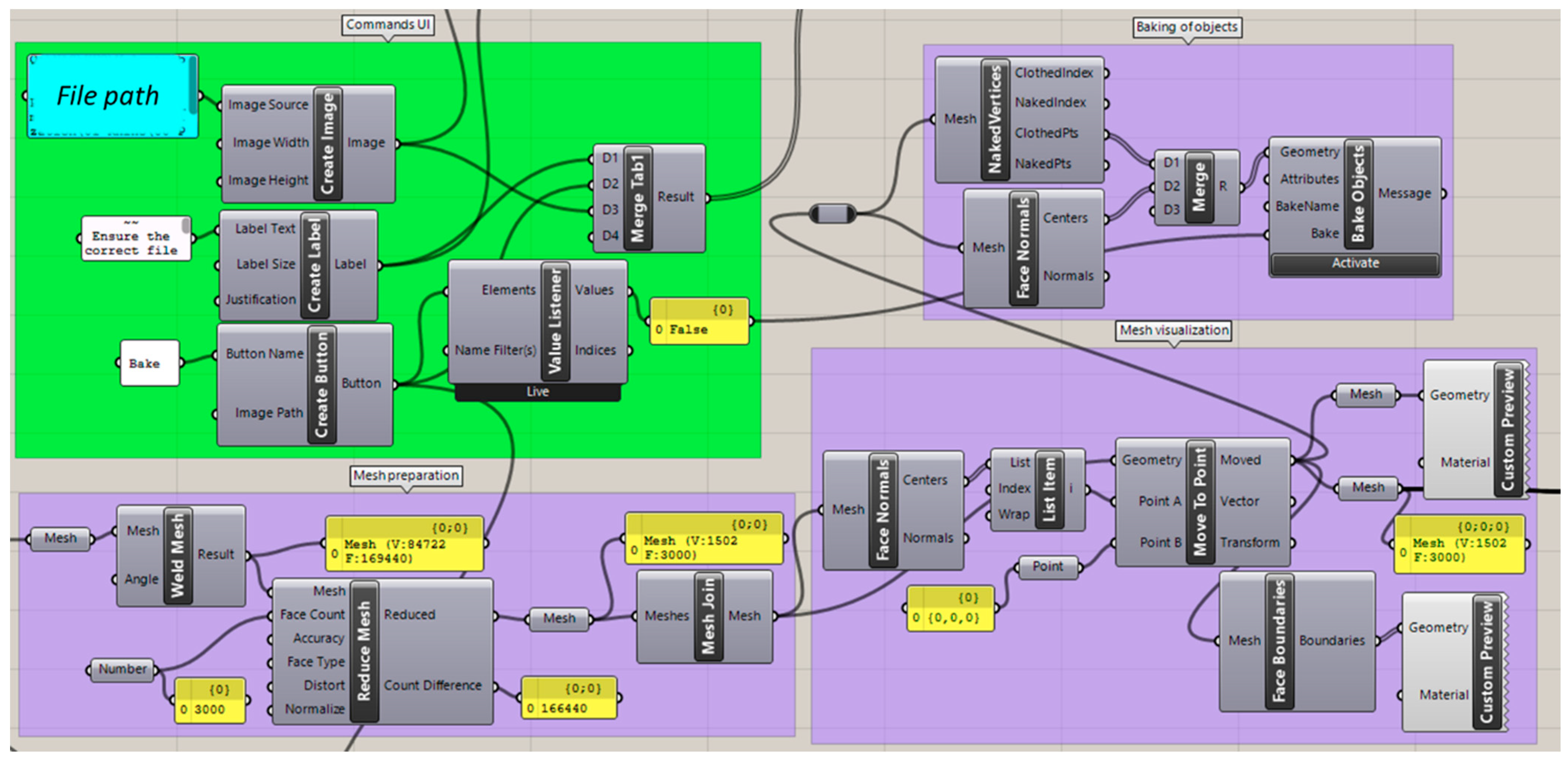
2.2.1. User Interface (UI) Creation and Editing Tools
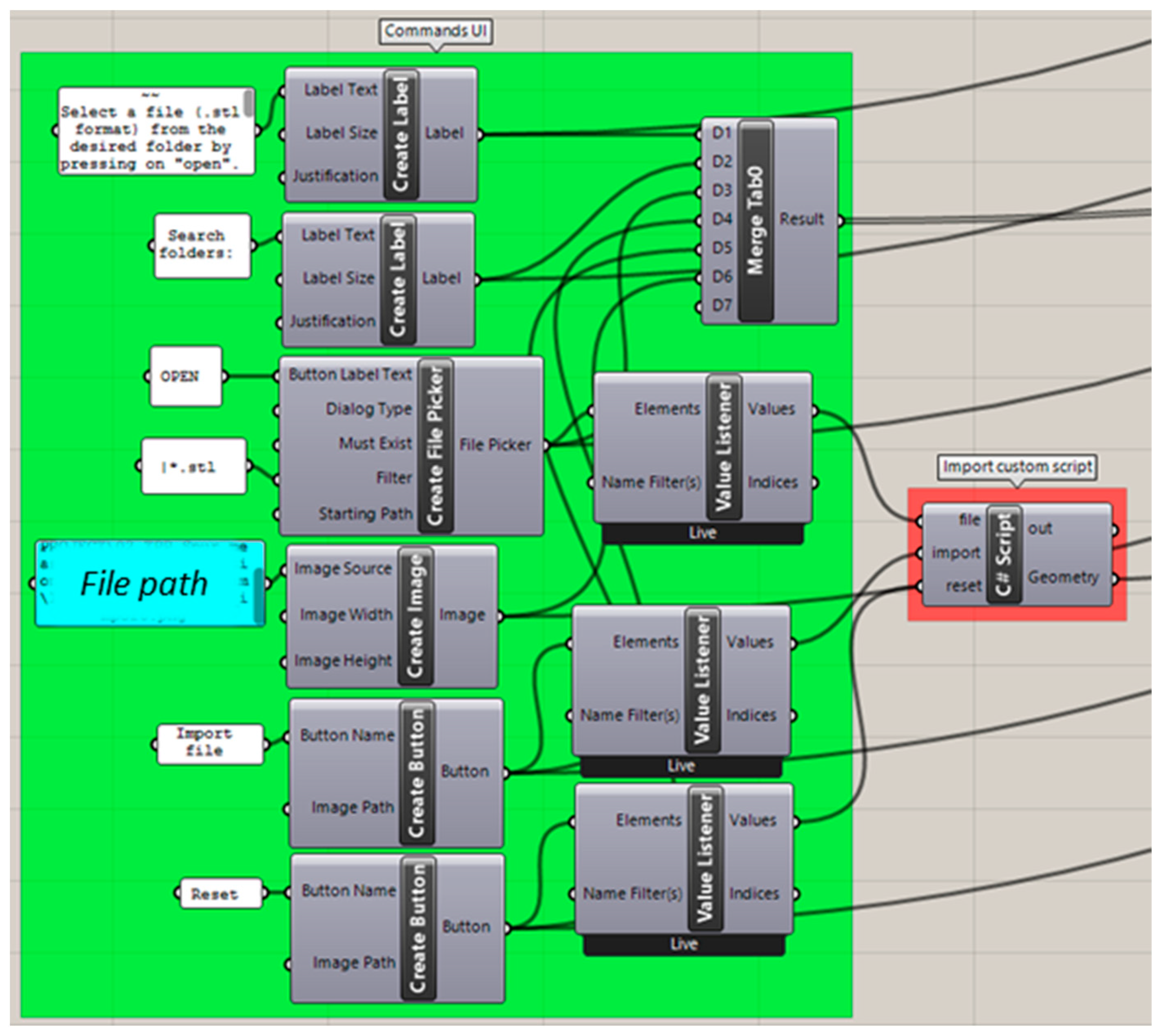
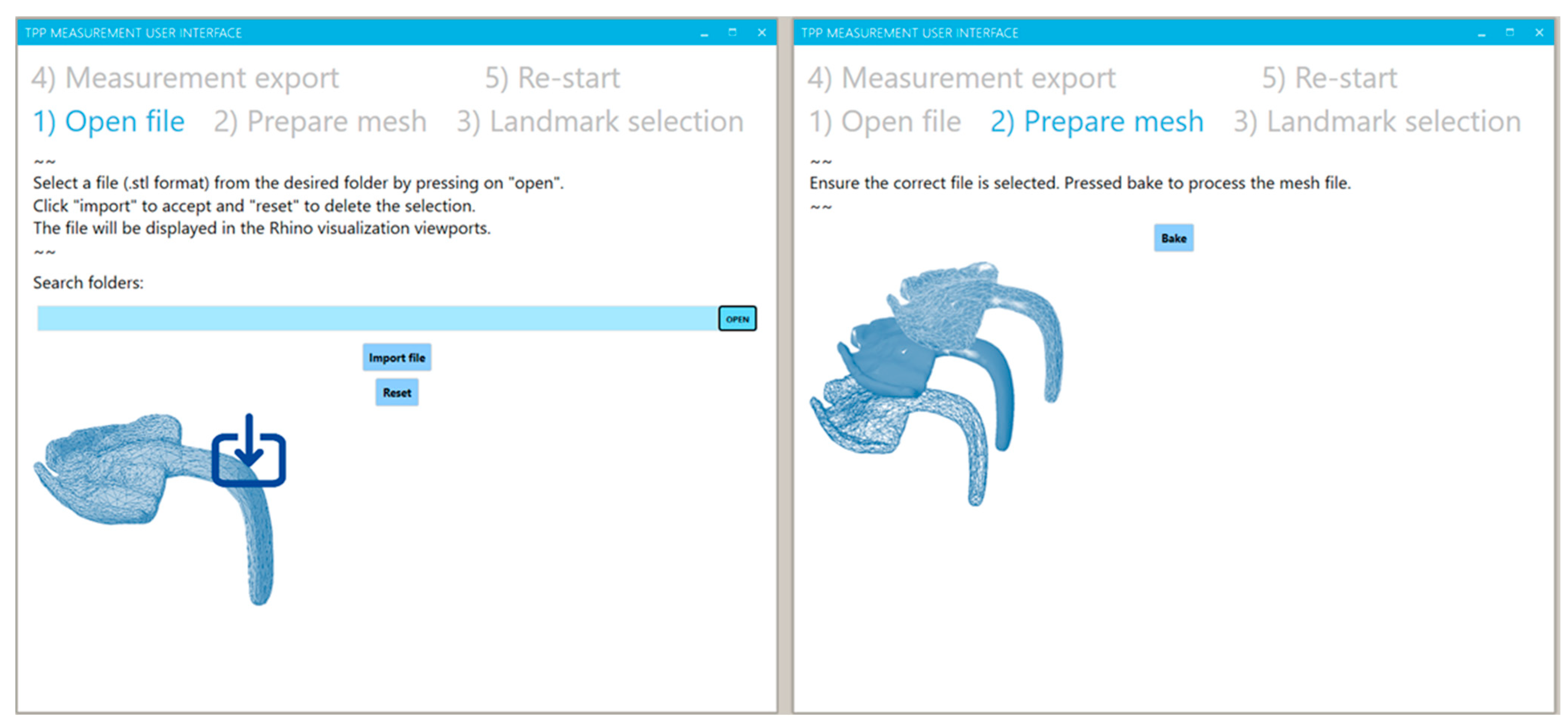
2.2.2. Importing Files
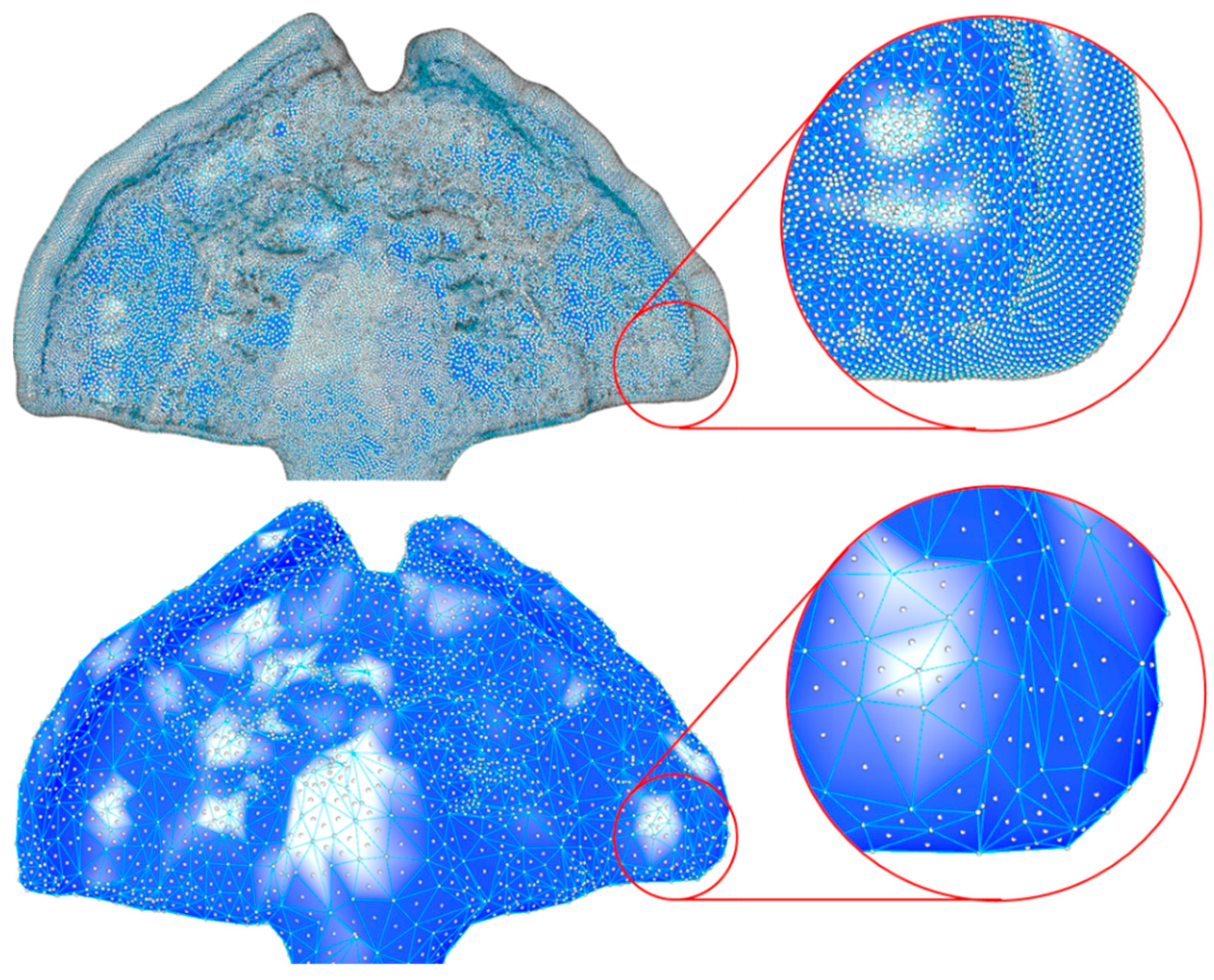
2.2.3. Mesh Preparation
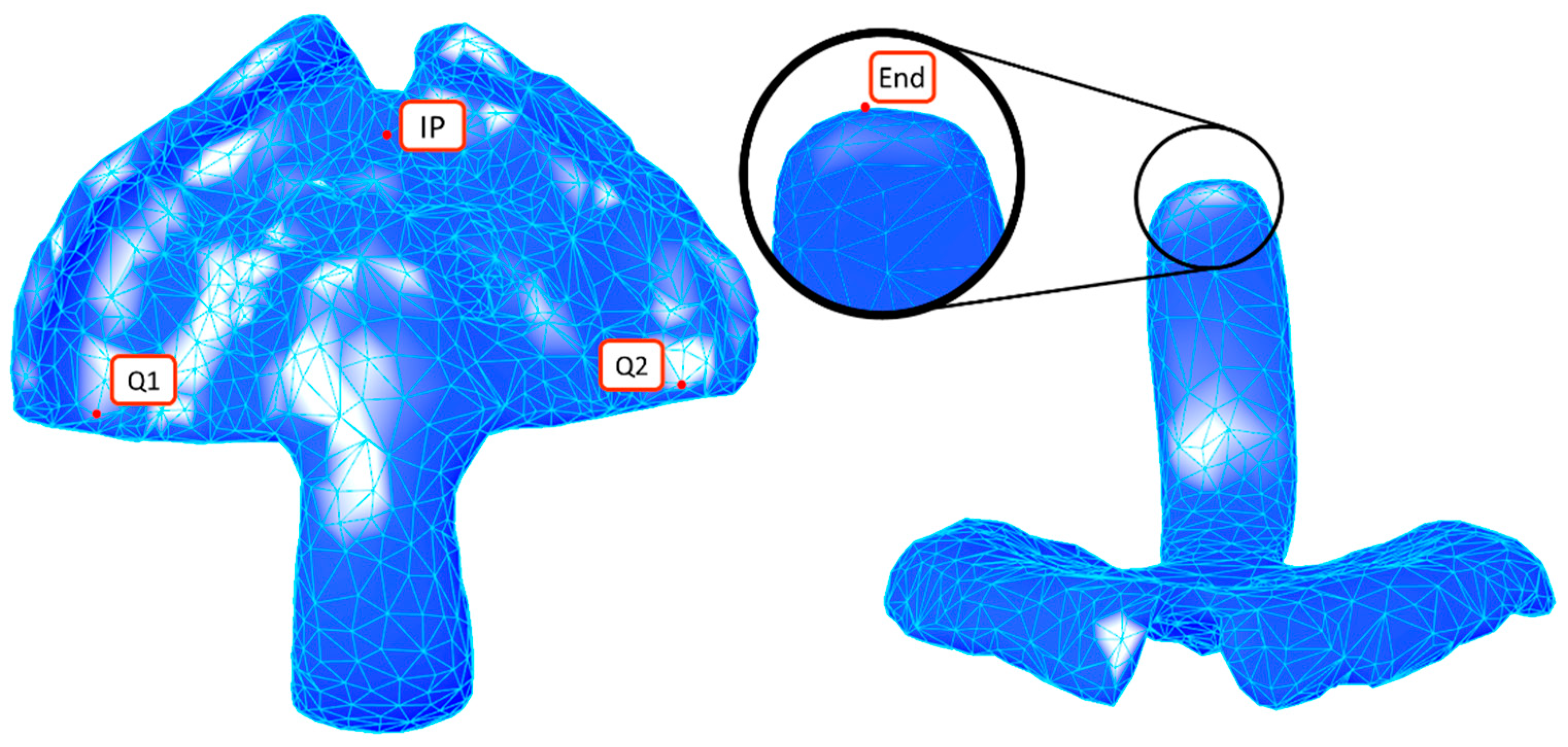
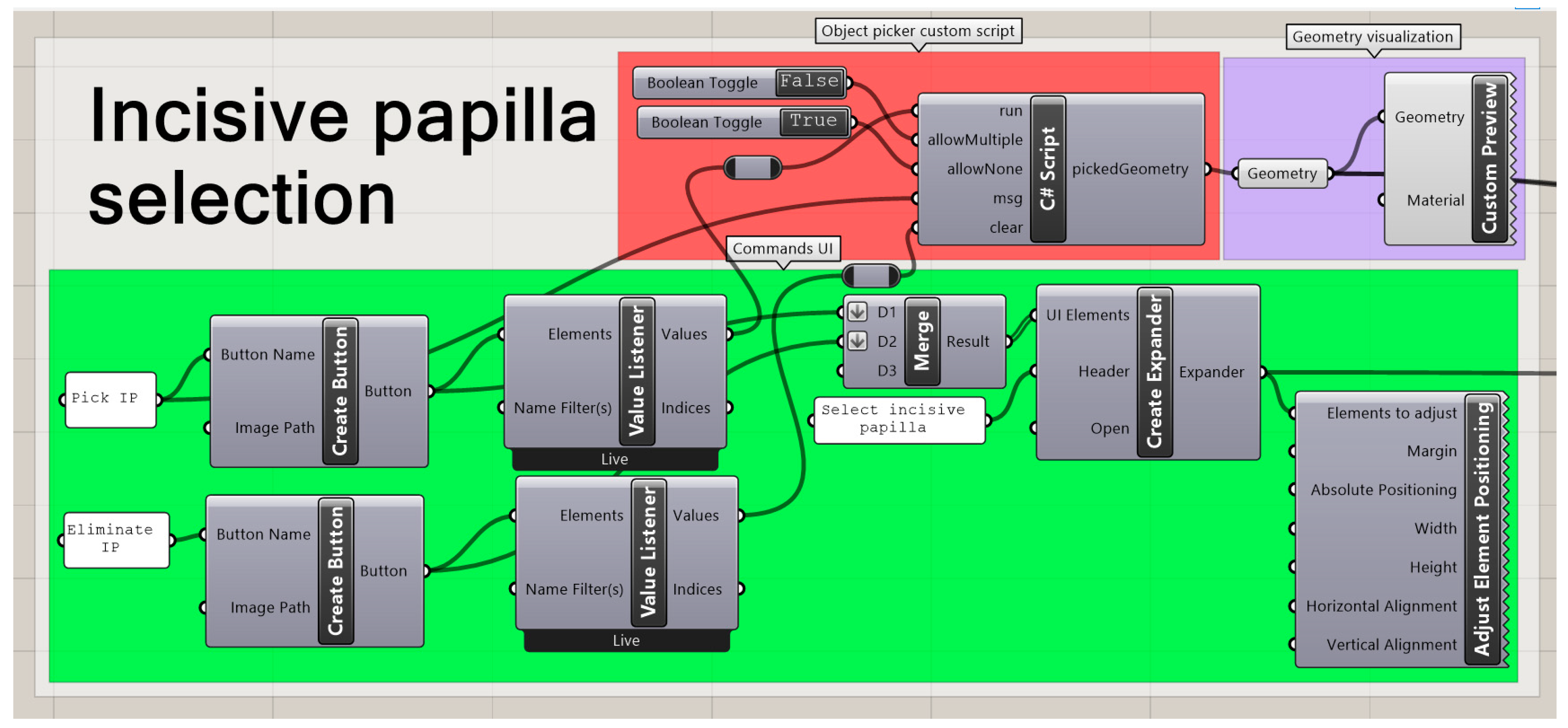
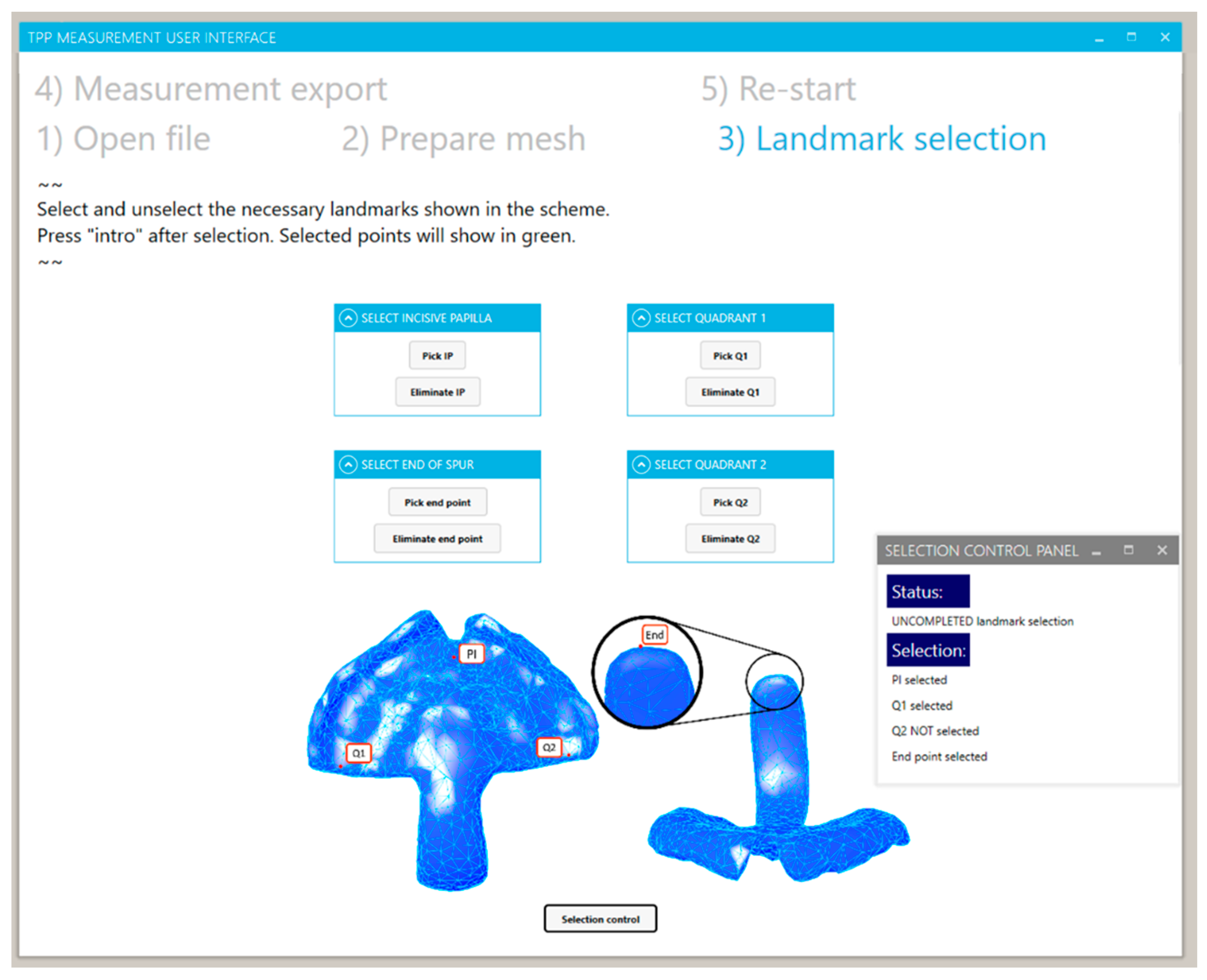
2.2.4. Landmark Selection
2.2.5. Automated Measurement
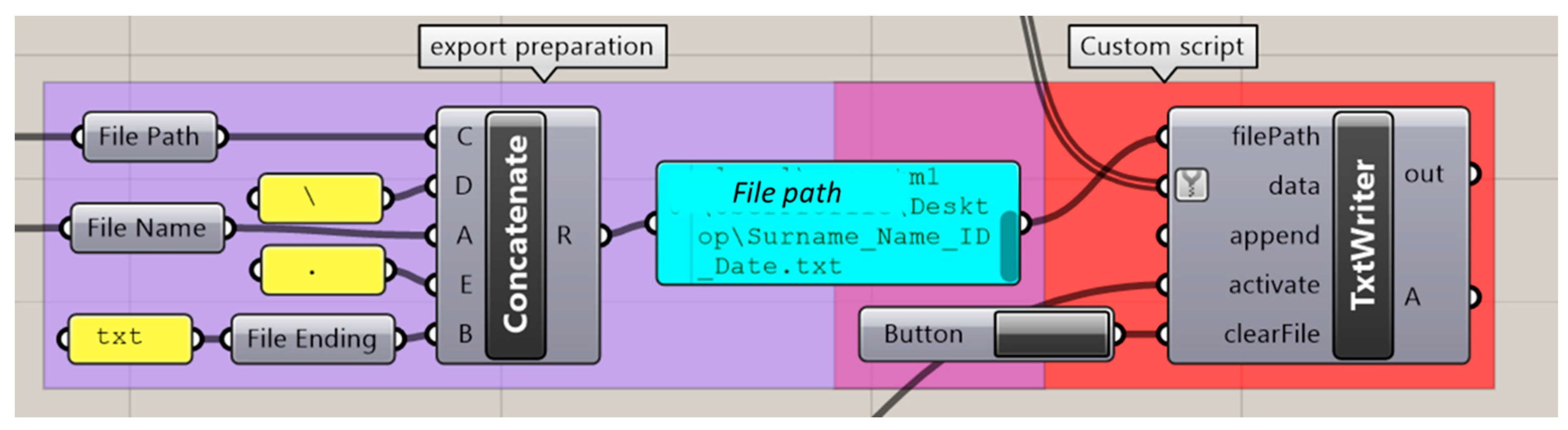
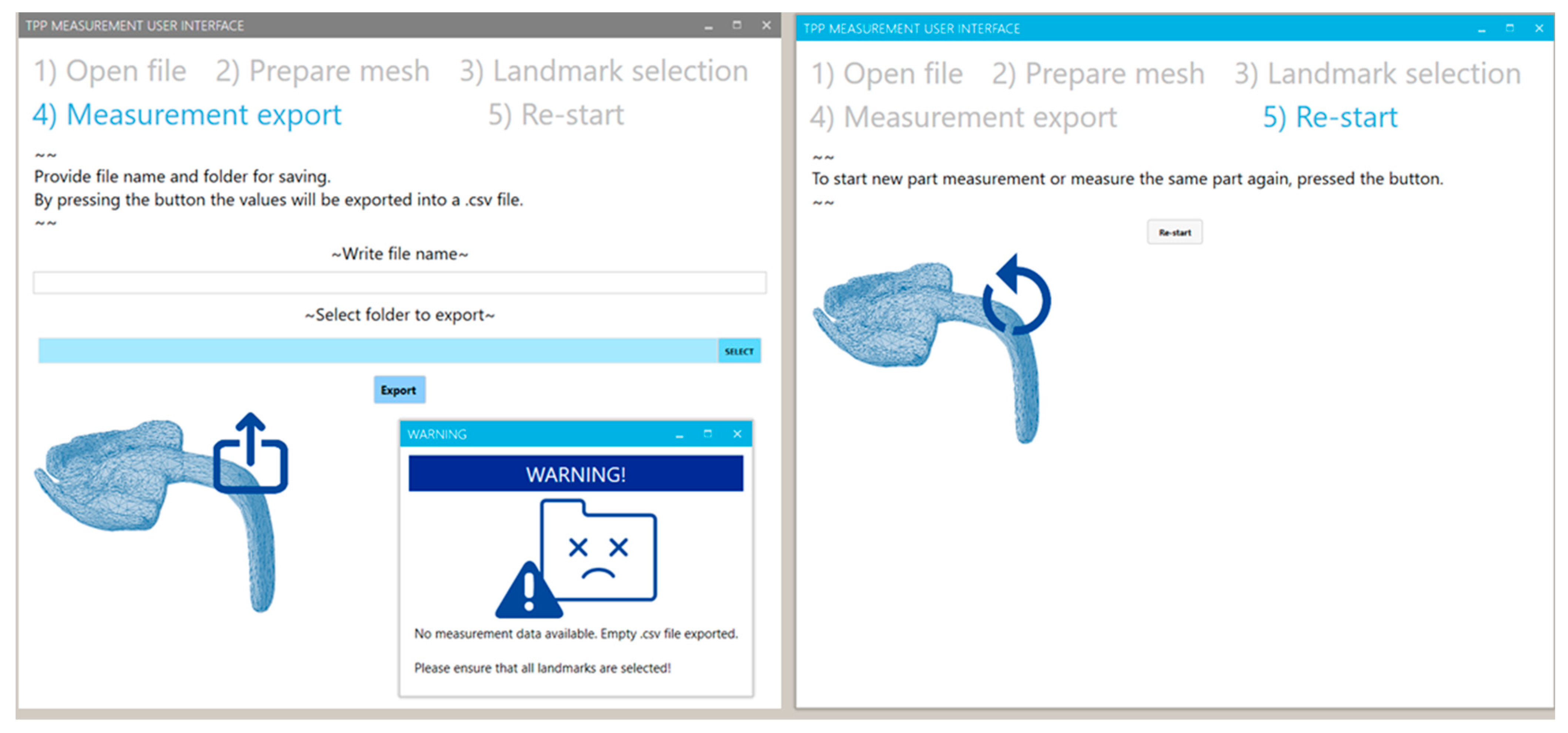
2.2.6. Exporting of Values
2.2.7. Re-Starting of the Program
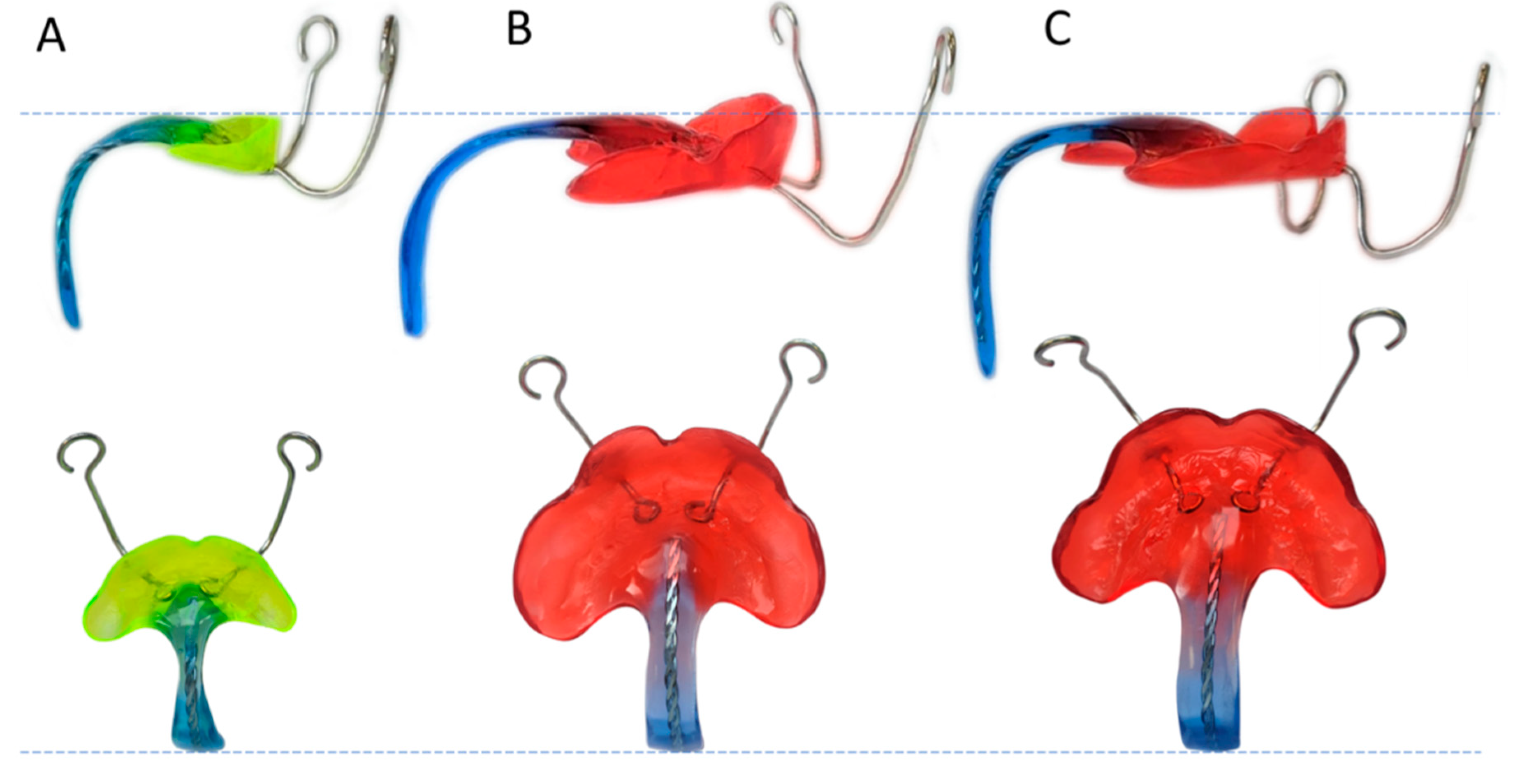
2.3. Case Study and Measurement Example
3. Results
3.1. Performance of the Measuring Program and UI
3.2. Results of Case Study
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Parthasarathy, J. 3D modeling, custom implants and its future perspectives in craniofacial surgery. Ann. Maxillofac. Surg. 2014, 4, 9. [Google Scholar] [CrossRef] [PubMed]
- Barrios-Muriel, J.; Romero-Sánchez, F.; Alonso-Sánchez, F.J.; Salgado, D.R. Advances in orthotic and prosthetic manufacturing: A technology review. Materials 2020, 13, 295. [Google Scholar] [CrossRef] [PubMed]
- Gualdrón, C.-I.L.; Ibarra, E.-R.B.; Bohórquez, A.-P.M.; Bohórquez, I.G. Present and future for technologies to develop patient-specific medical devices: A systematic review approach. Med. Devices 2019, 12, 253. [Google Scholar] [CrossRef] [PubMed]
- Memon, A.R.; Wang, E.; Hu, J.; Egger, J.; Chen, X. A review on computer-aided design and manufacturing of patient-specific maxillofacial implants. Expert Rev. Med. Devices 2020, 17, 345–356. [Google Scholar] [CrossRef]
- Aretxabaleta, M.; Xepapadeas, A.B.; Poets, C.F.; Koos, B.; Spintzyk, S. Comparison of additive and subtractive CAD/CAM materials for their potential use as Tübingen Palatal Plate: An in-vitro study on flexural strength. Addit. Manuf. 2021, 37, 101693. [Google Scholar] [CrossRef]
- Douglas, T.S. Image processing for craniofacial landmark identification and measurement: A review of photogrammetry and cephalometry. Comput. Med. Imaging Graph. 2004, 28, 401–409. [Google Scholar] [CrossRef] [PubMed]
- Koo, T.K.; Li, M.Y. A guideline of selecting and reporting intraclass correlation coefficients for reliability research. J. Chiropr. Med. 2016, 15, 155–163. [Google Scholar] [CrossRef]
- Wang, C.-W.; Huang, C.-T.; Hsieh, M.-C.; Li, C.-H.; Chang, S.-W.; Li, W.-C.; Vandaele, R.; Marée, R.; Jodogne, S.; Geurts, P. Evaluation and comparison of anatomical landmark detection methods for cephalometric x-ray images: A grand challenge. IEEE Trans. Med. Imaging 2015, 34, 1890–1900. [Google Scholar] [CrossRef]
- Kunz, F.; Stellzig-Eisenhauer, A.; Zeman, F.; Boldt, J. Artificial intelligence in orthodontics. J. Orofac. Orthop./Fortschr. Kieferorthop. 2020, 81, 52–68. [Google Scholar] [CrossRef]
- Aretxabaleta, M.; Unkovskiy, A.; Koos, B.; Spintzyk, S.; Xepapadeas, A.B. Accuracy Evaluation of Additively and Subtractively Fabricated Palatal Plate Orthodontic Appliances for Newborns and Infants—An In Vitro Study. Materials 2021, 14, 4103. [Google Scholar] [CrossRef]
- Lee, K.S.; Song, H.K. Automation of 3D average human body shape modeling using Rhino and Grasshopper Algorithm. Fash. Text. 2021, 8, 23. [Google Scholar] [CrossRef]
- Formisano, M.; Iuppariello, L.; Casaburi, A.; Guida, P.; Clemente, F. An industrial oriented workflow for 3D printed, patient specific orthopedic cast. SN Appl. Sci. 2021, 3, 830. [Google Scholar] [CrossRef]
- Ricotta, V.; Campbell, R.I.; Ingrassia, T.; Nigrelli, V. A new design approach for customised medical devices realized by additive manufacturing. Int. J. Interact. Des. Manu. 2020, 14, 1171–1178. [Google Scholar] [CrossRef]
- Giudice, A.; Barone, S.; Belhous, K.; Morice, A.; Soupre, V.; Bennardo, F.; Boddaert, N.; Vazquez, M.-P.; Abadie, V.; Picard, A. Pierre Robin sequence: A comprehensive narrative review of the literature over time. J. Stomatol. Oral Maxillofac. Surg. 2018, 119, 419–428. [Google Scholar] [CrossRef]
- Wiechers, C.; Arand, J.; Koos, B.; Poets, C.F. Evidence and practical aspects of treatment with the Tübingen palatal plate. Semin. Fetal Neonatal Med. 2021, 26, 101281. [Google Scholar] [CrossRef] [PubMed]
- Wiechers, C.; Buchenau, W.; Arand, J.; Oertel, A.-F.; Peters, K.; Müller-Hagedorn, S.; Koos, B.; Poets, C.F. Mandibular growth in infants with Robin sequence treated with the Tübingen palatal plate. Head Face Med. 2019, 15, 17. [Google Scholar] [CrossRef]
- Poets, C.F.; Koos, B.; Reinert, S.; Wiechers, C. The Tübingen palatal plate approach to Robin sequence: Summary of current evidence. J. Cranio-Maxillofac. Surg. 2019, 47, 1699–1705. [Google Scholar] [CrossRef]
- Poets, C.F.; Wiechers, C.; Koos, B.; Muzaffar, A.R.; Gozal, D. Pierre Robin and breathing: What to do and when? Pediatr. Pulmonol. 2022, 57, 1887–1896. [Google Scholar] [CrossRef]
- Buchenau, W.; Urschitz, M.S.; Sautermeister, J.; Bacher, M.; Herberts, T.; Arand, J.; Poets, C.F. A randomized clinical trial of a new orthodontic appliance to improve upper airway obstruction in infants with Pierre Robin sequence. J. Pediatr. 2007, 151, 145–149. [Google Scholar] [CrossRef] [PubMed]
- Müller-Hagedorn, S.; Arand, J.; Scholz, T.; Poets, C.F.; Wiechers, C. An innovative method for manufacturing the Tuebingen palatal plate for infants with Robin sequence. BMC Pediatr. 2020, 20, 103. [Google Scholar] [CrossRef] [PubMed]
- Bacher, M.; Linz, A.; Buchenau, W.; Arand, J.; Krimmel, M.; Poets, C. Therapeutisches Vorgehen bei Pierre-Robin-Sequenz. Laryngo-Rhino-Otol. 2010, 89, 621–629. [Google Scholar] [CrossRef]
- Wiechers, C.; Iffländer, R.; Gerdes, R.; Ciuffolotti, M.; Arand, J.; Weise, C.; Peters, K.; Grandke, B.; Reinert, S.; Koos, B. Retrospective study on growth in infants with isolated Robin sequence treated with the Tuebingen Palate Plate. Orphanet J. Rare Dis. 2021, 16, 338. [Google Scholar] [CrossRef]
- Naros, A.; Krimmel, M.; Zengerle, F.; Bacher, M.; Koos, B.; Mack, U.; Wiechers, C.; Poets, C.F.; Reinert, S. Perioperative complications in cleft palate repair with Robin sequence following Tuebingen palatal plate treatment. J. Cranio-Maxillofac. Surg. 2021, 49, 298–303. [Google Scholar] [CrossRef] [PubMed]
- Wiechers, C.; Koos, B.; Poets, C. Schlafmedizinische Diagnostik und therapeutisches Vorgehen bei der Robin-Sequenz. Atemwegs Lungenkrankh. 2019, 45, 39. [Google Scholar] [CrossRef]
- Müller-Hagedorn, S.; Buchenau, W.; Arand, J.; Bacher, M.; Poets, C.F. Treatment of infants with Syndromic Robin sequence with modified palatal plates: A minimally invasive treatment option. Head Face Med. 2017, 13, 4. [Google Scholar] [CrossRef] [PubMed]
- Bacher, M.; Sautermeister, J.; Urschitz, M.S.; Buchenau, W.; Arand, J.; Poets, C.F. An oral appliance with velar extension for treatment of obstructive sleep apnea in infants with Pierre Robin sequence. Cleft Palate-Craniofacial J. 2011, 48, 331–336. [Google Scholar] [CrossRef]
- Buchenau, W.; Wenzel, S.; Bacher, M.; Müller-Hagedorn, S.; Arand, J.; Poets, C.F. Functional treatment of airway obstruction and feeding problems in infants with Robin sequence. Arch. Dis. Child.-Fetal Neonatal Ed. 2017, 102, F142–F146. [Google Scholar] [CrossRef]
- Von Bodman, A.; Buchenau, W.; Bacher, M.; Arand, J.; Urschitz, M.; Poets, C. Treatment of infants with Pierre Robin Sequence using a new oral appliance. Wien. Klin. Wochenschr. 2003, 115, 871–873. [Google Scholar] [CrossRef]
- Buchenau, W.; Sautermeister, J.; Bacher, M.; Arand, J.; Urschitz, M.; Poets, C. Die Tübinger Gaumenplatte–Ein innovatives Therapiekonzept bei Pierre-Robin-Sequenz. Einfluss auf die schlafbezogene Atmungsstörung. Z. Für Geburtshilfe Neonatol. 2004, 208, 7. [Google Scholar] [CrossRef]
- Van Lieshout, M.J.; Joosten, K.F.; Mathijssen, I.M.; Koudstaal, M.J.; Wolvius, E.B.; van der Schroeff, M.P. Non-surgical and surgical interventions for airway obstruction in children with Robin Sequence. J. Cranio-Maxillofac. Surg. 2016, 44, 1871–1879. [Google Scholar] [CrossRef]
- Gerzanic, L.; Feichtinger, M.; Kärcher, H. The influence of the Tübingen soft palate plate and early cleft closure on the nasopharyngeal airway for the management of airway obstruction in an infant with Pierre Robin sequence: A case report. Int. J. Surg. Case Rep. 2012, 3, 608–610. [Google Scholar] [CrossRef]
- Ho, A.C.; Wong, R.W.; Cheung, T.; Ng, D.K.; Siu, K.; Fung, S. Orthodontic plate for management of obstructive sleep apnoea in infants with Pierre Robin sequence: Experience and protocol in Hong Kong. J. Orthod. 2019, 46, 367–373. [Google Scholar] [CrossRef] [PubMed]
- Choo, H.; Khosla, R.K.; Meister, K.D.; Wan, D.C.; Lin, H.-F.C.; Feczko, R.; Bruckman, K.; Hopkins, E.; Truong, M.T.; Lorenz, H.P. Nonsurgical orthodontic airway plate treatment for newborns with Robin sequence. Cleft Palate-Craniofacial J. 2022, 59, 403–410. [Google Scholar] [CrossRef]
- Schmidt, G.; Hirschfelder, A.; Heiland, M.; Matuschek, C. Customized pre-epiglottic baton plate—A practical guide for successful, patient-specific, noninvasive treatment of neonates with Robin Sequence. Cleft Palate-Craniofacial J. 2021, 58, 1063–1069. [Google Scholar] [CrossRef]
- Poets, C.F.; Maas, C.; Buchenau, W.; Arand, J.; Vierzig, A.; Braumann, B.; Müller-Hagedorn, S. Multicenter study on the effectiveness of the pre-epiglottic baton plate for airway obstruction and feeding problems in Robin sequence. Orphanet J. Rare Dis. 2017, 12, 46. [Google Scholar] [CrossRef]
- Goryachkina, Y.A.; Goryachkin, A. Treatment of infants with Pierre Robin syndrome using the orthodontic device PEBP (pre-epiglottic baton plate). Stomatologiia 2020, 99, 29–32. [Google Scholar] [CrossRef]
- Pang, S.L.; Ho, C.H.A.; Law, C.M.L.; Yang, Y.; Leung, Y.Y. Pre-epiglottic baton plate in the management of upper airway obstruction in an infant with femoral facial syndrome: A case report. Cleft Palate-Craniofacial J. 2021, 10556656211059705. [Google Scholar] [CrossRef]
- Weise, C.; Frank, K.; Wiechers, C.; Weise, H.; Reinert, S.; Koos, B.; Xepapadeas, A.B. Intraoral scanning of neonates and infants with craniofacial disorders: Feasibility, scanning duration, and clinical experience. Eur. J. Orthod. 2022, 44, 279–286. [Google Scholar] [CrossRef]
- Xepapadeas, A.B.; Weise, C.; Frank, K.; Spintzyk, S.; Poets, C.; Wiechers, C.; Arand, J.; Koos, B. Technical note on introducing a digital workflow for newborns with craniofacial anomalies based on intraoral scans-part I: 3D printed and milled palatal stimulation plate for trisomy 21. BMC Oral Health 2020, 20, 20. [Google Scholar] [CrossRef]
- Xepapadeas, A.B.; Weise, C.; Frank, K.; Spintzyk, S.; Poets, C.; Wiechers, C.; Arand, J.; Koos, B. Technical note on introducing a digital workflow for newborns with craniofacial anomalies based on intraoral scans—Part II: 3D printed Tübingen palatal plate prototype for newborns with Robin sequence. BMC Oral Health 2020, 20, 171. [Google Scholar] [CrossRef]
- Aretxabaleta, M.; Xepapadeas, A.B.; Poets, C.F.; Koos, B.; Spintzyk, S. Fracture load of an Orthodontic appliance for Robin Sequence Treatment in a digital workflow. Materials 2021, 14, 344. [Google Scholar] [CrossRef] [PubMed]
- Thurzo, A.; Šufliarsky, B.; Urbanová, W.; Čverha, M.; Strunga, M.; Varga, I. Pierre Robin Sequence and 3D Printed Personalized Composite Appliances in Interdisciplinary Approach. Polymers 2022, 14, 3858. [Google Scholar] [CrossRef] [PubMed]


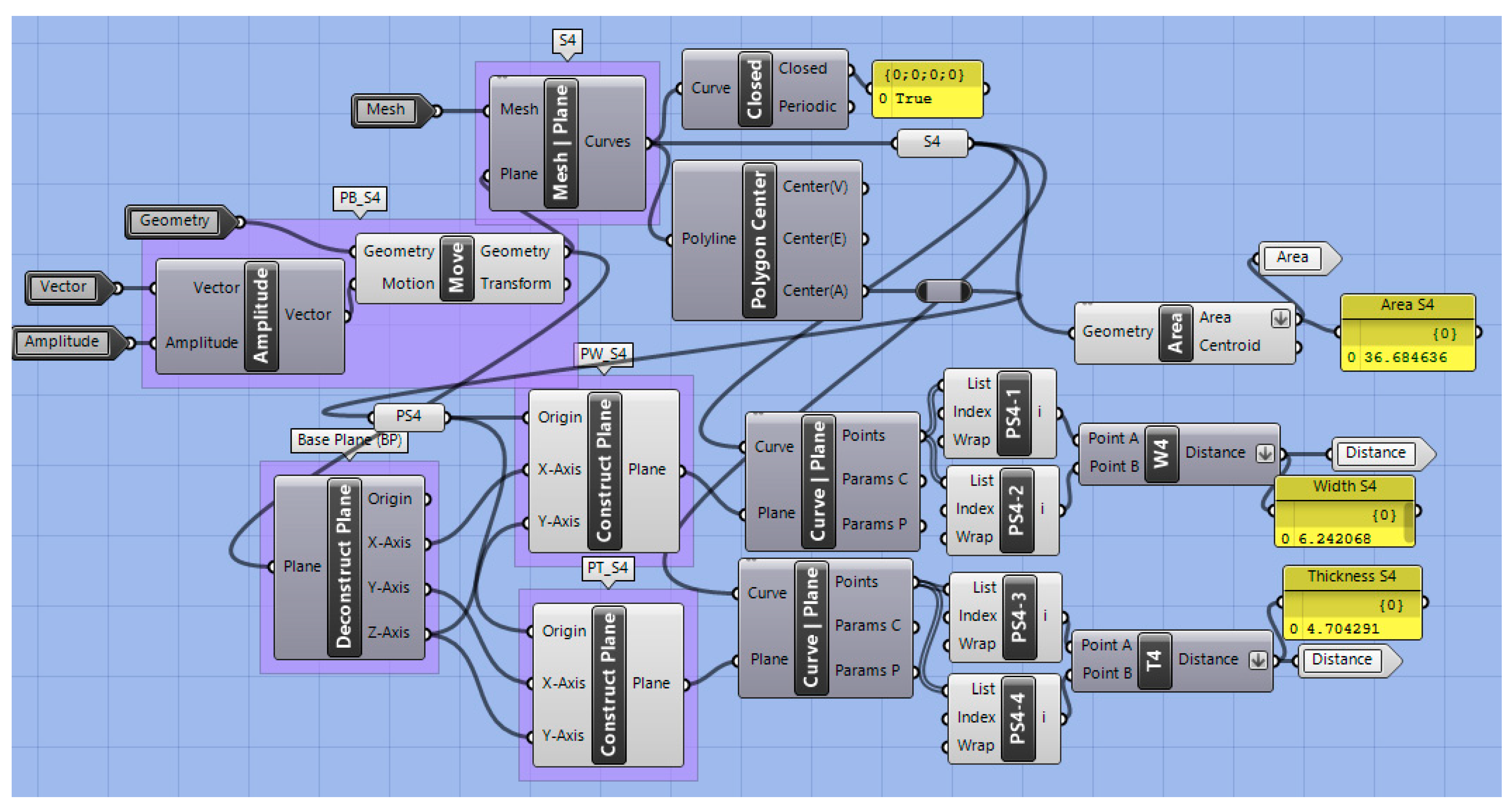
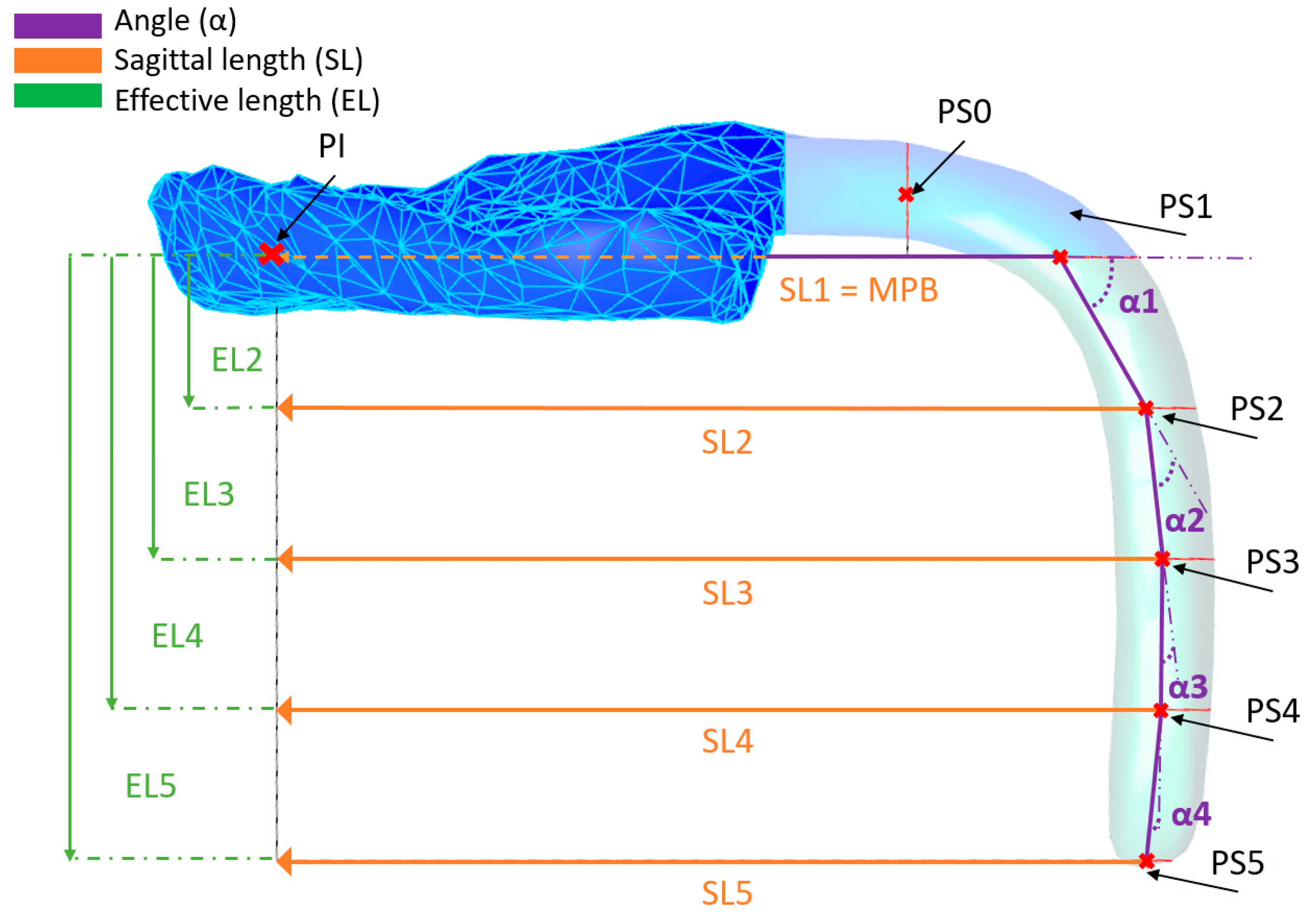
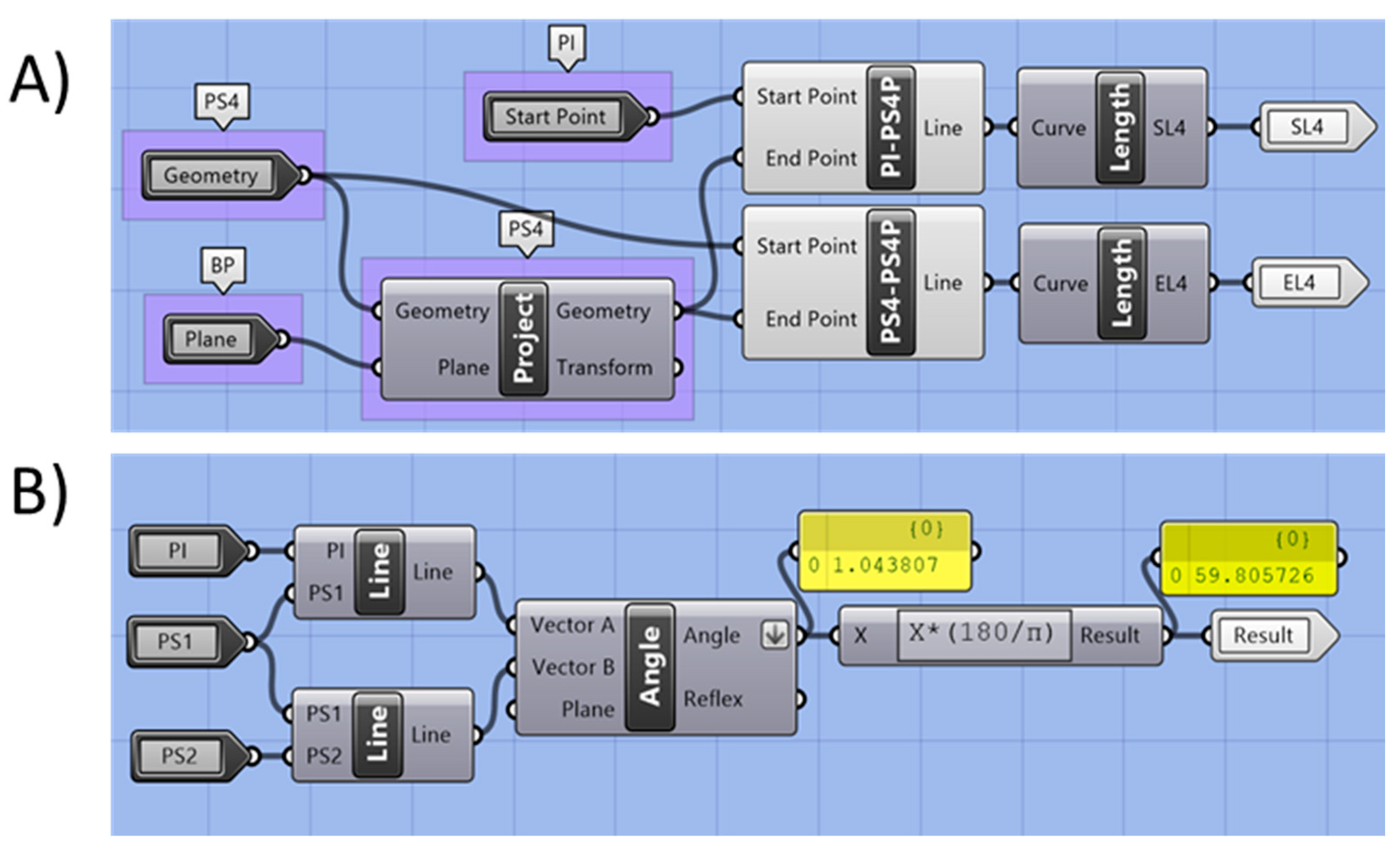
| Construction Names | Measurement Names | ||
|---|---|---|---|
| Acronym | Meaning | Acronym | Meaning |
| S4 | Curve Section 4 | A4 | Area of S4 (mm2) |
| PB4 | Base plane S4 | W4 | Width S4 (mm) |
| PS4 | Centroid point S4 | T4 | Thickness S4 (mm) |
| PW-S4 | Plane for width S4 | EL4 | Effective length to S4. Distance PI to PS4 (mm) |
| PT-T4 | Plane for thickness S4 | SL4 | Sagittal length to S4. Distance PI to PS4 (mm) |
| PS4-P | Projection of PS4 on main base plane | α4 | Angle (°) |
| Case | Gender | Age (Days) | Gestational Age | Head Circumference (cm) | Plate Number | |
|---|---|---|---|---|---|---|
| Weeks | Days | |||||
| Case A | Female | 18 | 37 | 6 | 34 | 1 |
| Case B | Male | 162 | 40 | 2 | 44 | 2 |
| Case C | Male | 165 | 38 | 1 | 43 | 2 |
| Values | Case A | Case B | Case C | Values | Case A | Case B | Case C | ||
|---|---|---|---|---|---|---|---|---|---|
| S0 (mm) | EL0 | 1.05 | 5.14 | 3.61 | S3 (mm) | EL3 | 10.57 | 9.32 | 13.29 |
| SL0 | 23.21 | 34.01 | 27.79 | SL3 | 28.63 | 45.59 | 37.83 | ||
| A0 | 7.17 | 17.04 | 15.18 | A3 | 19.67 | 28.28 | 22.19 | ||
| W0 | 3.38 | 7.93 | 6.10 | W3 | 8.00 | 5.25 | 2.97 | ||
| T0 | 2.53 | 2.50 | 2.74 | T3 | 2.79 | 3.31 | 4.14 | ||
| S1 (mm) | EL1 | 0.00 | 0.00 | 0.00 | S4 (mm) | EL4 | 15.86 | 13.98 | 19.93 |
| SL1 | 24.58 | 42.08 | 34.55 | SL4 | 29.00 | 45.97 | 37.42 | ||
| A1 | 14.01 | 26.16 | 20.95 | A4 | 21.45 | 24.04 | 24.07 | ||
| W1 | 4.77 | 7.94 | 6.97 | W4 | 4.17 | 3.83 | 3.99 | ||
| T1 | 3.51 | 3.52 | 4.73 | T4 | 3.59 | 3.99 | 4.36 | ||
| S2 (mm) | EL2 | 5.29 | 4.66 | 6.64 | S5 (mm) | EL5 | 21.14 | 18.63 | 26.58 |
| SL2 | 27.43 | 44.54 | 37.39 | SL5 | 29.12 | 45.91 | 35.83 | ||
| A2 | 16.74 | 29.25 | 18.72 | Angles (°) | α1 | 61.67 | 62.27 | 66.91 | |
| W2 | 6.64 | 5.66 | 2.69 | α2 | 8.78 | 8.17 | 7.44 | ||
| T2 | 2.85 | 3.56 | 3.81 | α3 | 6.11 | 10.4 | 4.45 | ||
| Veff (mm3) | 374.20 | 475.39 | 547.17 | α4 | 15.67 | 15.13 | 19.4 | ||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Aretxabaleta, M.; Roehler, A.; Poets, C.F.; Xepapadeas, A.B.; Koos, B.; Weise, C. Automation of Measurements for Personalized Medical Appliances by Means of CAD Software—Application in Robin Sequence Orthodontic Appliances. Bioengineering 2022, 9, 773. https://doi.org/10.3390/bioengineering9120773
Aretxabaleta M, Roehler A, Poets CF, Xepapadeas AB, Koos B, Weise C. Automation of Measurements for Personalized Medical Appliances by Means of CAD Software—Application in Robin Sequence Orthodontic Appliances. Bioengineering. 2022; 9(12):773. https://doi.org/10.3390/bioengineering9120773
Chicago/Turabian StyleAretxabaleta, Maite, Ariadne Roehler, Christian F. Poets, Alexander B. Xepapadeas, Bernd Koos, and Christina Weise. 2022. "Automation of Measurements for Personalized Medical Appliances by Means of CAD Software—Application in Robin Sequence Orthodontic Appliances" Bioengineering 9, no. 12: 773. https://doi.org/10.3390/bioengineering9120773
APA StyleAretxabaleta, M., Roehler, A., Poets, C. F., Xepapadeas, A. B., Koos, B., & Weise, C. (2022). Automation of Measurements for Personalized Medical Appliances by Means of CAD Software—Application in Robin Sequence Orthodontic Appliances. Bioengineering, 9(12), 773. https://doi.org/10.3390/bioengineering9120773