Design, Structural Characteristic and Antibacterial Performance of Silver-Containing Cotton Fiber Nanocomposite
Abstract
1. Introduction
2. Experimental Procedure
2.1. Materials
2.2. Modification of Cotton-OH
2.3. Preparation of Silver-Containing Cotton Fiber Nanocomposite
2.4. Characterization Techniques
2.5. Antibacterial Test
3. Results and Discussion
3.1. Description of the Synthesis Process
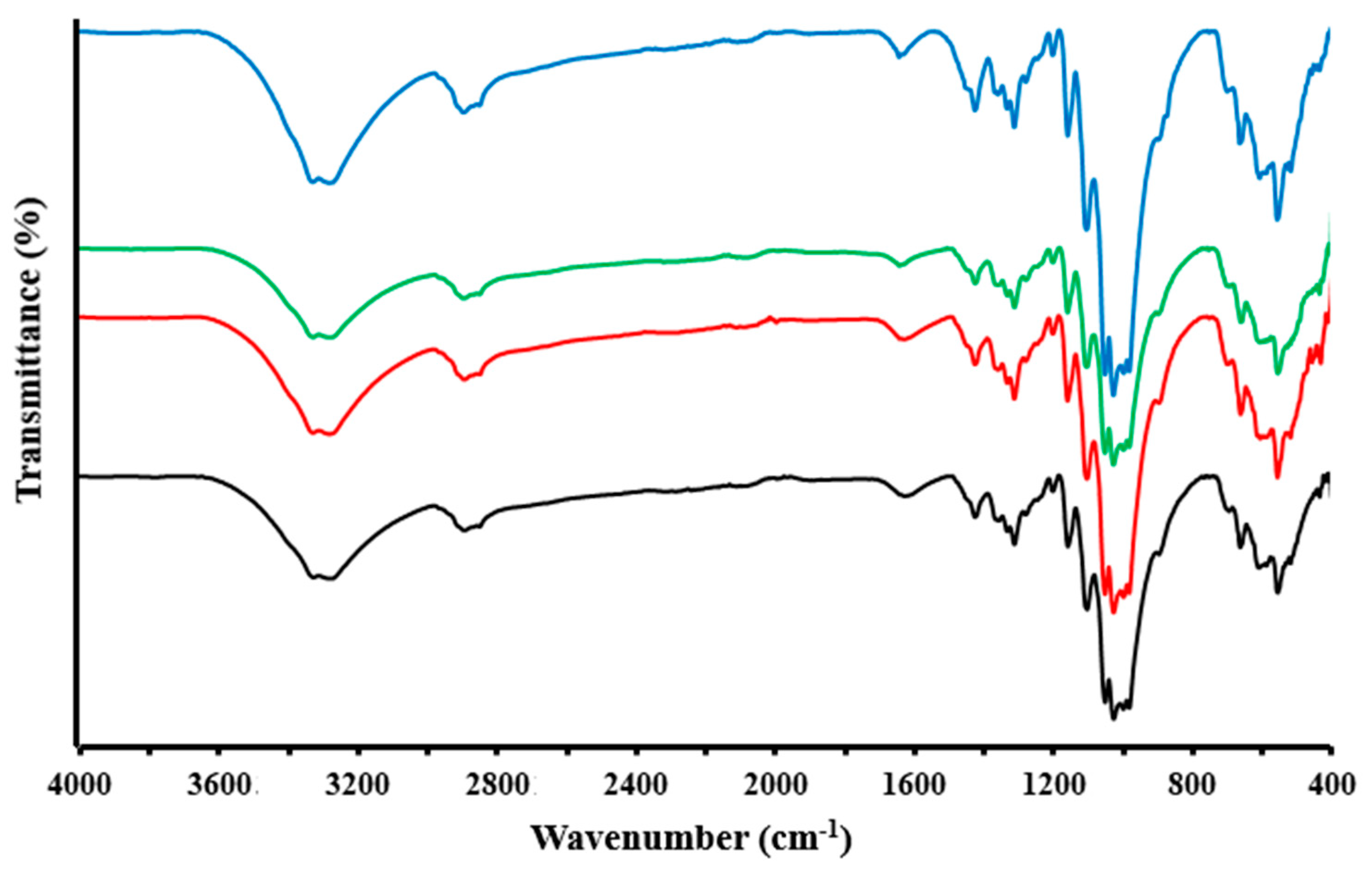
3.2. FTIR Spectroscopy
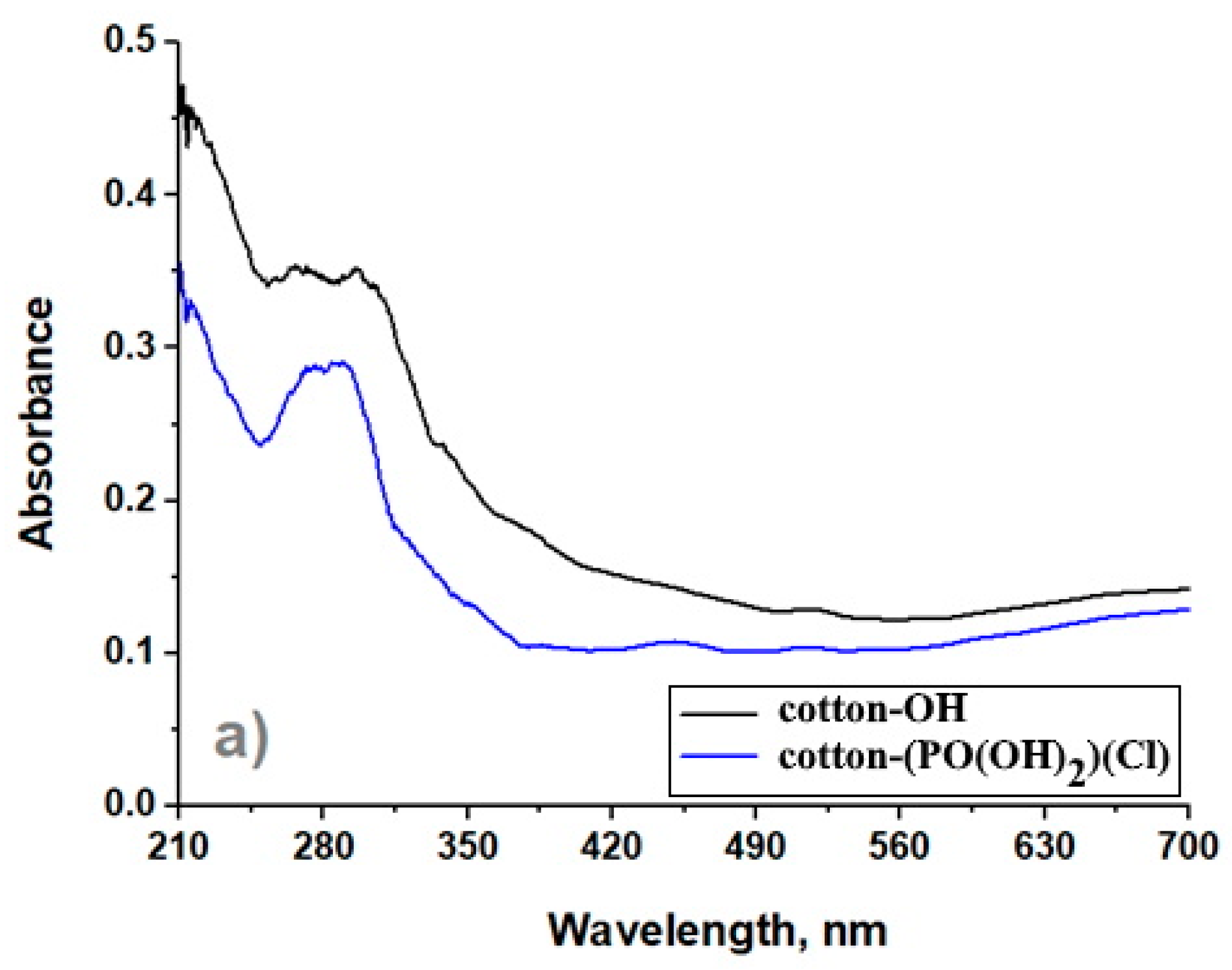
3.3. UV–Visible Spectroscopy
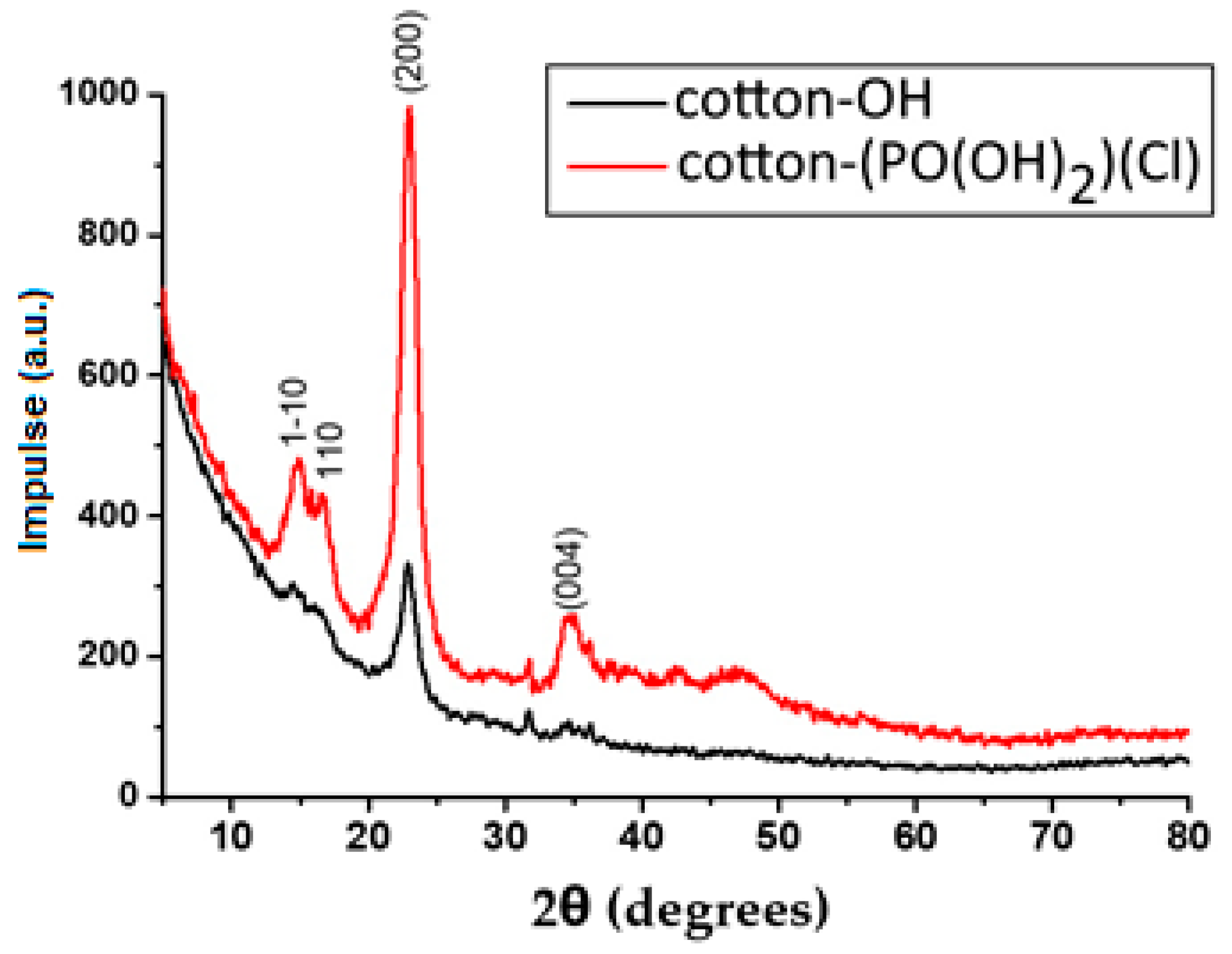
3.4. XRD Analysis
3.5. Thermal Analysis
3.6. SEM and EDX Micrographs
3.7. Antibacterial Properties
4. Conclusions
- -
- As a result of the chemical modification of the cotton-OH sample, a low degree of conversion could be observed, and the reaction product contained phosphate groups and chlorine atoms. In addition, non-cellulose compounds (pectin and wax) were removed from cotton-OH as amorphous domains, resulting in the higher crystallization of cotton-(PO(OH)2)(Cl) than cotton-OH. It should also be noted that after modification, the surface of the sample (cotton-(PO(OH)2)(Cl)) became less rough and smoother and its thermal stability decreased.
- -
- Silver-containing particles in the cotton-OH-Ag composite were present in the form of zero-valent metal and oxide, and, in the cotton-(PO(OH)2)(Cl)-Ag composite, in addition to the above, AgCl particles were present. In general, the amount of silver nanoparticles in the cotton-(PO(OH)2)(Cl)-Ag composite was slightly higher than in cotton-OH-Ag, and they were well dispersed. In both composites, silver nanoparticles had the shape of hollow spheres. In silver-containing samples, in comparison with the original sample, an increase in thermal stability could be observed.
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Climate Change and Health—Better Health Channel. Available online: https://www.betterhealth.vic.gov.au/health/healthyliving/climate-change-and-health#who-is-most-at-risk-of-health-effects-due-to-climate-change (accessed on 18 August 2022).
- Zibulewsky, J. Defining Disaster: The Emergency Department Perspective. Bayl. Univ. Med. Cent. Proc. 2001, 14, 144–149. [Google Scholar] [CrossRef] [PubMed]
- Karim, N.; Afroj, S.; Lloyd, K.; Oaten, L.C.; Andreeva, D.V.; Carr, C.; Farmery, A.D.; Kim, I.-D.; Novoselov, K.S. Sustainable Personal Protective Clothing for Healthcare Applications: A Review. ACS Nano 2020, 14, 12313–12340. [Google Scholar] [CrossRef] [PubMed]
- Revathi, T.; Thambidurai, S. Synthesis of chitosan incorporated neem seed extract (Azadirachta indica) for medical textiles. Int. J. Biol. Macromol. 2017, 104, 1890–1896. [Google Scholar] [CrossRef] [PubMed]
- Venkatesan, H.; Periyasamy, A.P. Eco-fibers in the Textile Industry BT. In Handbook of Eco-Materials; Martínez, L.M.T., Kharissova, O.V., Kharisov, B.I., Eds.; Springer International Publishing: Cham, Switzerland, 2019; pp. 1413–1433. [Google Scholar] [CrossRef]
- Zhao, C.; Okada, H.; Sugimoto, R. Chemical modification of cotton by methyl methacrylate via emulsion polymerization. Polym. J. 2019, 51, 1023–1028. [Google Scholar] [CrossRef]
- Cortese, B.; Caschera, D.; Padeletti, G.; Ingo, G.M.; Gigli, G. A brief review of surface-functionalized cotton fabrics. Surf. Innov. 2013, 1, 140–156. [Google Scholar] [CrossRef]
- Granados, A.; Pleixats, R.; Vallribera, A. Recent Advances on Antimicrobial and Anti-Inflammatory Cotton Fabrics Containing Nanostructures. Molecules 2021, 26, 3008. [Google Scholar] [CrossRef]
- Pan, Y.; Yang, P.; Moloney, M.G.; Wang, L.; Ma, F.; Wang, Y. Ag NP-Loaded Cotton Fiber Materials: Preparation, Surface Deposition, and Antibacterial Activity with Different Chemical Structures. ACS Appl. Bio Mater. 2019, 2, 510–517. [Google Scholar] [CrossRef]
- Emam, H.E.; Zahran, M. Ag0 nanoparticles containing cotton fabric: Synthesis, characterization, color data and antibacterial action. Int. J. Biol. Macromol. 2015, 75, 106–114. [Google Scholar] [CrossRef]
- Sirelkhatim, A.; Mahmud, S.; Seeni, A.; Kaus, N.H.M.; Ann, L.C.; Bakhori, S.K.M.; Hasan, H.; Mohamad, D. Review on Zinc Oxide Nanoparticles: Antibacterial Activity and Toxicity Mechanism. Nano-Micro Lett. 2015, 7, 219–242. [Google Scholar] [CrossRef]
- Alagarasan, D.; Harikrishnan, A.; Surendiran, M.; Indira, K.; Khalifa, A.S.; Elesawy, B.H. Synthesis and characterization of CuO nanoparticles and evaluation of their bactericidal and fungicidal activities in cotton fabrics. Appl. Nanosci. 2021, 11, 1–10. [Google Scholar] [CrossRef]
- Wang, L.; Wen, X.; Zhang, X.; Yuan, S.; Xu, Q.; Fu, F.; Diao, H.; Liu, X. Durable antimicrobial cotton fabric fabricated by carboxymethyl chitosan and quaternary ammonium salts. Cellulose 2021, 28, 5867–5879. [Google Scholar] [CrossRef]
- Raeisi, M.; Kazerouni, Y.; Mohammadi, A.; Hashemi, M.; Hejazi, I.; Seyfi, J.; Khonakdar, H.A.; Davachi, S.M. Superhydrophobic cotton fabrics coated by chitosan and titanium dioxide nanoparticles with enhanced antibacterial and UV-protecting properties. Int. J. Biol. Macromol. 2021, 171, 158–165. [Google Scholar] [CrossRef]
- Le Ouay, B.; Stellacci, F. Antibacterial activity of silver nanoparticles: A surface science insight. Nano Today 2015, 10, 339–354. [Google Scholar] [CrossRef]
- Srisod, S.; Motina, K.; Inprasit, T.; Pisitsak, P. A green and facile approach to durable antimicrobial coating of cotton with silver nanoparticles, whey protein, and natural tannin. Prog. Org. Coatings 2018, 120, 123–131. [Google Scholar] [CrossRef]
- Sambalova, O.; Thorwarth, K.; Heeb, N.V.; Bleiner, D.; Zhang, Y.; Borgschulte, A.; Kroll, A. Carboxylate Functional Groups Mediate Interaction with Silver Nanoparticles in Biofilm Matrix. ACS Omega 2018, 3, 724–733. [Google Scholar] [CrossRef] [PubMed]
- Zhou, J.; Cai, D.; Xu, Q.; Zhang, Y.; Fu, F.; Diao, H.; Liu, X. Excellent binding effect of l-methionine for immobilizing silver nanoparticles onto cotton fabrics to improve the antibacterial durability against washing. RSC Adv. 2018, 8, 24458–24463. [Google Scholar] [CrossRef] [PubMed]
- Alosmanov, R.; Wolski, K.; Matuschek, G.; Magerramov, A.; Azizov, A.; Zimmermann, R.; Aliyev, E.; Zapotoczny, S. Effect of functional groups on the thermal degradation of phosphorus- and phosphorus/nitrogen-containing functional polymers. J. Therm. Anal. 2017, 130, 799–812. [Google Scholar] [CrossRef]
- Aliyeva, S.; Alosmanov, R.; Buniyatzadeh, I.; Eyvazova, G.; Azizov, A.; Maharramov, A. Functionalized graphene nanoplatelets/modified polybutadiene hybrid composite. Colloid Polym. Sci. 2019, 297, 1529–1540. [Google Scholar] [CrossRef]
- Mulfinger, L.; Solomon, S.D.; Bahadory, M.; Jeyarajasingam, A.V.; Rutkowsky, S.A.; Boritz, C. Synthesis and Study of Silver Nanoparticles. J. Chem. Educ. 2007, 84, 322. [Google Scholar] [CrossRef]
- AATCC Test Method 100-2004, Antibacterial Finishes on Textile Materials: Assessment of AATCC Technical Manual; AATCC: Research Triangle, CA, USA, 2008; Volume 83, p. 145.
- Dochia, M.; Chambre, D.; Gavrilaş, S.; Moisă, C. Characterization of the complexing agents’ influence on bioscouring cotton fabrics by FT-IR and TG/DTG/DTA analysis. J. Therm. Anal. 2018, 132, 1489–1498. [Google Scholar] [CrossRef]
- Alosmanov, R.; Wolski, K.; Zapotoczny, S. Grafting of thermosensitive poly(N-isopropylacrylamide) from wet bacterial cellulose sheets to improve its swelling-drying ability. Cellulose 2016, 24, 285–293. [Google Scholar] [CrossRef]
- Ma, Z.; Liu, J.; Shen, G.; Zheng, X.; Pei, Y.; Tang, K. In-situ synthesis and immobilization of silver nanoparticles on microfibrillated cellulose for long-term antibacterial applications. Cellulose 2021, 28, 6287–6303. [Google Scholar] [CrossRef]
- Salama, A.; El-Sakhawy, M. Regenerated cellulose/wool blend enhanced biomimetic hydroxyapatite mineralization. Int. J. Biol. Macromol. 2016, 92, 920–925. [Google Scholar] [CrossRef] [PubMed]
- Yan, H.; Hua, Z.; Qian, G.; Wang, M.; Du, G.; Chen, J. Analysis of the chemical composition of cotton seed coat by Fourier-transform infrared (FT-IR) microspectroscopy. Cellulose 2009, 16, 1099–1107. [Google Scholar] [CrossRef]
- Fu, K.-L.; Liu, J.-P.; Lu, D.-N. The pectin substance used to detect the activity of pectinase suitable for bioscouring. J. Text. Inst. 2013, 105, 12–19. [Google Scholar] [CrossRef]
- Kokot, S.; Czarnik-Matusewicz, B.; Ozaki, Y. Two-dimensional correlation spectroscopy and principal component analysis studies of temperature-dependent IR spectra of cotton-cellulose. Biopolymers 2002, 67, 456–469. [Google Scholar] [CrossRef]
- Al Aboody, M.S. Silver/silver chloride (Ag/AgCl) nanoparticles synthesized from Azadirachta indica lalex and its antibiofilm activity against fluconazole resistant Candida tropicalis. Artif. Cells Nanomed. Biotechnol. 2019, 47, 2107–2113. [Google Scholar] [CrossRef]
- Ravichandran, S.; Paluri, V.; Kumar, G.; Loganathan, K.; Venkata, B.R.K. A novel approach for the biosynthesis of silver oxide nanoparticles using aqueous leaf extract of Callistemon lanceolatus (Myrtaceae) and their therapeutic potential. J. Exp. Nanosci. 2015, 11, 445–458. [Google Scholar] [CrossRef]
- Siddiqui, M.R.H.; Adil, S.; Assal, M.; Ali, R.; Al-Warthan, A.A. Synthesis and Characterization of Silver Oxide and Silver Chloride Nanoparticles with High Thermal Stability. Asian J. Chem. 2013, 25, 3405–3409. [Google Scholar] [CrossRef]
- Law Yong, N.; Ahmad, A.; Wahab Mohammad, A. Synthesis and Characterization of Silver Oxide Nanoparticles by a Novel Method. Int. J. Sci. Eng. Res. 2013, 4, 155–158. [Google Scholar]
- Suflet, D.M.; Chitanu, G.C.; Popa, V.I. Phosphorylation of polysaccharides: New results on synthesis and characterisation of phosphorylated cellulose. React. Funct. Polym. 2006, 66, 1240–1249. [Google Scholar] [CrossRef]
- Jia, Y.; Hu, Y.; Zheng, D.; Zhang, G.; Zhang, F.; Liang, Y. Synthesis and evaluation of an efficient, durable, and environmentally friendly flame retardant for cotton. Cellulose 2016, 24, 1159–1170. [Google Scholar] [CrossRef]
- Kashyap, M.; Samadhiya, K.; Ghosh, A.; Anand, V.; Shirage, P.M.; Bala, K. Screening of microalgae for biosynthesis and optimization of Ag/AgCl nano hybrids having antibacterial effect. RSC Adv. 2019, 9, 25583–25591. [Google Scholar] [CrossRef] [PubMed]
- Panchal, P.; Meena, P.; Nehra, S.P. A rapid green synthesis of Ag/AgCl-NC photocatalyst for environmental applications. Environ. Sci. Pollut. Res. 2021, 28, 3972–3982. [Google Scholar] [CrossRef] [PubMed]
- Krishnamoorthy, K.; Navaneethaiyer, U.; Mohan, R.; Lee, J.; Kim, S.-J. Graphene oxide nanostructures modified multifunctional cotton fabrics. Appl. Nanosci. 2011, 2, 119–126. [Google Scholar] [CrossRef]
- Wiley, B.J.; Im, S.H.; Li, Z.-Y.; McLellan, J.; Siekkinen, A.; Xia, Y. Maneuvering the Surface Plasmon Resonance of Silver Nanostructures through Shape-Controlled Synthesis. J. Phys. Chem. B 2006, 110, 15666–15675. [Google Scholar] [CrossRef]
- Makuła, P.; Pacia, M.; Macyk, W. How To Correctly Determine the Band Gap Energy of Modified Semiconductor Photocatalysts Based on UV–Vis Spectra. J. Phys. Chem. Lett. 2018, 9, 6814. [Google Scholar] [CrossRef]
- Liu, Q.; Zhang, Y.; Li, A.; Ren, E.; Cui, C.; Zhou, M.; Guo, R.; Xiao, H.; Jiang, S.; Qin, W. Reduced graphene oxide-coated carbonized cotton fabric wearable strain sensors with ultralow detection limit. J. Mater. Sci. Mater. Electron. 2020, 31, 17233–17248. [Google Scholar] [CrossRef]
- Palme, A.; Theliander, H.; Brelid, H. Acid hydrolysis of cellulosic fibres: Comparison of bleached kraft pulp, dissolving pulps and cotton textile cellulose. Carbohydr. Polym. 2016, 136, 1281–1287. [Google Scholar] [CrossRef]
- Ni, H.; Zeng, S.; Wu, J.; Cheng, X.; Luo, T.; Wang, W.; Zeng, W.; Chen, Y. Cellulose nanowhiskers: Preparation, characterization and cytotoxicity evaluation. Bio-Medical Mater. Eng. 2012, 22, 121–127. [Google Scholar] [CrossRef]
- Rajavel, K.; Gomathi, R.; Pandian, R.; Kumar, R.T.R. In situ attachment and its hydrophobicity of size- and shape-controlled silver nanoparticles on fabric surface for bioapplication. Inorg. Nano-Met. Chem. 2017, 47, 1196–1203. [Google Scholar] [CrossRef]
- Gao, L.; Shi, S.; Hou, W.; Wang, S.; Yan, Z.; Ge, C. NaOH/Urea Swelling Treatment and Hydrothermal Degradation of Waste Cotton Fiber. J. Renew. Mater. 2020, 8, 703–713. [Google Scholar] [CrossRef]
- Wang, J.; An, C.; Zhang, M.; Qin, C.; Ming, X.; Zhang, Q. Photochemical conversion of AgCl nanocubes to hybrid AgCl–Ag nanoparticles with high activity and long-term stability towards photocatalytic degradation of organic dyes. Can. J. Chem. 2012, 90, 858–864. [Google Scholar] [CrossRef]
- Fan, Z.; Di, L.; Zhang, X.; Wang, H. A Surface Dielectric Barrier Discharge Plasma for Preparing Cotton-Fabric-Supported Silver Nanoparticles. Nanomaterials 2019, 9, 961. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Xiang, Z.; Cao, D.; Liu, C.-J. Covalent organic polymer supported palladium catalysts for CO oxidation. Chem. Commun. 2013, 49, 5633–5635. [Google Scholar] [CrossRef]
- Pandi, N.; Sonawane, S.H.; Kishore, K.A. Synthesis of cellulose nanocrystals (CNCs) from cotton using ultrasound-assisted acid hydrolysis. Ultrason. Sonochem. 2020, 70, 105353. [Google Scholar] [CrossRef]
- Nam, S.; French, A.D.; Condon, B.D.; Concha, M. Segal crystallinity index revisited by the simulation of X-ray diffraction patterns of cotton cellulose Iβ and cellulose II. Carbohydr. Polym. 2016, 135, 1–9. [Google Scholar] [CrossRef]
- Barbour, J. Wood formation and properties. In Wood Quality; Burley, J., Ed.; Elsevier Amsterdam: Oxford, UK, 2004; pp. 1840–1846. [Google Scholar] [CrossRef]
- Peng, P.; Zhang, Z.; Yang, J.; Wu, M.; Wu, Q.; Liu, J.; Zhang, J. Grafted antimicrobial cotton fabrics with N-halamine groups via atom transfer radical polymerization. Cellulose 2021, 28, 8855–8866. [Google Scholar] [CrossRef]



| Samples | Stage II | Residual Mass at 600 °C, % | |
|---|---|---|---|
| DTGmax | Tonset | ||
| Cotton-OH | 351.0 | 313.0 | 18.00 |
| Cotton-(PO(OH)2)(Cl) | 314.8 | 285.0 | 24.74 |
| Cotton˗OH-Ag | 365.8 | 341.0 | 10.18 |
| Cotton-(PO(OH)2)(Cl)-Ag | 357.8 | 319.0 | 19.76 |
| Samples | Cotton-OH (Blank) | Cotton-OH-Ag | Cotton-(PO(OH)2)(Cl) | Cotton-(PO(OH)2)(Cl)-Ag |
|---|---|---|---|---|
| Escherichia coli | 0 | 27 | 8 | 150 |
| Staphylococcus aureus | 0 | 48 | 10 | 150 |
| Candida albicans | 0 | 24 | 26 | 150 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alosmanov, R.; Buniyat-zadeh, I.; Soylak, M.; Shukurov, A.; Aliyeva, S.; Turp, S.; Guliyeva, G. Design, Structural Characteristic and Antibacterial Performance of Silver-Containing Cotton Fiber Nanocomposite. Bioengineering 2022, 9, 770. https://doi.org/10.3390/bioengineering9120770
Alosmanov R, Buniyat-zadeh I, Soylak M, Shukurov A, Aliyeva S, Turp S, Guliyeva G. Design, Structural Characteristic and Antibacterial Performance of Silver-Containing Cotton Fiber Nanocomposite. Bioengineering. 2022; 9(12):770. https://doi.org/10.3390/bioengineering9120770
Chicago/Turabian StyleAlosmanov, Rasim, Irada Buniyat-zadeh, Mustafa Soylak, Azad Shukurov, Solmaz Aliyeva, Sinan Turp, and Gulnara Guliyeva. 2022. "Design, Structural Characteristic and Antibacterial Performance of Silver-Containing Cotton Fiber Nanocomposite" Bioengineering 9, no. 12: 770. https://doi.org/10.3390/bioengineering9120770
APA StyleAlosmanov, R., Buniyat-zadeh, I., Soylak, M., Shukurov, A., Aliyeva, S., Turp, S., & Guliyeva, G. (2022). Design, Structural Characteristic and Antibacterial Performance of Silver-Containing Cotton Fiber Nanocomposite. Bioengineering, 9(12), 770. https://doi.org/10.3390/bioengineering9120770






