Scaffold Fabrication Techniques of Biomaterials for Bone Tissue Engineering: A Critical Review
Abstract
1. Introduction
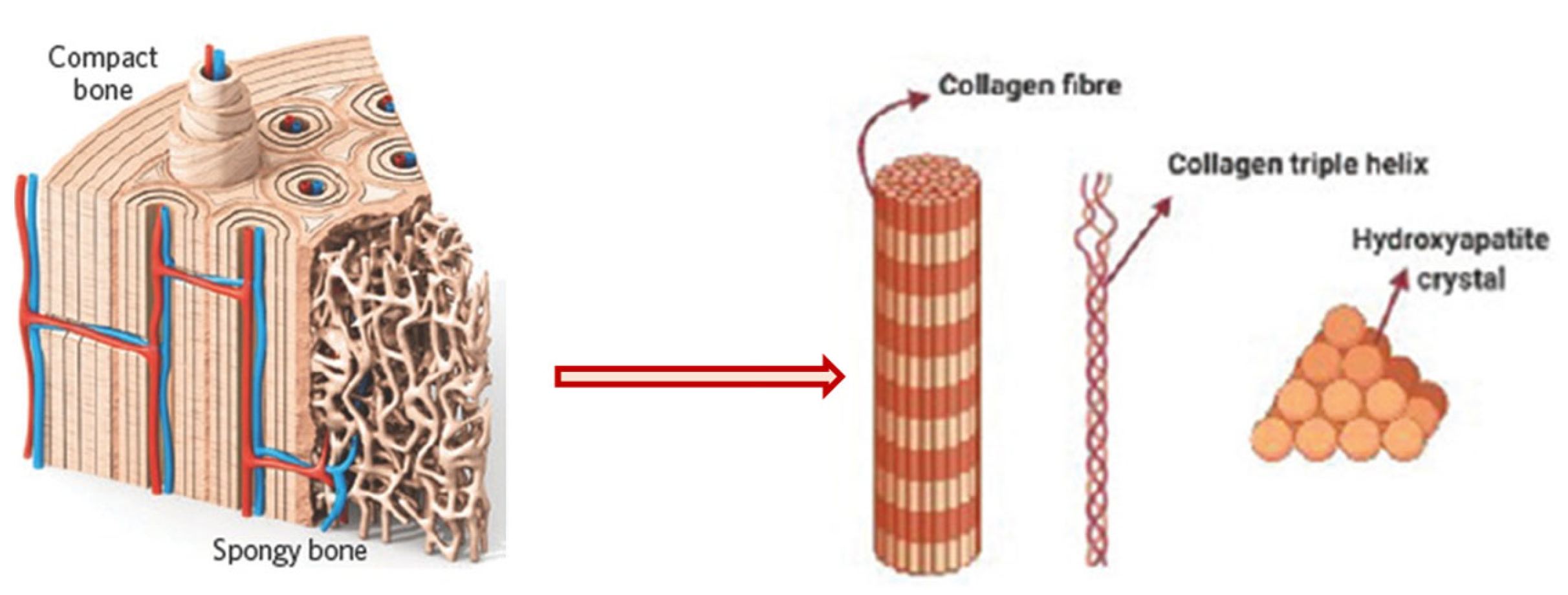
2. Conventional Methods
2.1. Solvent Casting
2.2. Freeze-Drying Method
2.3. Hydrogels
2.4. Cryogel Formation
2.5. Phase Separation Method
2.6. Gas Foaming Method

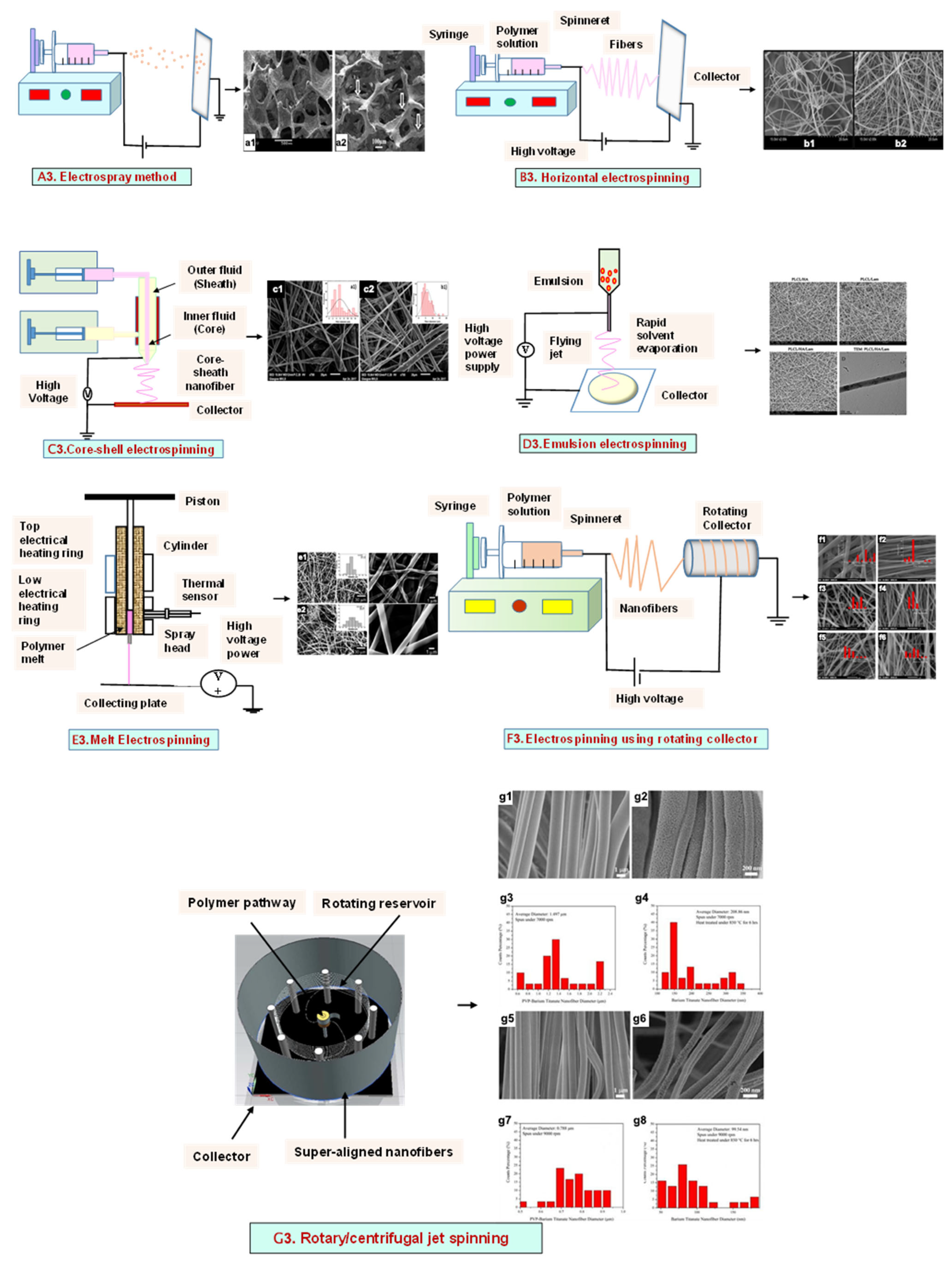
3. Electrohydrodynamic Technique
3.1. Electrospray Technique
3.2. Electrospinning
3.2.1. Horizontal Electrospinning
3.2.2. Core–Shell Electrospinning
3.2.3. Emulsion Electrospinning
3.2.4. Melt Electrospinning
3.2.5. Rotating Collector Electrospinning
3.2.6. Rotary/Centrifugal Jet Spinning
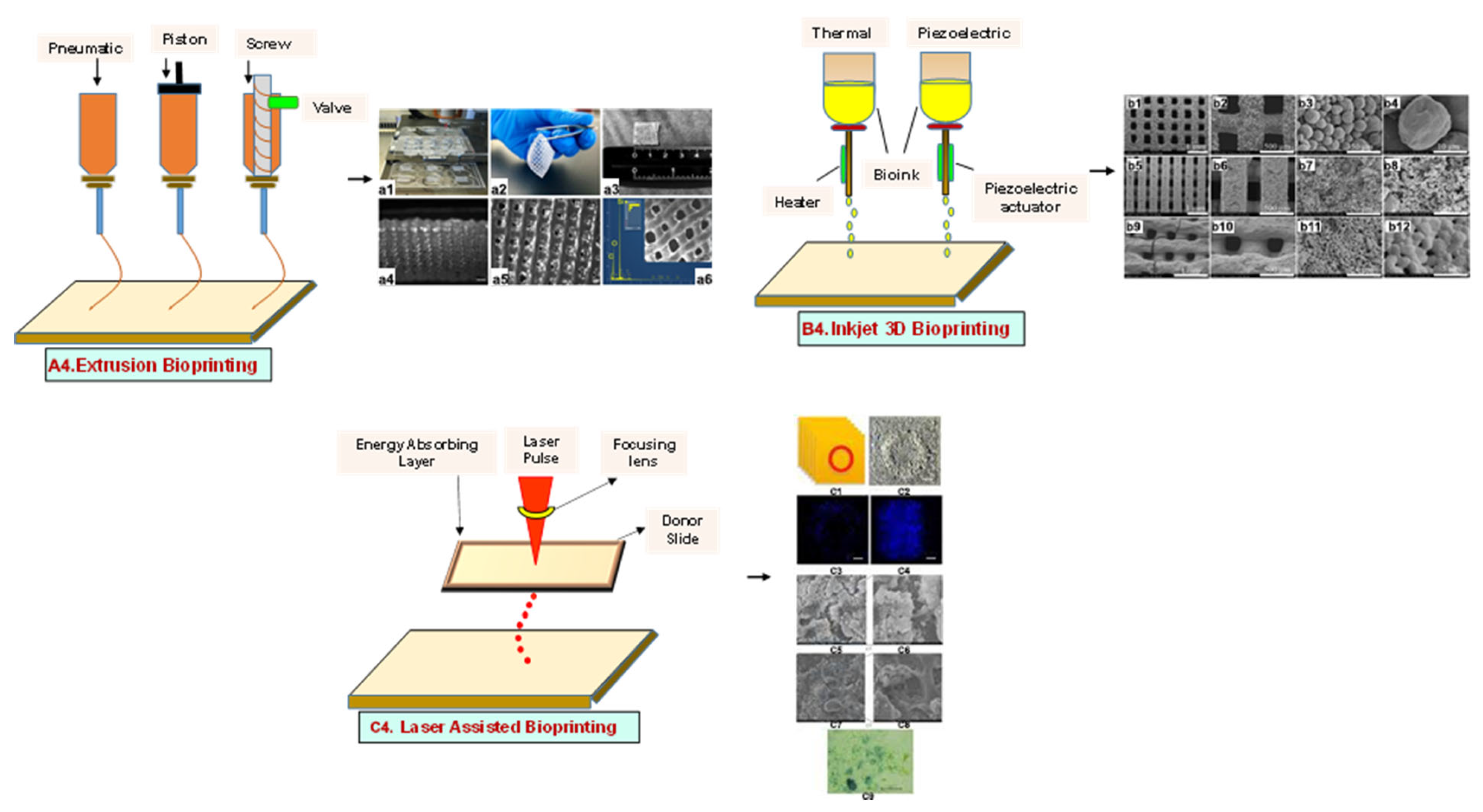
4. Additive Manufacturing (AM) Techniques
4.1. Three-Dimensional Printing
4.1.1. Extrusion-Based Bioprinting
4.1.2. Inkjet Bioprinting
4.1.3. Laser-Assisted Bioprinting
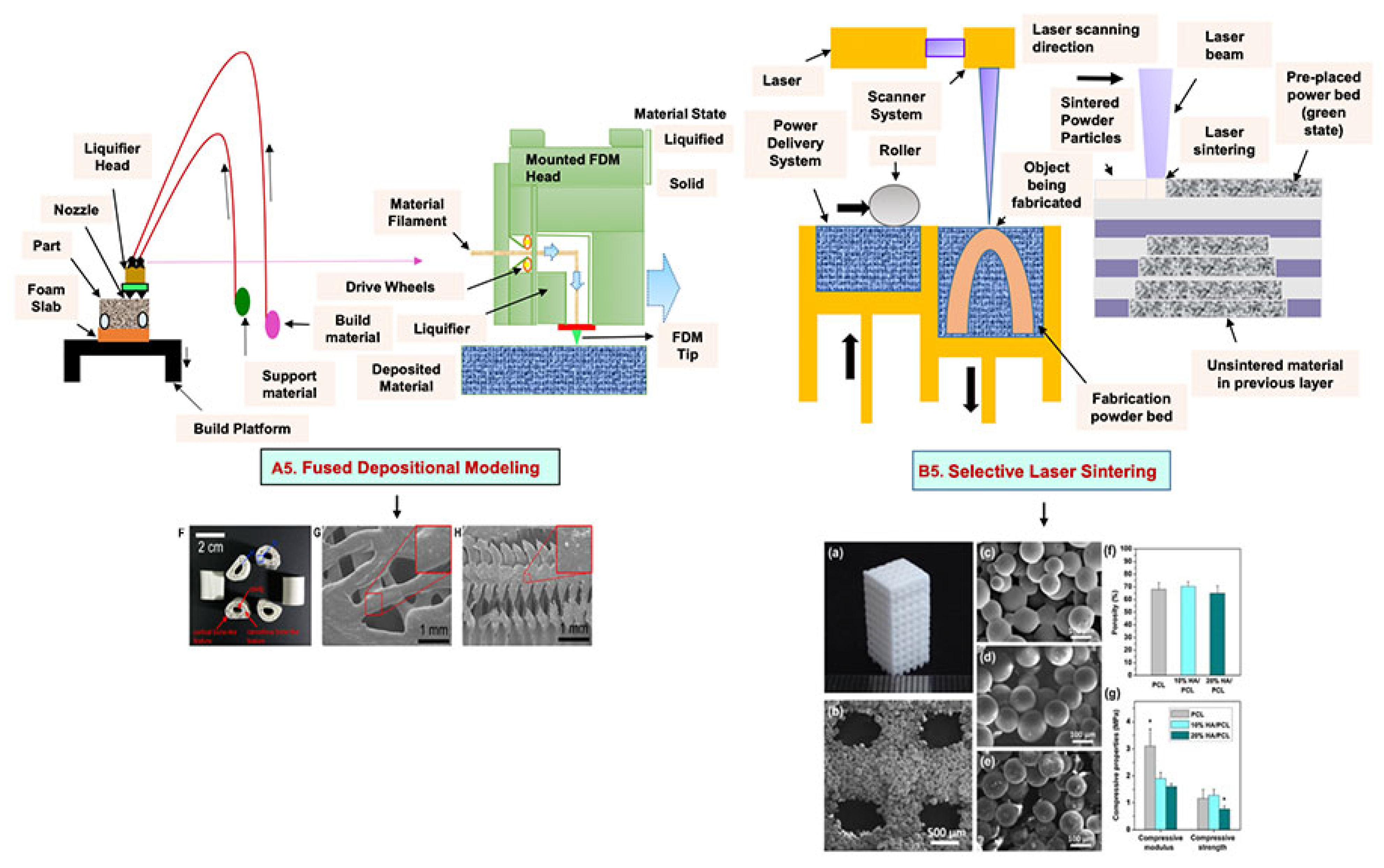
4.2. Fused Deposition Modeling
4.3. Selective Laser Sintering
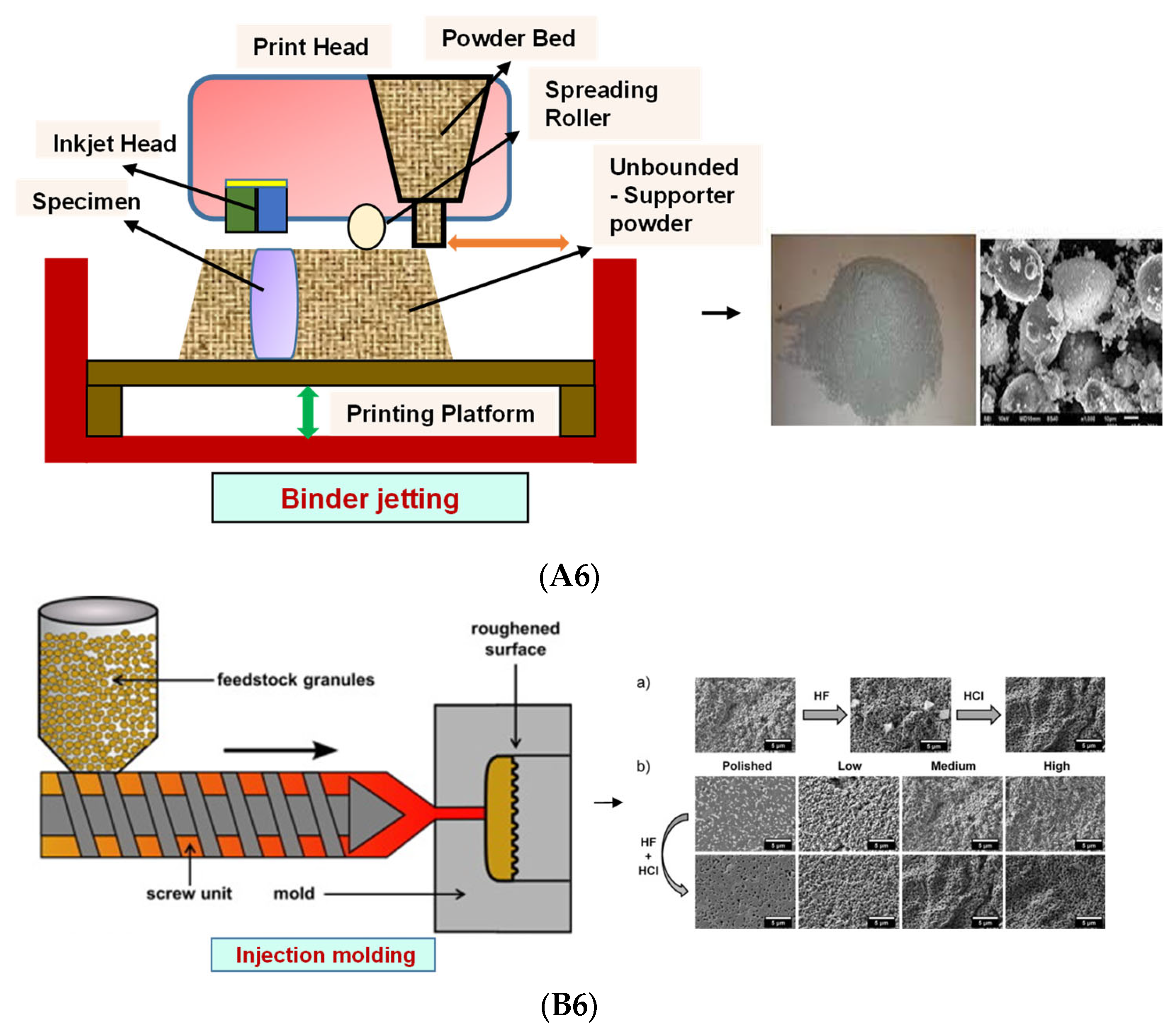
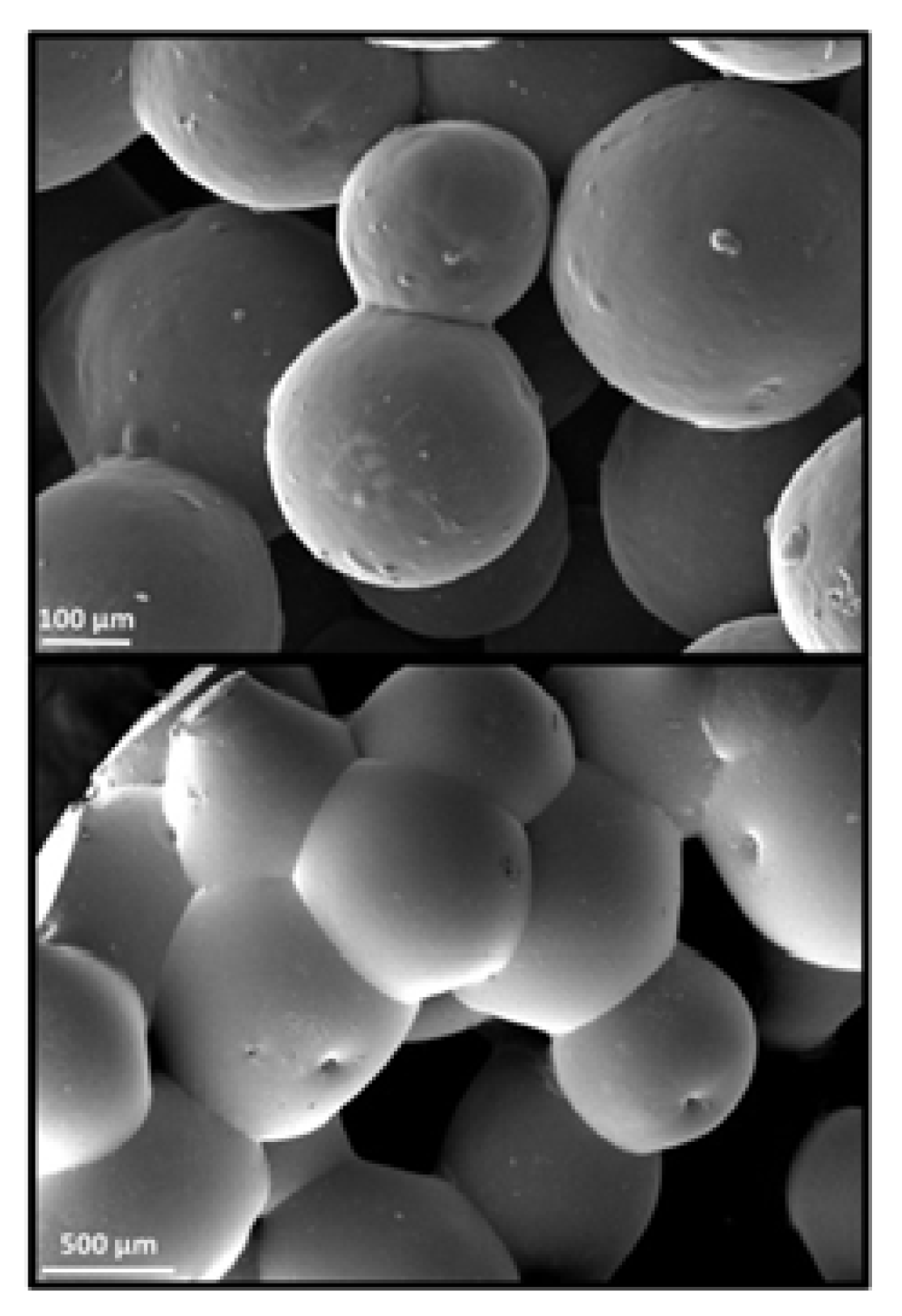
4.4. Binder Jetting
4.5. Injection Molding
5. Photolithography
5.1. Stereolithography Technique
5.2. Digital Light Processing
5.3. Continuous Liquid Interface Production/Digital Light Synthesis
5.4. Two-Photon Polymerization
5.5. Multiphoton Polymerization/Multiphoton Lithography

6. Microsphere-Based Sintering Method
7. Four-Dimensional Printing
8. Applications in Bone Tissue Engineering
8.1. Conventional Methods
8.2. Electrohydrodynamic Methods
8.3. Additive Manufacturing Method
8.4. Others
9. Conclusions and Future Perspective
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- McMahon, R.E.; Wang, L.; Skoracki, R.; Mathur, A.B. Development of nanomaterials for bone repair and regeneration. J. Biomed. Mater. Res. Part B Appl. Biomater. 2013, 101B, 387–397. [Google Scholar] [CrossRef]
- Reznikov, N.; Shahar, R.; Weiner, S. Three-dimensional structure of human lamellar bone: The presence of two different materials and new insights into the hierarchical organization. Bone 2014, 59, 93–104. [Google Scholar] [CrossRef]
- Bose, S.; Vahabzadeh, S.; Bandyopadhyay, A. Bone tissue engineering using 3D printing. Mater. Today 2013, 16, 496–504. [Google Scholar] [CrossRef]
- Buckwalter, J.A.; Glimcher, M.J.; Cooper, R.R.; Recker, R. Bone Biology. J. Bone Jt. Surg. 1995, 77, 1276–1289. [Google Scholar] [CrossRef]
- Remedios, A. Bone and bone healing. Vet. Clin. N. Am. Small Anim. Pract. 1999, 29, 1029–1044. [Google Scholar] [CrossRef]
- Väänänen, H.K.; Zhao, H.; Mulari, M.; Halleen, J.M. The cell biology of osteoclast function. J. Cell Sci. 2000, 113, 377–381. [Google Scholar] [CrossRef]
- Markel, M.D. Bone structure and the response of bone to stress. Equine Fract. Repair 2019, 3–11. [Google Scholar] [CrossRef]
- Liu, Y.; Luo, D.; Wang, T. Hierarchical Structures of Bone and Bioinspired Bone Tissue Engineering. Small 2016, 12, 4611–4632. [Google Scholar] [CrossRef]
- Marenzana, M.; Arnett, T.R. The Key Role of the Blood Supply to Bone. Bone Res. 2013, 1, 203–215. [Google Scholar] [CrossRef]
- Campana, V.; Milano, G.; Pagano, E.; Barba, M.; Cicione, C.; Salonna, G.; Lattanzi, W.; Logroscino, G. Bone substitutes in orthopaedic surgery: From basic science to clinical practice. J. Mater. Sci. Mater. Med. 2014, 25, 2445–2461. [Google Scholar] [CrossRef]
- Amini, A.R.; Laurencin, C.T.; Nukavarapu, S.P. Bone Tissue Engineering: Recent Advances and Challenges. Crit. Rev. Biomed. Eng. 2012, 40, 363–408. [Google Scholar] [CrossRef] [PubMed]
- Katari, R.S.; Peloso, A.; Orlando, G. Tissue engineering. Adv. Surg. 2014, 48, 137–154. [Google Scholar] [CrossRef] [PubMed]
- Feng, S.; He, F.; Ye, J. Hierarchically porous structure, mechanical strength and cell biological behaviors of calcium phosphate composite scaffolds prepared by combination of extrusion and porogen burnout technique and enhanced by gelatin. Mater. Sci. Eng. C 2018, 82, 217–224. [Google Scholar] [CrossRef] [PubMed]
- Johnson, T.; Bahrampourian, R.; Patel, A.; Mequanint, K. Fabrication of highly porous tissue-engineering scaffolds using selective spherical porogens. Biomed. Mater. Eng. 2010, 20, 107–118. [Google Scholar] [CrossRef] [PubMed]
- Liao, C.J.; Chen, C.F.; Chen, J.H.; Chiang, S.F.; Lin, Y.J.; Chang, K.Y. Fabrication of porous biodegradable polymer scaffolds using a solvent merging/particulate leaching method. J. Biomed. Mater. Res. 2002, 59, 676–681. [Google Scholar] [CrossRef]
- Yashaswini, Y.D.; Prabhu, A.; Anil, S.; Venkatesan, J. Preparation and characterization of dexamethasone loaded sodium alginate-graphene oxide microspheres for bone tissue engineering. J. Drug Deliv. Sci. Technol. 2021, 64, 102624. [Google Scholar] [CrossRef]
- Pikal, M.J.; Shah, S.; Roy, M.L.; Putman, R. The secondary drying stage of freeze drying: Drying kinetics as a function of temperature and chamber pressure. Int. J. Pharm. 1990, 60, 203–207. [Google Scholar] [CrossRef]
- Liapis, A.I.; Bruttini, R. A theory for the primary and secondary drying stages of the freeze-drying of pharmaceutical crystalline and amorphous solutes: Comparison between experimental data and theory. Sep. Technol. 1994, 4, 144–155. [Google Scholar] [CrossRef]
- Singh, S.; Dutt, D.; Kaur, P.; Singh, H.; Mishra, N.C. Microfibrous paper scaffold for tissue engineering application. J. Biomater. Sci. Polym. Ed. 2020, 31, 1091–1106. [Google Scholar] [CrossRef]
- Singh, S.; Dutt, D.; Mishra, N.C. Cotton pulp for bone tissue engineering. J. Biomater. Sci. Polym. Ed. 2020, 31, 2094–2113. [Google Scholar] [CrossRef]
- Sainitya, R.; Sriram, M.; Kalyanaraman, V.; Dhivya, S.; Saravanan, S.; Vairamani, M.; Sastry, T.P.; Selvamurugan, N. Scaffolds containing chitosan/carboxymethyl cellulose/mesoporous wollastonite for bone tissue engineering. Int. J. Biol. Macromol. 2015, 80, 481–488. [Google Scholar] [CrossRef]
- Lu, T.; Li, Y.; Chen, T. Techniques for fabrication and construction of three-dimensional scaffolds for tissue engineering. Int. J. Nanomed. 2013, 8, 337–350. [Google Scholar] [CrossRef]
- Qian, L.; Zhang, H. Controlled freezing and freeze drying: A versatile route for porous and micro-/nano-structured materials. J. Chem. Technol. Biotechnol. 2011, 86, 172–184. [Google Scholar] [CrossRef]
- Whang, K.; Thomas, C.H.; Healy, K.E.; Nuber, G. A novel method to fabricate bioabsorbable scaffolds. Polymer 1995, 36, 837–842. [Google Scholar] [CrossRef]
- Turnbull, G.; Clarke, J.; Picard, F.; Riches, P.; Jia, L.; Han, F.; Li, B.; Shu, W. 3D bioactive composite scaffolds for bone tissue engineering. Bioact. Mater. 2018, 3, 278–314. [Google Scholar] [CrossRef]
- Ahmed, E.M. Hydrogel: Preparation, characterization, and applications: A review. J. Adv. Res. 2015, 6, 105–121. [Google Scholar] [CrossRef]
- Castilho, M.; de Ruijter, M.; Beirne, S.; Villette, C.C.; Ito, K.; Wallace, G.G.; Malda, J. Multitechnology Biofabrication: A New Approach for the Manufacturing of Functional Tissue Structures? Trends Biotechnol. 2020, 38, 1316–1328. [Google Scholar] [CrossRef]
- Kumar, H.; Sakthivel, K.; Mohamed, M.G.A.; Boras, E.; Shin, S.R.; Kim, K. Designing Gelatin Methacryloyl (GelMA)-Based Bioinks for Visible Light Stereolithographic 3D Biofabrication. Macromol. Biosci. 2021, 21, 2000317. [Google Scholar] [CrossRef]
- Kirschner, C.M.; Anseth, K.S. Hydrogels in healthcare: From static to dynamic material microenvironments. Acta Mater. 2013, 61, 931–944. [Google Scholar] [CrossRef]
- Lee, K.Y.; Mooney, D.J. Hydrogels for tissue engineering. Chem. Rev. 2001, 101, 1869–1879. [Google Scholar] [CrossRef]
- Zhang, H.B.; Xing, T.L.; Yin, R.X.; Shi, Y.; Yang, S.M.; Zhang, W.J. Three-dimensional bioprinting is not only about cell-laden structures. Chin. J. Traumatol. 2016, 19, 187–192. [Google Scholar] [CrossRef] [PubMed]
- Kumar, A.; Mishra, R.; Reinwald, Y.; Bhat, S. Cryogels: Freezing unveiled by thawing. Mater. Today 2010, 13, 42–44. [Google Scholar] [CrossRef]
- Rodrigues, S.C.; Salgado, C.L.; Sahu, A.; Garcia, M.P.; Fernandes, M.H.; Monteiro, F.J. Preparation and characterization of collagen-nanohydroxyapatite biocomposite scaffolds by cryogelation method for bone tissue engineering applications. J. Biomed. Mater. Res. Part A 2013, 101A, 1080–1094. [Google Scholar] [CrossRef] [PubMed]
- Guillen, G.R.; Pan, Y.; Li, M.; Hoek, E.M.V. Preparation and characterization of membranes formed by nonsolvent induced phase separation: A review. Ind. Eng. Chem. Res. 2011, 50, 3798–3817. [Google Scholar] [CrossRef]
- Jung, J.T.; Kim, J.F.; Wang, H.H.; di Nicolo, E.; Drioli, E.; Lee, Y.M. Understanding the non-solvent induced phase separation (NIPS) effect during the fabrication of microporous PVDF membranes via thermally induced phase separation (TIPS). J. Memb. Sci. 2016, 514, 250–263. [Google Scholar] [CrossRef]
- Mi, H.Y.; Jing, X.; Turng, L.S. Fabrication of porous synthetic polymer scaffolds for tissue engineering. J. Cell. Plast. 2014, 51, 165–196. [Google Scholar] [CrossRef]
- Nam, Y.S.; Yoon, J.J.; Park, T.G. A novel fabrication method of macroporous biodegradable polymer scaffolds using gas foaming salt as a porogen additive. J. Biomed. Mater. Res. 2000, 53, 1–7. [Google Scholar] [CrossRef]
- Di Maio, E.; Mensitieri, G.; Iannace, S.; Nicolais, L.; Li, W.; Flumerfelt, R.W. Structure optimization of polycaprolactone foams by using mixtures of CO 2 and N 2 as blowing agents. Polym. Eng. Sci. 2005, 45, 432–441. [Google Scholar] [CrossRef]
- Barbetta, A.; Rizzitelli, G.; Bedini, R.; Pecci, R.; Dentini, M. Porous gelatin hydrogels by gas-in-liquid foam templating. Soft Matter 2010, 6, 1785–1792. [Google Scholar] [CrossRef]
- Zhu, N.; Che, X. Biofabrication of Tissue Scaffolds. Adv. Biomater. Sci. Biomed. Appl. 2013, 12, 315–328. [Google Scholar] [CrossRef]
- Barbetta, A.; Gumiero, A.; Pecci, R.; Bedini, R.; Dentini, M. Gas-in-liquid foam templating as a method for the production of highly porous scaffolds. Biomacromolecules 2009, 10, 3188–3192. [Google Scholar] [CrossRef]
- Quirk, R.A.; France, R.M.; Shakesheff, K.M.; Howdle, S.M. Supercritical fluid technologies and tissue engineering scaffolds. Curr. Opin. Solid State Mater. Sci. 2004, 8, 313–321. [Google Scholar] [CrossRef]
- Thadavirul, N.; Pavasant, P.; Supaphol, P. Development of polycaprolactone porous scaffolds by combining solvent casting, particulate leaching, and polymer leaching techniques for bone tissue engineering. J. Biomed. Mater. Res. Part A 2014, 102, 3379–3392. [Google Scholar] [CrossRef]
- Stokols, S.; Tuszynski, M.H. Freeze-dried agarose scaffolds with uniaxial channels stimulate and guide linear axonal growth following spinal cord injury. Biomaterials 2006, 27, 443–451. [Google Scholar] [CrossRef]
- Shahbazarab, Z.; Teimouri, A.; Chermahini, A.N.; Azadi, M. Fabrication and characterization of nanobiocomposite scaffold of zein/chitosan/nanohydroxyapatite prepared by freeze-drying method for bone tissue engineering. Int. J. Biol. Macromol. 2018, 108, 1017–1027. [Google Scholar] [CrossRef]
- Saekhor, K.; Udomsinprasert, W.; Honsawek, S.; Tachaboonyakiat, W. Preparation of an injectable modified chitosan-based hydrogel approaching for bone tissue engineering. Int. J. Biol. Macromol. 2019, 123, 167–173. [Google Scholar] [CrossRef]
- Bencherif, S.A.; Braschler, T.M.; Renaud, P. Advances in the design of macroporous polymer scaffolds for potential applications in dentistry. J. Periodontal Implant Sci. 2013, 43, 251–261. [Google Scholar] [CrossRef]
- Wu, S.; Ma, S.; Zhang, C.; Cao, G.; Wu, D.; Gao, C.; Lakshmanan, S. Cryogel biocomposite containing chitosan-gelatin/cerium–zinc doped hydroxyapatite for bone tissue engineering. Saudi J. Biol. Sci. 2020, 27, 2638–2644. [Google Scholar] [CrossRef]
- Seok, J.M.; Rajangam, T.; Jeong, J.E.; Cheong, S.; Joo, S.M.; Oh, S.J.; Shin, H.; Kim, S.H.; Park, S.A. Fabrication of 3D plotted scaffold with microporous strands for bone tissue engineering. J. Mater. Chem. B R. Soc. Chem. 2020, 8, 951–960. [Google Scholar] [CrossRef]
- Akbarzadeh, R.; Yousefi, A.M. Effects of processing parameters in thermally induced phase separation technique on porous architecture of scaffolds for bone tissue engineering. J. Biomed. Mater. Res. Part B Appl. Biomater. 2014, 102, 1304–1315. [Google Scholar] [CrossRef]
- Catanzano, O.; Soriente, A.; La Gatta, A.; Cammarota, M.; Ricci, G.; Fasolino, I.; Schiraldi, C.; Ambrosio, L.; Malinconico, M.; Laurienzo, P.; et al. Macroporous alginate foams crosslinked with strontium for bone tissue engineering. Carbohydr. Polym. 2018, 202, 72–83. [Google Scholar] [CrossRef] [PubMed]
- Webster Thomas, J. Nanotechnology for the Regeneration of Hard and Soft Tissues; World Scientific: Hackensack, NJ, USA, 2010; ISBN 9789812779656. [Google Scholar]
- Papadimitriou, L.; Manganas, P.; Ranella, A.; Stratakis, E. Biofabrication for neural tissue engineering applications. Mater. Today Bio 2020, 6, 100043. [Google Scholar] [CrossRef] [PubMed]
- Vijayavenkataraman, S. Nerve guide conduits for peripheral nerve injury repair: A review on design, materials and fabrication methods. Acta Biomater. 2020, 106, 54–69. [Google Scholar] [CrossRef] [PubMed]
- Reneker, D.H.; Yarin, A.L. Electrospinning jets and polymer nanofibers. Polymer 2008, 49, 2387–2425. [Google Scholar] [CrossRef]
- Deitzel, J.M.; Kleinmeyer, J.; Harris, D.; Beck Tan, N.C. The effect of processing variables on the morphology of electrospun. Polymer 2001, 42, 261–272. [Google Scholar] [CrossRef]
- Chen, D.R.; Pui, D.Y.H.; Kaufman, S.L. Electrospraying of conducting liquids for monodisperse aerosol generation in the 4 nm to 1.8 μm diameter range. J. Aerosol Sci. 1995, 26, 963–977. [Google Scholar] [CrossRef]
- Sung, K.; Lee, C.S. Factors influencing liquid breakup in electrohydrodynamic atomization. J. Appl. Phys. 2004, 96, 3956–3961. [Google Scholar] [CrossRef]
- Hartman, R.P.A.; Brunner, D.J.; Camelot, D.M.A.; Marijnissen, J.C.M.; Scarlett, B. Electrohydrodynamic atomization in the cone-jet mode physical modeling of the liquid cone and jet. J. Aerosol Sci. 1999, 30, 823–849. [Google Scholar] [CrossRef]
- Yao, J.; Kuang Lim, L.; Xie, J.; Hua, J.; Wang, C.H. Characterization of electrospraying process for polymeric particle fabrication. J. Aerosol Sci. 2008, 39, 987–1002. [Google Scholar] [CrossRef]
- Kim, G.H.; Park, J.H. A PMMA optical diffuser fabricated using an electrospray method. Appl. Phys. A Mater. Sci. Process. 2007, 86, 347–351. [Google Scholar] [CrossRef]
- Huang, Z.M.; Zhang, Y.Z.; Kotaki, M.; Ramakrishna, S. A review on polymer nanofibers by electrospinning and their applications in nanocomposites. Compos. Sci. Technol. 2003, 63, 2223–2253. [Google Scholar] [CrossRef]
- Munir, M.W.; Ali, U. Classification of Electrospinning Methods, Nanorods and Nanocomposites; IntechOpen: London, UK, 2020; Available online: https://books.google.co.in/books?hl=en&lr=&id=a0P8DwAAQBAJ&oi=fnd&pg=PA229&dq=Munir,+M.W.%3B+Ali,+U.+Classification+of+Electrospinning+Methods,+Nanorods+and+Nanocomposites%3B+IntechOpen+:+2020&ots=5NQEMM5Lh5&sig=QqN3DuEsTZRM-bzNQW8uTABbaLw&redir_esc=y#v=onepage&q&f=false,2020 (accessed on 19 September 2022).
- Sir, B.Y.; Taylor, G. Disintegration of Water Drops in an Electric Field. 1964. Available online: http://rspa.royalsocietypublishing.org/content/royprsa/280/1382/383.full.pdf (accessed on 19 September 2022).
- Rezvani, Z.; Venugopal, J.R.; Urbanska, A.M.; Mills, D.K.; Ramakrishna, S.; Mozafari, M. A bird’s eye view on the use of electrospun nanofibrous scaffolds for bone tissue engineering: Current state-of-the-art, emerging directions and future trends. Nanomed. Nanotechnol. Biol. Med. 2016, 12, 2181–2200. [Google Scholar] [CrossRef] [PubMed]
- Sun, Z.; Zussman, E.; Yarin, A.L.; Wendorff, J.H.; Greiner, A. Compound Core-Shell Polymer Nanofibers by Co-Electrospinning. Adv. Mater. 2003, 15, 1929–1932. [Google Scholar] [CrossRef]
- Sruthi, R.; Balagangadharan, K.; Selvamurugan, N. Polycaprolactone/polyvinylpyrrolidone coaxial electrospun fibers containing veratric acid-loaded chitosan nanoparticles for bone regeneration. Colloids Surf. B Biointerfaces 2020, 193, 111110. [Google Scholar] [CrossRef] [PubMed]
- Xie, X.; Chen, Y.; Wang, X.; Xu, X.; Shen, Y.; ur Rehman Khan, A.; Aldalbahi, A.; Fetz, A.E.; Bowlin, G.L.; El-Newehy, M.; et al. Electrospinning nanofiber scaffolds for soft and hard tissue regeneration. J. Mater. Sci. Technol. 2020, 59, 243–261. [Google Scholar] [CrossRef]
- Begum, H.A.; Khalilur, M.; Khan, R. Study on the Various Types of Needle Based and Needleless Electrospinning System for Nanofiber Production. Int. J. Text. Sci. 2017, 2017, 110–117. [Google Scholar] [CrossRef]
- Khang, A.; Ravishankar, P.; Krishnaswamy, A.; Anderson, P.K.; Cone, S.G.; Liu, Z.; Qian, X.; Balachandran, K. Engineering anisotropic biphasic Janus-type polymer nanofiber scaffold networks via centrifugal jet spinning. J. Biomed. Mater. Res. Part B Appl. Biomater. 2017, 105, 2455–2464. [Google Scholar] [CrossRef]
- Spano, F.; Quarta, A.; Martelli, C.; Ottobrini, L.; Rossi, R.M.; Gigli, G.; Blasi, L. Fibrous scaffolds fabricated by emulsion electrospinning: From hosting capacity to in vivo biocompatibility. Nanoscale 2016, 8, 9293–9303. [Google Scholar] [CrossRef]
- Wang, C.; Tong, S.N.; Tse, Y.H.; Wang, M. Conventional electrospinning vs. emulsion electrospinning: A comparative study on the development of nanofibrous drug/biomolecule delivery vehicles. Adv. Mater. Res. 2012, 410, 118–121. [Google Scholar] [CrossRef]
- Dalton, P.D.; Klinkhammer, K.; Salber, J.; Klee, D.; Möller, M. Direct in vitro electrospinning with polymer melts. Biomacromolecules 2006, 7, 686–690. [Google Scholar] [CrossRef]
- Dalton, P.D.; Joergensen, N.T.; Groll, J.; Moeller, M. Patterned melt electrospun substrates for tissue engineering. Biomed. Mater. 2008, 3, 034109. [Google Scholar] [CrossRef]
- Rogalski, J.J.; Botto, L.; Bastiaansen, C.W.M.; Peijs, T. A study of rheological limitations in rotary jet spinning of polymer nanofibers through modeling and experimentation. J. Appl. Polym. Sci. 2020, 137, 48963. [Google Scholar] [CrossRef]
- Nerurkar, N.L.; Han, W.; Mauck, R.L.; Elliott, D.M. Homologous structure-function relationships between native fibrocartilage and tissue engineered from MSC-seeded nanofibrous scaffolds. Biomaterials 2011, 32, 461–468. [Google Scholar] [CrossRef]
- Koepsell, L.; Remund, T.; Bao, J.; Neufeld, D.; Fong, H.; Deng, Y. Tissue engineering of annulus fibrosus using electrospun fibrous scaffolds with aligned polycaprolactone fibers. J. Biomed. Mater. Res. Part A 2011, 99A, 564–575. [Google Scholar] [CrossRef]
- Baker, B.M.; Mauck, R.L. The effect of nanofiber alignment on the maturation of engineered meniscus constructs. Biomaterials 2007, 28, 1967–1977. [Google Scholar] [CrossRef]
- Lin, F.R.; Kotha, S.P. Centrifugal jet spinning for highly efficient and large-scale fabrication of barium titanate nanofibers. Materials Letters 2014, 117, 153–157 . [Google Scholar] [CrossRef]
- López-Córdoba, A.; Castro, G.R.; Goyanes, S. Cellulose-containing scaffolds fabricated by electrospinning: Applicatioin tissue engineering and drug delivery. In Handbook of Composites from Renewable Materials; Wiley: New York, NY, USA, 2017; Volume 8, pp. 361–388. [Google Scholar] [CrossRef]
- Chen, Q.Z.; Boccaccini, A.R.; Zhang, H.B.; Wang, D.Z.; Edirisinghe, M.J. Improved mechanical reliability of bone tissue engineering (zirconia) scaffolds by electrospraying. J. Am. Ceram. Soc. 2006, 89, 1534–1539. [Google Scholar] [CrossRef]
- Rosman, N.; Salleh, W.N.W.; Mohamed, M.A.; Ismail, N.H.; Sazali, N.; Jaafar, J.; Hasbullah, H. Electrospun nanofiber-coated membrane: A review. J. Teknol. 2016, 78, 83–88. [Google Scholar] [CrossRef][Green Version]
- Chakraborty, P.K.; Adhikari, J.; Saha, P. Facile fabrication of electrospun regenerated cellulose nanofiber scaffold for potential bone-tissue engineering application. Int. J. Biol. Macromol. 2019, 122, 644–652. [Google Scholar] [CrossRef]
- Han, D.; Steckl, A.J. Coaxial Electrospinning Formation of Complex Polymer Fibers and their Applications. Chempluschem 2019, 84, 1453–1497. [Google Scholar] [CrossRef]
- Kareem, M.M.; Hodgkinson, T.; Sanchez, M.S.; Dalby, M.J. Hybrid Core—Shell Scaffolds for Bone Tissue Engineering; IOP Publishing Ltd.: Bristol, UK, 2019. [Google Scholar]
- Zhang, C.; Feng, F.; Zhang, H. Emulsion electrospinning: Fundamentals, food applications and prospects. Trends Food Sci. Technol. 2018, 80, 175–186. [Google Scholar] [CrossRef]
- Tian, L.; Prabhakaran, M.P.; Ding, X.; Ramakrishna, S. Biocompatibility evaluation of emulsion electrospun nanofibers using osteoblasts for bone tissue engineering. J. Biomater. Sci. Polym. Ed. 2013, 24, 1952–1968. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Zhao, F.; Zhang, C.; Zhang, J.; Yang, W. Solvent-free preparation of poly(lactic acid) fibers by melt electrospinning using an umbrella-like spray head and alleviation of the problematic thermal degradation. J. Serbian Chem. Soc. 2012, 77, 1071–1082. [Google Scholar] [CrossRef]
- Lee, H.; Ahn, S.; Choi, H.; Cho, D.; Kim, G. Fabrication, characterization, and in vitro biological activities of melt-electrospun PLA micro/nanofibers for bone tissue regeneration. J. Mater. Chem. B 2013, 1, 3670–3677. [Google Scholar] [CrossRef]
- Kadavil, H.; Zagho, M.; Elzatahry, A.; Altahtamouni, T. Sputtering of electrospun polymer-based nanofibers for biomedical applications: A perspective. Nanomaterials 2019, 9, 77. [Google Scholar] [CrossRef] [PubMed]
- Meng, Z.X.; Wang, Y.S.; Ma, C.; Zheng, W.; Li, L.; Zheng, Y.F. Electrospinning of PLGA/gelatin randomly-oriented and aligned nanofibers as potential scaffold in tissue engineering. Mater. Sci. Eng. C 2010, 30, 1204–1210. [Google Scholar] [CrossRef]
- Monzón, M.D.; Ortega, Z.; Martínez, A.; Ortega, F. Standardization in additive manufacturing: Activities carried out by international organizations and projects. Int. J. Adv. Manuf. Technol. 2015, 76, 1111–1121. [Google Scholar] [CrossRef]
- Jazayeri, H.E.; Rodriguez-Romero, M.; Razavi, M.; Tahriri, M.; Ganjawalla, K.; Rasoulianboroujeni, M.; Malekoshoaraie, M.H.; Khoshroo, K.; Tayebi, L. The cross-disciplinary emergence of 3D printed bioceramic scaffolds in orthopedic bioengineering. Ceram. Int. 2018, 44, 1–9. [Google Scholar] [CrossRef]
- Malda, J.; Visser, J.; Melchels, F.P.; Jüngst, T.; Hennink, W.E.; Dhert, W.J.A.; Groll, J.; Hutmacher, D.W. 25th anniversary article: Engineering hydrogels for biofabrication. Adv. Mater. 2013, 25, 5011–5028. [Google Scholar] [CrossRef]
- Bikas, H.; Stavropoulos, P.; Chryssolouris, G. Additive manufacturing methods and modeling approaches: A critical review. Int. J. Adv. Manuf. Technol. 2016, 83, 389–405. [Google Scholar] [CrossRef]
- Pati, F.; Jang, J.; Lee, J.W.; Cho, D.W. Extrusion Bioprinting. In Essentials of 3D Biofabrication and Translation; Elsevier Inc.: Amsterdam, The Netherlands, 2015; pp. 123–152. ISBN 9780128010150. [Google Scholar] [CrossRef]
- Bishop, E.S.; Mostafa, S.; Pakvasa, M.; Luu, H.H.; Lee, M.J.; Wolf, J.M.; Ameer, G.A.; He, T.C.; Reid, R.R. 3-D bioprinting technologies in tissue engineering and regenerative medicine: Current and future trends. Genes Dis. 2017, 4, 185–195. [Google Scholar] [CrossRef]
- Wijshoff, H. The dynamics of the piezo inkjet printhead operation. Phys. Rep. 2010, 491, 77–177. [Google Scholar] [CrossRef]
- Xu, T.; Jin, J.; Gregory, C.; Hickman, J.J.; Boland, T. Inkjet printing of viable mammalian cells. Biomaterials 2005, 26, 93–99. [Google Scholar] [CrossRef]
- Zhang, X.; Zhang, Y. Tissue Engineering Applications of Three-Dimensional Bioprinting. Cell Biochem. Biophys. 2015, 72, 777–782. [Google Scholar] [CrossRef]
- Gu, Z.; Fu, J.; Lin, H.; He, Y. Development of 3D bioprinting: From printing methods to biomedical applications. Asian J. Pharm. Sci. 2020, 15, 529–557. [Google Scholar] [CrossRef]
- Skardal, A.; Atala, A. Biomaterials for Integration with 3-D Bioprinting. Ann. Biomed. Eng. 2015, 43, 730–746. [Google Scholar] [CrossRef]
- Schiele, N.R.; Corr, D.T.; Huang, Y.; Raof, N.A.; Xie, Y.; Chrisey, D.B. Laser-based direct-write techniques for cell printing. Biofabrication 2010, 2, 032001. [Google Scholar] [CrossRef]
- Colina, M.; Serra, P.; Fernández-Pradas, J.M.; Sevilla, L.; Morenza, J.L. DNA deposition through laser induced forward transfer. Biosens. Bioelectron. 2005, 20, 1638–1642. [Google Scholar] [CrossRef]
- Guillotin, B.; Souquet, A.; Catros, S.; Duocastella, M.; Pippenger, B.; Bellance, S.; Bareille, R.; Rémy, M.; Bordenave, L.; Amédée, J.; et al. Laser assisted bioprinting of engineered tissue with high cell density and microscale organization. Biomaterials 2010, 31, 7250–7256. [Google Scholar] [CrossRef]
- Seol, Y.J.; Kang, H.W.; Lee, S.J.; Atala, A.; Yoo, J.J. Bioprinting technology and its applications. Eur. J. Cardio-Thorac. Surg. 2014, 46, 342–348. [Google Scholar] [CrossRef]
- Monavari, M.; Homaeigohar, S.; Fuentes-Chandía, M.; Nawaz, Q.; Monavari, M.; Venkatraman, A.; Boccaccini, A.R. 3D printing of alginate dialdehyde-gelatin (ADA-GEL) hydrogels incorporating phytotherapeutic icariin loaded mesoporous SiO2-CaO nanoparticles for bone tissue engineering. Mater. Sci. Eng. C 2021, 131, 112470. [Google Scholar] [CrossRef] [PubMed]
- Zhang, B.; Pei, X.; Song, P.; Sun, H.; Li, H.; Fan, Y.; Jiang, Q.; Zhou, C.; Zhang, X. Porous bioceramics produced by inkjet 3D printing: Effect of printing ink formulation on the ceramic macro and micro porous architectures control. Compos. Part B Eng. 2018, 155, 112–121. [Google Scholar] [CrossRef]
- Catros, S.; Fricain, J.C.; Guillotin, B.; Pippenger, B.; Bareille, R.; Remy, M.; Lebraud, E.; Desbat, B.; Amédée, J.; Guillemot, F. Laser-assisted bioprinting for creating on-demand patterns of human osteoprogenitor cells and nano-hydroxyapatite. Biofabrication 2011, 3, 025001. [Google Scholar] [CrossRef] [PubMed]
- Maina, R.M.; Barahona, M.J.; Finotti, M.; Lysyy, T.; Geibel, P.; D’Amico, F.; Mulligan, D.; Geibel, J.P. Generating vascular conduits: From tissue engineering to three-dimensional bioprinting. Innov. Surg. Sci. 2020, 3, 203–213. [Google Scholar] [CrossRef] [PubMed]
- Masood, S.H. Intelligent rapid prototyping with fused deposition modelling. Rapid Prototyp. J. 1996, 2, 24–33. [Google Scholar] [CrossRef]
- Boparai, K.S.; Singh, R. Advances in Fused Deposition Modeling. Ref. Modul. Mater. Sci. Mater. Eng. 2017, 1–10. [Google Scholar] [CrossRef]
- Ratheesh, G.; Venugopal, J.R.; Chinappan, A.; Ezhilarasu, H.; Sadiq, A.; Ramakrishna, S. 3D Fabrication of Polymeric Scaffolds for Regenerative Therapy. ACS Biomater. Sci. Eng. 2017, 3, 1175–1194. [Google Scholar] [CrossRef]
- Xu, N.; Ye, X.; Wei, D.; Zhong, J.; Chen, Y.; Xu, G.; He, D. 3D artificial bones for bone repair prepared by computed tomography-guided fused deposition modeling for bone repair. ACS Appl. Mater. Interfaces 2014, 6, 14952–14963. [Google Scholar] [CrossRef]
- Du, Y.; Liu, H.; Shuang, J.; Wang, J.; Ma, J.; Zhang, S. Microsphere-based selective laser sintering for building macroporous bone scaffolds with controlled microstructure and excellent biocompatibility. Colloids Surf. B Biointerfaces 2015, 135, 81–89. [Google Scholar] [CrossRef]
- Inzana, J.A.; Olvera, D.; Fuller, S.M.; Kelly, J.P.; Graeve, O.A.; Schwarz, E.M.; Kates, S.L.; Awad, H.A. 3D printing of composite calcium phosphate and collagen scaffolds for bone regeneration. Biomaterials 2014, 35, 4026–4034. [Google Scholar] [CrossRef]
- Kumar, A.; Mandal, S.; Barui, S.; Vasireddi, R.; Gbureck, U.; Gelinsky, M.; Basu, B. Low temperature additive manufacturing of three dimensional scaffolds for bone-tissue engineering applications: Processing related challenges and property assessment. Mater. Sci. Eng. R Rep. 2016, 103, 1–39. [Google Scholar] [CrossRef]
- Nooeaid, P.; Li, W.; Roether, J.A.; Mouriño, V.; Goudouri, O.-M.; Schubert, D.W.; Boccaccini, A.R. Development of bioactive glass based scaffolds for controlled antibiotic release in bone tissue engineering via biodegradable polymer layered coating. Biointerphases 2014, 9, 041001. [Google Scholar] [CrossRef]
- Renkó, J.B.; Kemény, D.M.; Nyiro, J.; Kovács, D. Comparison of cooling simulations of injection moulding tools created with cutting machining and additive manufacturing. Mater. Today Proc. 2019, 12, 462–469. [Google Scholar] [CrossRef]
- Hernandez, I.; Kumar, A.; Joddar, B. A bioactive hydrogel and 3d printed polycaprolactone system for bone tissue engineering. Gels 2017, 3, 26. [Google Scholar] [CrossRef]
- Singh, S.; Prakash, C.; Singh, M.; Mann, G.S.; Gupta, M.K.; Singh, R.; Ramakrishna, S. Poly-lactic-acid: Potential material for bio-printing applications. In Biomanufacturing; Springer: Cham, Switzerland, 2019; pp. 69–87. [Google Scholar] [CrossRef]
- Abbasi, N.; Hamlet, S.; Love, R.M.; Nguyen, N.T. Porous scaffolds for bone regeneration. J. Sci. Adv. Mater. Devices 2020, 5, 1–9. [Google Scholar] [CrossRef]
- Annabi, N.; Nichol, J.W.; Zhong, X.; Ji, C.; Koshy, S.; Khademhosseini, A.; Dehghani, F. Controlling the porosity and microarchitecture of hydrogels for tissue engineering. Tissue Eng. Part B Rev. 2010, 16, 371–383. [Google Scholar] [CrossRef]
- Wang, X.; Salick, M.R.; Gao, Y.; Jiang, J.; Li, X.; Liu, F.; Cordie, T.; Li, Q.; Turng, L.S. Interconnected porous poly(ε-caprolactone) tissue engineering scaffolds fabricated by microcellular injection molding. J. Cell. Plast. 2018, 54, 379–397. [Google Scholar] [CrossRef]
- İyibilgin, O.; Gepek, E. Characterization of CP-Titanium produced via binder jetting and conventional powder metallurgy. Rev. Metal. 2021, 57. [Google Scholar] [CrossRef]
- Agarwal, K.; Vangapally, S.; Sheldon, A. Binder jet additive manufacturing of stainless steel—Tricalcium phosphate biocomposite for bone scaffold and implant applications. In Proceedings of the 2017 International Solid Freeform Fabrication Symposium—An Additive Manufacturing Conference, Austin, TX, USA, 7–9 August 2020; pp. 2376–2388. [Google Scholar]
- Flamant, Q.; Caravaca, C.; Meille, S.; Gremillard, L.; Chevalier, J.; Biotteau-Deheuvels, K.; Kuntz, M.; Chandrawati, R.; Herrmann, I.K.; Spicer, C.D.; et al. Selective etching of injection molded zirconia-toughened alumina: Towards osseointegrated and antibacterial ceramic implants. Acta Biomater. 2016, 46, 308–322. [Google Scholar] [CrossRef]
- Dallas, A.J.; Graham, K.M.; Clarysse, M.; Fonderle, V. Characterization and control of organic airborne contamination in lithographic processing. In Proceedings of the Metrology, Inspection, and Process Control for Microlithography XVI 2002, Santa Clara, CA, USA, 4–7 March 2002; Volume 4689, pp. 1085–1109. [Google Scholar] [CrossRef]
- Rassaei, L.; Singh, P.S.; Lemay, S.G. Lithography-Based Nanoelectrochemistry. Anal. Chem. 2011, 83, 3974–3980. [Google Scholar] [CrossRef]
- Franssila, S. Introduction to Microfabrication; Wiley: New York, NY, USA, 2010; pp. 3–16. [Google Scholar] [CrossRef]
- Tran, K.T.M.; Nguyen, T.D. Lithography-based methods to manufacture biomaterials at small scales. J. Sci. Adv. Mater. Devices 2017, 2, 1–14. [Google Scholar] [CrossRef]
- Liang, R.; Gu, Y.; Wu, Y.; Bunpetch, V.; Zhang, S. Lithography-Based 3D Bioprinting and Bioinks for Bone Repair and Regeneration. ACS Biomater. Sci. Eng. 2021, 7, 806–816. [Google Scholar] [CrossRef] [PubMed]
- Terzaki, K.; Farsari, M. Polymer Processing Through Multiphoton Absorption. In Polymer and Photonic Materials towards Biomedical Breakthroughs; Springer: Cham, Switzerland, 2018; pp. 49–69. [Google Scholar] [CrossRef]
- Torgersen, J.; Qin, X.H.; Li, Z.; Ovsianikov, A.; Liska, R.; Stampfl, J. Hydrogels for two-photon polymerization: A toolbox for mimicking the extracellular matrix. Adv. Funct. Mater. 2013, 23, 4542–4554. [Google Scholar] [CrossRef]
- You, S.; Li, J.; Zhu, W.; Yu, C.; Mei, D.; Chen, S. Nanoscale 3D printing of hydrogels for cellular tissue engineering. J. Mater. Chem. B R. Soc. Chem. 2018, 6, 2187–2197. [Google Scholar] [CrossRef] [PubMed]
- LaFratta, C.N.; Fourkas, J.T.; Baldacchini, T.; Farrer, R.A. Multiphoton fabrication. Angew. Chem. Int. Ed. 2007, 46, 6238–6258. [Google Scholar] [CrossRef]
- Juodkazis, S.; Mizeikis, V.; Misawa, H. Three-dimensional microfabrication of materials by femtosecond lasers for photonics applications. J. Appl. Phys. 2009, 106, 051101. [Google Scholar] [CrossRef]
- Farsari, M.; Chichkov, B.N. Materials processing: Two-photon fabrication. Nat. Photonics 2009, 3, 450–452. [Google Scholar] [CrossRef]
- Satoshi, K.; Hong-Bo, S.; Tomokazu, T.; Kenji, T. Finer features for functional microdevices. Nature 2001, 412, 697–698. [Google Scholar]
- Dong, X.Z.; Zhao, Z.S.; Duan, X.M. Improving spatial resolution and reducing aspect ratio in multiphoton polymerization nanofabrication. Appl. Phys. Lett. 2008, 92, 90–93. [Google Scholar] [CrossRef]
- Guillaume, O.; Geven, M.A.; Sprecher, C.M.; Stadelmann, V.A.; Grijpma, D.W.; Tang, T.T.; Qin, L.; Lai, Y.; Alini, M.; de Bruijn, J.D.; et al. Surface-enrichment with hydroxyapatite nanoparticles in stereolithography-fabricated composite polymer scaffolds promotes bone repair. Acta Biomater. 2017, 54, 386–398. [Google Scholar] [CrossRef]
- Yang, Z.; Xie, L.; Zhang, B.; Zhang, G.; Huo, F.; Zhou, C.; Liang, X.; Fan, Y.; Tian, W.; Tan, Y. Preparation of BMP-2/PDA-BCP Bioceramic Scaffold by DLP 3D Printing and its Ability for Inducing Continuous Bone Formation. Front. Bioeng. Biotechnol. 2022, 10, 854693. [Google Scholar] [CrossRef]
- Ji, A.; Zhang, S.; Bhagia, S.; Yoo, C.G.; Ragauskas, A.J. 3D printing of biomass-derived composites: Application and characterization approaches. RSC Adv. 2020, 10, 21698–21723. [Google Scholar] [CrossRef]
- Soni, S. Research and Fabrication of 3D Printer Research and Fabrication of 3D Printer; Chhattisgarh Swami Vivekanand Technical University Bhilai: Bhilai, India, 2020; 59p, ISBN 3073714308. [Google Scholar]
- Deng, X.; Huang, B.; Hu, R.; Chen, L.; Tang, Y.; Lu, C.; Chen, Z.; Zhang, W.; Zhang, X. 3D printing of robust and biocompatible poly(ethylene glycol)diacrylate/nano-hydroxyapatite compositesviacontinuous liquid interface production. J. Mater. Chem. B 2021, 9, 1315–1324. [Google Scholar] [CrossRef]
- Paun, I.A.; Popescu, R.C.; Mustaciosu, C.C.; Zamfirescu, M.; Calin, B.S.; Mihailescu, M.; Dinescu, M.; Popescu, A.; Chioibasu, D.; Soproniy, M.; et al. Laser-direct writing by two-photon polymerization of 3D honeycomb-like structures for bone regeneration. Biofabrication 2018, 10, 025009. [Google Scholar] [CrossRef]
- Peltola, S.M.; Melchels, F.P.W.; Grijpma, D.W.; Kellomäki, M. A review of rapid prototyping techniques for tissue engineering purposes. Ann. Med. 2008, 40, 268–280. [Google Scholar] [CrossRef]
- Sharma, R.; Kuebler, S.M.; Grabill, C.N.; Digaum, J.L.; Kosan, N.R.; Cockerham, A.R.; Martinez, N.; Rumpf, R.C. Fabrication of Functional Nanophotonic Devices via Multiphoton Polymerization. ACS Symp. Ser. 2019, 1315, 151–171. [Google Scholar] [CrossRef]
- Jaklenec, A.; Wan, E.; Murray, M.E.; Mathiowitz, E. Novel scaffolds fabricated from protein-loaded microspheres for tissue engineering. Biomaterials 2008, 29, 185–192. [Google Scholar] [CrossRef]
- Borden, M.; Attawia, M.; Khan, Y.; El-Amin, S.F.; Laurencin, C.T. Tissue-engineered bone formation in vivo using a novel sintered polymeric microsphere matrix. J. Bone Jt. Surg. Ser. B 2004, 86, 1200–1208. [Google Scholar] [CrossRef]
- Yao, J.; Radin, S.; Leboy, P.S.; Ducheyne, P. The effect of bioactive glass content on synthesis and bioactivity of composite poly (lactic-co-glycolic acid)/bioactive glass substrate for tissue engineering. Biomaterials 2005, 26, 1935–1943. [Google Scholar] [CrossRef]
- Brown, J.L.; Nair, L.S.; Laurencin, C.T. Solvent/non-solvent sintering: A novel route to create porous microsphere scaffolds for tissue regeneration. J. Biomed. Mater. Res. Part B Appl. Biomater. 2008, 86, 396–406. [Google Scholar] [CrossRef]
- Nukavarapu, S.P.; Kumbar, S.G.; Brown, J.L.; Krogman, N.R.; Weikel, A.L.; Hindenlang, M.D.; Nair, L.S.; Allcock, H.R.; Laurencin, C.T. Polyphosphazene/nano-hydroxyapatite composite microsphere scaffolds for bone tissue engineering. Biomacromolecules 2008, 9, 1818–1825. [Google Scholar] [CrossRef] [PubMed]
- Singh, M.; Morris, C.P.; Ellis, R.J.; Detamore, M.S.; Berkland, C. Microsphere-based seamless scaffolds containing macroscopic gradients of encapsulated factors for tissue engineering. Tissue Eng. Part C Methods 2008, 14, 299–309. [Google Scholar] [CrossRef] [PubMed]
- Duarte, A.R.C.; Roy, C.; Vega-González, A.; Duarte, C.M.M.; Subra-Paternault, P. Preparation of acetazolamide composite microparticles by supercritical anti-solvent techniques. Int. J. Pharm. 2007, 332, 132–139. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.C.; Zhang, Y.S.; Akpek, A.; Shin, S.R.; Khademhosseini, A. 4D bioprinting: The next-generation technology for biofabrication enabled by stimuli-responsive materials. Biofabrication 2017, 9, 012001. [Google Scholar] [CrossRef] [PubMed]
- Gao, B.; Yang, Q.; Zhao, X.; Jin, G.; Ma, Y.; Xu, F. 4D Bioprinting for Biomedical Applications. Trends Biotechnol. 2016, 34, 746–756. [Google Scholar] [CrossRef]
- Suo, H.; Zhang, D.; Yin, J.; Qian, J.; Wu, Z.L.; Fu, J. Interpenetrating polymer network hydrogels composed of chitosan and photocrosslinkable gelatin with enhanced mechanical properties for tissue engineering. Mater. Sci. Eng. C 2018, 92, 612–620. [Google Scholar] [CrossRef]
- Khoshroo, K.; Jafarzadeh Kashi, T.S.; Moztarzadeh, F.; Tahriri, M.; Jazayeri, H.E.; Tayebi, L. Development of 3D PCL microsphere/TiO2 nanotube composite scaffolds for bone tissue engineering. Mater. Sci. Eng. C 2017, 70, 586–598. [Google Scholar] [CrossRef]
- Hwangbo, H.; Lee, H.; Roh, E.J.; Kim, W.; Joshi, H.P.; Kwon, S.Y.; Choi, U.Y.; Han, I.B.; Kim, G.H. Bone tissue engineering via application of a collagen/hydroxyapatite 4D-printed biomimetic scaffold for spinal fusion. Appl. Phys. Rev. 2021, 8, 021403. [Google Scholar] [CrossRef]
- Huang, H.Y.; Fan, F.Y.; Shen, Y.K.; Wang, C.H.; Huang, Y.T.; Chern, M.J.; Wang, Y.H.; Wang, L. 3D poly-ε-caprolactone/graphene porous scaffolds for bone tissue engineering. Colloids Surf. A Physicochem. Eng. Asp. 2020, 606, 125393. [Google Scholar] [CrossRef]
- Güney, E.; Emir, C.; Altan, D.; Yücel, S. Development of biocomposite tissue scaffolds of collagen/gelatin/boron-doped bioactive glass prepared through solvent casting/particulate leaching method for bone tissue engineering. J. Indian Chem. Soc. 2020, 97, 2006–2012. [Google Scholar]
- Moorthi, A.; Parihar, P.R.; Saravanan, S.; Vairamani, M.; Selvamurugan, N. Effects of silica and calcium levels in nanobioglass ceramic particles on osteoblast proliferation. Mater. Sci. Eng. C 2014, 43, 458–464. [Google Scholar] [CrossRef]
- Sola, A.; Bertacchini, J.; D’Avella, D.; Anselmi, L.; Maraldi, T.; Marmiroli, S.; Messori, M. Development of solvent-casting particulate leaching (SCPL) polymer scaffolds as improved three-dimensional supports to mimic the bone marrow niche. Mater. Sci. Eng. C 2019, 96, 153–165. [Google Scholar] [CrossRef]
- Kozaniti, F.K.; Deligianni, D.D.; Georgiou, M.D.; Portan, D.V. The Role of Substrate Topography and Stiffness on MSC Cells Functions: Key Material Properties for Biomimetic Bone Tissue Engineering. Biomimetics 2022, 7, 7. [Google Scholar] [CrossRef]
- Priya, G.; Madhan, B.; Narendrakumar, U.; Suresh Kumar, R.V.; Manjubala, I. In Vitro and in Vivo Evaluation of Carboxymethyl Cellulose Scaffolds for Bone Tissue Engineering Applications. ACS Omega 2021, 6, 1246–1253. [Google Scholar] [CrossRef]
- Valencia, C.; Valencia, C.H.; Zuluaga, F.; Valencia, M.E.; Mina, J.H.; Grande-Tovar, C.D. Synthesis and application of scaffolds of chitosan-graphene oxide by the freeze-drying method for tissue regeneration. Molecules 2018, 23, 2651. [Google Scholar] [CrossRef]
- Ju, J.; Peng, X.; Huang, K.; Li, L.; Liu, X.; Chitrakar, C.; Chang, L.; Gu, Z.; Kuang, T. High-performance porous PLLA-based scaffolds for bone tissue engineering: Preparation, characterization, and in vitro and in vivo evaluation. Polymer 2019, 180, 121707. [Google Scholar] [CrossRef]
- Nabavinia, M.; Khoshfetrat, A.B.; Naderi-Meshkin, H. Nano-hydroxyapatite-alginate-gelatin microcapsule as a potential osteogenic building block for modular bone tissue engineering. Mater. Sci. Eng. C 2019, 97, 67–77. [Google Scholar] [CrossRef]
- Sajadi-Javan, Z.S.; Varshosaz, J.; Mirian, M.; Manshaei, M.; Aminzadeh, A. Thermo-responsive hydrogels based on methylcellulose/Persian gum loaded with taxifolin enhance bone regeneration: An in vitro/in vivo study. Cellulose 2022, 29, 2413–2433. [Google Scholar] [CrossRef]
- Singh, B.N.; Veeresh, V.; Mallick, S.P.; Sinha, S.; Rastogi, A.; Srivastava, P. Generation of scaffold incorporated with nanobioglass encapsulated in chitosan/chondroitin sulfate complex for bone tissue engineering. Int. J. Biol. Macromol. 2020, 153, 1–16. [Google Scholar] [CrossRef]
- Enderami, S.E.; Shafiei, S.S.; Shamsara, M.; Enderami, S.E.; Rostamian Tabari, A. Evaluation of Osteogenic Differentiation of Bone Marrow-Derived Mesenchymal Stem Cell on Highly Porous Polycaprolactone Scaffold Reinforced With Layered Double Hydroxides Nanoclay. Front. Bioeng. Biotechnol. 2022, 10, 805969. [Google Scholar] [CrossRef]
- Buga, C.; Hunyadi, M.; Gácsi, Z.; Hegedűs, C.; Hakl, J.; Schmidt, U.; Ding, S.J.; Csík, A. Calcium silicate layer on titanium fabricated by electrospray deposition. Mater. Sci. Eng. C 2019, 98, 401–408. [Google Scholar] [CrossRef] [PubMed]
- Miszuk, J.; Liang, Z.; Hu, J.; Sanyour, H.; Hong, Z.; Fong, H.; Sun, H. Elastic Mineralized 3D Electrospun PCL Nanofibrous Scaffold for Drug Release and Bone Tissue Engineering. ACS Appl. Bio Mater. 2021, 4, 3639–3648. [Google Scholar] [CrossRef] [PubMed]
- Sedghi, R.; Sayyari, N.; Shaabani, A.; Niknejad, H.; Tayebi, T. Novel biocompatible zinc-curcumin loaded coaxial nanofibers for bone tissue engineering application. Polymer 2018, 142, 244–255. [Google Scholar] [CrossRef]
- Wang, Z.; Wang, H.; Xiong, J.; Li, J.; Miao, X.; Lan, X.; Liu, X.; Wang, W.; Cai, N.; Tang, Y. Fabrication and in vitro evaluation of PCL/gelatin hierarchical scaffolds based on melt electrospinning writing and solution electrospinning for bone regeneration. Mater. Sci. Eng. C 2021, 128, 112287. [Google Scholar] [CrossRef] [PubMed]
- Guler, E.; Baripoglu, Y.E.; Alenezi, H.; Arikan, A.; Babazade, R.; Unal, S.; Duruksu, G.; Alfares, F.S.; Yazir, Y.; Oktar, F.N.; et al. Vitamin D3/vitamin K2/magnesium-loaded polylactic acid/tricalcium phosphate/polycaprolactone composite nanofibers demonstrated osteoinductive effect by increasing Runx2 via Wnt/β-catenin pathway. Int. J. Biol. Macromol. 2021, 190, 244–258. [Google Scholar] [CrossRef]
- Jin, S.; Gao, J.; Yang, R.; Yuan, C.; Wang, R.; Zou, Q.; Zuo, Y.; Zhu, M.; Li, Y.; Man, Y.; et al. A baicalin-loaded coaxial nanofiber scaffold regulated inflammation and osteoclast differentiation for vascularized bone regeneration. Bioact. Mater. 2022, 8, 559–572. [Google Scholar] [CrossRef]
- Kang, Y.; Xu, C.; Meng, L.; Dong, X.; Qi, M.; Jiang, D. Exosome-functionalized magnesium-organic framework-based scaffolds with osteogenic, angiogenic and anti-inflammatory properties for accelerated bone regeneration. Bioact. Mater. 2022, 18, 26–41. [Google Scholar] [CrossRef]
- Hung, B.P.; Naved, B.A.; Nyberg, E.L.; Dias, M.; Holmes, C.A.; Elisseeff, J.H.; Dorafshar, A.H.; Grayson, W.L. Three-Dimensional Printing of Bone Extracellular Matrix for Craniofacial Regeneration. ACS Biomater. Sci. Eng. 2016, 2, 1806–1816. [Google Scholar] [CrossRef]
- Shuai, C.; Yang, Y.; Feng, P.; Peng, S.; Guo, W.; Min, A.; Gao, C. A multi-scale porous scaffold fabricated by a combined additive manufacturing and chemical etching process for bone tissue engineering. Int. J. Bioprint. 2018, 4, 133. [Google Scholar] [CrossRef]
- Hassanajili, S.; Karami-Pour, A.; Oryan, A.; Talaei-Khozani, T. Preparation and characterization of PLA/PCL/HA composite scaffolds using indirect 3D printing for bone tissue engineering. Mater. Sci. Eng. C 2019, 104, 109960. [Google Scholar] [CrossRef]
- Aliyu, A.A.A.; Abdul-Rani, A.M.; Ginta, T.L.; Rao, T.V.V.L.N.; Selvamurugan, N.; Roy, S. Hydroxyapatite mixed-electro discharge formation of bioceramic Lakargiite (CaZrO3) on Zr–Cu–Ni–Ti–Be for orthopedic application. Mater. Manuf. Process. 2018, 33, 1734–1744. [Google Scholar] [CrossRef]
- Cockerill, I.; Su, Y.; Sinha, S.; Qin, Y.X.; Zheng, Y.; Young, M.L.; Zhu, D. Porous zinc scaffolds for bone tissue engineering applications: A novel additive manufacturing and casting approach. Mater. Sci. Eng. C 2020, 110, 110738. [Google Scholar] [CrossRef]
- Chen, S.; Zhu, L.; Wen, W.; Lu, L.; Zhou, C.; Luo, B. Fabrication and Evaluation of 3D Printed Poly(l-lactide) Scaffold Functionalized with Quercetin-Polydopamine for Bone Tissue Engineering. ACS Biomater. Sci. Eng. 2019, 5, 2506–2518. [Google Scholar] [CrossRef]
- Xu, J.Z.; Ren, Y.; Yin, H.M.; Huang, Y.F.; Liu, W.; Zhao, B.; Gul, R.M.; Li, Z.M. Bone-like Polymeric Composites with a Combination of Bioactive Glass and Hydroxyapatite: Simultaneous Enhancement of Mechanical Performance and Bioactivity. ACS Biomater. Sci. Eng. 2018, 4, 4434–4442. [Google Scholar] [CrossRef]
- Jabbari, F.; Hesaraki, S.; Houshmand, B. The physical, mechanical, and biological properties of silk fibroin/chitosan/reduced graphene oxide composite membranes for guided bone regeneration. J. Biomater. Sci. Polym. Ed. 2019, 30, 1779–1802. [Google Scholar] [CrossRef]
- Bedair, T.M.; Lee, C.K.; Kim, D.S.; Baek, S.W.; Bedair, H.M.; Joshi, H.P.; Choi, U.Y.; Park, K.H.; Park, W.; Han, I.B.; et al. Magnesium hydroxide-incorporated PLGA composite attenuates inflammation and promotes BMP2-induced bone formation in spinal fusion. J. Tissue Eng. 2020, 11, 2041731420967591. [Google Scholar] [CrossRef]
- Narayanan, V.; Sumathi, S.; Narayanasamy, A.N.R. Tricomponent composite containing copper–hydroxyapatite/chitosan/polyvinyl pyrrolidone for bone tissue engineering. J. Biomed. Mater. Res. Part A 2020, 108, 1867–1880. [Google Scholar] [CrossRef]
- Valente, M.; Puiggalí, J.; Del Valle, L.J.; Titolo, G.; Sambucci, M. Recycled porcine bone powder as filler in thermoplastic composite materials enriched with chitosan for a bone scaffold application. Polymers 2021, 13, 2751. [Google Scholar] [CrossRef]
- Mahdavi, R.; Belgheisi, G.; Haghbin-Nazarpak, M.; Omidi, M.; Khojasteh, A.; Solati-Hashjin, M. Bone tissue engineering gelatin–hydroxyapatite/graphene oxide scaffolds with the ability to release vitamin D: Fabrication, characterization, and in vitro study. J. Mater. Sci. Mater. Med. 2020, 31, 97. [Google Scholar] [CrossRef]
- Stojkovska, J.; Zvicer, J.; Andrejevic, M.; Janackovic, D.; Obradovic, B.; Veljovic, D.N. Novel composite scaffolds based on alginate and Mg-doped calcium phosphate fillers: Enhanced hydroxyapatite formation under biomimetic conditions. J. Biomed. Mater. Res. Part B Appl. Biomater. 2021, 109, 2079–2090. [Google Scholar] [CrossRef]
- Singh, Y.P.; Dasgupta, S.; Bhaskar, R.; Agrawal, A.K. Monetite addition into gelatin based freeze-dried scaffolds for improved mechanical and osteogenic properties. Biomed. Mater. 2021, 16, 065030. [Google Scholar] [CrossRef] [PubMed]
- Genasan, K.; Mehrali, M.; Veerappan, T.; Talebian, S.; Raman, M.M.; Singh, S.; Swamiappan, S.; Mehrali, M.; Kamarul, T.; Raghavendran, H.R.B. Calcium-silicate-incorporated gellan-chitosan induced osteogenic differentiation in mesenchymal stromal cells. Polymers 2021, 13, 3211. [Google Scholar] [CrossRef] [PubMed]
- Bahrami, S.; Baheiraei, N.; Shahrezaee, M. Biomimetic reduced graphene oxide coated collagen scaffold for in situ bone regeneration. Sci. Rep. 2021, 11, 16783. [Google Scholar] [CrossRef] [PubMed]
- Thitiset, T.; Damrongsakkul, S.; Yodmuang, S.; Leeanansaksiri, W.; Apinun, J.; Honsawek, S. A novel gelatin/chitooligosaccharide/demineralized bone matrix composite scaffold and periosteum-derived mesenchymal stem cells for bone tissue engineering. Biomater. Res. 2021, 25, 19. [Google Scholar] [CrossRef] [PubMed]
- Han, S.K.; Song, M.; Choi, K.; Choi, S.W. Fabrication of Biodegradable Polyurethane Foam Scaffolds with Customized Shapes and Controlled Mechanical Properties by Gas Foaming Technique. Macromol. Mater. Eng. 2021, 306, 2100114. [Google Scholar] [CrossRef]
- Kim, S.E.; Tiwari, A.P. Three dimensional polycaprolactone/cellulose scaffold containing calcium-based particles: A new platform for bone regeneration. Carbohydr. Polym. 2020, 250, 116880. [Google Scholar] [CrossRef]
- Luo, K.; Wang, L.; Wang, Y.; Zhou, S.; Zhang, P.; Li, J. Porous 3D hydroxyapatite/polyurethane composite scaffold for bone tissue engineering and its in vitro degradation behavior. Ferroelectrics 2020, 566, 104–115. [Google Scholar] [CrossRef]
- Geng, M.; Zhang, Q.; Gu, J.; Yang, J.; Du, H.; Jia, Y.; Zhou, X.; He, C. Construction of a nanofiber network within 3D printed scaffolds for vascularized bone regeneration. Biomater. Sci. 2021, 9, 2631–2646. [Google Scholar] [CrossRef]
- Liu, S.; Wu, X.; Hu, J.; Wu, Z.; Zheng, Y. Preparation and characterisation of a novel polylactic acid/hydroxyapatite/graphene oxide/aspirin drug-loaded biomimetic composite scaffold. New J. Chem. 2021, 45, 10788–10797. [Google Scholar] [CrossRef]
- Wu, X.; Zhou, M.; Jiang, F.; Yin, S.; Lin, S.; Yang, G.; Lu, Y.; Zhang, W.; Jiang, X. Marginal sealing around integral bilayer scaffolds for repairing osteochondral defects based on photocurable silk hydrogels. Bioact. Mater. 2021, 6, 3976–3986. [Google Scholar] [CrossRef]
- Lantigua, D.; Wu, X.; Suvarnapathaki, S.; Nguyen, M.A.; Camci-Unal, G. Composite scaffolds from gelatin and bone meal powder for tissue engineering. Bioengineering 2021, 8, 169. [Google Scholar] [CrossRef]
- Zirak, N.; Maadani, A.M.; Salahinejad, E.; Abbasnezhad, N.; Shirinbayan, M. Fabrication, drug delivery kinetics and cell viability assay of PLGA-coated vancomycin-loaded silicate porous microspheres. Ceram. Int. 2022, 48, 48–54. [Google Scholar] [CrossRef]
- Liu, Y.; Zhang, T.; Li, M.; Ouyang, Z.; Gao, F.; Liu, C.; Li, C.; Liu, D.; Zhou, Z. PLGA hybrid porous microspheres as human periodontal ligament stem cell delivery carriers for periodontal regeneration. Chem. Eng. J. 2021, 420, 129703. [Google Scholar] [CrossRef]
- Kandel, R.; Jang, S.R.; Shrestha, S.; Ghimire, U.; Shrestha, B.K.; Park, C.H.; Kim, C.S. A bimetallic load-bearing bioceramics of TiO2 @ ZrO2 integrated polycaprolactone fibrous tissue construct exhibits anti bactericidal effect and induces osteogenesis in MC3T3-E1 cells. Mater. Sci. Eng. C 2021, 131, 112501. [Google Scholar] [CrossRef]
- Asl, M.A.; Karbasi, S.; Beigi-Boroujeni, S.; Zamanlui Benisi, S.; Saeed, M. Evaluation of the effects of starch on polyhydroxybutyrate electrospun scaffolds for bone tissue engineering applications. Int. J. Biol. Macromol. 2021, 191, 500–513. [Google Scholar] [CrossRef]
- Dimassi, S.; Tabary, N.; Chai, F.; Zobrist, C.; Hornez, J.C.; Cazaux, F.; Blanchemain, N.; Martel, B. Polydopamine treatment of chitosan nanofibers for the conception of osteoinductive scaffolds for bone reconstruction. Carbohydr. Polym. 2022, 276, 118774. [Google Scholar] [CrossRef]
- Li, K.; Zhang, Y.; Xu, J.; Wang, J.; Gu, X.; Li, P.; Fan, Y. Three-dimensional magnetic fibrous scaffold with icariin expanded by supercritical CO2 for bone tissue engineering under static magnetic field. Compos. Part B Eng. 2021, 226, 109304. [Google Scholar] [CrossRef]
- Abazari, M.F.; Zare Karizi, S.; Samadian, H.; Nasiri, N.; Askari, H.; Asghari, M.; Frootan, F.; Bakhtiari, H.; Mahboudi, H.; Mansouri, V. Poly (glycerol sebacate) and polyhydroxybutyrate electrospun nanocomposite facilitates osteogenic differentiation of mesenchymal stem cells. J. Drug Deliv. Sci. Technol. 2021, 66, 102796. [Google Scholar] [CrossRef]
- Pan, T.; Song, W.; Xin, H.; Yu, H.; Wang, H.; Ma, D.; Cao, X.; Wang, Y. MicroRNA-activated hydrogel scaffold generated by 3D printing accelerates bone regeneration. Bioact. Mater. 2022, 10, 1–14. [Google Scholar] [CrossRef]
- Hedayati, S.K.; Behravesh, A.H.; Hasannia, S.; Kordi, O.; Pourghaumi, M.; Saed, A.B.; Gashtasbi, F. Additive manufacture of PCL/nHA scaffolds reinforced with biodegradable continuous Fibers: Mechanical Properties, in-vitro degradation Profile, and cell study. Eur. Polym. J. 2022, 162, 110876. [Google Scholar] [CrossRef]
- Kim, D.; Lee, J.; Min Seok, J.; Jung, J.Y.; Hee Lee, J.; Sik Lee, J.; Lee, K.; Park, S.A. Three-dimensional bioprinting of bioactive scaffolds with thermally embedded abalone shell particles for bone tissue engineering. Mater. Des. 2021, 212, 110228. [Google Scholar] [CrossRef]
- Peng, B.; Li, J.; Wu, J.; Chen, N.; Chen, G. Preparation and performance of poly (vinyl alcohol)/polylactic acid/hydroxyapatite composite scaffolds based on 3D printing. J. Appl. Polym. Sci. 2022, 139, 51534. [Google Scholar] [CrossRef]
- Kim, W.J.; Lee, H.; Ji Roh, E.; Bae An, S.; Han, I.B.; Hyung Kim, G. A multicellular bioprinted cell construct for vascularized bone tissue regeneration. Chem. Eng. J. 2022, 431, 133882. [Google Scholar] [CrossRef]
- Alcala-Orozco, C.R.; Mutreja, I.; Cui, X.; Hooper, G.J.; Lim, K.S.; Woodfield, T.B.F. Hybrid biofabrication of 3D osteoconductive constructs comprising Mg-based nanocomposites and cell-laden bioinks for bone repair. Bone 2022, 154, 116198. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Song, Y.; Zhang, C.; Zhao, C.; He, C. Inverse CO2/C2H2 separation in a pillared-layer framework featuring a chlorine-modified channel by quadrupole-moment sieving. Sep. Purif. Technol. 2021, 279, 119608. [Google Scholar] [CrossRef]
- Xue, X.; Liu, H.; Wang, S.; Hu, Y.; Huang, B.; Li, M.; Gao, J.; Wang, X.; Su, J. Neutrophil-erythrocyte hybrid membrane-coated hollow copper sulfide nanoparticles for targeted and photothermal/anti-inflammatory therapy of osteoarthritis. Compos. Part B Eng. 2022, 237, 109855. [Google Scholar] [CrossRef]
- Li, H.; Zhang, Y.; Tai, Y.; Zhu, X.; Qi, X.; Zhou, L.; Li, Z.; Lan, H. Flexible transparent electromagnetic interference shielding films with silver mesh fabricated using electric-field-driven microscale 3D printing. Opt. Laser Technol. 2022, 148, 107717. [Google Scholar] [CrossRef]
- Khan, S.B.; Irfan, S.; Lam, S.S.; Sun, X.; Chen, S. 3D printed nanofiltration membrane technology for waste water distillation. J. Water Process Eng. 2022, 49, 102958. [Google Scholar] [CrossRef]
- Xue, X.; Zhang, H.; Liu, H.; Wang, S.; Li, J.; Zhou, Q.; Chen, X.; Ren, X.; Jing, Y.; Deng, Y.; et al. Rational Design of Multifunctional CuS Nanoparticle-PEG Composite Soft Hydrogel-Coated 3D Hard Polycaprolactone Scaffolds for Efficient Bone Regeneration. Adv. Funct. Mater. 2022, 32, 2202470. [Google Scholar] [CrossRef]
- Yang, Y.; Zhu, H.; Xu, X.; Bao, L.; Wang, Y.; Lin, H.; Zheng, C. Construction of a novel lanthanum carbonate-grafted ZSM-5 zeolite for effective highly selective phosphate removal from wastewater. Microporous Mesoporous Mater. 2021, 324, 111289. [Google Scholar] [CrossRef]




| (A) | ||||||
|---|---|---|---|---|---|---|
| Scaffolds | Polymers | Structures | Cells Used | Advantages | Limitations | References |
| Chitosan(CS)/silk fibroin(SF)/reduced graphene oxide (rGO) composite membranes | CS SF rGO | 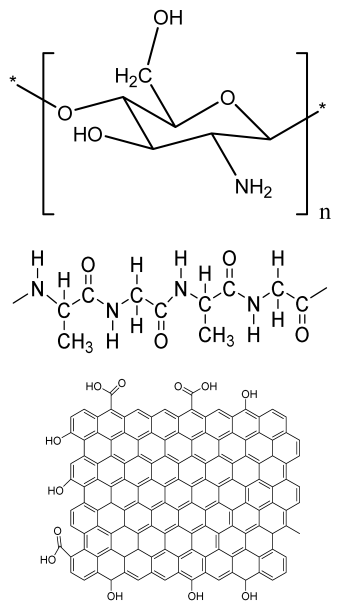 | G-292 cells | Decrease in hydrophilicity, swelling, and degradability with an increase in SF content, increase in tensile strength and hydrophilicity due to increase in rGO concentration | No in vivo study Osteoconductivity study of scaffolds should be analyzed. In vitro study should be analyzed using other bone cell lines such as MG-63 and MC3T3-E1 cells. | [187] |
| PDA-modified BMP2- immobilized PLGA/MH composite scaffold | PLGA Magnesium hydroxide (MH) PDA | 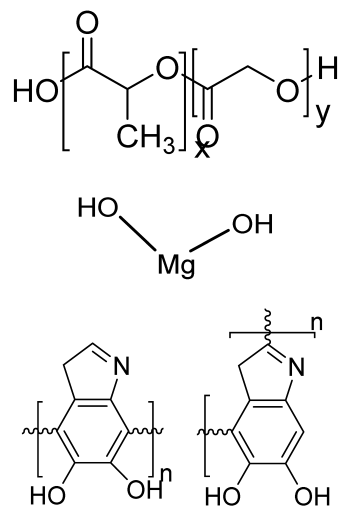 | MC3T3-E1 cells | Better hydrophilicity, neutralization effects, and degradation performance and enhanced BMP2 loading efficiency supported proliferation and osteogenic differentiation and BMP2-induced bone formation. | Short-term in vivo study (4 weeks) In vitro study for a short duration (7 days) using PLGA (synthetic polymer) | [188] |
| Copper-hydroxyapatite/ chitosan/polyvinyl pyrrolidone composite | Chitosan Polyvinyl pyrrolidone Copper-hydroxyapatite |  | Human osteosarcoma cell line | High porosity and tensile strength, lower swelling percentage, possesses antimicrobial activity and hemocompatibility, helps in the formation of apatite, good biocompatibility and cell attachment | No in vivo study The effects of osteogenic markers using the developed scaffold should be studied. In vitro biodegradation study using the scaffolds is required. | [189] |
| PCL–porcine bone powder (BP) composites reinforced with PLA-CS microfibers | BP PLA PCL CS | 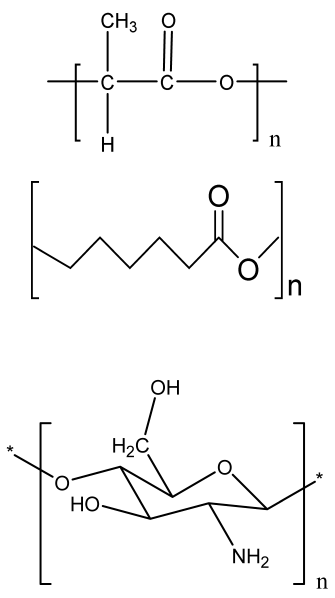 | - | Suitable mechanical properties and effective bactericidal efficiency | Possibility to fabricate electrospun composites using PCL-BP reinforced with CS-PLA microfibers In vitro studies required Needs animal sacrifice Ethical issues in some countries | [190] |
| Gelatin (G)–HA scaffolds containing vitamin D (VD)-loaded graphene oxide | G HA GO | 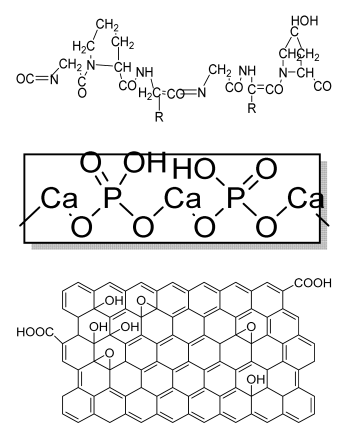 | Buccal fat pad derived stem cells (BFPSCs) | Better encapsulation efficiency and mechanical properties, porosity percentage and density comparable to spongy bone, good cell adhesion and cell viability, possesses ALP activity | Developed scaffolds should be examined on animal models. Toxicity of graphene oxide at high concentration (2%) | [191] |
| (B) | ||||||
| Scaffolds | Polymers | Structures | Cells Used | Advantages | Limitations | References |
| Alginate and Mg-doped calcium phosphate fillers | Sodium alginate HAP Magnesium Nitrate Hexahydrate |  | - | Highly porous and open connected pores; Better mechanical property due to Ca2+ ions compared to the previous scaffolds | In vitro and in vivo study needed Swelling and degradation behavior required | [192] |
| Monetite-nanoparticle-impregnated gelatin-based composite scaffold | Gelatin Monetite | 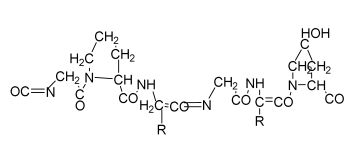 | MG-63 | Increase in compressive strength and better bioactivity compared to gelatin scaffolds; higher biomineralization ability, exhibits osteoinduction | Long processing time The developed scaffolds should be analyzed in animal models. Osteogenic differentiation using scaffold should be analyzed. | [193] |
| Gellan–chitosan scaffolds modified with calcium silicate | Calcium silicate Gellan CS |  | HBMSCs cells | Good cell attachment, increased proliferation and viability, supported bone mineralization, showed osteoinduction potential | In vivo experiments required for further human applications Mechanical strength using the scaffold is needed. | [194] |
| Collagen (Col)–rGO scaffolds | Col rGO |  | hBMSCs cells | Increased mechanical strength due to rGO; nontoxic, with better viability and proliferation of cells; increased bone formation in mouse models within 12 weeks of implantation | In vivo study should be performed on higher animal models. The effects of developed scaffolds should be analyzed using growth factors. | [195] |
|
Gelatin/chitooligosaccharide/ demineralized bone matrix composite scaffold |
G Chitooligosaccharide (COS) bone matrix (BM) | 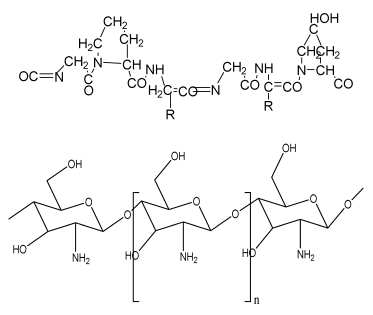 | Mesenchymal stem cells | Improved cell attachment with proliferation on scaffold, mineralization until 8 weeks, supported in vivo ectopic bone formation | Osteoconductive and osteoinductive features required for the confirmation of increased bone formation. | [196] |
| (C) | ||||||
| Scaffolds | Polymers | Structures | Cells Used | Advantages | Limitations | References |
| PCL-based PU foam scaffolds | PCL triol PU Gelatin | 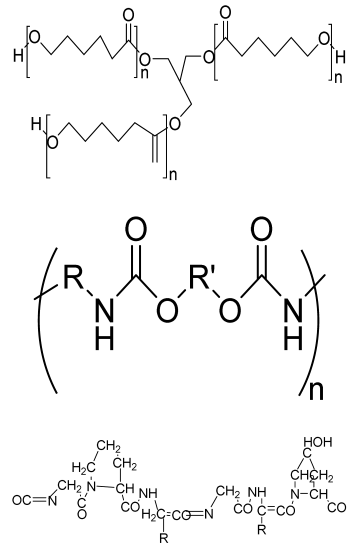 | MC3T3-E1 cells | Highly porous structure, high compression strength, ductile and flexible, low toxicity, high ALP activity | The application of optimized PU scaffold for specific tissue is needed in an animal model for future analysis | [197] |
| Ca-3D@PCL-CL24 (3D multilayered polycaprolactone/cellulose (CL) scaffold) | PCL CL Calcium hydroxide particles | 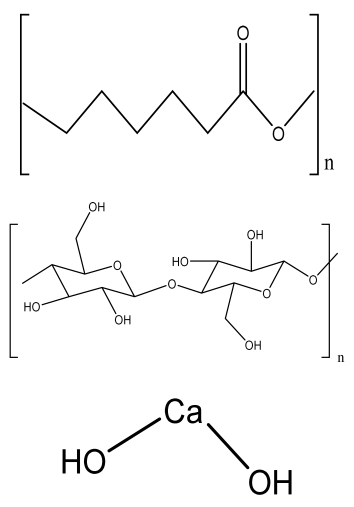 | MC3T3-E1 cells | Better mechanical and thermal properties compared to the control, enhanced cell growth and mineralization | The function of the scaffold in animal models should be analyzed in a future study. Long degradation time using synthetic polymer-based scaffold | [198] |
| HA/PU composite porous 3D scaffold | HA PU | 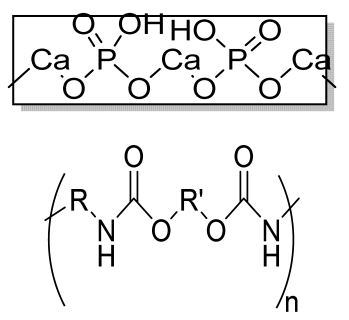 | - | Promotes bioactivity, superior mechanical properties, satisfactory degradation time (12 weeks) | In vitro studies are required on bone cells. Osteoinductive behavior should be analyzed. | [199] |
| (D) | ||||||
| Scaffolds | Polymers | Structures | Cells Used | Advantages | Limitations | References |
| Deferoxamine (DFO)-loaded poly (glycerol-co-sebacic acid-co-L-lactic acid-co-polyethylene glycol) (PGSLP)-based composite scaffolds | Poly (glycerol-co-sebacic acid-co-L-lactic acid-co-polyethylene glycol deferoxamine Gelatin | 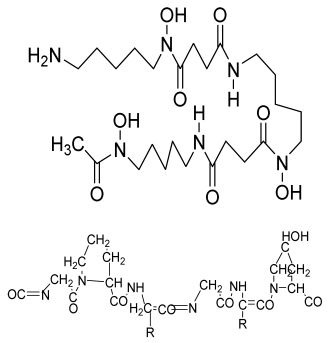 | Human umbilical vein endothelial cells (HUVECs) | Supported vascular formation, enhanced bone regeneration, supported cell adhesion and migration, promoted osteogenesis and angiogenesis, enhanced mineral nodule formation and vascular formation and promoted bone formation in rat model | Optimization of the scaffold is needed in a higher animal model in a future study. | [200] |
| HA/PLA/ASA/GO (hydroxyapatite/polylactic acid/aspiringraphene oxide/) drug-loaded biomimetic composite scaffold | PLA HA GO aspirin | 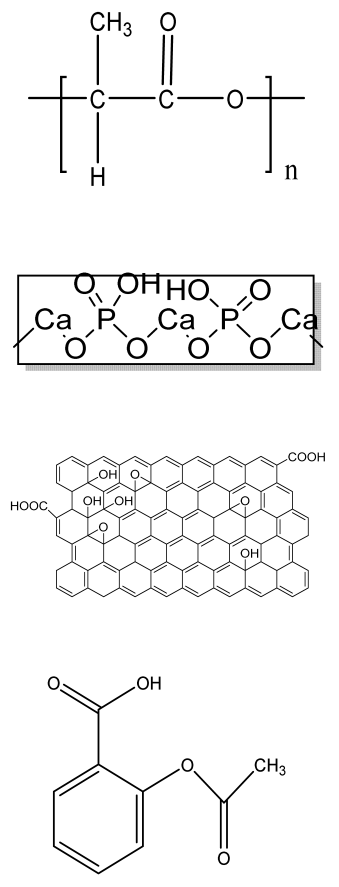 | MC3T3-E1 | Hydrophilic scaffold with good bioactivity, hemocompatibility, cytocompatibility, and sustained drug release | The inhibitory effect of ASA on bone cells may hinder bone regeneration. | [201] |
| (E) | ||||||
| Scaffolds | Polymers | Structures | Cells Used | Advantages | Limitations | References |
| Transforming growth factor-β3-loaded Sil-MA (methacrylated silk fibroin) hydrogel | SF | 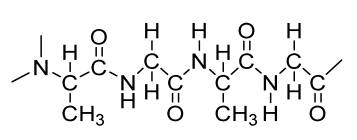 | BMSCs | Osteochondral regeneration, better lateral integration, good adhesive property, marginal sealing effect, promotes chondrocyte migration and differentiation | In vivo analysis should be performed on higher animal models with long duration. Hydrophilicity and degradation study required | [202] |
| Gelatin methacrylate (GelMA)/Bone meal powder (BP) composite hydrogels | GelMA BP | 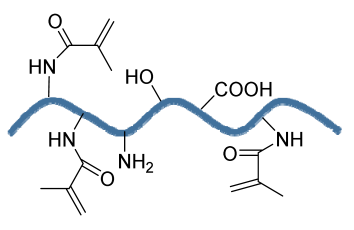 | MC3T3-E1 | Improves osteoinductivity and mechanical performance and supports cell differentiation, biocompatibility, and biodegradation properties | The effect of the hydrogel should be analyzed in higher animal models. The developed scaffold should be analyzed for the delivery of small molecules, for example, therapeutics and growth factors. | [203] |
| (F) | ||||||
| Scaffolds | Polymers | Structures | Cells Used | Advantages | Limitations | References |
| Mg-Ca silicate microspheres encapsulated in PLGA | Sodium alginate PLGA | 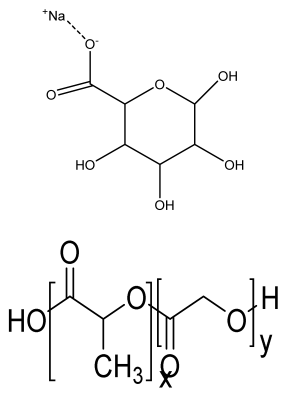 | Dental pulp stem cells | Limited the burst release of the vancomycin and drug concentration was above the critical value inhibiting S.aureus growth. PLGA-coated akermanite microspheres showed highest cell viability | The effects of developed microspheres should be analyzed in animal models and should also be optimized using other drugs. | [204] |
| HA-SF-PLGA hybrid porous microspheres | HA PLGA SF | 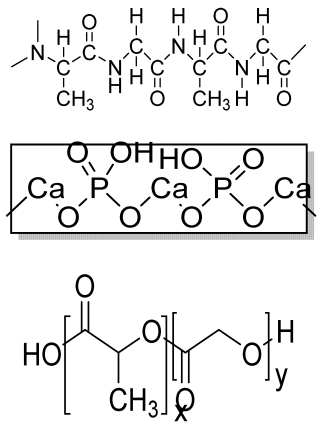 | Human periodontal ligament stem cells (hPDLSCs) | Enhanced viability, proliferation, and osteogenic differentiation and better tissue repair efficacy | In vivo study required. Osteoinductive behavior should be analyzed. | [205] |
| (G) | ||||||
| Scaffolds | Polymers | Structures | Cells Used | Advantages | Limitations | References |
| Bioceramic PCL scaffold containing metallic oxides | PCL Hybrid TiO2@ZrO2 composite | 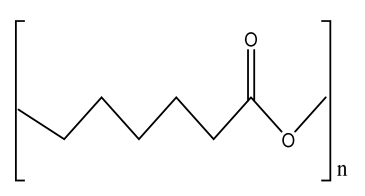 | MC3T3-E1 cells | Excellent antibacterial activity, improved load-bearing ability, hydrophilicity, and biomineralization, better cell-to-cell interactions, enhanced proliferation and regeneration, and good biocompatibility with osteoinductive abilities | No in vivo study Long-term degradation of PCL Short-term in vitro study (5 days), as PCL was used, which is a synthetic polymer | [206] |
| Poly (3-hydroxybutyrate(PHB)/starch electrospun scaffold | PHB Starch | 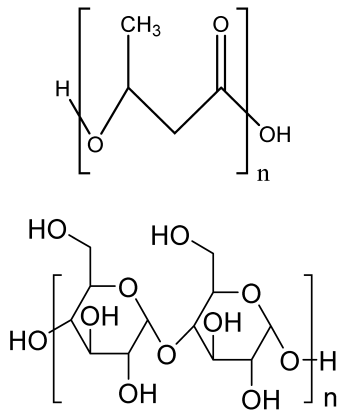 | MG-63 cells | Improved tensile strength, degradation, and hydrophilicity due to starch, better viability and proliferation of the electrospun scaffolds than PHB scaffold | The effect of growth-factor-incorporated electrospun scaffold should be analyzed in future study. | [207] |
| Chitosan nanofibrous scaffolds modified by polydopamine (NFs-PDA) | Chitosan Dopamine hydrochloride | 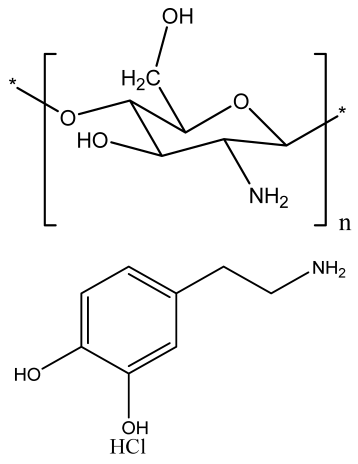 | MT3C3-E1 cells | Supported structural stability of fabricated nanofibers in PBS and improved cytocompatibility and in vitro biomineralization | Investigation of the in vivo process for biological action with replacement of NF with newly formed bone | [208] |
| Porous magnetic PCL/Fe3O4/icariin (ICA) 3D scaffold | Fe3O4 MNPs ICA PCL fibers | 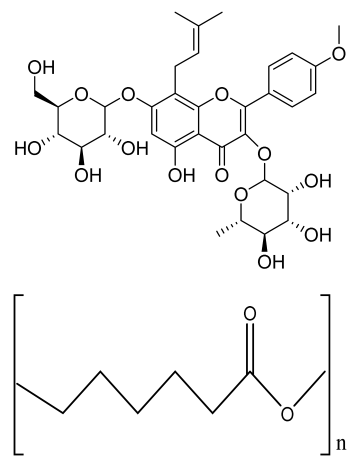 | MC3T3-E1 | Improved cell proliferation viability compared to 2D membrane, increased cell viability, and superior cell infiltration, internal collagen deposition, and angiogenesis | The developed scaffold should be analyzed in higher animal models. Mechanical strength and degradation behavior required of developed scaffold | [209] |
| PGS-PHB scaffold | Poly (glycerol sebacate) PHB | 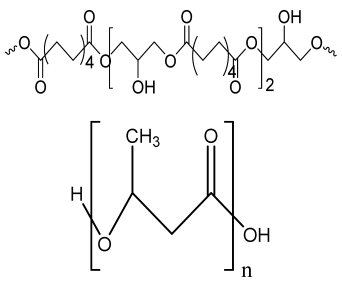 | ADSCs | Good porosity, higher protein adsorption capacity than control, biocompatible, better ALP activity, calcium production, and expression level of bone-related genes | The effect of functionalized PGS-PHB scaffold should be analyzed in future study. | [210] |
| (H) | ||||||
| Scaffolds | Polymers | Structures | Cells Used | Advantages | Limitations | References |
| miRNA-activated hydrogel scaffolds (MAHSs) | Gelatin Alginate |  | hMSCs (human bone mesenchymal stem cells) | Sustained release of miR-29b, accelerated bone regeneration, induced osteogenesis and new bone formation | Short term in vivo study (4 weeks) Use of glutaraldehyde as a cross-linker may be toxic to cells. | [211] |
| PCL/nHA scaffolds | PCL nHA Polyglycolic acid | 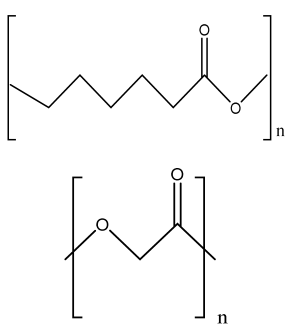 | Mouse fibroblast cells ((L-929) | Improved tensile properties and compressive strength, increased hydrophilicity, increased adhesion and mechanical property, and nontoxic, suitable for bone tissue engineering | In vivo study required for the developed scaffold. Osteoinductive and osteoconductive behavior should be analyzed. Mineralization study required. | [212,213,214] |
| ASP (abalone shell particles)-embedded PCL scaffolds | PCL | 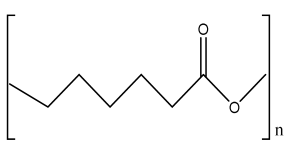 | MG63 cells | Increased mechanical properties, improved absorption of cell proteins, supported cell viability and proliferation, high ALP activity, supported bone regeneration | The developed method is costly. In vitro biodegradation study required. | [213,215,216] |
| Poly (vinyl alcohol)/ polylactic acid/hydroxyapatite composite scaffolds | PVA PLA HA | 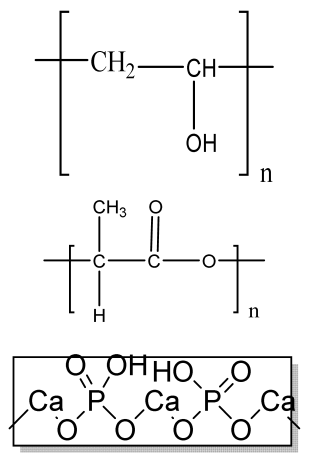 | L929 cells | Increased compressive strength and compressive modulus, good bone formation and mineralization ability, excellent biocompatibility | In vitro study performed for very short duration (72 h). In vivo study should be performed in future study. | [214,217,218] |
| Biomimetic 3D cell-laden construct | Collagen β-TCP |  | hASCs, HUVECs | Positive angiogenic phenotype; improved new bone formation and angiogenesis. | The effect of other cell lines on 3D construct should be analyzed. The biomimetic scaffolds should be analyzed for clinical trials in higher animal models. | [219,220,221] |
| Magnesium-based nanocomposite bioink material | Magnesium hydroxide nanoparticles (Mg) PCL | 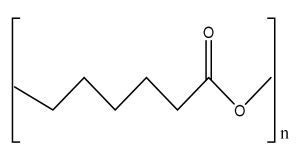 | Human mesenchymal stromal cells (hMSCs) | Enhanced osteogenic differentiation and bone-specific matrix deposition, accelerates degradation rate of scaffold compared to PCL, and supported bone ECM deposition | The developed scaffolds should be analyzed in animal models in future study. | [216,222] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bhushan, S.; Singh, S.; Maiti, T.K.; Sharma, C.; Dutt, D.; Sharma, S.; Li, C.; Tag Eldin, E.M. Scaffold Fabrication Techniques of Biomaterials for Bone Tissue Engineering: A Critical Review. Bioengineering 2022, 9, 728. https://doi.org/10.3390/bioengineering9120728
Bhushan S, Singh S, Maiti TK, Sharma C, Dutt D, Sharma S, Li C, Tag Eldin EM. Scaffold Fabrication Techniques of Biomaterials for Bone Tissue Engineering: A Critical Review. Bioengineering. 2022; 9(12):728. https://doi.org/10.3390/bioengineering9120728
Chicago/Turabian StyleBhushan, Sakchi, Sandhya Singh, Tushar Kanti Maiti, Chhavi Sharma, Dharm Dutt, Shubham Sharma, Changhe Li, and Elsayed Mohamed Tag Eldin. 2022. "Scaffold Fabrication Techniques of Biomaterials for Bone Tissue Engineering: A Critical Review" Bioengineering 9, no. 12: 728. https://doi.org/10.3390/bioengineering9120728
APA StyleBhushan, S., Singh, S., Maiti, T. K., Sharma, C., Dutt, D., Sharma, S., Li, C., & Tag Eldin, E. M. (2022). Scaffold Fabrication Techniques of Biomaterials for Bone Tissue Engineering: A Critical Review. Bioengineering, 9(12), 728. https://doi.org/10.3390/bioengineering9120728






