Abstract
The attention on orthopedic biomaterials has shifted from their direct osteogenic properties to their osteoimmunomodulation, especially the modulation of macrophage polarization. Presently, advanced technologies endow polyetheretherketone (PEEK) with good osteoimmunomodulation by modifying PEEK surface characteristics or incorporating bioactive substances with regulating macrophage polarization. Recent studies have demonstrated that the fabrication of a hydrophilic surface and the incorporation of bioactive substances into PEEK (e.g., zinc, calcium, and phosphate) are good strategies to promote osteogenesis by enhancing the polarization of M2 macrophages. Furthermore, the modification by other osteoimmunomodulatory composites (e.g., lncRNA-MM2P, IL-4, IL-10, and chitosan) and their controlled and desired release may make PEEK an optimal bio-activated implant for regulating and balancing the osteogenic system and immune system. The purpose of this review is to comprehensively evaluate the potential of bio-activated PEEK in polarizing macrophages into M2 phenotype to improve osteogenesis. For this objective, we retrieved and discussed different kinds of bio-activated PEEK regarding improving osteogenesis through modulating macrophage polarization. Meanwhile, the relevant challenges and outlook were presented. We hope that this review can shed light on the development of bio-activated PEEK with more favorable osteoimmunomodulation.
1. Introduction
In clinic, bone losses and defects are always caused by traumatic fracture, osteolysis, bone-involved tumor resection, aseptic or infected osteonecrosis, spinal fusion, periodontitis, etc., and are typically repaired and improved by using the advanced osteogenic biomaterials by surgeries. In these processes, effective bone regeneration is important for prognosis [,,,,,]. In addition, the development of these biomaterials, for decades, has focused on the role that biomaterials play in directly regulating the osteogenic differentiation of the osteoblastic lineage cells, such as osteoblasts, osteoclasts, and bone mesenchymal stem cells (BMSCs) [,,]. Therefore, a series of advanced osteogenic biomaterials have been developed to gain excellent osteogenesis by carrying out various strategies, such as the desired fabrication of biomaterials themselves (from two-dimensional to three-dimensional structure, from hydrophobic to hydrophilic surface, from nano to micro even macro size, etc.) [,,], and the advanced integration between biomaterials and bioactive substances (from metallic to non-metallic elements, from inorganic to organic compounds, from genes to proteins, etc.) [,,,,]. Based on this fruitful theory research, the application of these biomaterials seems to have a promising medical prospect.
However, it is very disappointing that the majority of these biomaterials are not used in clinic. Of the possible reasons, one is that we only focus on the direct relationships between the properties of biomaterials and the osteogenic differentiation of the osteoblastic lineage cells but neglect the role of other cells from other systems (such as the immune cells) during the process of bone formation and healing [,,,,]. It is concluded that a study is probably imperfect or outdated if only following the traditional principles of enhancing osteogenesis. Therefore, an advanced osteogenic biomaterial could be better fabricated and put into clinical use when the bone biologies of all system cells are taken into consideration as much as possible.
As we know, when biomaterials are implanted into host, the immune system instantly recognizes the foreign bodies, the immune cells are activated, and a series of immune responses are triggered in turn [,,]. An undesirable immune response could lead to the failure of the implantation by negatively influencing the bio-behavior of bone cells to compromise osseointegration between the implant and host bone [,]. In other words, the immune cells play an important role in the development of host bone, and there is a close crosstalk between the skeletal system and the immune system, which is linked by a series of cytokines, signaling molecules and proteins, receptors, etc. [,,,]. Of the immune cells, the biological behavior of macrophages is regarded as a critical factor because the different polarization phenotypes of macrophages result in different effects on the modulation of osteogenesis [,,,]. Therefore, in order to create a desirable environment of macrophage polarization for improving osteogenesis, some studies on developing biomaterial implants have been carried out [,,].
As one of biomaterial implants, polyetheretherketone (PEEK) is a semi-crystalline, non-resorbable, high-performance, and thermoplastic material, and is being paid more and more attention in trauma, orthopedic, and spinal surgeries owing to its several excellent advantages [,,,,,]. For instance, there is a similar elastic modulus between PEEK (3–4 Gpa) and human cortical bone (18 Gpa), which contributes to reducing stress shielding. Because of its radiolucency, the new bone formation surrounding the PEEK implant can be better evaluated through imageological examinations (such as X-ray) during the postoperative follow-ups. Additionally, biochemical corrosion can be avoided when PEEK is implanted into the host because of its environmental resistance. However, the bio-inertness of pure PEEK, to some extent, limits its application in the field of medicine. In order to improve the bioactivity of PEEK, a porous surface, on one hand, usually needs to be developed. The porous structure not only benefits the induction of shifts in local macrophage polarization but is only used as a carrier where some bioactive substances are loaded []. Presently, in order to combine with the bioactive substances to improve its osteogenesis through polarizing macrophage, the porous structure on the surface of PEEK can be fabricated through several special techniques (see Table 1). On the other hand, bioactive substances can also be combined with pure PEEK through some special intermedi, such as catecholamines which include dopamine and norepinephrine (NE). Furthermore, in weakly alkaline solution, these intermedi polymerize to form small aggregates which can bind to a variety of materials including ceramics, metals, and polymers [,].

Table 1.
Technologies of developing porous PEEK.
With the development and design of PEEK, it has been proved that PEEK is fabricated into the porous implant or used as a carrier loading some bioactive substances (e.g., zinc and calcium) to improve the osteogenic process by inducing a shift of local macrophage polarization in the foreign body reaction, and the use of them has extensively increased [,]. To better understand the relationships among PEEK, macrophage polarization, and osteogenesis, we retrieved the featured research on this issue and detailed several important topics. Firstly, the synthesis and characteristics of porous PEEK were addressed briefly through reviewing previous studies. Secondly, the relationship between host osteogenesis and macrophage polarization was detailed. Thirdly, the enhanced osteogenesis of bio-activated PEEK through inducing M2 macrophage polarization was emphasized. Finally, challenges and outlook were summarized.
2. Fabrication and Characteristics of Porous PEEK
Just as described in Table 1, a porous structure on PEEK surface can be fabricated through special physio-chemical techniques or computer techniques. Take the sulfonation technique for example: PEEK is first immersed in concentrated sulfuric acid (about 98%) while being stirred through magnetic stirring machine in order to fabricate a uniform porous surface. Next, the residues of sulfuric acid on PEEK surface are gotten rid of through immersion in deionized water for 10 min. Then, the sulfur concentration is decreased as much as possible through a hydrothermal treatment (120 °C for 4 h). Finally, the sulfonated PEEK (SPEEK) is gently washed using deionized water and dried at room temperature. According to the results of our work [], Figure 1 shows the SEM images of PEEK before and after sulfonation. On the other hand, according to the results of our previous study (Figure 2), the thickness of the porous layer is about 5–6 μm after PEEK is sulfonated, meaning it is too thin to compromise its bulk mechanical properties, which is proved by the results of the mechanical tests that the porous surface is not destroyed even if pressed down by external force and the results of the stress–strain curve which show that their mechanical properties (e.g., breaking point, elasticity modulus, and fracture strength) are similar between PEEK and sulfonated PEEK (Table 2). This is consistent with the results from previous studies [,].
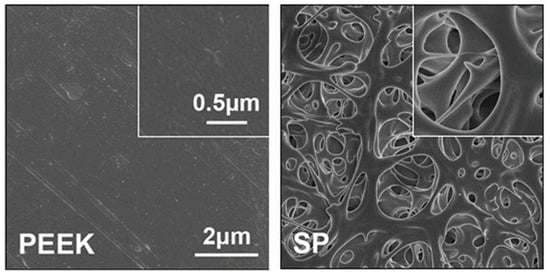
Figure 1.
Surface morphology of PEEK and sulfonated PEEK (SP) detected by SEM. Reprinted with permission from Ref. []. Copyright 2022, Elsevier.
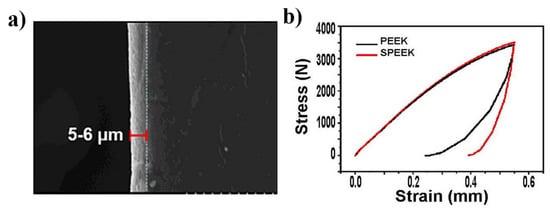
Figure 2.
(a) Thickness of the sulfonation layer (5–6 μm). (b) Stress–strain curve for PEEK and SPEEK. Reprinted with permission from Ref. []. Copyright 2022, Elsevier.

Table 2.
The mechanical properties of PEEK and SPEEK.
3. Development of Bio-Activated PEEK
With regard to the techniques of combination between the bioactive substances and PEEK, they can be generally divided into three categories. (1) Direct surface modification: plasma surface treatment; wet chemical treatment; ultraviolet/ozone surface treatment; accelerated neutral atom beam surface treatment; nanofiber fabrication; laser surface modification [,,,,,,,]. (2) Deposition techniques: vacuum plasma spray; chemical deposition; plasma spray; arc ion plating; radio frequency magnetron sputtering; electron beam deposition [,,,,,]. (3) Fabricating bioactive composites: selective laser sintering; injection molding; in situ synthetic method [,,]. Though the preparation methods of combination between the bioactive substances and PEEK are significantly different, all designs and preparations require a win–win combination that not only can PEEK keep its own excellent properties, but that it also plays a desirable and durable role in host osteogenesis through the controlled release of the bioactive substances.
4. Macrophage Polarization for Osteogenesis
Of all the immune cells, macrophages presently have received a large amountof attention because of their high plasticity and different functional phenotypes in the period of the interaction between implants and host. In response to the local micro-environment signals caused by implants, macrophages can be polarized into different macrophage phenotypes which are mainly divided into M1 (classically activated/inflammatory phenotype) and M2 (alternatively activated/regenerative phenotype), just as T helper cells are named as Th1 and Th2 []. Briefly, lipopolysaccharide (LPS), interferon-γ (IFN-γ), and tumor necrosis factor-α (TNF-α) can induce macrophages into M1 polarization with anti-tumoral and pro-inflammatory properties, whereas macrophage exposure to the immune complexes, interleukin-4 (IL-4) and IL-10, can transform into M2 polarization, which has an anti-inflammatory property and improves tissue repair []. On the other hand, when exposed to different stimulating signals, M2 macrophage can be divided into M2a (induced by IL-13 and IL-4), M2b (induced by toll-like receptors (TLRs) or IL-1R agonists and immune complexes), and M2c (induced by IL-10) which has different functions including Th2 inflammation, allergy, immunoregulation, tissue repair, etc. []. Because macrophages can be switched into different functional phenotypes when responding to the local environment signals, it may be feasible that a desirable osteogenesis could be enhanced by regulating and controlling macrophage phenotypes.
Presently, in view of the different functions of M1 and M2 macrophages, there is a general consensus that the M2 phenotype is more favorable to improve osteogenesis when compared to M1 phenotype. It is well known that M1 macrophage secretes a series of proinflammatory cytokines (IL-6, IL-1β, and TNF-α) and proteinase (matrix metalloproteinases (MMPs)) which could accelerate the activity of osteoclasts, and results in bone resorption and the subsequent aseptic loosening of implants []. Nevertheless, M2 macrophage can express and secrete some helpful factors which induce and promote osteogenesis, such as vascular endothelial growth factor (VEGF), transforming growth factor-β (TGF-β), and, especially, bone morphogenetic protein-2 (BMP-2) [,,]. Therefore, it is suggested that M2 macrophage plays an indispensable role in improving osteogenesis, and this point paves a new way for the development of the innovative biomaterial implants which not only directly focus on osteoblastic lineage cells but also emphasiz the role of macrophage polarization in osteogenesis.
When implanted into host bone, the innovative biomaterial is recognized as a foreign body by the host immune system, arousing immune responses and activating immune cells (e.g., macrophages). Then, macrophages, in response to the biomaterial implants, could be regulated and induced into M2 phenotype. The latter further produces a series of osteoinductive and osteogenic factors (TGF-β, VEGF, BMP-2, etc.) to enhance osteogenesis around implants. This effect is attributed to the shared mechanisms and cross-talks between immunology and osteology, which is probably defined as osteoimmunomodulation []. Based on the understanding of both systems, a new perspective will probably shed light on for the design and development of medical biomaterials (e.g., PEEK) to significantly improve osteogenesis through regulating a required macrophage polarization.
5. Bio-Activated PEEK for Osteoimmunomodulation
Presently, there are a great many studies which have demonstrated that the immune environment for inducing osteogenesis generated by biomaterial implants can be modulated through their surface characteristics and bioactive composites, such as topography, porosity, wettability, released ions, etc. For example, Chen and coworkers reported that nano-topography of biomaterials could modulate the immune response to improve osteogenesis surrounding the implants through possible underlying mechanisms [,]. This is consistent with the results of the previous study []. On the other hand, Lee CH et al. provided similar insight into the surface modulation of biomaterials that divalent cation chemistry (e.g., calcium and strontium) could regulate the cell shapes of adherent macrophages and significantly up-regulate the expression of M2 macrophage phenotypes when combined with the nanostructured titanium implant surfaces []. However, studies on bio-activated PEEK with improving osteogenesis by modulating macrophage polarization are very few. It is necessary to make a summary by reviewing relevant references to guide the osteoimmunomodulatory design and the clinical application of PEEK.
5.1. Hydrophilicity-Modified PEEK
Of the biomaterial surface characteristics, wettability plays an important role in the modulation of host cell adhesion, proliferation, and differentiation, and many studies showed that, on the highly hydrophobic biomaterial surface, proteins are adsorbed in denatured and rigid forms which inhibit cell adhesion; whereas, on the highly hydrophilic biomaterial surface, the proteins are prevented from adsorption or bound very weakly which leads to a higher cell adhesion and modulates cell bio-behavior [,,,]. Therefore, it is probably feasible that surface modification endows PEEK with a hydrophilic surface which induces a higher M2 macrophage polarization to improve the osteogenic process. As suggested by Lv L [], when compared with the hydrophobic surface, the hydrophilic surface could enhance the anti-inflammatory and pro-healing performances of macrophages, which is attributed to the mechanism by which, on the hydrophilic surface, integrin β1 interacts with the adsorbed fibronectin (FN) and then stimulates macrophages into M2 phenotype, likely through the PI3K/Akt signaling pathway, while integrin β2 interacts with fibrinogen (FG) absorbed on the hydrophobic surface and, next, induces a higher M1 macrophage phenotype, probably via activating the NF-κB signaling pathway.
Through surface plasma treatment and phosphorylation, the PEEK surface is endowed with hydrophilicity which triggers M2 polarization []. This is mainly because the hydrophobic surface can induce protein denaturation, and the latter stimulates macrophages to produce pro-inflammatory cytokines and nitric oxide which are the typical markers of M1 macrophages [,,]. On the other hand, it is suggested that a hydrophilic surface is more important for enhancing M2 polarization when compared to the roughness of the PEEK surface []. However, the roughness of the biomaterial surface contributes to improving osseointegration between host bone and the implant [,]. Therefore, for the sake of the optimized osseointegration, the biomaterial surface characteristics (e.g., roughness and wettability) need to be thoroughly taken into account.
5.2. Zinc-Modified PEEK
It is well known that zinc is classified as one of the micro-nutrients in the essential trace elements and also the only metal that is an important component in at least 3000 proteins and six classes of enzymes, including transferases, isomerases, hydrolases, oxidoreductases, lyases, and ligases, and plays a vital role in the maintenance of internal environment homeostasis, such as reproductive health, physical growth and development, neuro-behavioral development, sensory function, and functioning of immune system [,,,].
In terms of zinc ions for improving osteoimmunomodulation, it has been well documented that the immune regulation of zinc is probably regulated by the NF-κB signaling pathway. That is, zinc can induce the expression of A20 zinc finger protein and the latter plays a positive role in the anti-inflammatory response through inhibiting the NF-κB canonical signaling pathway in macrophages [,]. In addition, Ooi TC et al. suggested that this anti-inflammatory effect of zinc is also attributed to the activated Nrf2/HO-1 signaling pathway, but independent of the MAPKs signaling pathway [,]. Its anti-inflammatory effect could result from the increase in M2 polarization and the decrease in M1 polarization. For instance, Liu and his partners evaluated the immunomodulatory function for guiding cell fate and bone regeneration among PEEK, porous PEEK, and zinc-coated porous PEEK, and their results showed that, when compared with PEEK, zinc-coated porous PEEK, mainly owing to the controllable release of zinc, could significantly induce macrophages into an anti-inflammatory phenotype (M2 macrophage); the latter then produced a set of osteogenic cytokines and osteoinductive ones which significantly enhanced osseointegration between the implant and host bone, and next to porous PEEK, probably because of its porous structure [].
However, there are still some controversies. They suggested that zinc supplementation plays an inhibitory effect on M2 polarization and could switch macrophages toward the M1 phenotype to enhance anticancer activity [,]. Therefore, in order to derive a definite therapeutic potential of zinc, more detailed studies need to be carried out. For example, the influence of zinc content in PEEK on macrophage must be taken into consideration.
5.3. Calcium-Modified PEEK
Calcium (Ca), an indispensable macro-element of life, is mostly distributed in bones and teeth. It is involved in enzyme reaction, blood coagulation, cardiac muscle contraction, and cell membrane integrity, and influences the osteogenic differentiation of mesenchymal stem cells [,,,,]. Furthermore, certain inflammatory signaling pathways are also impacted by calcium, which, in a high concentration, could activate the calcium sensing receptor (CaSR) signaling cascade and then result in the production of Wnt5a, which reduces inflammation by inhibiting the expression of TNF-α through down-regulating NF-kB and TNFR1 via the Wnt5a/ /Ror2 signaling pathway [,,,,,].
Furthermore, calcium is also proved to be involved in macrophage polarization [,,]. Through the mediation of an intermedium (such as norepinephrine), calcium ions were bound on the PEEK surface. Toita R et al. proved that Ca-modified PEEK could induce LPS-stimulated RAW264.7 macrophages towards an anti-inflammatory/wound healing type (M2 phenotype), producing higher levels of anti-inflammatory cytokines but causing lower levels of pro-inflammatory ones when compared to pure PEEK, and subsequently activate osteoblastic cell proliferation and differentiation []. This is partly attributed to the calmodulin (CaM) signaling pathway activated by Ca ions, or it is the induction of M2 macrophage polarization by calcium ion modification that is beneficial to enhance the capacities of chemotaxis and the osteoinductivity of BMSCs [].
Apart from its direct potential in improving newborn formation [,], it should be emphasized that the indirect osteogenic function of calcium incorporated in PEEK also results from its osteoimmunomodulation by inducing M2 macrophage polarization. In order to simultaneously obtain and balanc the optimal effects of both, the porous PEEK is probably an excellent carrier which not only avoids the rapid release of calcium by chemical bonding but also the porous structure itself can improve osteogenesis. However, it is necessary to further study how to obtain the maximum osteogenic effect by including the combination of calcium content, porosity, etc.
5.4. Phosphate-Modified PEEK
Phosphorus plays an indispensable role in a series of biological processes, such as energy metabolism, bone mineralization, nucleic acid synthesis, phosphorylated enzymes, cell membranes, and cell signaling [,]. As reported in the previous studies, β-tricalcium phosphate is an excellent osteoinductive biomaterial which can stimulate macrophages into the M2 phenotype through releasing calcium, cobalt, and magnesium ions, and phosphate may improve the biocompatibility of implants by enhancing osteoblast attachment and differentiation [,,,]. However, what role phosphate ions play in regulating macrophage polarization has been neglected.
A recent study carried out by Villa-Bellosta R et al. showed that phosphate ions could induce a higher differentiation of an M2-anti-inflammatory macrophage phenotype which was evidenced by the higher expression of a typical M2 macrophage marker (arginase-1) []. In addition, Fukuda N et al. developed a novel PEEK modified with phosphate though plasma treatment and a subsequent phosphorylation reaction []. Their results showed that phosphorylated PEEK could lower the levels of TNF-α (a pro-inflammatory cytokine) but raise the levels of IL-10 (an anti-inflammatory cytokine) produced by macrophages when compared with the bare and plasma treated PEEK. Furthermore, they supposed that phosphate ions may play a positive role in inducing M2 polarization. However, the detailed mechanism involved in the phosphate-induced M2 polarization is unclear, which needs to be further explored.
6. Challenges and Outlook
The advent of PEEK has had a great influence in the field of medical biomaterial implants, and bio-implants based on PEEK have been considered as a good alternative to titanium-based and ceramic-based implants in spinal, cranial, dental, traumatic, and orthopedic surgeries owing to their excellent radiolucency, stable physicochemical properties, bio-compatibility, and elastic modulus similar to normal human bones [,,,,]. However, it is the fact that PEEK is biologically inert that, to some extent, limits the clinical application of PEEK due to preventing an excellent bonding with surrounding bone cells and tissues when implanted into host bone []. Presently, direct surface modification, deposition techniques, and bioactive composite fabrication play important roles in improving the bioactivity of PEEK, which provides PEEK with the required osteoimmunomodulation involved in regulating immune cells including macrophages, T cells, B cells, mast cells, etc. [,,,,]. Disappointingly, as far as the authors know, the majority of PEEK-based biomaterials have not been approved and put into clinical use. With regard to this, there exist three major barriers to the translation of PEEK-based biomaterials from the bench to the bedside. Firstly, it is a question of whether the excellent mechanical properties of PEEK are compromised by the presence of porous structure and additional bioactive substances after being processed by the aforementioned techniques [,,]. Secondly, how to controllably release bioactive composites from PEEK substrate for obtaining a durable and desirable effect deserves to be studied. Thirdly, it is discouraging that the clinical approval and the preliminary researches of novel PEEK-based biomaterial implants are very time-consuming and costly [].
Recently, in order to address these issues, many efforts have been made. For example, the incorporation of carbon fiber provides PEEK material with a good mechanical strength comparable to that of human cancellous bone, which is relatively strong and safe to be implanted in vivo [,], which may be a better choice than the binding between PEEK and bioactive composites through some intermedi without changing the inherent structure of PEEK []. On the other hand, the layer-by-layer assembled strategy can allow PEEK-based biomaterials a controlled and durable release of bioactive composites [,]. Therefore, through advanced technologies, PEEK not only keeps the acceptable mechanical properties but is also endowed with the needed bioactive potential, and it is promising that PEEK, with the development of advanced technology, could pave the way for the advent of medical biomaterial implants.
Apart from the aforementioned studies focusing on the osteogenic effect of bioactive substances in combination with PEEK by regulating macrophage polarization, the phenotypes of macrophages are also modulated by biomaterial topography, bioactive elements, cytokines, RNAs, and chemical substances, such as nanostructure surface, strontium (Sr), IL-10, IL-4, lncRNA-MM2P, chitosan, curcumin, etc. [,,,,,,]. If these are used as the modification of PEEK, PEEK may have worldwide application in the medical field because of its great potential for modulating M2 polarization to improve osteogenesis. However, this issue related to bioactive substance incorporation is highly complicated and still needs to be addressed.
In the next few years, two strategies need to be implemented. On one hand, seeking a novel technique provides PEEK with a perfect design that guarantees its own good properties and endows it with more excellent osteoimmunomodulation. On the other hand, the complex mechanisms of macrophage polarization of bio-activated PEEK need to be further understood, which contributes to guiding the design of bio-activated PEEK in improving osteoimmunomodulation.
7. Conclusions
PEEK can cause a macrophage response, and it is clear that different macrophage phenotypes play different roles in the tissue repairing process. The timely modulation of macrophage phenotypes seems to be an important aspect of improving osteogenesis through the fabrication of PEEK topography and the incorporation of bioactive substances. Presently, many bioactive substances have been studied and have shown their osteoimmunomodulation by modulating macrophage polarization. In addition, of these bioactive substances, several have been used to modify PEEK, and excellently bio-activated PEEK can enhance M2 macrophage phenotypes to promote new bone formation surrounding implants. In the future, bio-activated PEEK with modulation of the type of immune environment to induce osteogenesis should be focused on, and the specific mechanisms should also be better understood. This will shed light on the development of an optimally bio-activated PEEK with more favorable osteoimmunomodulation.
Funding
This work was supported by the National Natural Science Foundation of China (Grant NO. 81902183).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Hoberg, M.; Holzapfel, B.M.; Steinert, A.F.; Kratzer, F.; Walcher, M.; Rudert, M. Treatment of acetabular bone defects in revision hip arthroplasty using the Revisio-System. Der Orthop. 2017, 46, 126–132. [Google Scholar] [CrossRef] [PubMed]
- Li, D.; Xie, X.; Yang, Z.; Wang, C.; Wei, Z.; Kang, P. Enhanced bone defect repairing effects in glucocorticoid-induced osteonecrosis of the femoral head using a porous nano-lithium-hydroxyapatite/gelatin microsphere/erythropoietin composite scaffold. Biomater. Sci. 2018, 6, 519–537. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Wang, W.; Zhu, Y.; Lu, Y.; Wan, P.; Yang, K.; Zhang, Y.; Mao, C. Molecular and cellular mechanisms for zoledronic acid-loaded magnesium-strontium alloys to inhibit giant cell tumors of bone. Acta Biomater. 2018, 77, 365–379. [Google Scholar] [CrossRef] [PubMed]
- Pankaj, D.; Sahu, I.; Kurian, I.G.; Pradeep, A.R. Comparative evaluation of subgingivally delivered 1.2% rosuvastatin and 1% metformin gel in treatment of intrabony defects in chronic periodontitis: A randomized controlled clinical trial. J. Periodontol. 2018, 89, 1318–1325. [Google Scholar] [CrossRef]
- Srnec, R.; Divin, R.; Skoric, M.; Snasil, R.; Krbec, M.; Necas, A. Use of the Peptigel with Nanofibres in the Bone Defects Healing. Acta Chir. Orthop. Et Traumatol. Cechoslov. 2018, 85, 359–365. [Google Scholar]
- Wang, W.; Yeung, K.W.K. Bone grafts and biomaterials substitutes for bone defect repair: A review. Bioact. Mater. 2017, 2, 224–247. [Google Scholar] [CrossRef]
- Lee, M.S.; Lee, D.H.; Jeon, J.; Oh, S.H. Topographically Defined, Biodegradable Nanopatterned Patches to Regulate Cell Fate and Acceleration of Bone Regeneration. ACS Appl. Mater. Interfaces 2018, 10, 45. [Google Scholar] [CrossRef]
- Onder, S.; Calikoglu-Koyuncu, A.C.; Kazmanli, K.; Urgen, M.; Kok, F.N.; Torun-Kose, G. Magnesium doping on TiN coatings affects mesenchymal stem cell differentiation and proliferation positively in a dose-dependent manner. Bio-Med. Mater. Eng. 2018, 29, 427–438. [Google Scholar] [CrossRef]
- Zhang, Y.; Chen, S.E.; Shao, J.; van den Beucken, J. Combinatorial Surface Roughness Effects on Osteoclastogenesis and Osteogenesis. ACS Appl. Mater. Interfaces 2018, 10, 36652–36663. [Google Scholar] [CrossRef]
- Toita, R.; Rashid, A.N.; Tsuru, K.; Ishikawa, K. Modulation of the osteoconductive property and immune response of poly(ether ether ketone) by modification with calcium ions. Biotechnol. Adv. 2015, 3, 8738–8746. [Google Scholar] [CrossRef]
- George, P.A.; Quinn, K.; Cooper-White, J.J. Hierarchical scaffolds via combined macro- and micro-phase separation. Biomaterials 2010, 31, 641–647. [Google Scholar] [CrossRef]
- Dusseiller, M.R.; Schlaepfer, D.; Koch, M.; Kroschewski, R.; Textor, M. An inverted microcontact printing method on topographically structured polystyrene chips for arrayed micro-3-D culturing of single cells. Biomaterials 2005, 26, 5917–5925. [Google Scholar] [CrossRef]
- Kong, L.; Wu, Z.; Zhao, H.; Cui, H.; Shen, J.; Chang, J. Bioactive Injectable Hydrogels Containing Desferrioxamine and Bioglass for Diabetic Wound Healing. ACS Appl. Mater. Interfaces 2018, 10, 30103–30114. [Google Scholar] [CrossRef] [PubMed]
- Zhao, S.; Zhang, J.; Zhu, M.; Zhang, Y.; Liu, Z.; Tao, C.; Zhu, Y.; Zhang, C. Three-dimensional printed strontium-containing mesoporous bioactive glass scaffolds for repairing rat critical-sized calvarial defects. Acta Biomater. 2015, 12, 270–280. [Google Scholar] [CrossRef] [PubMed]
- Zhang, B.J.; Han, Z.W.; Duan, K.; Mu, Y.D.; Weng, J. Multilayered pore-closed PLGA microsphere delivering OGP and BMP-2 in sequential release patterns for the facilitation of BMSCs osteogenic differentiation. J. Biomed. Mater. Res. A 2018, 106, 95–105. [Google Scholar] [CrossRef]
- Subbiah, R.; Hwang, M.P.; Van, S.Y.; Do, S.H.; Park, H.; Lee, K.; Kim, S.H.; Yun, K.; Park, K. Osteogenic/angiogenic dual growth factor delivery microcapsules for regeneration of vascularized bone tissue. Adv. Healthc. Mater. 2015, 4, 1982–1992. [Google Scholar] [CrossRef]
- Kharaghani, D.; Kurniwan, E.B.; Khan, M.Q.; Yoshiko, Y. MiRNA-Nanofiber, the Next Generation of Bioactive Scaffolds for Bone Regeneration: A Review. Micromachines 2021, 12, 1472. [Google Scholar] [CrossRef]
- Liu, W.; Li, J.; Cheng, M.; Wang, Q.; Yeung, K.W.K.; Chu, P.K.; Zhang, X. Zinc-Modified Sulfonated Polyetheretherketone Surface with Immunomodulatory Function for Guiding Cell Fate and Bone Regeneration. Adv. Sci. 2018, 5, 1800749. [Google Scholar] [CrossRef]
- Takayanagi, H. Osteoimmunology: Shared mechanisms and crosstalk between the immune and bone systems. Nat. Rev. Immunol. 2007, 7, 292–304. [Google Scholar] [CrossRef]
- Li, Y.; Toraldo, G.; Li, A.; Yang, X.; Zhang, H.; Qian, W.P.; Weitzmann, M.N. B cells and T cells are critical for the preservation of bone homeostasis and attainment of peak bone mass in vivo. Blood 2007, 109, 3839–3848. [Google Scholar] [CrossRef]
- Tanaka, Y. Clinical immunity in bone and joints. J. Bone Min. Metab. 2018, 37, 2–8. [Google Scholar] [CrossRef] [PubMed]
- Fouilloux, I.; Duplan, M.B.; Baroukh, B.; Cherruau, M.; Saffar, J.L.; Lesclous, P. Mast cell activation and degranulation occur early during induction of periosteal bone resorption. Bone 2006, 38, 59–66. [Google Scholar] [CrossRef] [PubMed]
- Wooley, P.H.; Nasser, S.; Fitzgerald, R.H., Jr. The immune response to implant materials in humans. Clin. Orthop. Relat. Res. 1996, 326, 63–70. [Google Scholar] [CrossRef] [PubMed]
- Chung, L.; Maestas, D.R., Jr.; Housseau, F.; Elisseeff, J.H. Key players in the immune response to biomaterial scaffolds for regenerative medicine. Adv. Drug Deliv. Rev. 2017, 114, 184–192. [Google Scholar] [CrossRef] [PubMed]
- Julier, Z.; Park, A.J.; Briquez, P.S.; Martino, M.M. Promoting tissue regeneration by modulating the immune system. Acta Biomater. 2017, 53, 13–28. [Google Scholar] [CrossRef]
- Trindade, R.; Albrektsson, T.; Tengvall, P.; Wennerberg, A. Foreign Body Reaction to Biomaterials: On Mechanisms for Buildup and Breakdown of Osseointegration. Clin. Implant Dent. Relat. Res. 2016, 18, 192–203. [Google Scholar] [CrossRef]
- Uchihara, Y.; Grammatopoulos, G.; Munemoto, M.; Matharu, G.; Inagaki, Y.; Pandit, H.; Tanaka, Y.; Athanasou, N.A. Implant failure in bilateral metal-on-metal hip resurfacing arthroplasties: A clinical and pathological study. J. Mater. Sci. Mater. Med. 2018, 29, 28. [Google Scholar] [CrossRef]
- Champagne, C.M.; Takebe, J.; Offenbacher, S.; Cooper, L.F. Macrophage cell lines produce osteoinductive signals that include bone morphogenetic protein-2. Bone 2002, 30, 26–31. [Google Scholar] [CrossRef]
- Freytes, D.O.; Kang, J.W.; Marcos-Campos, I.; Vunjak-Novakovic, G. Macrophages modulate the viability and growth of human mesenchymal stem cells. J. Cell. Biochem. 2013, 114, 220–229. [Google Scholar] [CrossRef]
- Rifas, L. T-cell cytokine induction of BMP-2 regulates human mesenchymal stromal cell differentiation and mineralization. J. Cell. Biochem. 2006, 98, 706–714. [Google Scholar] [CrossRef]
- Ono, T.; Takayanagi, H. Osteoimmunology in Bone Fracture Healing. Curr. Osteoporos. Rep. 2017, 15, 367–375. [Google Scholar] [CrossRef] [PubMed]
- Wynn, T.A.; Vannella, K.M. Macrophages in Tissue Repair, Regeneration, and Fibrosis. Immunity 2016, 44, 450–462. [Google Scholar] [CrossRef] [PubMed]
- Oishi, Y.; Manabe, I. Macrophages in inflammation, repair and regeneration. Int. Immunol. 2018, 30, 511–528. [Google Scholar] [CrossRef] [PubMed]
- Yuan, X.; Cao, H.; Wang, J.; Tang, K.; Li, B.; Zhao, Y.; Cheng, M.; Qin, H.; Liu, X.; Zhang, X. Immunomodulatory Effects of Calcium and Strontium Co-Doped Titanium Oxides on Osteogenesis. Front. Immunol. 2017, 8, 1196. [Google Scholar] [CrossRef]
- Kersten, R.F.; van Gaalen, S.M.; de Gast, A.; Oner, F.C. Polyetheretherketone (PEEK) cages in cervical applications: A systematic review. Spine J. 2015, 15, 1446–1460. [Google Scholar] [CrossRef]
- Kurtz, S.M.; Devine, J.N. PEEK biomaterials in trauma, orthopedic, and spinal implants. Biomaterials 2007, 28, 4845–4869. [Google Scholar] [CrossRef]
- Panayotov, I.V.; Orti, V.; Cuisinier, F.; Yachouh, J. Polyetheretherketone (PEEK) for medical applications. J. Mater. Sci. Mater. Med. 2016, 27, 118. [Google Scholar] [CrossRef]
- Horak, Z.; Pokorny, D.; Fulin, P.; Slouf, M.; Jahoda, D.; Sosna, A. [Polyetheretherketone (PEEK). Part I: Prospects for use in orthopaedics and traumatology]. Acta Chir. Orthop. Et Traumatol. Cechoslov. 2010, 77, 463–469. [Google Scholar]
- Pokorny, D.; Fulin, P.; Slouf, M.; Jahoda, D.; Landor, I.; Sosna, A. [Polyetheretherketone (PEEK). Part II: Application in clinical practice]. Acta Chir. Orthop. Et Traumatol. Cechoslov. 2010, 77, 470–478. [Google Scholar]
- Abdullah, M.R.; Goharian, A.; Abdul Kadir, M.R.; Wahit, M.U. Biomechanical and bioactivity concepts of polyetheretherketone composites for use in orthopedic implants-a review. J. Biomed. Mater. Res. Part A 2015, 103, 3689–3702. [Google Scholar] [CrossRef]
- Sussman, E.M.; Halpin, M.C.; Muster, J.; Moon, R.T.; Ratner, B.D. Porous implants modulate healing and induce shifts in local macrophage polarization in the foreign body reaction. Ann. Biomed. Eng. 2014, 42, 1508–1516. [Google Scholar] [CrossRef] [PubMed]
- Kang, S.M.; Rho, J.; Choi, I.S.; Messersmith, P.B.; Lee, H. Norepinephrine: Material-independent, multifunctional surface modification reagent. J. Am. Chem. Soc. 2009, 131, 13224–13225. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.; Dellatore, S.M.; Miller, W.M.; Messersmith, P.B. Mussel-inspired surface chemistry for multifunctional coatings. Science 2007, 318, 426–430. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Wong, H.M.; Wang, W.; Li, P.; Xu, Z.; Chong, E.Y.; Yan, C.H.; Yeung, K.W.; Chu, P.K. Cytocompatibility, osseointegration, and bioactivity of three-dimensional porous and nanostructured network on polyetheretherketone. Biomaterials 2013, 34, 9264–9277. [Google Scholar] [CrossRef]
- Ouyang, L.; Zhao, Y.; Jin, G.; Lu, T.; Li, J.; Qiao, Y.; Ning, C.; Zhang, X.; Chu, P.K.; Liu, X. Influence of sulfur content on bone formation and antibacterial ability of sulfonated PEEK. Biomaterials 2016, 83, 115–126. [Google Scholar] [CrossRef] [PubMed]
- Ouyang, L.; Sun, Z.; Wang, D.; Qiao, Y.; Zhu, H.; Ma, X.; Liu, X. Smart release of doxorubicin loaded on polyetheretherketone (PEEK) surface with 3D porous structure. Colloids Surf. B Biointerfaces 2018, 163, 175–183. [Google Scholar] [CrossRef] [PubMed]
- Edwards, S.L.; Werkmeister, J.A. Mechanical evaluation and cell response of woven polyetheretherketone scaffolds. J. Biomed. Mater. Res. A 2012, 100, 3326–3331. [Google Scholar] [CrossRef]
- Landy, B.C.; Vangordon, S.B.; McFetridge, P.S.; Sikavitsas, V.I.; Jarman-Smith, M. Mechanical and in vitro investigation of a porous PEEK foam for medical device implants. J. Appl. Biomater. Funct. Mater. 2013, 11, e35–e44. [Google Scholar] [CrossRef]
- Torstrick, F.B.; Evans, N.T.; Stevens, H.Y.; Gall, K.; Guldberg, R.E. Do Surface Porosity and Pore Size Influence Mechanical Properties and Cellular Response to PEEK? Clin. Orthop. Relat. Res. 2016, 474, 2373–2383. [Google Scholar] [CrossRef]
- Evans, N.T.; Torstrick, F.B.; Lee, C.S.; Dupont, K.M.; Safranski, D.L.; Chang, W.A.; Macedo, A.E.; Lin, A.S.; Boothby, J.M.; Whittingslow, D.C.; et al. High-strength, surface-porous polyether-ether-ketone for load-bearing orthopedic implants. Acta Biomater. 2015, 13, 159–167. [Google Scholar] [CrossRef]
- Evans, N.T.; Torstrick, F.B.; Safranski, D.L.; Guldberg, R.E.; Gall, K. Local deformation behavior of surface porous polyether-ether-ketone. J. Mech. Behav. Biomed. Mater. 2017, 65, 522–532. [Google Scholar] [CrossRef] [PubMed]
- Yuan, B.; Cheng, Q.; Zhao, R.; Zhu, X.; Yang, X.; Yang, X.; Zhang, K.; Song, Y.; Zhang, X. Comparison of osteointegration property between PEKK and PEEK: Effects of surface structure and chemistry. Biomaterials 2018, 170, 116–126. [Google Scholar] [CrossRef] [PubMed]
- Siddiq, A.R.; Kennedy, A.R. Porous poly-ether ether ketone (PEEK) manufactured by a novel powder route using near-spherical salt bead porogens: Characterisation and mechanical properties. Mater. Sci. Eng. C Mater. Biol. Appl. 2015, 47, 180–188. [Google Scholar] [CrossRef] [PubMed]
- Ahn, H.; Patel, R.R.; Hoyt, A.J.; Lin, A.S.P.; Torstrick, F.B.; Guldberg, R.E.; Frick, C.P.; Carpenter, R.D.; Yakacki, C.M.; Willett, N.J. Biological evaluation and finite-element modeling of porous poly(para-phenylene) for orthopaedic implants. Acta Biomater. 2018, 72, 352–361. [Google Scholar] [CrossRef]
- Roskies, M.; Jordan, J.O.; Fang, D.; Abdallah, M.N.; Hier, M.P.; Mlynarek, A.; Tamimi, F.; Tran, S.D. Improving PEEK bioactivity for craniofacial reconstruction using a 3D printed scaffold embedded with mesenchymal stem cells. J. Biomater. Appl. 2016, 31, 132–139. [Google Scholar] [CrossRef]
- Deng, L.; Deng, Y.; Xie, K. AgNPs-decorated 3D printed PEEK implant for infection control and bone repair. Colloids Surf. B Biointerfaces 2017, 160, 483–492. [Google Scholar] [CrossRef]
- Yuan, X.; Ouyang, L.; Luo, Y.; Sun, Z.; Yang, C.; Wang, J.; Liu, X.; Zhang, X. Multifunctional Sulfonated Polyetheretherketone Coating with Beta-defensin-14 for Yielding Durable and Broad-spectrum Antibacterial Activity and Osseointegration. Acta Biomater. 2019, 86, 323–337. [Google Scholar] [CrossRef]
- Fukuda, N.; Tsuchiya, A.; Toita, R.; Tsuru, K.; Mori, Y.; Ishikawa, K. Surface plasma treatment and phosphorylation enhance the biological performance of poly(ether ether ketone). Colloids Surf. B Biointerfaces 2018, 173, 36–42. [Google Scholar] [CrossRef]
- Becker, M.; Lorenz, S.; Strand, D.; Vahl, C.F.; Gabriel, M. Covalent grafting of the RGD-peptide onto polyetheretherketone surfaces via Schiff base formation. Sci. World J. 2013, 2013, 616535. [Google Scholar] [CrossRef]
- Ajami, S.; Coathup, M.J.; Khoury, J.; Blunn, G.W. Augmenting the bioactivity of polyetheretherketone using a novel accelerated neutral atom beam technique. J. Biomed. Mater. Res. B Appl. Biomater. 2017, 105, 1438–1446. [Google Scholar] [CrossRef]
- Khoury, J.; Maxwell, M.; Cherian, R.E.; Bachand, J.; Kurz, A.C.; Walsh, M.; Assad, M.; Svrluga, R.C. Enhanced bioactivity and osseointegration of PEEK with accelerated neutral atom beam technology. J. Biomed. Mater. Res. B Appl. Biomater. 2017, 105, 531–543. [Google Scholar] [CrossRef] [PubMed]
- Akkan, C.K.; Hammadeh, M.E.; May, A.; Park, H.W.; Abdul-Khaliq, H.; Strunskus, T.; Aktas, O.C. Surface topography and wetting modifications of PEEK for implant applications. Lasers Med. Sci. 2014, 29, 1633–1639. [Google Scholar] [CrossRef] [PubMed]
- Amdjadi, P.; Nojehdehian, H.; Najafi, F.; Ghasemi, A.; Seifi, M.; Dashtimoghadam, E.; Fahimipour, F.; Tayebi, L. Ultraviolet-induced surface grafting of octafluoropentyl methacrylate on polyether ether ketone for inducing antibiofilm properties. J. Biomater. Appl. 2017, 32, 3–11. [Google Scholar] [CrossRef] [PubMed]
- Gao, H.; Zhang, J.; Liu, F.; Ao, Z.; Liu, S.; Zhu, S.; Han, D.; Yang, B. Fabrication of polyaniline nanofiber arrays on poly(etheretherketone) to induce enhanced biocompatibility and controlled behaviours of mesenchymal stem cells. J. Mater. Chem. B 2014, 2, 7192–7200. [Google Scholar] [CrossRef] [PubMed]
- Kizuki, T.; Matsushita, T.; Kokubo, T. Apatite-forming PEEK with TiO2 surface layer coating. J. Mater. Sci. Mater. Med. 2015, 26, 5359. [Google Scholar] [CrossRef]
- Mahjoubi, H.; Buck, E.; Manimunda, P.; Farivar, R.; Chromik, R.; Murshed, M.; Cerruti, M. Surface phosphonation enhances hydroxyapatite coating adhesion on polyetheretherketone and its osseointegration potential. Acta Biomater. 2017, 47, 149–158. [Google Scholar] [CrossRef]
- Vogel, D.; Dempwolf, H.; Baumann, A.; Bader, R. Characterization of thick titanium plasma spray coatings on PEEK materials used for medical implants and the influence on the mechanical properties. J. Mech. Behav. Biomed. Mater. 2018, 77, 600–608. [Google Scholar] [CrossRef]
- Tsou, H.K.; Chi, M.H.; Hung, Y.W.; Chung, C.J.; He, J.L. In Vivo Osseointegration Performance of Titanium Dioxide Coating Modified Polyetheretherketone Using Arc Ion Plating for Spinal Implant Application. BioMed Res. Int. 2015, 2015, 328943. [Google Scholar] [CrossRef]
- Rabiei, A.; Sandukas, S. Processing and evaluation of bioactive coatings on polymeric implants. J. Biomed. Mater. Res. A 2013, 101, 2621–2629. [Google Scholar] [CrossRef] [PubMed]
- Han, C.M.; Lee, E.J.; Kim, H.E.; Koh, Y.H.; Kim, K.N.; Ha, Y.; Kuh, S.U. The electron beam deposition of titanium on polyetheretherketone (PEEK) and the resulting enhanced biological properties. Biomaterials 2010, 31, 3465–3470. [Google Scholar] [CrossRef]
- Hoppe, S.; Albers, C.E.; Elfiky, T.; Deml, M.C.; Milavec, H. First Results of a New Vacuum Plasma Sprayed (VPS) Titanium-Coated Carbon/PEEK Composite Cage for Lumbar Interbody Fusion. J. Funct. Biomater. 2018, 9, 23. [Google Scholar] [CrossRef] [PubMed]
- Tan, K.H.; Chua, C.K.; Leong, K.F.; Cheah, C.M.; Cheang, P.; Abu Bakar, M.S.; Cha, S.W. Scaffold development using selective laser sintering of polyetheretherketone-hydroxyapatite biocomposite blends. Biomaterials 2003, 24, 3115–3123. [Google Scholar] [CrossRef] [PubMed]
- Ma, R.; Tang, S.; Tan, H.; Qian, J.; Lin, W.; Wang, Y.; Liu, C.; Wei, J.; Tang, T. Preparation, characterization, in vitro bioactivity, and cellular responses to a polyetheretherketone bioactive composite containing nanocalcium silicate for bone repair. ACS Appl. Mater. Interfaces 2014, 6, 12214–12225. [Google Scholar] [CrossRef] [PubMed]
- Abu Bakar, M.S.; Cheng, M.H.; Tang, S.M.; Yu, S.C.; Liao, K.; Tan, C.T.; Khor, K.A.; Cheang, P. Tensile properties, tension-tension fatigue and biological response of polyetheretherketone-hydroxyapatite composites for load-bearing orthopedic implants. Biomaterials 2003, 24, 2245–2250. [Google Scholar] [CrossRef] [PubMed]
- Mosser, D.M.; Edwards, J.P. Exploring the full spectrum of macrophage activation. Nat. Rev. Immunol. 2008, 8, 958–969. [Google Scholar] [CrossRef]
- Mantovani, A.; Sica, A.; Sozzani, S.; Allavena, P.; Vecchi, A.; Locati, M. The chemokine system in diverse forms of macrophage activation and polarization. Trends Immunol. 2004, 25, 677–686. [Google Scholar] [CrossRef]
- Ingham, E.; Fisher, J. The role of macrophages in osteolysis of total joint replacement. Biomaterials 2005, 26, 1271–1286. [Google Scholar] [CrossRef]
- Li, T.; Peng, M.; Yang, Z.; Zhou, X.; Deng, Y.; Jiang, C.; Xiao, M.; Wang, J. 3D-printed IFN-gamma-loading calcium silicate-beta-tricalcium phosphate scaffold sequentially activates M1 and M2 polarization of macrophages to promote vascularization of tissue engineering bone. Acta Biomater. 2018, 71, 96–107. [Google Scholar] [CrossRef]
- Chen, Z.; Mao, X.; Tan, L.; Friis, T.; Wu, C.; Crawford, R.; Xiao, Y. Osteoimmunomodulatory properties of magnesium scaffolds coated with beta-tricalcium phosphate. Biomaterials 2014, 35, 8553–8565. [Google Scholar] [CrossRef]
- Chen, Z.; Bachhuka, A.; Wei, F.; Wang, X.; Liu, G.; Vasilev, K.; Xiao, Y. Nanotopography-based strategy for the precise manipulation of osteoimmunomodulation in bone regeneration. Nanoscale 2017, 9, 18129–18152. [Google Scholar] [CrossRef]
- Chen, Z.; Bachhuka, A.; Han, S.; Wei, F.; Lu, S.; Visalakshan, R.M.; Vasilev, K. Tuning Chemistry and Topography of Nanoengineered Surfaces to Manipulate Immune Response for Bone Regeneration Applications. ACS Nano 2017, 11, 4494–4506. [Google Scholar] [CrossRef] [PubMed]
- Hotchkiss, K.M.; Reddy, G.B.; Hyzy, S.L.; Schwartz, Z.; Boyan, B.D.; Olivares-Navarrete, R. Titanium surface characteristics, including topography and wettability, alter macrophage activation. Acta Biomater. 2016, 31, 425–434. [Google Scholar] [CrossRef] [PubMed]
- Lee, C.H.; Kim, Y.J.; Jang, J.H.; Park, J.W. Modulating macrophage polarization with divalent cations in nanostructured titanium implant surfaces. Nanotechnology 2016, 27, 085101. [Google Scholar] [CrossRef] [PubMed]
- Guo, S.; Zhu, X.; Li, M.; Shi, L.; Ong, J.L.; Janczewski, D.; Neoh, K.G. Parallel Control over Surface Charge and Wettability Using Polyelectrolyte Architecture: Effect on Protein Adsorption and Cell Adhesion. ACS Appl. Mater. Interfaces 2016, 8, 30552–30563. [Google Scholar] [CrossRef]
- Bacakova, L.; Filova, E.; Parizek, M.; Ruml, T.; Svorcik, V. Modulation of cell adhesion, proliferation and differentiation on materials designed for body implants. Biotechnol. Adv. 2011, 29, 739–767. [Google Scholar] [CrossRef]
- Okada, Y.; Furumatsu, T.; Miyazawa, S.; Fujii, M.; Takahashi, H.; Kimura, H.; Ozaki, T.; Abe, N. Modification of the cell adhesion and hydrophilic characteristics of poly(ether-ether-ketone) by 172-nm xenon excimer radiation. Bio-Med. Mater. Eng. 2015, 25, 169–175. [Google Scholar] [CrossRef]
- Lv, L.; Xie, Y.; Li, K.; Hu, T.; Lu, X.; Cao, Y.; Zheng, X. Unveiling the Mechanism of Surface Hydrophilicity-Modulated Macrophage Polarization. Adv. Healthc. Mater. 2018, 7, e1800675. [Google Scholar] [CrossRef]
- Roach, P.; Farrar, D.; Perry, C.C. Interpretation of protein adsorption: Surface-induced conformational changes. J. Am. Chem. Soc. 2005, 127, 8168–8173. [Google Scholar] [CrossRef]
- Jozefowski, S.; Marcinkiewicz, J. Aggregates of denatured proteins stimulate nitric oxide and superoxide production in macrophages. Inflamm. Res. 2010, 59, 277–289. [Google Scholar] [CrossRef]
- Deng, Z.J.; Liang, M.; Monteiro, M.; Toth, I.; Minchin, R.F. Nanoparticle-induced unfolding of fibrinogen promotes Mac-1 receptor activation and inflammation. Nat. Nanotechnol. 2011, 6, 39–44. [Google Scholar] [CrossRef]
- Tsuchiya, A.; Fukuda, N.; Toita, R. Effect of micro-roughening of poly(ether ether ketone) on bone marrow derived stem cell and macrophage responses, and osseointegration. J. Biomater. Sci. Polym. Ed. 2018, 29, 1375–1388. [Google Scholar] [CrossRef] [PubMed]
- Deng, Y.; Liu, X.; Xu, A.; Wang, L.; Luo, Z.; Zheng, Y.; Deng, F.; Wei, J.; Tang, Z.; Wei, S. Effect of surface roughness on osteogenesis in vitro and osseointegration in vivo of carbon fiber-reinforced polyetheretherketone-nanohydroxyapatite composite. Int. J. Nanomed. 2015, 10, 1425–1447. [Google Scholar] [CrossRef]
- Zhao, Y.; Wong, H.M.; Lui, S.C.; Chong, E.Y.; Wu, G.; Zhao, X.; Wang, C.; Pan, H.; Cheung, K.M.; Wu, S.; et al. Plasma Surface Functionalized Polyetheretherketone for Enhanced Osseo-Integration at Bone-Implant Interface. ACS Appl. Mater. Interfaces 2016, 8, 3901–3911. [Google Scholar] [CrossRef] [PubMed]
- Wessels, I.; Maywald, M.; Rink, L. Zinc as a Gatekeeper of Immune Function. Nutrients 2017, 9, 1286. [Google Scholar] [CrossRef] [PubMed]
- Sharma, A.; Patni, B.; Shankhdhar, D.; Shankhdhar, S.C. Zinc—An indispensable micronutrient. Physiol. Mol. Biol. Plants Int. J. Funct. Plant Biol. 2013, 19, 11–20. [Google Scholar] [CrossRef]
- Maret, W. Zinc and human disease. Met. Ions Life Sci. 2013, 13, 389–414. [Google Scholar] [CrossRef]
- Hojyo, S.; Fukada, T. Roles of Zinc Signaling in the Immune System. J. Immunol. Res. 2016, 2016, 6762343. [Google Scholar] [CrossRef]
- Yan, Y.W.; Fan, J.; Bai, S.L.; Hou, W.J.; Li, X.; Tong, H. Zinc Prevents Abdominal Aortic Aneurysm Formation by Induction of A20-Mediated Suppression of NF-kappaB Pathway. PLoS ONE 2016, 11, e0148536. [Google Scholar] [CrossRef]
- Liu, B.; Jiang, D.; Ou, Y.; Hu, Z.; Jiang, J.; Lei, X. An anti-inflammatory role of A20 zinc finger protein during trauma combined with endotoxin challenge. J. Surg. Res. 2013, 185, 717–725. [Google Scholar] [CrossRef]
- Ooi, T.C.; Chan, K.M.; Sharif, R. Zinc L-carnosine suppresses inflammatory responses in lipopolysaccharide-induced RAW 264.7 murine macrophages cell line via activation of Nrf2/HO-1 signaling pathway. Immunopharmacol. Immunotoxicol. 2017, 39, 259–267. [Google Scholar] [CrossRef]
- Ooi, T.C.; Chan, K.M.; Sharif, R. Zinc Carnosine Inhibits Lipopolysaccharide-Induced Inflammatory Mediators by Suppressing NF-kappab Activation in Raw 264.7 Macrophages, Independent of the MAPKs Signaling Pathway. Biol. Trace Elem. Res. 2016, 172, 458–464. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Lee, J.S.; Kim, D. Exploration of Zinc Oxide Nanoparticles as a Multitarget and Multifunctional Anticancer Nanomedicine. ACS Appl. Mater. Interfaces 2017, 9, 39971–39984. [Google Scholar] [CrossRef] [PubMed]
- Dierichs, L.; Kloubert, V.; Rink, L. Cellular zinc homeostasis modulates polarization of THP-1-derived macrophages. Eur. J. Nutr. 2018, 57, 2161–2169. [Google Scholar] [CrossRef] [PubMed]
- Lima, G.A.; Lima, P.D.; Barros Mda, G.; Vardiero, L.P.; Melo, E.F.; Paranhos-Neto Fde, P.; Madeira, M.; Farias, M.L. Calcium intake: Good for the bones but bad for the heart? An analysis of clinical studies. Arch. Endocrinol. Metab. 2016, 60, 252–263. [Google Scholar] [CrossRef] [PubMed]
- Sawada, R.; Kono, K.; Isama, K.; Haishima, Y.; Matsuoka, A. Calcium-incorporated titanium surfaces influence the osteogenic differentiation of human mesenchymal stem cells. J. Biomed. Mater. Res. A 2013, 101, 2573–2585. [Google Scholar] [CrossRef]
- Tankeu, A.T.; Ndip Agbor, V. Calcium supplementation and cardiovascular risk: A rising concern. J. Clin. Hypertens 2017, 19, 640–646. [Google Scholar] [CrossRef]
- Nakashima, T. [Bone and Calcium Research Update 2015. Regulation of bone remodeling by osteocytes]. Clin. Calcium 2015, 25, 21–28. [Google Scholar]
- Goltzman, D.; Hendy, G.N. The calcium-sensing receptor in bone—mechanistic and therapeutic insights. Nat. Rev. Endocrinol. 2015, 11, 298–307. [Google Scholar] [CrossRef]
- Desai, B.N.; Leitinger, N. Purinergic and calcium signaling in macrophage function and plasticity. Front. Immunol. 2014, 5, 580. [Google Scholar] [CrossRef]
- Hamlet, S.; Ivanovski, S. Inflammatory cytokine response to titanium chemical composition and nanoscale calcium phosphate surface modification. Acta Biomater. 2011, 7, 2345–2353. [Google Scholar] [CrossRef]
- Park, J.W.; Han, S.H.; Hanawa, T. Effects of Surface Nanotopography and Calcium Chemistry of Titanium Bone Implants on Early Blood Platelet and Macrophage Cell Function. BioMed Res. Int. 2018, 2018, 1362958. [Google Scholar] [CrossRef] [PubMed]
- De, A. Wnt/Ca2+ signaling pathway: A brief overview. Acta Biochim. Et Biophys. Sin. 2011, 43, 745–756. [Google Scholar] [CrossRef] [PubMed]
- Kelly, J.C.; Lungchukiet, P.; Macleod, R.J. Extracellular Calcium-Sensing Receptor Inhibition of Intestinal EpithelialTNF Signaling Requires CaSR-Mediated Wnt5a/Ror2 Interaction. Front. Physiol. 2011, 2, 17. [Google Scholar] [CrossRef] [PubMed]
- Kelly, E.K.; Wang, L.; Ivashkiv, L.B. Calcium-activated pathways and oxidative burst mediate zymosan-induced signaling and IL-10 production in human macrophages. J. Immunol. 2010, 184, 5545–5552. [Google Scholar] [CrossRef]
- Lu, T.; Qian, S.; Meng, F.; Ning, C.; Liu, X. Enhanced osteogenic activity of poly ether ether ketone using calcium plasma immersion ion implantation. Colloids Surf. B Biointerfaces 2016, 142, 192–198. [Google Scholar] [CrossRef]
- Oyane, A.; Nakamura, M.; Sakamaki, I.; Shimizu, Y.; Miyata, S.; Miyaji, H. Laser-assisted wet coating of calcium phosphate for surface-functionalization of PEEK. PLoS ONE 2018, 13, e0206524. [Google Scholar] [CrossRef]
- Berndt, T.J.; Schiavi, S.; Kumar, R. “Phosphatonins” and the regulation of phosphorus homeostasis. Am. J. Physiol. Ren. Physiol. 2005, 289, F1170–F1182. [Google Scholar] [CrossRef]
- Berndt, T.; Kumar, R. Phosphatonins and the regulation of phosphate homeostasis. Annu. Rev. Physiol. 2007, 69, 341–359. [Google Scholar] [CrossRef]
- Chen, Z.; Wu, C.; Gu, W.; Klein, T.; Crawford, R.; Xiao, Y. Osteogenic differentiation of bone marrow MSCs by beta-tricalcium phosphate stimulating macrophages via BMP2 signalling pathway. Biomaterials 2014, 35, 1507–1518. [Google Scholar] [CrossRef]
- Chen, Z.; Yuen, J.; Crawford, R.; Chang, J.; Wu, C.; Xiao, Y. The effect of osteoimmunomodulation on the osteogenic effects of cobalt incorporated beta-tricalcium phosphate. Biomaterials 2015, 61, 126–138. [Google Scholar] [CrossRef]
- Park, J.W.; Kim, Y.J.; Jang, J.H.; Kwon, T.G.; Bae, Y.C.; Suh, J.Y. Effects of phosphoric acid treatment of titanium surfaces on surface properties, osteoblast response and removal of torque forces. Acta Biomater. 2010, 6, 1661–1670. [Google Scholar] [CrossRef] [PubMed]
- Villa-Bellosta, R.; Hamczyk, M.R.; Andres, V. Novel phosphate-activated macrophages prevent ectopic calcification by increasing extracellular ATP and pyrophosphate. PLoS ONE 2017, 12, e0174998. [Google Scholar] [CrossRef] [PubMed]
- Rahmitasari, F.; Ishida, Y.; Kurahashi, K.; Matsuda, T.; Watanabe, M.; Ichikawa, T. PEEK with Reinforced Materials and Modifications for Dental Implant Applications. Dent. J. 2017, 5, 35. [Google Scholar] [CrossRef] [PubMed]
- Najeeb, S.; Zafar, M.S.; Khurshid, Z.; Siddiqui, F. Applications of polyetheretherketone (PEEK) in oral implantology and prosthodontics. J. Prosthodont. Res. 2016, 60, 12–19. [Google Scholar] [CrossRef] [PubMed]
- Punchak, M.; Chung, L.K.; Lagman, C.; Bui, T.T.; Lazareff, J.; Rezzadeh, K.; Jarrahy, R.; Yang, I. Outcomes following polyetheretherketone (PEEK) cranioplasty: Systematic review and meta-analysis. J. Clin. Neurosci. 2017, 41, 30–35. [Google Scholar] [CrossRef] [PubMed]
- Montazerian, M.; Zanotto, E.D. Bioactive and inert dental glass-ceramics. J. Biomed. Mater. Res. A 2017, 105, 619–639. [Google Scholar] [CrossRef]
- Konnecke, I.; Serra, A.; El Khassawna, T.; Schlundt, C.; Schell, H.; Hauser, A.; Ellinghaus, A.; Volk, H.D.; Radbruch, A.; Duda, G.N.; et al. T and B cells participate in bone repair by infiltrating the fracture callus in a two-wave fashion. Bone 2014, 64, 155–165. [Google Scholar] [CrossRef]
- Kovach, T.K.; Dighe, A.S.; Lobo, P.I.; Cui, Q. Interactions between MSCs and immune cells: Implications for bone healing. J. Immunol. Res. 2015, 2015, 752510. [Google Scholar] [CrossRef]
- Zhang, L.; Yang, G.; Johnson, B.N.; Jia, X. Three-dimensional (3D) Printed Scaffold and Material Selection for Bone Repair. Acta Biomater 2018, 84, 16–33. [Google Scholar] [CrossRef]
- Yabutsuka, T.; Fukushima, K.; Hiruta, T.; Takai, S.; Yao, T. Fabrication of Bioactive Fiber-reinforced PEEK and MXD6 by Incorporation of Precursor of Apatite. J. Biomed. Mater. Res. B Appl. Biomater. 2018, 106, 2254–2265. [Google Scholar] [CrossRef]
- Fernandez-Moure, J.S. Lost in Translation: The Gap in Scientific Advancements and Clinical Application. Front. Bioeng. Biotechnol. 2016, 4, 43. [Google Scholar] [CrossRef] [PubMed]
- Schwitalla, A.D.; Zimmermann, T.; Spintig, T.; Kallage, I.; Muller, W.D. Fatigue limits of different PEEK materials for dental implants. J. Mech. Behav. Biomed. Mater. 2017, 69, 163–168. [Google Scholar] [CrossRef] [PubMed]
- Li, C.S.; Vannabouathong, C.; Sprague, S.; Bhandari, M. The Use of Carbon-Fiber-Reinforced (CFR) PEEK Material in Orthopedic Implants: A Systematic Review. Clin. Med. Insights. Arthritis Musculoskelet. Disord. 2015, 8, 33–45. [Google Scholar] [CrossRef] [PubMed]
- Du, Z.J.; Bi, G.Q.; Cui, X.T. Electrically Controlled Neurochemical Release from Dual-Layer Conducting Polymer Films for Precise Modulation of Neural Network Activity in Rat Barrel Cortex. Adv. Funct. Mater. 2018, 28, 1703988. [Google Scholar] [CrossRef] [PubMed]
- Deng, Y.; Yang, L.; Huang, X.; Chen, J.; Shi, X.; Yang, W.; Hong, M.; Wang, Y.; Dargusch, M.S.; Chen, Z.G. Dual Ag/ZnO-Decorated Micro-/Nanoporous Sulfonated Polyetheretherketone with Superior Antibacterial Capability and Biocompatibility via Layer-by-Layer Self-Assembly Strategy. Macromol. Biosci. 2018, 18, e1800028. [Google Scholar] [CrossRef] [PubMed]
- Vasconcelos, D.P.; Fonseca, A.C.; Costa, M.; Amaral, I.F.; Barbosa, M.A.; Aguas, A.P.; Barbosa, J.N. Macrophage polarization following chitosan implantation. Biomaterials 2013, 34, 9952–9959. [Google Scholar] [CrossRef] [PubMed]
- Gao, S.; Zhou, J.; Liu, N.; Wang, L.; Gao, Q.; Wu, Y.; Zhao, Q.; Liu, P.; Wang, S.; Liu, Y.; et al. Curcumin induces M2 macrophage polarization by secretion IL-4 and/or IL-13. J. Mol. Cell. Cardiol. 2015, 85, 131–139. [Google Scholar] [CrossRef]
- Ma, Q.L.; Zhao, L.Z.; Liu, R.R.; Jin, B.Q.; Song, W.; Wang, Y.; Zhang, Y.S.; Chen, L.H.; Zhang, Y.M. Improved implant osseointegration of a nanostructured titanium surface via mediation of macrophage polarization. Biomaterials 2014, 35, 9853–9867. [Google Scholar] [CrossRef]
- Cao, J.; Dong, R.; Jiang, L.; Gong, Y.; Yuan, M.; You, J.; Meng, W.; Chen, Z.; Zhang, N.; Weng, Q.; et al. LncRNA-MM2P identified as a Modulator of Macrophage M2 Polarization. Cancer Immunol. Res. 2019, 7, 292–305. [Google Scholar] [CrossRef]
- Deng, B.; Wehling-Henricks, M.; Villalta, S.A.; Wang, Y.; Tidball, J.G. IL-10 triggers changes in macrophage phenotype that promote muscle growth and regeneration. J. Immunol. 2012, 189, 3669–3680. [Google Scholar] [CrossRef]
- Hachim, D.; LoPresti, S.T.; Yates, C.C.; Brown, B.N. Shifts in macrophage phenotype at the biomaterial interface via IL-4 eluting coatings are associated with improved implant integration. Biomaterials 2017, 112, 95–107. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).