Abstract
Freezing of gait (FOG) is a sudden episodic inability to produce effective stepping despite the intention to walk. It typically occurs during gait initiation (GI) or modulation and may lead to falls. We studied the anticipatory postural adjustments (imbalance, unloading, and stepping phase) at GI in 23 patients with Parkinson’s disease (PD) and FOG (PDF), 20 patients with PD and no previous history of FOG (PDNF), and 23 healthy controls (HCs). Patients performed the task when off dopaminergic medications. The center of pressure (CoP) displacement and velocity during imbalance showed significant impairment in both PDNF and PDF, more prominent in the latter patients. Several measurements were specifically impaired in PDF patients, especially the CoP displacement along the anteroposterior axis during unloading. The pattern of segmental center of mass (SCoM) movements did not show differences between groups. The standing postural profile preceding GI did not correlate with outcome measurements. We have shown impaired motor programming at GI in Parkinsonian patients. The more prominent deterioration of unloading in PDF patients might suggest impaired processing and integration of somatosensory information subserving GI. The unaltered temporal movement sequencing of SCoM might indicate some compensatory cerebellar mechanisms triggering time-locked models of body mechanics in PD.
1. Introduction
Freezing of gait (FOG) is a dramatic phenomenon frequently affecting patients with Parkinson’s disease (PD) [1], causing falls, mobility restrictions, and poor quality of life [2,3,4]. FOG is defined as a brief, episodic absence or marked reduction of forward progression of the feet despite the intention to walk [5], which typically occurs when initiating or modulating gait (e.g., turning, obstacle crossing, and so on).
Gait initiation (GI) is a highly challenging task for the balance control system and is of particular interest in the study of neural control of upright posture maintenance during whole-body movement [6]. Specifically, this task allows the precise assessment of anticipatory postural adjustments (APAs; i.e., muscular synergies that precede GI), aiming to destabilize the antigravity postural set by shifting the center of pressure (CoP) to generate a gravitational moment favoring the center of mass (CoM) forward acceleration [7]. APAs are considered a motor program controlled by feedforward mechanisms regulated by the supraspinal locomotor network [8,9,10,11,12]. The selection and scaling of appropriate APAs rely on the ability to use sensory information to determine the body positioning relative to the environment prior to step execution [13,14] and on the intended forthcoming movement (natural, slow, fast, obstacle, and so on) [7,15,16,17]. Striatal dopamine loss, a pathophysiological hallmark of PD, greatly impacts the production of APAs at GI and particularly the CoP displacement and velocity [12]. Only a few studies have specifically investigated the GI task in Parkinsonian patients with a history of FOG (PDF), non-implanted for deep brain stimulation (DBS), and after withdrawal of dopaminergic medication (meds-off state). The stimulation and medication condition should be carefully considered, as both DBS and dopaminergic drugs can variably influence posture and gait in PD [12,18,19,20,21,22,23,24,25,26]. Overall, these studies showed conflicting results, with APAs being reported as normal [27,28,29] or multiple and hypometric [10,30]. Several methodological discrepancies may account for such different findings, including a non-standardized meds-off state [27], imposed (predefined) feet positioning [29], cueing [10,29,30], and specific instructions on the execution of the GI task (e.g., to start walking as quickly as possible [30,31] or while performing a cognitive task [28]). All of these factors can significantly impact and alter APA expression at GI. Specifically, a cued start signal influences motor programming towards normalization, especially in PDF [9,18], similar to the improvements seen with the administration of levodopa for self-generated step initiation [18]. Moreover, the initial feet position [12,22,32] and posture [33,34,35] can significantly impact the biomechanical features of APAs at GI.
Postural changes in particular would have a detrimental impact on APA production. An altered representation of the body position (egocentric representation) may determine a functional re-organization of the supplementary motor area (SMA)-proper, hampering selection and re-scaling of APAs to adapt to the altered postural framework and bradykinetic stepping [33,34,36,37,38].
Our study aims to describe GI alteration in patients with PD and FOG, accounting for the influence of anthropometric measurements (AMs) and the base of support (BoS) and investigating their relationship with the initial posture. We have also addressed the relative timing and movement sequence of each body segment subserving GI.
2. Materials and Methods
2.1. Subjects
We recruited 23 patients with idiopathic PD (according to the U.K. Brain Bank criteria) and an unambiguous, previous history of FOG (PDF; i.e., patients reporting episodes of FOG on a daily basis prior to the experiment). On the day of the experiment, the presence of FOG was confirmed with a clinical evaluation by an experienced neurologist (I.U.I.). In addition, 20 patients with PD and no previous history of FOG (PDNF) and 23 healthy controls (HCs) were also included. HCs and PDNF patients were chosen to match in terms of demographic and clinical data with the PDF group. Subjects with neurological diseases other than PD, including cognitive decline (i.e., Mini-Mental State Examination score < 27), vestibular disorders, and orthopedic impairments that could interfere with gait were excluded. Disease severity was evaluated with the Unified Parkinson’s Disease Rating Scale motor part (UPDRS-III).
2.2. Experimental Protocol
Patients were investigated in practical meds-off state, i.e., in the morning after overnight withdrawal (>12 h) of all dopaminergic drugs.
Kinematic data were recorded using an optoelectronic system with six cameras (sampling rate 60 Hz, SMART 1.10, BTS, Garbagnate Milanese, Italy) and a set of 29 markers placed on anatomical landmarks (temples, acromions, lateral humeral condyles, ulnar styloids, anterior superior iliac spines, middle thighs, lateral femoral condyles, fibula heads, tibial anterior side, lateral malleoli, Achilles tendon insertion, fifth metatarsal heads, halluxes, the seventh cervical vertebra [C7], point of maximum kyphosis, and middle point between the posterior superior iliac spines) [39,40]. Eight additional technical markers were placed on the trochanters, the medial condyles, the medial malleoli, and the first metatarsi for a short calibration trial, which allowed the computation of the AMs and BoS measurements [11,12,41]. Markers traces were filtered with a fifth-order lowpass Butterworth filter (cut-off frequency: 10 Hz [41]). Dynamic measurements were recorded with a force plate working at a sampling rate of 960 Hz (KISTLER 9286A, Winterthur, Switzerland). The resulting signal was low-pass filtered (fifth-order lowpass Butterworth filter) with a cut-off frequency of 30 Hz [11,42].
At the beginning of each trial, subjects stood upright on the force platform at a comfortable stance position for about 30 s. The initial stance position was not standardized to prevent modification of the subject’s usual motor strategy to initiate gait [12].
Participants were instructed to start walking after a self-selected period from a verbal signal, in order to avoid any effect of cueing on GI. The instruction given was as follows: “Start walking at the moment of your choice”. Subjects were not instructed on the stepping leg to use and they moved at their own pace until the end of the walkway. After a training session, at least three consecutive trials were recorded. The principal investigator supervised all participants during the experiment.
2.3. Biomechanical Measurements
2.3.1. Anthropometric Measurements and Base of Support
For each subject, we measured the following AMs (Table 1): body height, inter anterior-superior iliac spine distance, limb length, foot length, body mass, and body mass index. The AMs were recorded over a period of 5 s of standing using eight additional markers, as described in [12]. The AMs were used for the estimation of the CoM of each body segment (SCoM), according to the anthropometric tables and regression equations proposed by [43]. For each trial, the BoS area and BoS width were calculated. We also accounted for feet position asymmetry by measuring the foot alignment, the difference between feet extra-rotation angles, and the BoS opening angle [11,12].

Table 1.
Biomechanical measurements. Abbreviations: AP, anteroposterior; C7, seventh cervical vertebra; CoM, center of mass; CoP, center of pressure; ML, mediolateral; PSIS, posterior superior iliac spine.
2.3.2. Postural Profile
The standing postural profile was characterized by means of trunk, thigh, and shank sagittal angles (Figure 1) [20] computed shortly before the GI execution (during a 1 s window before the onset of the APAs). The trunk angle was defined as the inclination of the line passing through the markers placed on the middle point between the two posterior superior iliac spines and the seventh cervical vertebra with respect to the vertical axis of the laboratory. The thigh angle was calculated as the angle between the vector connecting the knee and hip center of rotation and the vertical axis of the laboratory. The shank angle was computed between the line connecting the joint centers of the knee and ankle and the vertical axis of the laboratory.
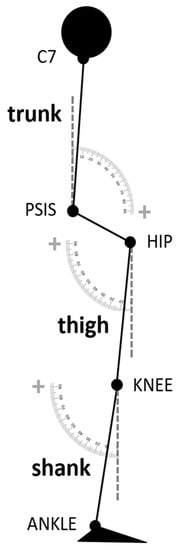
Figure 1.
Scheme of the postural angles analyzed in the study. The trunk angle was defined as the inclination of the line passing through the markers placed on the middle point between the two posterior superior iliac spines and the seventh cervical vertebra with respect to the vertical axis of the laboratory. The thigh angle was calculated as the angle between the vector connecting the knee and hip center of rotation and the vertical axis of the laboratory. The shank angle was computed between the line connecting the joint centers of the knee and ankle and the vertical axis of the laboratory.
2.3.3. Anticipatory Postural Adjustments and Gait Initiation
GI variables were defined based on the displacement of the CoP, recorded by the force platform. The CoM was estimated as the weighted mean of the SCoM [44]. GI variables were calculated by dedicated algorithms in Matlab ambient (Matlab® R2018b, The MathWorks Inc., Natick, MA, USA) (as in [11,12]). All GI measurements computed in the study are listed and described in Table 1. Briefly, four reference instants were automatically identified on the CoP track and checked by visual inspection using an interactive software: the onset of the APAs, the heel-off of the swing foot (HOSW), the toe-off of the swing foot (TOSW), and the toe-off of the stance foot (TOST). The APA onset (APAONSET) was detected as the instant at which the CoP started moving consistently backward and toward the swing foot; HOSW was defined as the time at which CoP reached the most lateral position toward the swing foot; TOSW was defined as the moment at which the CoP shifted from lateral to anterior motion; and TOST was defined as the last frame of the force platform signal (Figure 2). The APAs were divided into two periods: the imbalance phase (IMB), from APAONSET to HOSW, and the unloading phase (UNL), from HOSW to TOSW [12,20,40,45]. The following measurements were calculated for both the IMB and UNL periods: duration and anteroposterior and mediolateral CoP displacement, average velocity, and maximal velocity (Table 1). Of note, the mediolateral CoP displacement during the imbalance phase was considered positive when the shift of the CoP was towards the swing foot, while the mediolateral CoP displacement during the unloading phase was considered positive when the CoP was moving towards the stance foot. The IMB and UNL anteroposterior CoP displacement were both defined as positive when the CoP movement was oriented backwards. We additionally defined the stepping phase, from HOSW to the subsequent heel contact of the swing foot, by means of markers placed on the feet. The first step was characterized in terms of step length and average and maximal velocity (Table 1). Velocity and acceleration of the CoM were defined at the end of the IMB and UNL phases and at the instant of TOST. Additionally, the position of the CoM with respect to the CoP and the inclination of the vector connecting the two points in the transversal plane were computed at the end of IMB and UNL and at the TOST (Table 1) [12].
2.3.4. Segmental Centers of Mass
To describe the temporal pattern of segmental movements during GI, we computed the latency of movement onset of the following 16 SCoM: head, chest, abdomen, pelvis, swing arm, stance arm, swing forearm, stance forearm, swing hand, stance hand, swing thigh, stance thigh, swing shank, stance shank, swing foot, and stance foot (similarly to [46]). For each trial, the movement onset latency of each SCoM was computed as the movement time from the onset of the APAs and normalized for the total GI time (from APAONSET to the toe-off of the swing foot). For each subject, we rank-ordered the SCoM onset times and computed the following for each group: (i) the movement time from APAONSET normalized for the total GI time and (ii) the relative frequency of each SCoM onset time to appear as events 1–16 of GI. To improve the readability of the data, we repeated the analysis after combining the SCoM into six groups (upper trunk: head and chest; lower trunk: abdomen and pelvis; swing arm: swing arm, forearm, and hand; stance arm: stance arm, forearm, and hand; swing leg: swing thigh, shank, and foot; and stance leg: stance thigh, shank, and foot).
2.4. Statistical Analysis
For each subject, all measurements were averaged over GI trials executed with the same swing foot. Each participant performed at least three GI trials with the same swing foot. Single trials and average values were inspected and outliers were removed from further analyses based on the Mahalanobis distance [47,48].
First, we verified matching between groups for demographic, clinic, BoS, and AM features with a Mann–Whitney U-test (p-value set at 0.05). Before comparing the GI measurements across groups, we investigated their relationship with the BoS and AMs with two partial correlation analyses [12]. For each group, we correlated the GI measurements first with the BoS measurements controlling for the AMs, and then with the AMs controlling for the BoS. In agreement with [11], GI variables that significantly correlated (Spearman’s ρ > 0.5 and p-value < 0.01) with the BoS in at least one group were excluded from further analyses. We opted for this conservative approach because the BoS was freely chosen by the subjects and may have been influenced by both the disease and compensatory mechanisms. The GI variables that correlated (Spearman’s ρ > 0.5 and p-value < 0.01) with the AMs were instead corrected by means of the decorrelation normalization technique, as described by O’Malley [49]. This correction was applicable as AMs were not influenced by the disease (no patient had camptocormia, skeletal deformities, and so on).
GI variables not dependent on the BoS and decorrelated from the influence of the AMs were then compared between groups using a Dunn’s test (p-value set at 0.05, adjusted with Bonferroni correction for multiple comparisons).
We then investigated alterations of the initial postural condition. As for the GI measurements, we assessed the correlation of the AMs and the BoS with the postural angles with partial correlation analyses (Spearman’s ρ > 0.5 and p-value < 0.01), before comparing the postural angles across groups (Dunn’s test, p-value set at 0.05, adjusted with Bonferroni correction for multiple comparisons).
As we found differences in the postural profiles across groups, we investigated whether altered GI measurements in the PD groups were related to postural changes rather than to impaired motor programming. We performed a partial correlation analysis between the GI outcome measurements and the postural angles correcting for the group variable. We considered a correlation significant when Spearman’s ρ > 0.5 and p-value < 0.01.
Differences across groups in the SCoM movement onset were analyzed with a Dunn’s test (p-value < 0.05, adjusted with Bonferroni correction for multiple comparisons).
All statistical analyses, except partial correlation analyses performed in Matlab, were performed with the JMP package (JMP® Pro 14.0.0, SAS Institute Inc., Cary, NC, USA).
3. Results
Demographic features, AM measurements, and BoS measurements did not significantly differ between groups (Table 2). Clinical data were similar between PDNF and PDF patients (Table 2).

Table 2.
Demographic, clinical, anthropometric, and base of support features. Data are shown as mean (standard deviation). No statistically significant difference was found across groups (Mann–Whitney U-test, p-value set at 0.05). Abbreviations: HC, healthy controls; LEDD, levodopa equivalent daily dose; PDF, Parkinson’s disease with freezing of gait; PDNF, Parkinson’s disease with no freezing of gait; UPDRS-III, Unified Parkinson’s Disease Rating scale, part III. Refer to Table 1 for a list of other abbreviations used.
Of note, none of the patients showed freezing episodes during GI recordings. Therefore, our results define primarily the impact of APA alterations and postural features in favoring FOG in PD and not a causal correlation with the occurrence of gait freezing episodes at GI.
3.1. Selection of GI Variables
The BoS did not correlate with most of the biomechanical measures of the IMB and stepping phases, but did correlate with the UNL. The results are consistent with our previous findings [12]. The GI variables that were independent from the BoS are listed in Table 3. The BoS and the AMs showed no correlations with the trunk, thigh, and shank angles.

Table 3.
Gait initiation measurements: comparison between groups. Only biomechanical variables not correlated with the base of support are listed. Data are shown as mean (standard deviation). The mediolateral CoP displacement during imbalance and unloading was considered positive when the shift in the CoP was towards the swing and the stance foot, respectively. The anteroposterior CoP displacement during imbalance and unloading phase were both defined as positive when the CoP movement was oriented backwards. Abbreviations: HC, healthy controls; PDF, Parkinson’s disease with freezing of gait; PDNF, Parkinson’s disease with no freezing of gait; refer to Table 1 for a list of other acronyms used.
Table 3.
Gait initiation measurements: comparison between groups. Only biomechanical variables not correlated with the base of support are listed. Data are shown as mean (standard deviation). The mediolateral CoP displacement during imbalance and unloading was considered positive when the shift in the CoP was towards the swing and the stance foot, respectively. The anteroposterior CoP displacement during imbalance and unloading phase were both defined as positive when the CoP movement was oriented backwards. Abbreviations: HC, healthy controls; PDF, Parkinson’s disease with freezing of gait; PDNF, Parkinson’s disease with no freezing of gait; refer to Table 1 for a list of other acronyms used.
| HC | PDNF | ||
|---|---|---|---|
| IMB duration (s) | 0.39 (0.08) | 0.38 (0.08) | 0.33 (0.09) |
| IMB displacement (mm) | 61.23 + (20.32) | 35.54 (19.71) | 23.67 + (9.94) |
| IMB displacement ML (mm) | 42.07 #,+ (13.04) | 24.04 # (13.61) | 17.76 + (8.59) |
| IMB displacement AP (mm) | 36.53 + (16.17) | 18.79 (14.75) | 9.16 + (5.93) |
| IMB average velocity (mm/s) | 163.40 #,+ (62.29) | 90.79 # (47.29) | 84.03 + (46.81) |
| IMB average velocity ML (mm/s) | 110.94 # (34.90) | 62.14 # (34.80) | 67.53 (41.47) |
| IMB average velocity AP (mm/s) | 103.36 #,+ (53.78) | 47.74 # (34.39) | 40.19 + (30.45) |
| IMB maximal velocity (mm/s) | 344.22 #,+ (149.41) | 189.88 # (113.54) | 150.41 + (64.26) |
| IMB maximal velocity ML (mm/s) | 238.29 #,+ (77.82) | 137.25 # (72.76) | 124.97 + (56.96) |
| IMB maximal velocity AP (mm/s) | 225.81 + (110.67) | 124.78 (65.52) | 101.41 + (55.74) |
| IMB end CoM velocity (m/s) | 0.09 + (0.03) | 0.06 (0.03) | 0.04 + (0.02) |
| IMB end CoP–CoM distance (m) | 0.07 + (0.02) | 0.04 (0.02) | 0.03 + (0.01) |
| UNL duration (s) | 0.36 (0.08) | 0.40 (0.08) | 0.45 (0.19) |
| UNL displacement AP (mm) | −9.67 + (15.30) | −6.25 * (18.22) | 14.69 +,* (14.70) |
| UNL average velocity (mm/s) | 465.61 (162.21) | 323.94 (131.02) | 320.34 (150.20) |
| UNL average velocity ML (mm/s) | 422.79 (148.96) | 289.26 (121.24) | 290.67 (140.81) |
| UNL average velocity AP (mm/s) | 53.11 (20.97) | 37.29 (17.16) | 46.04 (34.21) |
| UNL maximal velocity AP (mm/s) | 344.48 (154.35) | 388.76 (169.88) | 359.19 (178.76) |
| UNL end CoM velocity (m/s) | 0.21 + (0.06) | 0.16 (0.07) | 0.11 + (0.04) |
| UNL end CoM acceleration (m/s2) | 1.29 (0.33) | 1.08 (0.41) | 1.12 (0.25) |
| UNL end CoP–CoM distance (m) | 0.08 (0.03) | 0.07 (0.03) | 0.07 (0.02) |
| ST toe-off CoM velocity (m/s) | 0.86 #,+ (0.13) | 0.63 # (0.24) | 0.53 + (0.18) |
| ST toe-off CoM acceleration (m/s2) | 1.73 + (0.38) | 1.28 (0.42) | 1.08 + (0.33) |
| ST toe CoP–CoM distance (m) | 0.48 (0.32) | 0.51 (0.29) | 0.34 (0.28) |
| First step length (m) | 0.56 + (0.07) | 0.43 (0.14) | 0.33 + (0.13) |
Dunn’s test, significant p-value after Bonferroni correction: # HC vs. PDNF, + HC vs. PDF, * PDNF vs. PDF.
3.2. Postural Features
The trunk and thigh angles, but not the shank angle, were significantly altered in both PDNF and PDF patients compared with HCs (Table 4). Parkinsonian patients showed increased forward trunk bending associated with a reduced thigh angle. The trunk was more flexed in PDF patients than in PDNF patients, although this difference did not reach statistical significance. The thigh angle showed a negative average value only in the PDF group.

Table 4.
Postural angles were computed shortly before the gait initiation execution (during a 1 s window before the onset of the anticipatory postural adjustments). Data are shown as mean (standard deviation). Abbreviations: HC, healthy controls; PDF, Parkinson’s disease with freezing of gait; PDNF, Parkinson’s disease with no freezing of gait.
3.3. Effect of PD and History of FOG on GI
We observed significant alterations in the GI execution in both PDNF and PDF patients, with the latter group showing overall more severely altered APA measurements (Table 3, Figure 2).
The CoP displacement and velocity during IMB showed a progressive and significant reduction from HCs to PDNF to PDF groups along both the mediolateral and anteroposterior axes.
The UNL and stepping phases were also altered in PDNF and PDF patients (Table 3, Figure 2). Of most relevance, in PDF patients, the anteroposterior displacement of the CoP during UNL was backwards in most of the trials.
PDF patients had a significantly reduced first step length and both PD groups had a lower first step average velocity compared with HCs.
The CoM forward propulsion (velocity and acceleration) progressively decreased from HCs to PDNF to PDF.
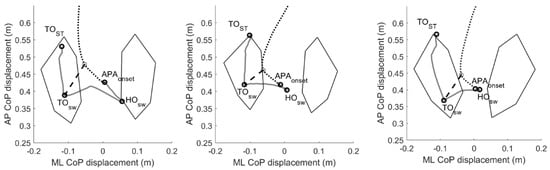
Figure 2.
Two-dimensional center of pressure and center of mass trajectories during gait initiation. Example of the pathway of the center of pressure (CoP, grey solid line) and center of mass (CoM, black dotted line) during a gait initiation trial of one healthy subject (left panel) and one Parkinsonian patient without (PDNF, central panel) and one patient with (PDF, right panel) a positive history of freezing of gait. We defined the imbalance (IMB) phase as the interval between the onset of the APAs (APAONSET) and the heel-off of the swing foot (HOSW), and the unloading phase (UNL) as the interval between the HOSW and the toe-off of the swing foot (TOSW). The black dashed line represents the CoP–CoM vector at the end of the unloading (UNL) phase. With respect to healthy controls, the CoP displacement during the IMB phase was reduced for both PD and PDF patients. The CoP displacement during the UNL phase was in most cases backwards for the PDF patients only. Please see Table 3 for further details. Abbreviations: APAs, anticipatory postural adjustments; AP, anterior–posterior; CoP, center of pressure; HC, healthy controls; HO, heel off; ML, mediolateral; PDF, Parkinson’s disease with freezing of gait; PDNF, Parkinson’s disease with no freezing of gait; TO, toe-off.
3.4. Relationship between the Standing Postural Profile and the GI
We did not find any significant correlation between the postural angles and the GI measurements. However, when not correcting for multiple comparisons, the shank angle was predictive for velocity variables of the IMB phase. The results are shown in Table 5.

Table 5.
Correlation between the shank angle and gait initiation measurements. Only significant partial correlations between postural angles and gait initiation measurements corrected for the influence of the group variable are shown (Spearman’s ρ, p-value < 0.05). No correlation was significant after Bonferroni correction for multiple comparisons.
3.5. Pattern of Movements during GI
The overall pattern of segmental movements during GI did not show clear differences between groups (Table 6 and Table 7). However, PDF showed shorter times of movement onset for almost all ranked segments (Table 7), possibly suggesting tight inter-segmental coupling [50]. All groups started preferably with the swing or stance arm, especially the swing hand for HCs and PDNF and the stance hand for PDF (Figures S1 and S2). The abdomen was often the last body segment moved by HCs and PDNF, but not by PDF (Figure S1). Of note, we observed a remarkable inter-subject variability of SCoM onset times, especially for PD, as shown by the high value of the standard deviation (Table 6) and the large dispersion of the temporal order of SCoM movement onsets (Figure S1), which probably prevented us from capturing statistically significant differences.

Table 6.
Onset of segmental movements at gait initiation. Data are shown as mean (standard deviation). Time of movement onset of each segmental center of mass was expressed as the percentage with respect to total gait initiation duration (i.e., from the onset of the anticipatory postural adjustments to the heel contact of the swing foot) and compared across groups (Dunn’s test, no difference was significant after Bonferroni correction for multiple comparisons). Abbreviations: HC, healthy controls; PDF, Parkinson’s disease with freezing of gait; PDNF, Parkinson’s disease with no freezing of gait; ST: stance limb; SW: swing limb.

Table 7.
Onset of rank-ordered segmental movements at gait initiation. Data are shown as mean (standard deviation). Time of movement onset of rank-ordered segmental centers of mass was expressed as the percentage with respect to total gait initiation duration (i.e., from the onset of the anticipatory postural adjustments to the heel contact of the swing foot) and compared across groups (Dunn’s test, no difference was significant after Bonferroni correction for multiple comparisons). Abbreviations: HC, healthy controls; PDF, Parkinson’s disease with freezing of gait; PDNF, Parkinson’s disease with no freezing of gait.
4. Discussion
This study aimed to evaluate the specific biomechanical alterations of APAs at GI in PD patients with a positive history of FOG, accounting for known confounders such as medication condition, anthropometric measurements, base of support, and initial stance posture. The CoP displacement and velocity during the imbalance phase were altered in both PDNF and PDF patients, but more prominently in the latter group. The CoP displacement along the anteroposterior axis during the unloading phase was impaired only in PDF patients. The order of SCoM movements was unaltered in the two patient groups. The postural profile did not correlate with GI outcome measurements.
Our findings are in line with previous studies in PD that showed an impairment in APAs’ production at GI [20,51]. However, a direct comparison with earlier works is limited because we aimed to minimize possible bias from cueing or imposed postural constraints that are known to affect the execution of the GI task [9,11,12,18,22,52,53,54].
We have now shown that there is a profound alteration of APA execution in PDF patients, which cannot be attributed to specific demographic or clinical features (such as disease severity and duration, medication dose, and efficacy) as the patient groups were matched for all of these features [55].
The IMB phase of APA execution was significantly altered in all PD patients, particularly in PDF (Table 3). Increasing evidence suggests that this GI phase is governed by centrally mediated feedforward signals and involves the cortico-basal ganglia loop, with the SMA-proper and the striatum chiefly contributing to the execution of these pre-programmed movements [6,8,9,11,12,56,57,58,59,60]. In PD, we have previously shown a detrimental effect of striatal dopamine loss in the IMB execution at GI [12]. Recent studies in Parkinsonian patients suggested that striatal dopamine may in part enable normal movement by encoding sensitivity to the energy cost of a movement [61,62,63,64]. Therefore, from the perspective of motor planning, especially of patterned and consolidated motor actions such as APAs, a reduced tonic dopaminergic activity could reframe the coding of the expected energetic costs and impair motor control [63].
In our study, we also showed a prominent alteration in the AP displacement of the CoP during the UNL phase in PDF patients. We interpret this result as a possible alteration, mainly of PDF patients, in the processing and integration of somatosensory information prior to stepping [6,14,65,66]. A chief contribution to integrate proprioceptive and voluntary components for a proper weight transfer during GI can be expected from the premotor–parietal–cerebellar loop [14,58,67,68,69,70,71]. An impaired ability to inhibit stance postural control and initiate stepping and poor set-shifting is also included in pathophysiological hypotheses of FOG in PD [5,10,57,72,73,74,75,76,77].
Despite impaired APA execution, the sequencing of the movement did not show major alterations in the PD groups. We speculate that additional inputs from the cerebellum could overcome impaired information processing by favoring internal movement timing [78]. The efficacy of an online compensatory role of the cerebellum [70,78] is suggested in our study by the relatively preserved SCoM temporal movement sequencing [79], which could have also prevented the appearance of any gait freezing episode during our acquisitions. Relative timing of segmental movements was also described as unaltered in patients with PD by Rosin and colleagues (1997), further suggesting a compensatory rather than detrimental role of the cerebellum in Parkinsonian patients with FOG and balance disturbances [60,78,80]. Of relevance, the high variability in the SCoM movement onsets might have prevented us from detecting differences across groups. Further studies with larger cohorts might further explore this aspect to definitively rule out the presence of PD-related alterations in the movement sequencing.
We envisioned a significant impact of postural abnormalities on GI in PD, but our results did not support this hypothesis. Interestingly, our findings instead confirmed previous physiological studies reporting no correlation between APA execution at GI and the natural inclination of the trunk [33] or of a forward leaning up to 30% of the maximum voluntary lean [35].
Our study suffers from some limitations. First, although we reduced as much as possible the influence of known confounders (i.e., initial feet position and posture, anthropometric parameters, and cues), we cannot fully exclude a residual influence of Parkinsonian symptoms such as bradykinesia and rigidity on the task performance [37]. However, in our previous work [12], we showed that levodopa intake, by improving bradykinesia and rigidity, increases the length and speed of the first step at GI, but does not affect the AP shift during UNL. We can thus hypothesize that the alterations in AP displacement during UNL in the PDF group are not related to akinetic-rigid symptoms, but to impairment of the motor program itself. Future studies are needed to better clarify this aspect. Second, the limited sample size and very stringent statistics may have limited the detection of differences between groups (e.g., SCoM onset times). Third, the lack of a brain imaging evaluation in this study prevents any firm conclusions about our pathophysiological interpretation of the kinematic and dynamic findings, but they match well with the brain metabolic activity changes [66,68] and network derangements [81,82,83,84] described during actual gait and gait freezing episodes in Parkinsonian patients.
In conclusion, our data demonstrate substantial impairment of feedforward motor programming mechanisms at GI in Parkinsonian patients. The deterioration of the UNL and stepping in PDF patients would suggest an additional impaired integration of postural and locomotor programs subserving gait initiation and modulation, which might be partly compensated by cerebellar mechanisms triggering time-locked models of body movement. Postural alterations seem to play a minor role in GI impairment in patients with PD. Last, but not least, our results suggest the potential clinical utility of recording the CoP displacement during GI, and particularly its AP shift during the UNL to identify patients at risk of FOG and to monitor the efficacy of therapeutic strategies. Future longitudinal studies may support this assumption.
Supplementary Materials
The following supporting information can be downloaded at https://www.mdpi.com/article/10.3390/bioengineering9110639/s1, Figure S1: Rank order of segmental CoM onset times; Figure S2: Rank order of onset times of groups of segmental CoM.
Author Contributions
Conceptualization, C.P. and I.U.I.; methodology, C.P. and I.U.I.; software, C.P. and S.H.; formal analysis, C.P., L.B., S.H. and I.U.I.; investigation, C.P. and I.U.I.; resources, C.P., G.P. and I.U.I.; data curation, C.P. and L.B.; writing—original draft preparation, C.P. and L.B.; writing—review and editing, J.V. and I.U.I.; visualization, C.P.; supervision, S.H., J.V. and I.U.I.; project administration, J.V. and I.U.I.; funding acquisition, J.V., G.P. and I.U.I. All authors have read and agreed to the published version of the manuscript.
Funding
The study was sponsored by the Deutsche Forschungsgemeinschaft (DFG, German Research Foundation)—Project-ID 424778381-TRR 295 and the Fondazione Grigioni per il Morbo di Parkinson. C.P. was supported by a grant from the German Excellence Initiative to the Graduate School of Life Sciences, University of Würzburg. L.B. was supported by a grant from New York University School of Medicine and The Marlene and Paolo Fresco Institute for Parkinson’s and Movement Disorders, which was made possible with support from Marlene and Paolo Fresco. This publication was supported by the Open Access Publication Fund of the University of Würzburg.
Institutional Review Board Statement
The study was conducted in accordance with the Declaration of Helsinki and approved by the Ethics Committee of the Università degli Studi di Milano (5/16, 15.02.2016).
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study.
Data Availability Statement
The data presented in this study are available upon request from the corresponding author. The data are not publicly available for privacy reasons.
Acknowledgments
We would like to thank all patients and caregivers for their participation. Our special thanks go to Paolo Cavallari for his help in conducting the study and to Monica Norcini for study management and administrative support. The study was sponsored by the Deutsche Forschungsgemeinschaft (DFG, German Research Foundation)—Project-ID 424778381-TRR 295 and the Fondazione Grigioni per il Morbo di Parkinson. C.P. was supported by a grant from the German Excellence Initiative to the Graduate School of Life Sciences, University of Würzburg. L.B. was supported by a grant from New York University School of Medicine and The Marlene and Paolo Fresco Institute for Parkinson’s and Movement Disorders, which was made possible with support from Marlene and Paolo Fresco. This publication was supported by the Open Access Publication Fund of the University of Würzburg.
Conflicts of Interest
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
References
- Perez-Lloret, S.; Negre-Pages, L.; Damier, P.; Delval, A.; Derkinderen, P.; Destée, A.; Meissner, W.G.; Schelosky, L.; Tison, F.; Rascol, O. Prevalence, Determinants, and Effect on Quality of Life of Freezing of Gait in Parkinson Disease. JAMA Neurol. 2014, 71, 884–890. [Google Scholar] [CrossRef] [PubMed]
- Kerr, G.K.; Worringham, C.J.; Cole, M.H.; Lacherez, P.F.; Wood, J.M.; Silburn, P.A. Predictors of Future Falls in Parkinson Disease. Neurology 2010, 75, 116–124. [Google Scholar] [CrossRef] [PubMed]
- Okada, Y.; Fukumoto, T.; Takatori, K.; Nagino, K.; Hiraoka, K. Abnormalities of the First Three Steps of Gait Initiation in Patients with Parkinson’s Disease with Freezing of Gait. Parkinsons. Dis. 2011, 2011, 202937. [Google Scholar] [CrossRef] [PubMed]
- Pelykh, O.; Klein, A.-M.; Bötzel, K.; Kosutzka, Z.; Ilmberger, J. Dynamics of Postural Control in Parkinson Patients with and without Symptoms of Freezing of Gait. Gait Posture 2015, 42, 246–250. [Google Scholar] [CrossRef]
- Nutt, J.G.; Bloem, B.R.; Giladi, N.; Hallett, M.; Horak, F.B.; Nieuwboer, A. Freezing of Gait: Moving Forward on a Mysterious Clinical Phenomenon. Lancet Neurol. 2011, 10, 734–744. [Google Scholar] [CrossRef]
- Yiou, E.; Caderby, T.; Delafontaine, A.; Fourcade, P.; Honeine, J.-L.L. Balance Control during Gait Initiation: State-of-the-Art and Research Perspectives. World J. Orthop. 2017, 8, 815–828. [Google Scholar] [CrossRef]
- Crenna, P.; Frigo, C. A Motor Programme for the Initiation of Foroward-Oriented Movements in Humans. J. Physiol. 1991, 437, 635–653. [Google Scholar] [CrossRef]
- Petersen, N.T.; Butler, J.E.; Marchand-Pauvert, V.; Fisher, R.; Ledebt, A.; Pyndt, H.S.; Hansen, N.L.; Nielsen, J.B. Suppression of EMG Activity by Transcranial Magnetic Stimulation in Human Subjects during Walking. J. Physiol. 2001, 537, 651–656. [Google Scholar] [CrossRef]
- Hiraoka, K.; Matuo, Y.; Iwata, A.; Onishi, T.; Abe, K. The Effects of External Cues on Ankle Control during Gait Initiation in Parkinson’s Disease. Park. Relat. Disord. 2006, 12, 97–102. [Google Scholar] [CrossRef]
- Jacobs, J.V.; Nutt, J.G.; Carlson-Kuhta, P.; Stephens, M.; Horak, F.B. Knee Trembling during Freezing of Gait Represents Multiple Anticipatory Postural Adjustments. Exp. Neurol. 2009, 215, 334–341. [Google Scholar] [CrossRef]
- Palmisano, C.; Todisco, M.; Marotta, G.; Volkmann, J.; Pacchetti, C.; Frigo, C.A.; Pezzoli, G.; Isaias, I.U. Gait Initiation in Progressive Supranuclear Palsy: Brain Metabolic Correlates. NeuroImage Clin. 2020, 28, 102408. [Google Scholar] [CrossRef]
- Palmisano, C.; Brandt, G.; Vissani, M.; Pozzi, N.G.; Canessa, A.; Brumberg, J.; Marotta, G.; Volkmann, J.; Mazzoni, A.; Pezzoli, G.; et al. Gait Initiation in Parkinson’s Disease: Impact of Dopamine Depletion and Initial Stance Condition. Front. Bioeng. Biotechnol. 2020, 8, 137. [Google Scholar] [CrossRef]
- Inglis, J.T.; Horak, F.B.; Shupert, C.L.; Jones-Rycewicz, C. The Importance of Somatosensory Information in Triggering and Scaling Automatic Postural Responses in Humans. Exp. Brain Res. 1994, 101, 159–164. [Google Scholar] [CrossRef] [PubMed]
- Mouchnino, L.; Fontan, A.; Tandonnet, C.; Perrier, J.; Saradjian, A.; Blouin, J.; Simoneau, M. Facilitation of Cutaneous Inputs during the Planning Phase of Gait Initiation. J. Neurophysiol. 2015, 114, 301–308. [Google Scholar] [CrossRef] [PubMed]
- Brenière, Y.; Cuong Do, M.; Bouisset, S. Are Dynamic Phenomena Prior to Stepping Essential to Walking? J. Mot. Behav. 1987, 19, 62–76. [Google Scholar] [CrossRef] [PubMed]
- Lepers, R.; Brenière, Y. The Role of Anticipatory Postural Adjustments and Gravity in Gait Initiation. Exp. Brain Res. 1995, 107, 118–124. [Google Scholar] [CrossRef] [PubMed]
- Caderby, T.; Yiou, E.; Peyrot, N.; Begon, M.; Dalleau, G. Influence of Gait Speed on the Control of Mediolateral Dynamic Stability during Gait Initiation. J. Biomech. 2014, 47, 417–423. [Google Scholar] [CrossRef]
- Burleigh-Jacobs, A.; Horak, F.B.; Nutt, J.G.; Obeso, J.A. Step Initiation in Parkinson’s Disease: Influence of Levodopa and External Sensory Triggers. Mov. Disord. 1997, 12, 206–215. [Google Scholar] [CrossRef]
- Frank, J.S.; Horak, F.B.; Nutt, J. Centrally Initiated Postural Adjustments in Parkinsonian Patients on and off Levodopa. J. Neurophysiol. 2000, 84, 2440–2448. [Google Scholar] [CrossRef]
- Crenna, P.; Carpinella, I.; Rabuffetti, M.; Rizzone, M.; Lopiano, L.; Lanotte, M.; Ferrarin, M. Impact of Subthalamic Nucleus Stimulation on the Initiation of Gait in Parkinson’s Disease. Exp. Brain Res. 2006, 172, 519–532. [Google Scholar] [CrossRef]
- Liu, W.; McIntire, K.; Kim, S.H.; Zhang, J.; Dascalos, S.; Lyons, K.E.; Pahwa, R. Bilateral Subthalamic Stimulation Improves Gait Initiation in Patients with Parkinson’s Disease. Gait Posture 2006, 23, 492–498. [Google Scholar] [CrossRef]
- Rocchi, L.; Chiari, L.; Mancini, M.; Carlson-Kuhta, P.; Gross, A.; Horak, F.B. Step Initiation in Parkinson’s Disease: Influence of Initial Stance Conditions. Neurosci. Lett. 2006, 406, 128–132. [Google Scholar] [CrossRef] [PubMed]
- Chastan, N.; Do, M.C.; Bonneville, F.; Torny, F.; Bloch, F.; Westby, G.W.M.; Dormont, D.; Agid, Y.; Welter, M.L. Gait and Balance Disorders in Parkinson’s Disease: Impaired Active Braking of the Fall of Centre of Gravity. Mov. Disord. 2009, 24, 188–195. [Google Scholar] [CrossRef] [PubMed]
- Pötter-Nerger, M.; Volkmann, J. Deep Brain Stimulation for Gait and Postural Symptoms in Parkinson’s Disease. Mov. Disord. 2013, 28, 1609–1615. [Google Scholar] [CrossRef]
- Mazzone, P.; Paoloni, M.; Mangone, M.; Santilli, V.; Insola, A.; Fini, M.; Scarnati, E. Unilateral Deep Brain Stimulation of the Pedunculopontine Tegmental Nucleus in Idiopathic Parkinson’s Disease: Effects on Gait Initiation and Performance. Gait Posture 2014, 40, 357–362. [Google Scholar] [CrossRef] [PubMed]
- Curtze, C.; Nutt, J.G.; Carlson-Kuhta, P.; Mancini, M.; Horak, F.B. Levodopa Is a Double-Edged Sword for Balance and Gait in People With Parkinson’s Disease. Mov. Disord. 2015, 30, 1361–1370. [Google Scholar] [CrossRef]
- Nonnekes, J.; Geurts, A.C.H.; Nijhuis, L.B.O.; van Geel, K.; Snijders, A.H.; Bloem, B.R.; Weerdesteyn, V. Reduced StartReact Effect and Freezing of Gait in Parkinson’s Disease: Two of a Kind? J. Neurol. 2014, 261, 943–950. [Google Scholar] [CrossRef]
- de Souza Fortaleza, A.C.; Mancini, M.; Carlson-Kuhta, P.; King, L.A.; Nutt, J.G.; Chagas, E.F.; Freitas, I.F.; Horak, F.B. Dual Task Interference on Postural Sway, Postural Transitions and Gait in People with Parkinson’s Disease and Freezing of Gait. Gait Posture 2017, 56, 76–81. [Google Scholar] [CrossRef]
- Schlenstedt, C.; Mancini, M.; Nutt, J.; Hiller, A.P.; Maetzler, W.; Deuschl, G.; Horak, F. Are Hypometric Anticipatory Postural Adjustments Contributing to Freezing of Gait in Parkinson’s Disease? Front. Aging Neurosci. 2018, 10, 36. [Google Scholar] [CrossRef]
- Cohen, R.G.; Nutt, J.G.; Horak, F.B. Recovery from Multiple APAs Delays Gait Initiation in Parkinson’s Disease. Front. Hum. Neurosci. 2017, 11, 60. [Google Scholar] [CrossRef]
- Bayot, M.; Delval, A.; Moreau, C.; Defebvre, L.; Hansen, C.; Maetzler, W.; Schlenstedt, C. Initial Center of Pressure Position Prior to Anticipatory Postural Adjustments during Gait Initiation in People with Parkinson’s Disease with Freezing of Gait. Park. Relat. Disord. 2021, 84, 8–14. [Google Scholar] [CrossRef] [PubMed]
- Dalton, E.; Bishop, M.; Tillman, M.D.; Hass, C.J. Simple Change in Initial Standing Position Enhances the Initiation of Gait. Med. Sci. Sports Exerc. 2011, 43, 2352–2358. [Google Scholar] [CrossRef] [PubMed]
- Leteneur, S.; Simoneau, E.; Gillet, C.; Dessery, Y.; Barbier, F. Trunk’s Natural Inclination Influences Stance Limb Kinetics, but Not Body Kinematics, during Gait Initiation in Able Men. PLoS ONE 2013, 8, e55256. [Google Scholar] [CrossRef]
- Fortin, A.P.; Dessery, Y.; Leteneur, S.; Barbier, F.; Corbeil, P. Effect of Natural Trunk Inclination on Variability in Soleus Inhibition and Tibialis Anterior Activation during Gait Initiation in Young Adults. Gait Posture 2015, 41, 378–383. [Google Scholar] [CrossRef] [PubMed]
- Fawver, B.; Roper, J.A.; Sarmento, C.; Hass, C.J. Forward Leaning Alters Gait Initiation Only at Extreme Anterior Postural Positions. Hum. Mov. Sci. 2018, 59, 1–11. [Google Scholar] [CrossRef]
- Yoshii, F.; Moriya, Y.; Ohnuki, T.; Ryo, M.; Takahashi, W. Postural Deformities in Parkinson’s Disease–Mutual Relationships among Neck Flexion, Fore-Bent, Knee-Bent and Lateral-Bent Angles and Correlations with Clinical Predictors. J. Clin. Mov. Disord. 2016, 3, 1. [Google Scholar] [CrossRef]
- Delafontaine, A.; Gagey, O.; Colnaghi, S.; Do, M.C.; Honeine, J.L. Rigid Ankle Foot Orthosis Deteriorates Mediolateral Balance Control and Vertical Braking during Gait Initiation. Front. Hum. Neurosci. 2017, 11, 214. [Google Scholar] [CrossRef]
- Stansfield, B.; Hawkins, K.; Adams, S.; Church, D. Spatiotemporal and Kinematic Characteristics of Gait Initiation across a Wide Speed Range. Gait Posture 2018, 61, 331–338. [Google Scholar] [CrossRef]
- Ferrari, A.; Benedetti, M.G.; Pavan, E.; Frigo, C.; Bettinelli, D.; Rabuffetti, M.; Crenna, P.; Leardini, A. Quantitative Comparison of Five Current Protocols in Gait Analysis. Gait Posture 2008, 28, 207–216. [Google Scholar] [CrossRef]
- Isaias, I.U.; Dipaola, M.; Michi, M.; Marzegan, A.; Volkmann, J.; Roidi, M.L.R.; Frigo, C.A.; Cavallari, P. Gait Initiation in Children with Rett Syndrome. PLoS ONE 2014, 9, e92736. [Google Scholar] [CrossRef]
- Palmisano, C.; Brandt, G.; Pozzi, N.G.; Alice, L.; Maltese, V.; Andrea, C.; Jens, V.; Pezzoli, G.; Frigo, C.A.; Isaias, I.U. Sit-to-Walk Performance in Parkinson’s Disease: A Comparison between Faller and Non-Faller Patients. Clin. Biomech. 2019, 63, 140–146. [Google Scholar] [CrossRef]
- Muniz, A.M.S.; Nadal, J.; Lyons, K.E.; Pahwa, R.; Liu, W. Long-Term Evaluation of Gait Initiation in Six Parkinson’s Disease Patients with Bilateral Subthalamic Stimulation. Gait Posture 2012, 35, 452–457. [Google Scholar] [CrossRef]
- Zatsiorsky, V.M. Kinematics of Human Motion; Human Kinetics: Champaign, IL, USA, 1998. [Google Scholar]
- Dipaola, M.; Pavan, E.E.; Cattaneo, A.; Frazzitta, G.; Pezzoli, G.; Cavallari, P.; Frigo, C.A.; Isaias, I.U. Mechanical Energy Recovery during Walking in Patients with Parkinson Disease. PLoS ONE 2016, 11, e0156420. [Google Scholar] [CrossRef]
- Martin, M.; Shinberg, M.; Kuchibhatla, M.; Ray, L.; Carollo, J.J.; Schenkman, M.L. Gait Initiation in Community-Dwelling Adults with Parkinson Disease: Comparison with Older and Younger Adults without the Disease. Phys. Ther. 2002, 82, 566–577. [Google Scholar] [CrossRef]
- Rosin, R.; Topka, H.; Dichgans, J. Gait Initiation in Parkinson’s Disease. Mov. Disord. 1997, 12, 682–690. [Google Scholar] [CrossRef]
- Mahalanobis, P.C. On the Generalized Distance in Statistics. Proc. Natl. Inst. Sci. India 1936, 2, 49–55. [Google Scholar]
- Farinelli, V.; Hosseinzadeh, L.; Palmisano, C.; Frigo, C. An Easily Applicable Method to Analyse the Ankle-Foot Power Absorption and Production during Walking. Gait Posture 2019, 71, 56–61. [Google Scholar] [CrossRef]
- O’Malley, M.J. Normalization of Temporal-Distance Parameters in Pediatric Gait. J. Biomech. 1996, 29, 619–625. [Google Scholar] [CrossRef]
- Crenna, P.; Carpinella, I.; Rabuffetti, M.; Calabrese, E.; Mazzoleni, P.; Nemni, R.; Ferrarin, M. The Association between Impaired Turning and Normal Straight Walking in Parkinson’s Disease. Gait Posture 2007, 26, 172–178. [Google Scholar] [CrossRef]
- Halliday, S.E.; Winter, D.A.; Frank, J.S.; Patla, A.E.; Prince, F. The Initiation of Gait in Young, Elderly, and Parkinson’s Disease Subjects. Gait Posture 1998, 8, 8–14. [Google Scholar] [CrossRef]
- Dibble, L.E.; Nicholson, D.E.; Shultz, B.; MacWilliams, B.A.; Marcus, R.L.; Moncur, C. Sensory Cueing Effects on Maximal Speed Gait Initiation in Persons with Parkinson’s Disease and Healthy Elders. Gait Posture 2004, 19, 215–225. [Google Scholar] [CrossRef]
- Plate, A.; Klein, K.; Pelykh, O.; Singh, A.; Bötzel, K. Anticipatory Postural Adjustments Are Unaffected by Age and Are Not Absent in Patients with the Freezing of Gait Phenomenon. Exp. Brain Res. 2016, 234, 2609–2618. [Google Scholar] [CrossRef]
- Schlenstedt, C.; Mancini, M.; Horak, F.; Peterson, D. Anticipatory Postural Adjustment During Self-Initiated, Cued, and Compensatory Stepping in Healthy Older Adults and Patients With Parkinson Disease. Arch. Phys. Med. Rehabil. 2017, 98, 1316–1324.e1. [Google Scholar] [CrossRef]
- Heilbron, M.; Scholten, M.; Schlenstedt, C.; Mancini, M.; Schöllmann, A.; Cebi, I.; Pötter-Nerger, M.; Gharabaghi, A.; Weiss, D. Anticipatory Postural Adjustmens Are Modulated by Substantia Nigra Stimulation in People with Parkinson’s Disease and Freezing of Gait. Park. Relat. Disord. 2019, 66, 34–39. [Google Scholar] [CrossRef]
- Lee, K.M.; Chang, K.H.; Roh, J.K. Subregions within the Supplementary Motor Area Activated at Different Stages of Movement Preparation and Execution. Neuroimage 1999, 9, 117–123. [Google Scholar] [CrossRef]
- Jacobs, J.V.; Lou, J.S.; Kraakevik, J.A.; Horak, F.B. The Supplementary Motor Area Contributes to the Timing of the Anticipatory Postural Adjustment during Step Initiation in Participants with and without Parkinson’s Disease. Neuroscience 2009, 164, 877–885. [Google Scholar] [CrossRef]
- Bolzoni, F.; Bruttini, C.; Esposti, R.; Castellani, C.; Cavallari, P. Transcranial Direct Current Stimulation of SMA Modulates Anticipatory Postural Adjustments without Affecting the Primary Movement. Behav. Brain Res. 2015, 291, 407–413. [Google Scholar] [CrossRef]
- Varghese, J.P.; Merino, D.M.; Beyer, K.B.; McIlroy, W.E. Cortical Control of Anticipatory Postural Adjustments Prior to Stepping. Neuroscience 2016, 313, 99–109. [Google Scholar] [CrossRef]
- Richard, A.; Van Hamme, A.; Drevelle, X.; Golmard, J.L.; Meunier, S.; Welter, M.L. Contribution of the Supplementary Motor Area and the Cerebellum to the Anticipatory Postural Adjustments and Execution Phases of Human Gait Initiation. Neuroscience 2017, 358, 181–189. [Google Scholar] [CrossRef]
- Morris, G.; Nevet, A.; Arkadir, D.; Vaadia, E.; Bergman, H. Midbrain Dopamine Neurons Encode Decisions for Future Action. Nat. Neurosci. 2006, 9, 1057–1063. [Google Scholar] [CrossRef]
- Mazzoni, P.; Hristova, A.; Krakauer, J.W. Why Don’t We Move Faster? Parkinson’s Disease, Movement Vigor, and Implicit Motivation. J. Neurosci. 2007, 27, 7105–7116. [Google Scholar] [CrossRef]
- Gepshtein, S.; Li, X.; Snider, J.; Plank, M.; Lee, D.; Poizner, H. Dopamine Function and the Efficiency of Human Movement. J. Cogn. Neurosci. 2014, 26, 645–657. [Google Scholar] [CrossRef]
- Schultz, W. Multiple Dopamine Functions at Different Time Courses. Annu. Rev. Neurosci. 2007, 30, 259–288. [Google Scholar] [CrossRef]
- Ruget, H.; Blouin, J.; Teasdale, N.; Mouchnino, L. Can Prepared Anticipatory Postural Adjustments Be Updated by Proprioception? Neuroscience 2008, 155, 640–648. [Google Scholar] [CrossRef]
- Lhomond, O.; Teasdale, N.; Simoneau, M.; Mouchnino, L. Supplementary Motor Area and Superior Parietal Lobule Restore Sensory Facilitation Prior to Stepping When a Decrease of Afferent Inputs Occurs. Front. Neurol. 2018, 9, 1132. [Google Scholar] [CrossRef]
- Picard, N.; Strick, P.L. Imaging the Premotor Areas. Curr. Opin. Neurobiol. 2001, 11, 663–672. [Google Scholar] [CrossRef]
- Tard, C.; Delval, A.; Devos, D.; Lopes, R.; Lenfant, P.; Dujardin, K.; Hossein-Foucher, C.; Semah, F.; Duhamel, A.; Defebvre, L.; et al. Brain Metabolic Abnormalities during Gait with Freezing in Parkinson’s Disease. Neuroscience 2015, 307, 281–301. [Google Scholar] [CrossRef]
- Wolbers, T.; Hegarty, M.; Büchel, C.; Loomis, J.M. Spatial Updating: How the Brain Keeps Track of Changing Object Locations during Observer Motion. Nat. Neurosci. 2008, 11, 1223–1230. [Google Scholar] [CrossRef]
- Hanakawa, T.; Fukuyama, H.; Katsumi, Y.; Honda, M.; Shibasaki, H. Enhanced Lateral Premotor Activity during Paradoxical Gait in Parkinson’s Disease. Ann. Neurol. 1999, 45, 329–336. [Google Scholar] [CrossRef]
- Voss, M.; Ingram, J.N.; Haggard, P.; Wolpert, D.M. Sensorimotor Attenuation by Central Motor Command Signals in the Absence of Movement. Nat. Neurosci. 2006, 9, 26–27. [Google Scholar] [CrossRef]
- Jacobs, J.V.; Horak, F.B. External Postural Perturbations Induce Multiple Anticipatory Postural Adjustments When Subjects Cannot Pre-Select Their Stepping Foot. Exp. brain Res. 2007, 179, 29–42. [Google Scholar] [CrossRef]
- Amboni, M.; Cozzolino, A.; Longo, K.; Picillo, M.; Barone, P. Freezing of Gait and Executive Functions in Patients with Parkinson’s Disease. Mov. Disord. 2008, 23, 395–400. [Google Scholar] [CrossRef]
- Naismith, S.L.; Shine, J.M.; Lewis, S.J.G. The Specific Contributions of Set-Shifting to Freezing of Gait in Parkinson’s Disease. Mov. Disord. 2010, 25, 1000–1004. [Google Scholar] [CrossRef]
- Heremans, E.; Nieuwboer, A.; Vercruysse, S. Freezing of Gait in Parkinson’s Disease: Where Are We Now? Topical Collection on Movement Disorders. Curr. Neurol. Neurosci. Rep. 2013, 13, 350. [Google Scholar] [CrossRef]
- Cohen, R.G.; Klein, K.A.; Nomura, M.; Fleming, M.; Mancini, M.; Giladi, N.; Nutt, J.G.; Horak, F.B. Inhibition, Executive Function, and Freezing of Gait. J. Parkinsons. Dis. 2014, 4, 111–122. [Google Scholar] [CrossRef]
- Lira, J.L.O.; Ugrinowitsch, C.; Coelho, D.B.; Teixeira, L.A.; de Lima-Pardini, A.C.; Magalhães, F.H.; Barbosa, E.R.; Horak, F.B.; Silva-Batista, C. Loss of Presynaptic Inhibition for Step Initiation in Parkinsonian Individuals with Freezing of Gait. J. Physiol. 2020, 598, 1611–1624. [Google Scholar] [CrossRef]
- Drucker, J.H.; Sathian, K.; Crosson, B.; Krishnamurthy, V.; McGregor, K.M.; Bozzorg, A.; Gopinath, K.; Krishnamurthy, L.C.; Wolf, S.L.; Hart, A.R.; et al. Internally Guided Lower Limb Movement Recruits Compensatory Cerebellar Activity in People with Parkinson’s Disease. Front. Neurol. 2019, 10, 573. [Google Scholar] [CrossRef]
- Avanzino, L.; Pelosin, E.; Vicario, C.M.; Lagravinese, G.; Abbruzzese, G.; Martino, D. Time Processing and Motor Control in Movement Disorders. Front. Hum. Neurosci. 2016, 10, 631. [Google Scholar] [CrossRef]
- Isaias, I.U.; Brumberg, J.; Pozzi, N.G.; Palmisano, C.; Canessa, A.; Marotta, G.; Volkmann, J.; Pezzoli, G. Brain Metabolic Alterations Herald Falls in Patients with Parkinson’s Disease. Ann. Clin. Transl. Neurol. 2020, 7, 579–583. [Google Scholar] [CrossRef]
- Lipski, W.J.; Wozny, T.A.; Alhourani, A.; Kondylis, E.D.; Turner, R.S.; Crammond, D.J.; Richardson, R.M. Dynamics of Human Subthalamic Neuron Phase-Locking to Motor and Sensory Cortical Oscillations during Movement. J. Neurophysiol. 2017, 118, 1472–1487. [Google Scholar] [CrossRef]
- Arnulfo, G.; Pozzi, N.G.; Palmisano, C.; Leporini, A.; Canessa, A.; Brumberg, J.; Pezzoli, G.; Matthies, C.; Volkmann, J.; Isaias, I.U. Phase Matters: A Role for the Subthalamic Network during Gait. PLoS ONE 2018, 13, e0198691. [Google Scholar] [CrossRef]
- Georgiades, M.J.; Shine, J.M.; Gilat, M.; McMaster, J.; Owler, B.; Mahant, N.; Lewis, S.J.G. Hitting the Brakes: Pathological Subthalamic Nucleus Activity in Parkinson’s Disease Gait Freezing. Brain 2019, 142, 3906–3916. [Google Scholar] [CrossRef]
- Pozzi, N.G.; Canessa, A.; Palmisano, C.; Brumberg, J.; Steigerwald, F.; Reich, M.M.; Minafra, B.; Pacchetti, C.; Pezzoli, G.; Volkmann, J.; et al. Freezing of Gait in Parkinson’s Disease Reflects a Sudden Derangement of Locomotor Network Dynamics. Brain 2019, 142, 2037–2050. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).