Applications of Biotechnology to the Craniofacial Complex: A Critical Review
Abstract
1. Introduction
2. Relevant Sections
2.1. Craniofacial Defects
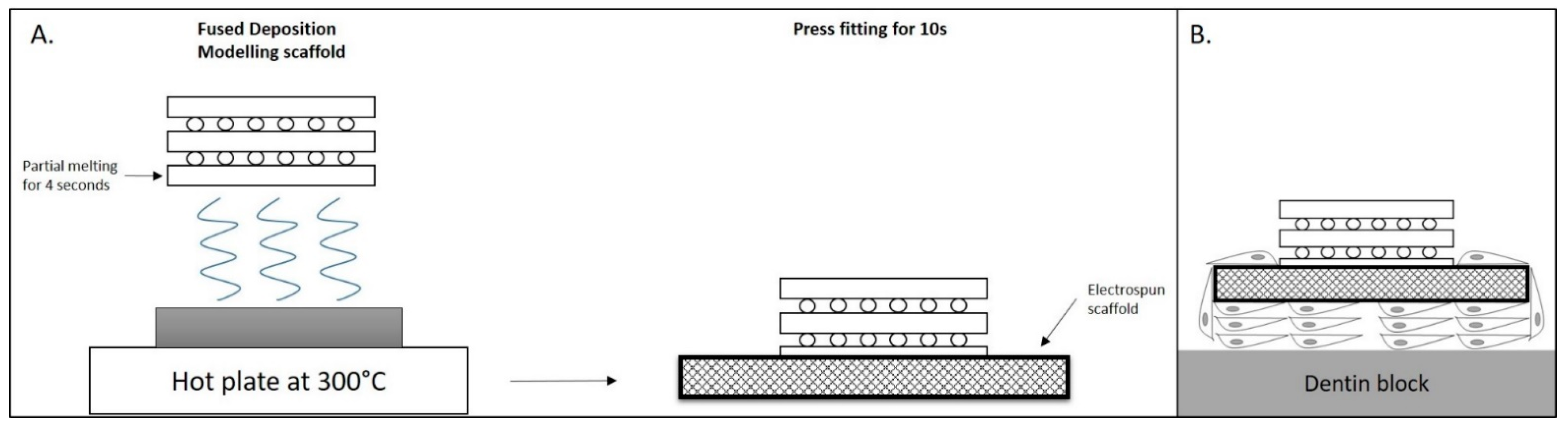
2.2. 3D Printing
2.3. Biomarkers
2.4. TMJ Regeneration
2.5. Tooth Tissue Regeneration
2.6. Polymers
2.6.1. Natural Polymers
2.6.2. Clear Overlay Appliances
3. Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
References
- King, G. Biomedicine in orthodontics: From tooth movement to facial growth. Orthod. Craniofac. Res. 2009, 12, 53–58. [Google Scholar] [CrossRef] [PubMed]
- Sharma, P.; Kumar, P.; Sharma, R.; Bhatt, V.D.; Dhot, P.S. Tissue Engineering; Current Status & Futuristic Scope. J. Med. Life 2019, 12, 225–229. [Google Scholar] [PubMed]
- Miura, M.; Miura, Y.; Sonoyama, W.; Yamaza, T.; Gronthos, S.; Shi, S. Bone marrow-derived mesenchymal stem cells for regenerative medicine in craniofacial region. Oral Dis. 2006, 12, 514–522. [Google Scholar] [CrossRef] [PubMed]
- Zhao, D.W.; Ren, B.; Wang, H.W.; Zhang, X.; Yu, M.Z.; Cheng, L.; Sang, Y.H.; Cao, S.S.; Thieringer, F.M.; Zhang, D.; et al. 3D-printed titanium implant combined with interleukin 4 regulates ordered macrophage polarization to promote bone regeneration and angiogenesis. Bone Jt. Res. 2021, 10, 411–424. [Google Scholar] [CrossRef] [PubMed]
- Friedenstein, A.J.; Petrakova, K.V.; Kurolesova, A.I.; Frolova, G.P. Heterotopic of bone marrow. Analysis of precursor cells for osteogenic and hematopoietic tissues. Transplantation 1968, 6, 230–247. [Google Scholar] [CrossRef]
- Friedenstein, A.J. Precursor cells of mechanocytes. Int. Rev. Cytol. 1976, 47, 327–359. [Google Scholar]
- Owen, M.; Friedenstein, A.J. Stromal stem cells: Marrow-derived osteogenic precursors. Ciba Found Symp. 1988, 136, 42–60. [Google Scholar]
- Asahina, I.; Kagami, H.; Agata, H.; Honda, M.J.; Sumita, Y.; Inoue, M.; Nagamura-Inoue, T.; Tojo, A. Clinical Outcome and 8-Year Follow-Up of Alveolar Bone Tissue Engineering for Severely Atrophic Alveolar Bone Using Autologous Bone Marrow Stromal Cells with Platelet-Rich Plasma and β-Tricalcium Phosphate Granules. J. Clin. Med. 2021, 10, 5231. [Google Scholar] [CrossRef]
- Hollý, D.; Klein, M.; Mazreku, M.; Zamborský, R.; Polák, Š.; Danišovič, Ľ.; Csöbönyeiová, M. Stem Cells and Their Derivatives-Implications for Alveolar Bone Regeneration: A Comprehensive Review. Int. J. Mol. Sci. 2021, 22, 11746. [Google Scholar] [CrossRef]
- Grayson, W.L.; Bunnell, B.A.; Martin, E.; Frazier, T.; Hung, B.P.; Gimble, J.M. Stromal cells and stem cells in clinical bone regeneration. Nat. Rev. Endocrinol. 2015, 11, 140–150. [Google Scholar] [CrossRef]
- Shang, Q.; Wang, Z.; Liu, W.; Shi, Y.; Cui, L.; Cao, Y. Tissue-engineered bone repair of sheep cranial defects with autologous bone marrow stromal cells. J. Craniofac. Surg. 2001, 12, 586–593. [Google Scholar] [CrossRef]
- Abukawa, H.; Zhang, W.; Young, C.S.; Asrican, R.; Vacanti, J.P.; Kaban, L.B.; Troulis, M.J.; Yelick, P.C. Reconstructing mandibular defects using autologous tissue-engineered tooth and bone constructs. J. Oral Maxillofac. Surg. 2009, 67, 335–347. [Google Scholar] [CrossRef]
- Zou, D.; Zhang, Z.; Ye, D.; Tang, A.; Deng, L.; Han, W.; Zhao, J.; Wang, S.; Zhang, W.; Zhu, C.; et al. Repair of critical-sized rat calvarial defects using genetically engineered bone marrow-derived mesenchymal stem cells overexpressing hypoxia-inducible factor-1α. Stem. Cells 2011, 29, 1380–1390. [Google Scholar] [CrossRef]
- Lin, C.Y.; Chang, Y.H.; Kao, C.Y.; Lu, C.H.; Sung, L.Y.; Yen, T.C.; Lin, K.J.; Hu, Y.C. Augmented healing of critical-size calvarial defects by baculovirus-engineered MSCs that persistently express growth factors. Biomaterials 2012, 33, 3682–3692. [Google Scholar] [CrossRef]
- Kim, I.S.; Cho, T.H.; Lee, Z.H.; Hwang, S.J. Bone regeneration by transplantation of human mesenchymal stromal cells in a rabbit mandibular distraction osteogenesis model. Tissue Eng. Part A 2013, 19, 66–78. [Google Scholar] [CrossRef]
- Qi, M.; Hu, J.; Zou, S.; Zhou, H.; Han, L. Mandibular distraction osteogenesis enhanced by bone marrow mesenchymal stem cells in rats. J. Craniomaxillofac. Surg. 2006, 34, 283–289. [Google Scholar] [CrossRef]
- Lai, Q.G.; Yuan, K.F.; Xu, X.; Li, D.R.; Li, G.J.; Wei, F.L.; Yang, Z.J.; Luo, S.L.; Tang, X.P.; Li, S. Transcription factor osterix modified bone marrow mesenchymal stem cells enhance callus formation during distraction osteogenesis. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. Endodontol. 2011, 111, 412–419. [Google Scholar] [CrossRef]
- Hu, J.; Qi, M.C.; Zou, S.J.; Li, J.H.; Luo, E. Callus formation enhanced by BMP-7 ex vivo gene therapy during distraction osteogenesis in rats. J. Orthop. Res. 2007, 25, 241–251. [Google Scholar] [CrossRef]
- Lai, Q.G.; Sun, S.L.; Zhou, X.H.; Zhang, C.P.; Yuan, K.F.; Yang, Z.J.; Luo, S.L.; Tang, X.P.; Ci, J.B. Adipose-derived stem cells transfected with pEGFP-OSX enhance bone formation during distraction osteogenesis. J. Zhejiang Univ. Sci. B 2014, 15, 482–490. [Google Scholar] [CrossRef]
- Long, J.; Li, P.; Du, H.M.; Liu, L.; Zheng, X.H.; Lin, Y.F.; Wang, H.; Jing, W.; Tang, W.; Chen, W.H.; et al. Effects of bone morphogenetic protein 2 gene therapy on new bone formation during mandibular distraction osteogenesis at rapid rate in rabbits. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. Endodontol. 2011, 112, 50–57. [Google Scholar] [CrossRef]
- Khojasteh, A.; Kheiri, L.; Motamedian, S.R.; Nadjmi, N. Regenerative medicine in the treatment of alveolar cleft defect: A systematic review of the literature. J. Craniomaxillofac. Surg. 2015, 43, 1608–1613. [Google Scholar] [CrossRef] [PubMed]
- Lee, D.K.; Ki, M.R.; Kim, E.H.; Park, C.J.; Ryu, J.J.; Jang, H.S.; Pack, S.P.; Jo, Y.K.; Jun, S.H. Biosilicated collagen/β-tricalcium phosphate composites as a BMP-2-delivering bone-graft substitute for accelerated craniofacial bone regeneration. Biomater. Res. 2021, 25, 13. [Google Scholar] [CrossRef]
- Behnia, H.; Khojasteh, A.; Soleimani, M.; Tehranchi, A.; Khoshzaban, A.; Keshel, S.H.; Atashi, R. Secondary repair of alveolar clefts using human mesenchymal stem cells. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. Endodontol. 2009, 108, e1–e6. [Google Scholar] [CrossRef] [PubMed]
- Behnia, H.; Khojasteh, A.; Soleimani, M.; Tehranchi, A.; Atashi, A. Repair of alveolar cleft defect with mesenchymal stem cells and platelet derived growth factors: A preliminary report. J. Craniomaxillofac. Surg. 2012, 40, 2–7. [Google Scholar] [CrossRef] [PubMed]
- Hibi, H.; Yamada, Y.; Ueda, M.; Endo, Y. Alveolar cleft osteoplasty using tissue-engineered osteogenic material. Int. J. Oral Maxillofac. Surg. 2006, 35, 551–555. [Google Scholar] [CrossRef]
- Pradel, W.; Lauer, G. Tissue-engineered bone grafts for osteoplasty in patients with cleft alveolus. Ann. Anat. 2012, 194, 545–548. [Google Scholar] [CrossRef]
- Conejero, J.A.; Lee, J.A.; Parrett, B.M.; Terry, M.; Wear-Maggitti, K.; Grant, R.T.; Breitbart, A.S. Repair of palatal bone defects using osteogenically differentiated fat-derived stem cells. Plast. Reconstr. Surg. 2006, 117, 857–863. [Google Scholar] [CrossRef]
- Rizzo, M.I.; Tomao, L.; Tedesco, S.; Cajozzo, M.; Esposito, M.; De Stefanis, C.; Ferranti, A.M.; Mezzogori, D.; Palmieri, A.; Pozzato, G.; et al. Engineered mucoperiosteal scaffold for cleft palate regeneration towards the non-immunogenic transplantation. Sci. Rep. 2021, 11, 14570. [Google Scholar] [CrossRef]
- Gimbel, M.; Ashley, R.K.; Sisodia, M.; Gabbay, J.S.; Wasson, K.L.; Heller, J.; Wilson, L.; Kawamoto, H.K.; Bradley, J.P. Repair of alveolar cleft defects: Reduced morbidity with bone marrow stem cells in a resorbable matrix. J. Craniofac. Surg. 2007, 18, 895–901. [Google Scholar] [CrossRef]
- Ho, T.V.; Iwata, J.; Ho, H.A.; Grimes, W.C.; Park, S.; Sanchez-Lara, P.A.; Chai, Y. Integration of comprehensive 3D micro-CT and signaling analysis reveals differential regulatory mechanisms of craniofacial bone development. Dev. Biol. 2015, 400, 180–190. [Google Scholar] [CrossRef]
- Derby, B. Printing and prototyping of tissues and scaffolds. Science 2015, 338, 921–926. [Google Scholar] [CrossRef]
- Murphy, S.V.; Atala, A. 3D bioprinting of tissues and organs. Nat. Biotechnol. 2014, 32, 773–785. [Google Scholar] [CrossRef]
- Schek, R.M.; Taboas, J.M.; Hollister, S.J.; Krebsbach, P.H. Tissue engineering osteochondral implants for temporomandibular joint repair. Orthod. Craniofac. Res. 2015, 8, 313–319. [Google Scholar] [CrossRef]
- Reichert, J.C.; Cipitria, A.; Epari, D.R.; Saifzadeh, S.; Krishnakanth, P.; Berner, A.; Woodruff, M.A.; Schell, H.; Mehta, M.; Schuetz, M.A.; et al. A tissue engineering solution for segmental defect regeneration in load-bearing long bones. Sci. Transl. Med. 2012, 4, 141ra93. [Google Scholar] [CrossRef]
- Vaquette, C.; Fan, W.; Xiao, Y.; Hamlet, S.; Hutmacher, D.W.; Ivanovski, S. A biphasic scaffold design combined with cell sheet technology for simultaneous regeneration of alveolar bone/periodontal ligament complex. Biomaterials 2012, 33, 5560–5573. [Google Scholar] [CrossRef]
- Lee, U.L.; Yun, S.; Cao, H.L.; Ahn, G.; Shim, J.H.; Woo, S.H.; Choung, P.H. Bioprinting on 3D Printed Titanium Scaffolds for Periodontal Ligament Regeneration. Cells 2021, 10, 1337. [Google Scholar] [CrossRef]
- Pavek, A.; Nartker, C.; Saleh, M.; Kirkham, M.; Khajeh Pour, S.; Aghazadeh-Habashi, A.; Barrott, J.J. Tissue Engineering Through 3D Bioprinting to Recreate and Study Bone Disease. Biomedicines 2021, 9, 551. [Google Scholar] [CrossRef]
- Kim, K.; Lee, C.H.; Kim, B.K.; Mao, J.J. Anatomically shaped tooth and periodontal regeneration by cell homing. J. Dent. Res. 2010, 89, 842–847. [Google Scholar] [CrossRef]
- Kapoor, P.; Kharbanda, O.P.; Monga, N.; Miglani, R.; Kapila, S. Effect of orthodontic forces on cytokine and receptor levels in gingival crevicular fluid: A systematic review. Prog. Orthod. 2014, 15, 65. [Google Scholar] [CrossRef]
- Kapoor, P.; Monga, N.; Kharbanda, O.P.; Kapila, S.; Miglani, R.; Moganty, R. Effect of orthodontic forces on levels of enzymes in gingival crevicular fluid (GCF): A systematic review. Dent. Press J. Orthod. 2019, 24, 40.e1–40.e22. [Google Scholar] [CrossRef]
- Zhang, B.; Yang, L.; Zheng, W.; Lin, T. MicroRNA-34 expression in gingival crevicular fluid correlated with orthodontic tooth movement. Angle Orthod. 2020, 90, 702–706. [Google Scholar] [CrossRef] [PubMed]
- Atsawasuwan, P.; Lazari, P.; Chen, Y.; Zhou, X.; Viana, G.; Evans, C.A. Secretory microRNA-29 expression in gingival crevicular fluid during orthodontic tooth movement. PLoS ONE 2018, 13, e0194238. [Google Scholar] [CrossRef]
- Wu, L.; Su, Y.; Lin, F.; Zhu, S.; Wang, J.; Hou, Y.; Du, J.; Liu, Y.; Guo, L. MicroRNA-21 promotes orthodontic tooth movement by modulating the RANKL/OPG balance in T cells. Oral Dis. 2020, 26, 370–380. [Google Scholar] [CrossRef] [PubMed]
- Taraslia, V.; Lymperi, S.; Pantazopoulou, V.; Anagnostopoulos, A.K.; Papassideri, I.S.; Basdra, E.K.; Bei, M.; Kontakiotis, E.G.; Tsangaris, G.T.; Stravopodis, D.J.; et al. A High-Resolution Proteomic Landscaping of Primary Human Dental Stem Cells: Identification of SHED- and PDLSC-Specific Biomarkers. Int. J. Mol. Sci. 2018, 19, 158. [Google Scholar] [CrossRef] [PubMed]
- Jones, B.A.; Pei, M. Synovium-derived stem cells: A tissue-specific stem cell for cartilage engineering and regeneration. Tissue Eng. Part B Rev. 2012, 4, 301–311. [Google Scholar] [CrossRef]
- Sakaguchi, Y.; Sekiya, I.; Yagishita, K.; Muneta, T. Comparison of human stem cells derived from various mesenchy- mal tissues: Superiority of synovium as a cell source. Arthritis Rheum. 2005, 8, 2521–2529. [Google Scholar] [CrossRef]
- Pei, M.; He, F.; Boyce, B.M.; Kish, V.L. Repair of full- thickness femoral condyle cartilage defects using allogeneic syno- vial cell-engineered tissue constructs. Osteoarthr. Cartil. 2009, 6, 714–722. [Google Scholar] [CrossRef]
- Yoshimura, H.; Muneta, T.; Nimura, A.; Yokoyama, A.; Koga, H.; Sekiya, I. Comparison of rat mesen- chymal stem cells derived from bone marrow, synovium, periosteum, adipose tissue, and muscle. Cell Tissue Res. 2007, 3, 449–462. [Google Scholar] [CrossRef]
- Wu, Y.; Gong, Z.; Li, J.; Meng, Q.; Fang, W.; Long, X. The pilot study of fibrin with temporomandibular joint derived synovial stem cells in repairing TMJ disc perforation. BioMed. Res. Int. 2014, 2014, 454021. [Google Scholar] [CrossRef]
- Chen, K.; Man, C.; Zhang, B.; Hu, J.; Zhu, S.S. Effect of in vitro chondrogenic differentiation of autologous mesenchy- mal stem cells on cartilage and subchondral cancellous bone repair in osteoarthritis of temporomandibular joint. Int. J. Oral Maxillofac. Surg. 2013, 2, 240–248. [Google Scholar] [CrossRef]
- GGrayson, W.L.; Fröhlich, M.; Yeager, K.; Bhumiratana, S.; Chan, M.E.; Cannizzaro, C.; Vunjak-Novakovic, G. Engineering anatomically shaped human bone grafts. Proc. Natl. Acad. Sci. USA 2010, 8, 3299–3304. [Google Scholar] [CrossRef]
- Murphy, M.K.; MacBarb, R.F.; Wong, M.E.; Athanasiou, K.A. Temporomandibular disorders: A review of etiology, clinical management, and tissue engineering strategies. Int. J. Oral Maxillofac. Implant. 2012, 6, e393–e414. [Google Scholar] [CrossRef]
- Tanaka, E.; Detamore, M.S.; Mercuri, L.G. Degenerative disorders of the temporomandibular joint: Etiology, diagnosis, and treatment. J. Dent. Res. 2008, 4, 296–307. [Google Scholar] [CrossRef]
- Allen, K.D.; Athanasiou, K.A. Scaffold and growth factor selection in temporomandibular joint disc engineering. J. Dent. Res. 2008, 2, 180–185. [Google Scholar] [CrossRef]
- Almarza, A.J.; Athanasiou, K.A. Seeding techniques and scaffolding choice for tissue engineering of the temporoman- dibular joint disc. Tissue Eng. 2004, 10, 1787–1795. [Google Scholar] [CrossRef]
- Chung, C.; Burdick, J.A. Engineering cartilage tissue. Adv. Drug. Deliv. Rev. 2008, 2, 243–262. [Google Scholar] [CrossRef]
- Hagandora, C.K.; Gao, J.; Wang, Y.; Almarza, A.J. Poly (glycerol sebacate): A novel scaffold material for temporo- mandibular joint disc engineering. Tissue Eng. Part A 2013, 19, 729–737. [Google Scholar] [CrossRef]
- Toh, W.S.; Lim, T.C.; Kurisawa, M.; Spector, M. Modulation of mesenchymal stem cell chondrogenesis in a tunable hyaluronic acid hydrogel microenvironment. Biomaterials 2012, 15, 3835–3845. [Google Scholar] [CrossRef]
- Wu, M.; Cai, J.; Yu, Y.; Hu, S.; Wang, Y.; Wu, M. Therapeutic Agents for the Treatment of Temporomandibular Joint Disorders: Progress and Perspective. Front. Pharmacol. 2021, 11, 596099. [Google Scholar] [CrossRef]
- Mitsiadis, T.A.; Orsini, G.; Jimenez-Rojo, L. Stem cell-based approaches in dentistry. Eur. Cell Mater. 2015, 30, 248–257. [Google Scholar] [CrossRef]
- Hu, L.; Liu, Y.; Wang, S. Stem cell-based tooth and periodontal regeneration. Oral Dis. 2018, 5, 696–705. [Google Scholar] [CrossRef] [PubMed]
- Battistella, E.; Varoni, E.M.; Cochis, A.; Palazzo, B.; Rimondini, L. Degradable polymers may improve dental practice. J. Appl. Biomater. Biomech. 2011, 9, 223–231. [Google Scholar] [CrossRef]
- Chung, J.-H.; Kim, Y.K.; Kim, K.-H.; Kwon, T.-Y.; Vaezmomeni, S.Z.; Samiei, M.; Aghazadeh, M.; Davaran, S.; Mahkam, M.; Asadi, G.; et al. Synthesis, characterization, biocompatibility of hydroxyapatite–natural polymers nanocomposites for dentistry applications. Artif. Cells Nanomed. Biotechnol. 2014, 44, 277–284. [Google Scholar] [CrossRef]
- Williams, D.F. On the mechanisms of biocompatibility. Biomaterials 2008, 29, 2941–2953. [Google Scholar] [CrossRef] [PubMed]
- Park, S.; Kim, H.-H.; Bin Yang, S.; Moon, J.-H.; Ahn, H.-W.; Hong, J. A Polysaccharide-Based Antibacterial Coating with Improved Durability for Clear Overlay Appliances. ACS Appl. Mater. Interfaces 2018, 10, 17714–17721. [Google Scholar] [CrossRef]
- Fraile-Martínez, O.; García-Montero, C.; Coca, A.; Álvarez-Mon, M.A.; Monserrat, J.; Gómez-Lahoz, A.M.; Coca, S.; Álvarez-Mon, M.; Acero, J.; Bujan, J.; et al. Applications of Polymeric Composites in Bone Tissue Engineering and Jawbone Regeneration. Polymers 2021, 13, 3429. [Google Scholar] [CrossRef] [PubMed]
- Dupont, K.M.; Sharma, K.; Stevens, H.Y.; Boerckel, J.D.; Garcia, A.J.; Guldberg, R.E. Human stem cell delivery for treatment of large segmental bone defects. Proc. Natl. Acad. Sci. USA 2010, 107, 3305–3310. [Google Scholar] [CrossRef]
- Habisch, H.J.; Janowski, M.; Binder, D.; Kuzma-Kozakiewicz, M.; Widmann, A.; Habich, A.; Schwalenstöcker, B.; Hermann, A.; Brenner, R.; Lukomska, B.; et al. Intrathecal application of neuroectodermally converted stem cells into a mouse model of ALS: Limited intraparenchymal migration and survival narrows therapeutic effects. J. Neural Transm. 2007, 114, 1395–1406. [Google Scholar] [CrossRef]
- Sodek, J.; McKee, M.D. Molecular and cellular biology of alveolar bone. Periodontology 2000, 24, 99–126. [Google Scholar] [CrossRef]
- Bara, J.J.; Herrmann, M.; Menzel, U.; Benneker, L.; Alini, M.; Stoddart, M.J. Three-dimensional culture and characterization of mononuclear cells from human bone marrow. Cytotherapy 2015, 17, 458–472. [Google Scholar] [CrossRef]
- Xiong, Y.; He, J.; Zhang, W.; Zhou, G.; Cao, Y.; Liu, W. Retention of the stemness of mouse adipose-derived stem cells by their expansion on human bone marrow stromal cell-derived extracellular matrix. Tissue Eng. Part A 2015, 21, 1886–1894. [Google Scholar] [CrossRef]
- Thesleff, I.; Tummers, M. Stem cells and tissue engineering: Prospects for regenerating tissue in dental practice. Med. Princ. Pract. 2003, 12 (Suppl. S1), 43–50. [Google Scholar] [CrossRef]
- Ueda, M. Regeneration of tooth and periodontal tissue using tissue engineering concepts. Nippon Rinsho 2003, 61, 439–447. [Google Scholar]
- Mitsiadis, T.A.; Trubiani, O. Editorial—Brothers in arms: Regenerative biology and dentistry. Eur. Cell Mater. 2022, 3, 4–5. [Google Scholar] [CrossRef]
- Young, C.S.; Terada, S.; Vacanti, J.P.; Honda, M.; Barthlett, J.D.; Yelick, P.C. Tissue engineering of complex tooth structures on biodgradable polymer scaffolds. J. Dent. Res. 2002, 81, 695–700. [Google Scholar] [CrossRef] [PubMed]
- Hasani-Sadrabadi, M.M.; Sarrion, P.; Pouraghaei, S.; Chau, Y.; Ansari, S.; Li, S.; Aghaloo, T.; Moshaverinia, A. An engineered cell-laden adhesive hydrogel promotes craniofacial bone tissue regeneration in rats. Sci. Transl. Med. 2020, 534, 12. [Google Scholar] [CrossRef]
- Ouchi, T.; Nakagawa, T. Tissue Regeneration and Physiological Functional Recovery in Dental and Craniofacial Fields. Biomolecules 2021, 11, 1644. [Google Scholar] [CrossRef] [PubMed]
- Young, M.F. Skeletal biology: Where matrix meets mineral. Matrix Biol. 2016, 52–54, 1–6. [Google Scholar] [CrossRef]
- Bajestan, M.N.; Rajan, A.; Edwards, S.P.; Aronovich, S.; Cevidanes, L.H.S.; Polymeri, A.; Travan, S.; Kaigler, D. Stem cell therapy for reconstruction of alveolar cleft and trauma defects in adults: A randomized controlled, clinical trial. Clin. Implant. Dent. Relat. Res. 2017, 5, 793–801. [Google Scholar] [CrossRef]
- Pagella, P.; Cordiale, A.; Marconi, G.D.; Trubiani, O.; Rasponi, M.; Mitsiadis, T.A. Bioengineered tooth emulation systems for regenerative and pharmacological purposes. Eur. Cell Mater. 2021, 10, 502–516. [Google Scholar] [CrossRef] [PubMed]
| Stem Cells | Scaffolds | 3D Printing | Natural or Synthetic Polymers | |
|---|---|---|---|---|
| Craniofacial defects | + | + | + | |
| CLP | + | + | + | |
| TMJ regeneration | + | + | + | |
| Tooth tissue regeneration | + |
| Craniofacial Component | Application |
|---|---|
| Bone Regeneration | MSCs mediate and enhance bone regeneration. They are suitable cells for craniofacial bone repair. BMSCs contribute to the healing of cranial lesions. Stem cells from teeth and distraction osteogenesis are promising factors for face reconstruction. Composite scaffolds of demineralized bone material and calcium phosphate loaded with MSCs result in bone regeneration in cleft areas. |
| Temporomandibular Joint (TMJ) Regeneration | Scaffolds from natural and/or synthetic polymers loaded with MSCs in the TMJ area enable the differentiation of encapsulated MSCs into distinct fibrocartilage formations, and as a result, various types of cartilage tissues are formed. |
| Tooth Regeneration | Dental mesenchymal stem cells are suitable as dental pulp stem cells, and periodontal ligament stem cells and exfoliated deciduous teeth stem cells enhance pulp and periodontal regeneration. |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tsolakis, I.A.; Christopoulou, I.; Papadopoulou, E.; Papaioannou, W.; Alexiou, K.-E.; Lyros, I.; Rontogianni, A.; Souliou, C.-E.; Tsolakis, A.I. Applications of Biotechnology to the Craniofacial Complex: A Critical Review. Bioengineering 2022, 9, 640. https://doi.org/10.3390/bioengineering9110640
Tsolakis IA, Christopoulou I, Papadopoulou E, Papaioannou W, Alexiou K-E, Lyros I, Rontogianni A, Souliou C-E, Tsolakis AI. Applications of Biotechnology to the Craniofacial Complex: A Critical Review. Bioengineering. 2022; 9(11):640. https://doi.org/10.3390/bioengineering9110640
Chicago/Turabian StyleTsolakis, Ioannis A., Isidora Christopoulou, Erofili Papadopoulou, William Papaioannou, Konstantina-Eleni Alexiou, Ioannis Lyros, Aliki Rontogianni, Christina-Efthymia Souliou, and Apostolos I. Tsolakis. 2022. "Applications of Biotechnology to the Craniofacial Complex: A Critical Review" Bioengineering 9, no. 11: 640. https://doi.org/10.3390/bioengineering9110640
APA StyleTsolakis, I. A., Christopoulou, I., Papadopoulou, E., Papaioannou, W., Alexiou, K.-E., Lyros, I., Rontogianni, A., Souliou, C.-E., & Tsolakis, A. I. (2022). Applications of Biotechnology to the Craniofacial Complex: A Critical Review. Bioengineering, 9(11), 640. https://doi.org/10.3390/bioengineering9110640










