UV Sterilization Effects and Osteoblast Proliferation on Amorphous Carbon Films Classified Based on Optical Constants
Abstract
:1. Introduction
2. Materials and Methods
2.1. Amorphous Carbon Film Deposition Conditions
2.2. Spectroscopic Ellipsometer Analysis
2.3. Ultraviolet Sterilization Treatment
2.4. Structural Analysis
2.5. Surface Analysis
2.6. Cell Culture Test
2.7. Statistical Analysis
3. Results and Discussion
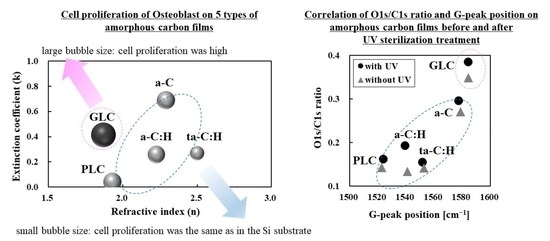
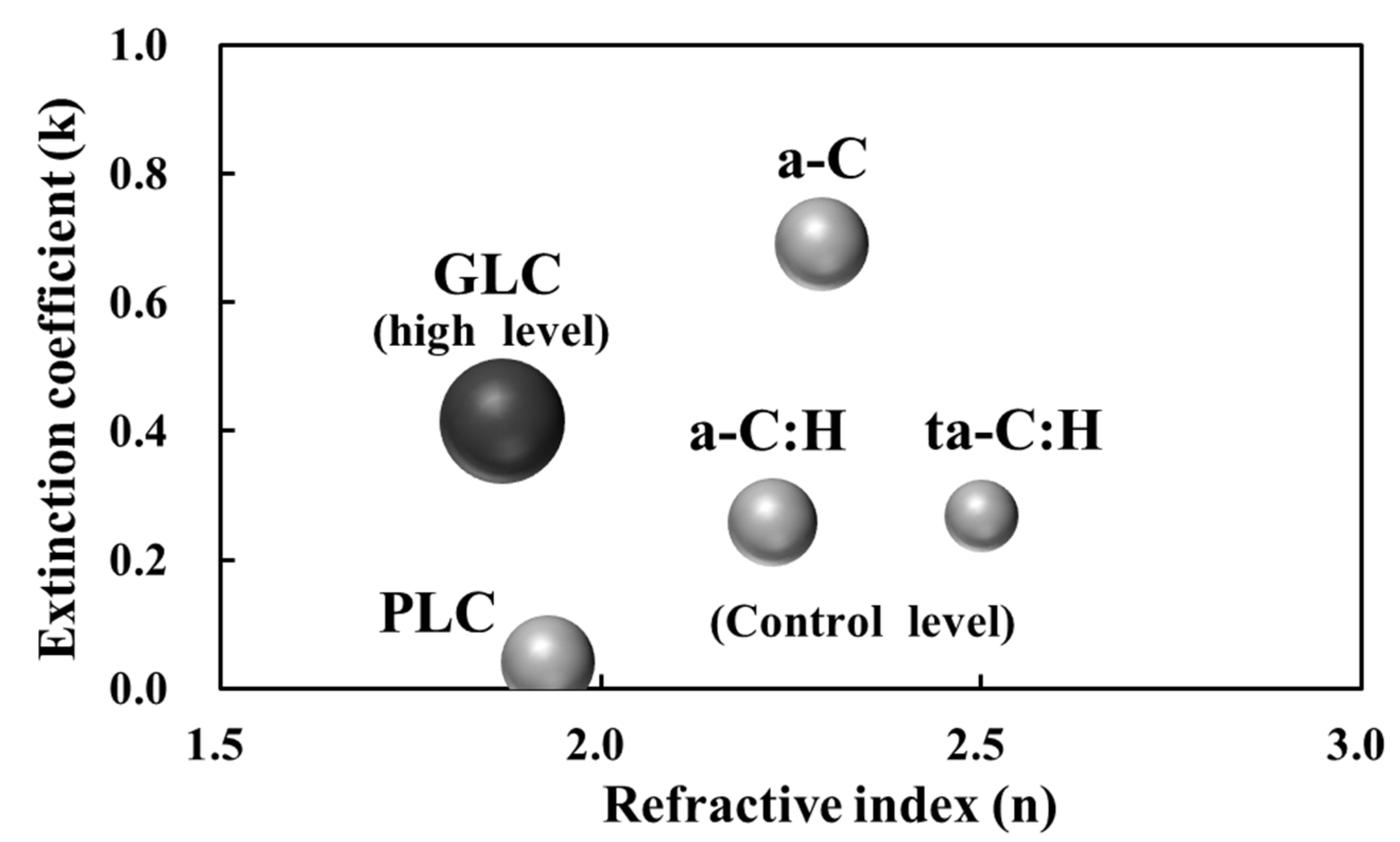
3.1. Classification of Amorphous Carbon Films Based on Optical Constants
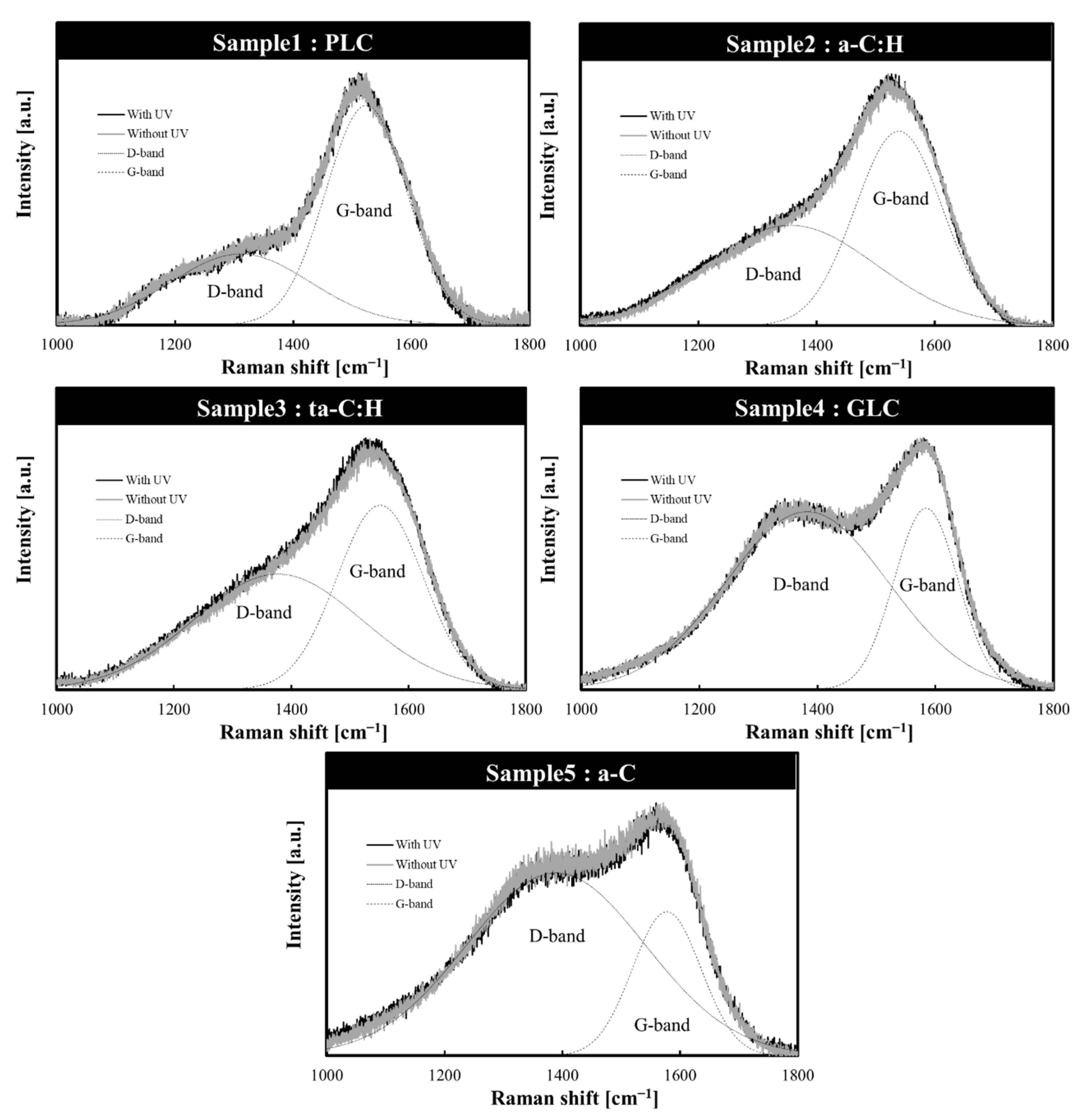
3.2. Structural Changes in Amorphous Carbon Films Following UV Sterilization Treatment
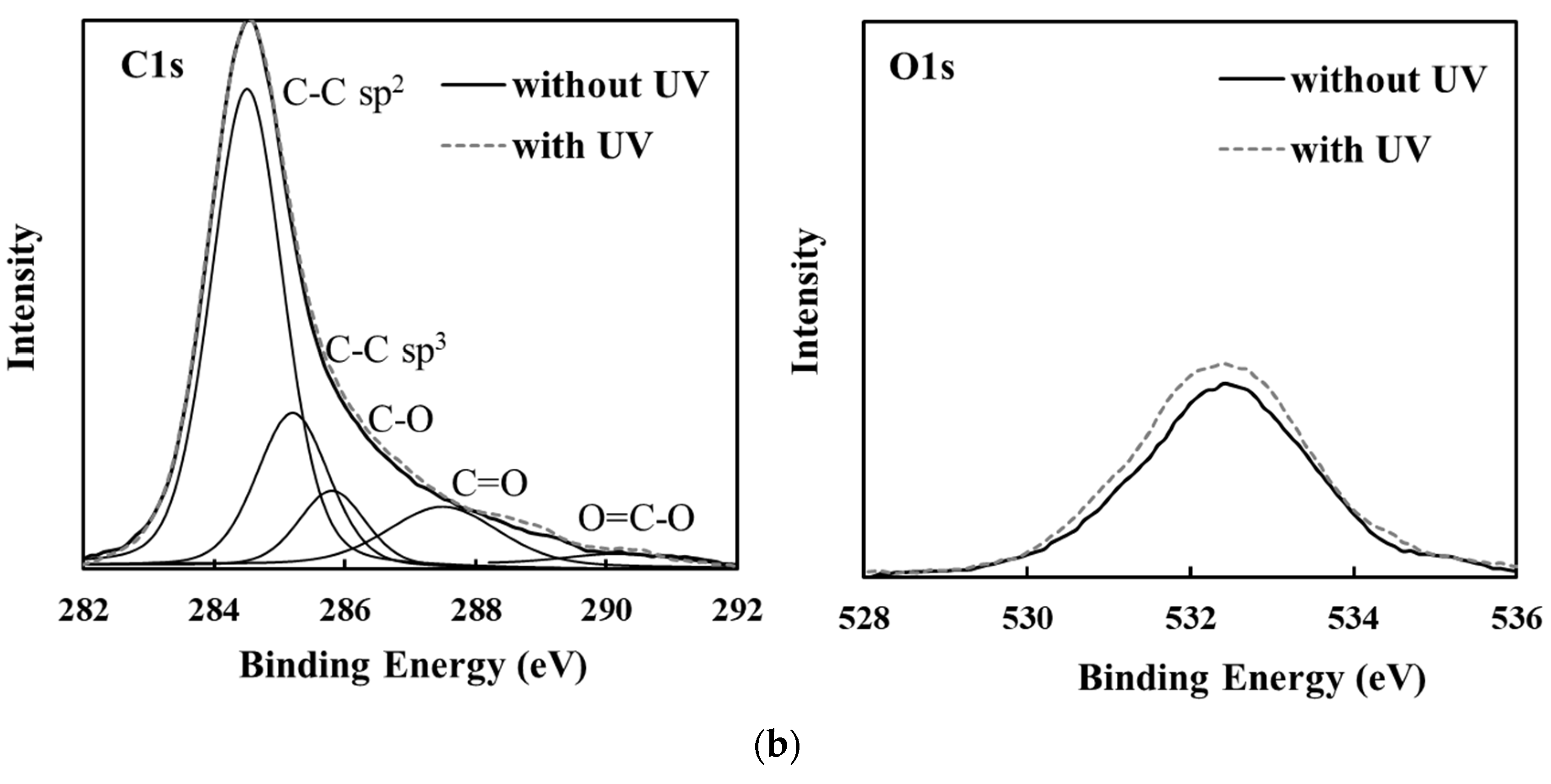
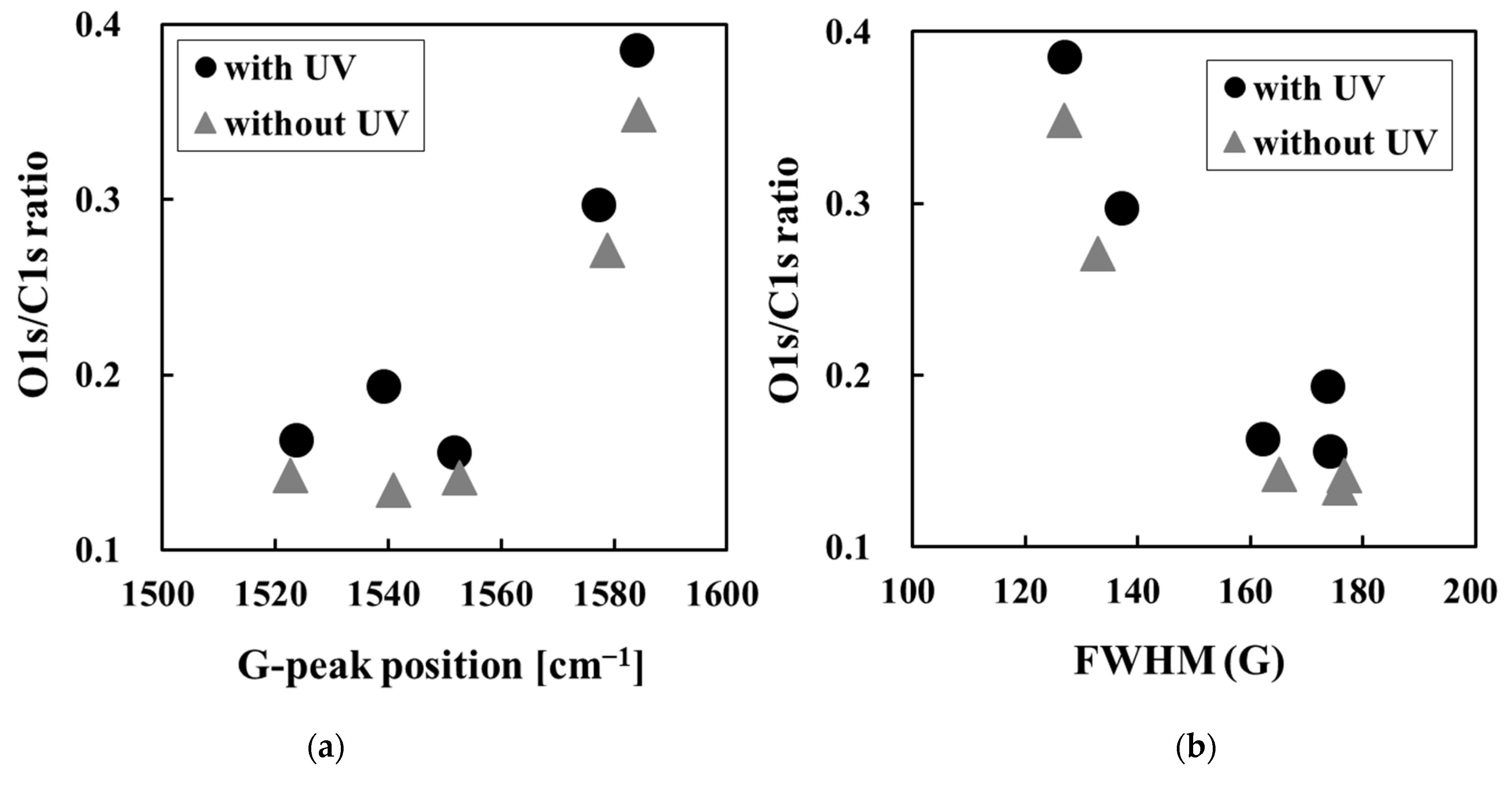
3.3. Changes in Amorphous Carbon Film Surface Properties Following UV Sterilization Treatment
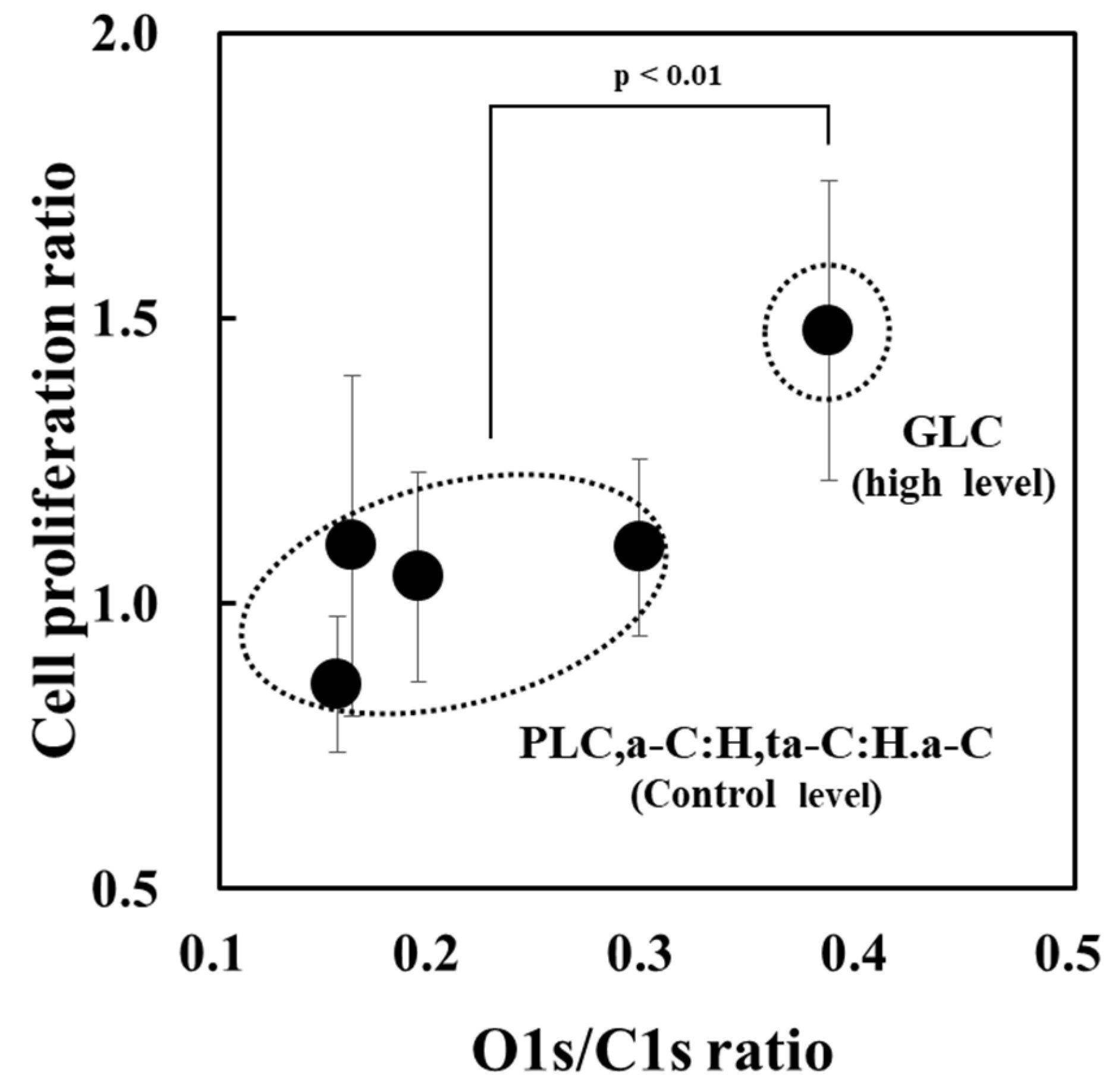
3.4. Classification of Amorphous Carbon for Osteoblast Proliferation Based on Optical Constants
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Robertson, J. Diamond-like amorphous carbon. Mater. Sci. Eng. R Rep. 2002, 37, 129–281. [Google Scholar] [CrossRef]
- Joska, L.; Fojt, J.; Mestek, O.; Cvrcek, L.; Brezina, V. The effect of a DLC coating adhesion layer on the corrosion behavior of titanium and the Ti6Al4V alloy for dental implants. Surf. Coat. Technol. 2012, 206, 4899–4906. [Google Scholar] [CrossRef]
- Grill, A. Diamond-like carbon coatings as biocompatible materials—An overview. Diam. Relat. Mater. 2003, 12, 166–170. [Google Scholar] [CrossRef]
- Dearnaley, G.; Arps, J.H. Biomedical applications of diamond-like carbon (DLC) coatings: A review. Surf. Coat. Technol. 2004, 200, 2518–2524. [Google Scholar] [CrossRef]
- Hauert, R. A review of modified DLC coatings for biological applications. Diam. Relat. Mater. 2003, 12, 583–589. [Google Scholar] [CrossRef]
- Ohtake, N.; Hiratsuka, M.; Kanda, K.; Akasaka, H.; Tsujioka, M.; Hirakuri, K.; Hirata, A.; Ohana, T.; Inaba, H.; Kano, M.; et al. Properties and classification of diamond-like carbon films. Materials 2021, 14, 315. [Google Scholar] [CrossRef] [PubMed]
- Ohgoe, Y.; Hirakuri, K.; Saitoh, H.; Nakahigashi, T.; Ohtake, N.; Hirata, A.; Kanda, K.; Hiratsuka, M.; Fukui, Y. Classification of DLC films in terms of biological response. Surf. Coat. Technol. 2012, 207, 350–354. [Google Scholar] [CrossRef]
- Hiratsuka, M.; Nakamori, H.; Kogo, Y.; Sakurai, M.; Ohtake, N.; Saitoh, H. Correlation between optical properties and hardness of diamond-like carbon films. J. Solid Mech. Mater. Eng. 2013, 7, 187–198. [Google Scholar] [CrossRef]
- Zhou, X.; Suzuki, T.; Nakajima, H.; Komatsu, K.; Kanda, K.; Ito, H.; Saitoh, H. Structural analysis of amorphous carbon films by spectroscopic ellipsometry, RBS/ERDA, and NEXAFS. Appl. Phys. Lett. 2017, 110, 201902. [Google Scholar] [CrossRef]
- Horaguchi, N.; Ishimoto, T.; Kurachi, K.; Umehara, N.; Young, L.W. Sliding surface improvement technology using low friction surface treatment to reduce friction loss and improve efficiency of products. Mitsubishi Heavy Ind. Technol. Rev. 2021, 58, 1–5. [Google Scholar]
- Kanasugi, K.; Ohgoe, Y.; Hiratsuka, M.; Nakamori, H.; Homma, A.; Hirakuri, K. Classification of DLC films for cell proliferation based on optical constants. Diam. Relat. Mater. 2021, 113, 108266. [Google Scholar] [CrossRef]
- Kanasugi, K.; Eguchi, H.; Ohgoe, Y.; Manome, Y.; Alanazi, A.; Hirakuri, K. Correlation of cell proliferation with surface properties of polymer-like carbon films of different thicknesses prepared by a radio-frequency plasma CVD process. Materials 2022, 15, 4466. [Google Scholar] [CrossRef] [PubMed]
- Liao, T.T.; Zhang, T.F.; Li, S.S.; Deng, Q.Y.; Wu, B.J.; Zhang, Y.Z.; Zhou, Y.J.; Guo, Y.B.; Leng, Y.X.; Huang, N. Biological responses of diamond-like carbon (DLC) films with different structures in biomedical application. Mater. Sci. Eng. C Mater. Biol. Appl. 2016, 69, 751–759. [Google Scholar] [CrossRef] [PubMed]
- Ban, M.; Tobe, S.; Takeuchi, L. Effects of diamond-like carbon thin film and wrinkle microstructure on cell proliferation. Diam. Relat. Mater. 2018, 90, 194–201. [Google Scholar] [CrossRef]
- Gotzmann, G.; Beckmann, J.; Wetzel, C.; Scholz, B.; Herrmann, U.; Neunzehn, J. Electron-beam modification of DLC coatings for biomedical applications. Surf. Coat. Technol. 2017, 311, 248–256. [Google Scholar] [CrossRef]
- Karlina, H.; Rina, S.; Warta, D. Bactericidal efficacy of ultraviolet irradiation on Staphylococcus aureus. Asian J. Oral Maxillofac. Surg. 2005, 17, 157–161. [Google Scholar] [CrossRef]
- Cutler, T.; Zimmerman, J. Ultraviolet irradiation and the mechanisms underlying its inactivation of infectious agents. Anim. Health Res. Rev. 2011, 12, 15–23. [Google Scholar] [CrossRef]
- Kim, S.H.; Lee, Y.L.; Koo, Y.J.; Pack, E.C.; Lim, K.M.; Choi, D.W. Migration of monomers, plastic additives, and non-intentionally added substances from food utensils made of melamine–formaldehyde resin following ultraviolet sterilization. Food Control 2021, 125, 107981. [Google Scholar] [CrossRef]
- Shi, P.; Sun, J.; Yan, W.; Zhou, N.; Zhang, J.; Zhang, J.; Chen, L.; Qian, L. Roles of phase transition and surface property evolution in nanotribological behaviors of H-DLC: Effects of thermal and UV irradiation treatments. Appl. Surf. Sci. 2020, 514, 145960. [Google Scholar] [CrossRef]
- Bewilogua, K.; Hofmann, D. History of diamond-like carbon films—From first experiments to worldwide applications. Surf. Coat. Technol. 2014, 242, 214–225. [Google Scholar] [CrossRef]
- Abbas, G.A.; McLaughlin, J.A.; Harkin-Jones, E. A study of ta-C, a-C:H and Si-a:C:H thin films on polymer substrates as a gas barrier. Diam. Relat. Mater. 2004, 13, 1342–1345. [Google Scholar] [CrossRef]
- Fernández-Lizárraga, M.; García-López, J.; Rodil, S.E.; Ribas-Aparicio, R.M.; Silva-Bermudez, P. Evaluation of the biocompatibility and osteogenic properties of metal oxide coatings applied by magnetron sputtering as potential biofunctional surface modifications for orthopedic implants. Materials 2022, 15, 5240. [Google Scholar] [CrossRef] [PubMed]
- Manome, Y.; Tachibana, T.; Hiratsuka, M.; Sato, K.; Ohgoe, Y.; Hirakuri, K. Osteoblastic MC3T3-E1 cells on diamond-like carbon-coated silicon plates: Field emission scanning electron microscopy data. Data Brief. 2021, 38, 107385. [Google Scholar] [CrossRef] [PubMed]
- Kanasugi, K.; Ohgoe, Y.; Hirakuri, K.K.; Fukui, Y. Cytocompatibility of modified a-C:H film deposited on complicated polymeric medical apparatus. J. Appl. Phys. 2009, 105, 094702. [Google Scholar] [CrossRef]
- Lin, J.; Sproul, W.D.; Wei, R.; Chistyakov, R. Diamond like carbon films deposited by HiPIMS using oscillatory voltage pulses. Surf. Coat. Technol. 2014, 258, 1212–1222. [Google Scholar] [CrossRef]
- ISO 23216:2021(E); Carbon Based Films—Determination of Optical Properties of Amorphous Carbon Films by Spectroscopic Ellipsometry. International Organization for Standardization: Geneva, Switzerland, 2021.
- Greczynski, G.; Hultman, L. X-ray photoelectron spectroscopy: Towards reliable binding energy referencing. Prog. Mater. Sci. 2020, 107, 100591. [Google Scholar] [CrossRef]
- Nitta, Y.; Okamoto, K.; Nakatani, T.; Hoshi, H.; Homma, A.; Tatsumi, E.; Taenaka, Y. Diamond-like carbon thin film with controlled zeta potential for medical material application. Diam. Relat. Mater. 2008, 17, 1972–1976. [Google Scholar] [CrossRef]
- Liu, B.; Zhang, T.F.; Wu, B.J.; Leng, Y.X.; Huang, N. In vitro cytocompatibility evaluation of hydrogenated and unhydrogenated carbon films. Surf. Coat. Technol. 2014, 258, 913–920. [Google Scholar] [CrossRef]
- Chai, F.; Mathis, N.; Blanchemain, N.; Meunier, C.; Hildebrand, H.F. Osteoblast interaction with DLC-coated Si substrates. Acta Biomater. 2008, 4, 1369–1381. [Google Scholar] [CrossRef] [PubMed]
- Moriguchi, H.; Ohara, H.; Tsujioka, M. History and applications of diamond-like carbon manufacturing processes. SEI Technol. Rev. 2016, 82, 52–57. [Google Scholar]
- Saitoh, H. Classification of diamond-like carbon films. Jpn. J. Appl. Phys. 2012, 51, 090120. [Google Scholar] [CrossRef]
- Piazza, F.; Morell, G. Wettability of hydrogenated tetrahedral amorphous carbon. Diam. Relat. Mater. 2009, 18, 43–50. [Google Scholar] [CrossRef]
- Ferrari, A.C. Determination of bonding in diamond-like carbon by Raman spectroscopy. Diam. Relat. Mater. 2002, 11, 1053–1061. [Google Scholar] [CrossRef]
- Wang, Y.; Li, H.; Ji, L.; Zhao, F.; Kong, Q.; Wang, Y.; Liu, X.; Quan, W.; Zhou, H.; Chen, J. Microstructure, mechanical and tribological properties of graphite-like amorphous carbon films prepared by unbalanced magnetron sputtering. Surf. Coat. Technol. 2011, 205, 3058–3065. [Google Scholar] [CrossRef]
- Artemenko, A.; Ižák, T.; Marton, M.; Ukraintsev, E.; Stuchlík, J.; Hruška, K.; Vojs, M.; Kromka, A. Stability of the surface termination of nanocrystalline diamond and diamond-like carbon films exposed to open air conditions. Diam. Relat. Mater. 2019, 100, 107562. [Google Scholar] [CrossRef]
- Caro, M.A.; Aarva, A.; Deringer, V.L.; Cs’anyi, G.; Laurila, T. Reactivity of amorphous carbon surfaces: Rationalizing the role of structural motifs in functionalization using machine learning. Chem. Mater. 2018, 30, 7446–7455. [Google Scholar] [CrossRef]
- Nitta, Y.; Okamoto, K.; Nakatani, T.; Shinohara, M. Effect of the surface bond states in as-deposited DLC films on the incorporation of nitrogen and oxygen atoms. IEEE Trans. Plasma Sci. 2012, 40, 2073–2078. [Google Scholar] [CrossRef]
- Sugawara, H. Optical Cleaning. USHIO Inc. Available online: https://www.ushio.co.jp/jp/technology/lightedge/200611/100330.html (accessed on 28 August 2022).
- Ji, L.; Li, H.; Zhao, F.; Quan, W.; Chen, J.; Zhou, H. Influences of ultraviolet irradiation on structure and tribological properties of diamond-like carbon films. Appl. Surf. Sci. 2009, 255, 8409–8413. [Google Scholar] [CrossRef]
- Zhou, H.-L.; Tunmee, S.; Suzuki, T.; Phothongkam, P.; Kanda, K.; Komatsu, K.; Kawahara, S.; Ito, H.; Saitoh, H. Quantitative NEXAFS and solid-state NMR studies of sp3/(sp2+sp3) ratio in the hydrogenated DLC films. Diam. Relat. Mater. 2017, 73, 232–240. [Google Scholar] [CrossRef]
- Lomon, J.; Chaiyabin, P.; Songsiriritthigul, P.; Saisopa, T.; Seawsakul, K.; Saowiang, N.; Poolcharuansin, P.; Chingsungnoen, A.; Supruangnet, R.; Chanlek, N.; et al. Diamond-like carbon films prepared by high power impulse magnetron sputtering. Mater. Today Proc. 2019, 17, 1549–1554. [Google Scholar] [CrossRef]



| Sample No. | Equipment | Deposition Method | Precursor | Gas Pressure (Pa) | Substrate Bias Voltage (kV) | Target Voltage (kV) | |
|---|---|---|---|---|---|---|---|
| 1 | ISDD4, Nanotec Co., Chiba, Japan | CVD | RF-PCVD | Hydrogenated gas (C6H6: 13 sccm) | 0.46 | - | - |
| 2 | NPS330, Nanotec Co., Chiba, Japan | Pulsed DC-PCVD | Hydrogenated gas (C6H6: 20 sccm) | 1.70 | 1.5 | - | |
| 3 | PVD | Ionized evaporation | Hydrogenated gas (C6H6: 1.4 sccm) | 0.12 | 1.0 | - | |
| 4 | HiPIMS | Graphite solid target Ar gas (50 sccm) | 0.82 | 0.0 | 0.75 | ||
| 5 | HiPIMS | Graphite solid target Ar gas (40 sccm) | 0.70 | 0.0 | 0.90 | ||
| Cell Line | Osteoblasts (MC-3T3) |
| Seeding density | 1.0 × 104 cells/cm2 |
| Medium | MEM-α |
| CO2 concentration | 5.0% |
| Temperature | 37.0 ℃ |
| Incubation time | 72 h |
| pH | 6.8–7.2 |
| Sample No. | χ2 | Film Thickness (nm) | n | k | Amorphous Carbon Type |
|---|---|---|---|---|---|
| λ = 550 nm | |||||
| 1 | 0.10 | 108 | 1.930 | 0.040 | PLC (not DLC) |
| 2 | 0.02 | 150 | 2.225 | 0.258 | a-C:H (DLC) |
| 3 | 0.05 | 90 | 2.500 | 0.268 | ta-C:H (DLC) |
| 4 | 0.04 | 175 | 1.870 | 0.415 | GLC (not DLC) |
| 5 | 0.03 | 120 | 2.290 | 0.690 | a-C (DLC) |
| Sample No. | Amorphous Carbon Type | UV Sterilization at 253.7 nm | D-Peak Position (cm−1) | G-Peak Position (cm−1) | FWHM (G) | ID/IG Intensity Ratio |
|---|---|---|---|---|---|---|
| 1 | PLC | Without UV | 1305 ± 1.3 | 1523 ± 0.3 | 165.2 ± 0.5 | 0.31 |
| With UV | 1311 ± 1.6 | 1524 ± 0.3 | 162.4 ± 0.5 | 0.32 | ||
| 2 | a-C:H | Without UV | 1361 ± 2.0 | 1541 ± 0.2 | 175.8 ± 0.7 | 0.52 |
| With UV | 1355 ± 2.1 | 1539 ± 0.2 | 173.9 ± 0.7 | 0.52 | ||
| 3 | ta-C:H | Without UV | 1378 ± 2.6 | 1553 ± 0.3 | 176.6 ± 1.0 | 0.61 |
| With UV | 1377 ± 2.6 | 1552 ± 0.2 | 174.2 ± 1.1 | 0.63 | ||
| 4 | GLC | Without UV | 1384 ± 0.5 | 1584 ± 0.1 | 127.0 ± 0.5 | 1.00 |
| With UV | 1383 ± 0.5 | 1584 ± 0.1 | 127.1 ± 0.5 | 0.98 | ||
| 5 | a-C | Without UV | 1395 ± 0.7 | 1579 ± 0.2 | 132.8 ± 0.8 | 1.35 |
| With UV | 1389 ± 0.8 | 1577 ± 0.2 | 137.2 ± 0.9 | 1.27 |
| Sample No. | Amorphous Carbon Type | UV Sterilization at 253.7 nm | Pure Water Contact Angle (Degree) (n = 10) | AFM |
|---|---|---|---|---|
| Surface Roughness (nm) | ||||
| 1 | PLC | Without UV | 71 ± 4 | 1.2 ± 0.7 (n = 4) |
| With UV | 70 ± 3 | 2.5 ± 2.0 (n = 4) | ||
| 2 | a-C:H | Without UV | 71 ± 5 | 1.1 ± 0.1 (n = 3) |
| With UV | 68 ± 1 | 2.0 ± 0.1 (n = 3) | ||
| 3 | ta-C:H | Without UV | 65 ± 3 | 1.4 ± 0.1 (n = 3) |
| With UV | 63 ± 2 | 2.1 ± 0.1 (n = 3) | ||
| 4 | GLC | Without UV | 78 ± 2 | 2.1 ± 0.0 (n = 3) |
| With UV | 75 ± 4 | 2.0 ± 0.2 (n = 3) | ||
| 5 | a-C | Without UV | 77 ± 5 | 2.2 ± 0.2 (n = 3) |
| With UV | 71 ± 5 | 2.1 ± 0.1 (n = 3) | ||
| Control (Si) | - | Without UV | - | 0.5 ± 0.4 (n = 3) |
| With UV | - | 0.5 ± 0.2 (n = 3) |
| Sample No. | Amorphous Carbon Type | UV Sterilization at 253.7 nm | O1s/C1s Ratio | C1s Curve Fitting Area | ||||
|---|---|---|---|---|---|---|---|---|
| C-C sp2 | C-C sp3 | C-O | C=O | O=C-O | ||||
| 1 | PLC | Without UV | 0.14 | 0.92 | 0.22 | 0.04 | 0.02 | 0.00 |
| With UV | 0.16 | 0.95 | 0.20 | 0.05 | 0.02 | 0.00 | ||
| 2 | a-C:H | Without UV | 0.13 | 0.90 | 0.29 | 0.06 | 0.03 | 0.00 |
| With UV | 0.19 | 0.86 | 0.20 | 0.07 | 0.03 | 0.01 | ||
| 3 | ta-C:H | Without UV | 0.14 | 0.95 | 0.12 | 0.06 | 0.05 | 0.01 |
| With UV | 0.16 | 0.93 | 0.22 | 0.05 | 0.04 | 0.00 | ||
| 4 | GLC | Without UV | 0.35 | 0.87 | 0.28 | 0.14 | 0.11 | 0.03 |
| With UV | 0.39 | 0.87 | 0.27 | 0.16 | 0.12 | 0.03 | ||
| 5 | a-C | Without UV | 0.27 | 0.90 | 0.18 | 0.10 | 0.08 | 0.01 |
| With UV | 0.30 | 0.91 | 0.19 | 0.11 | 0.08 | 0.02 | ||
| Sample No. | Amorphous Carbon Type | Osteoblast Proliferation (n = 6 × 5 Cycles) 1 | p-Value for Control |
|---|---|---|---|
| 1 | PLC | 1.1 ± 0.3 | p > 0.05 (control level) |
| 2 | a-C:H | 1.0 ± 0.2 | p > 0.05 (control level) |
| 3 | ta-C:H | 0.9 ± 0.1 | p > 0.05 (control level) |
| 4 | GLC | 1.5 ± 0.3 | p < 0.01 (high) |
| 5 | a-C | 1.1 ± 0.2 | p > 0.05 (control level) |
| Control (Si) | - | 1.0 ± 0.1 | - |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kanasugi, K.; Arimura, K.; Alanazi, A.; Ohgoe, Y.; Manome, Y.; Hiratsuka, M.; Hirakuri, K. UV Sterilization Effects and Osteoblast Proliferation on Amorphous Carbon Films Classified Based on Optical Constants. Bioengineering 2022, 9, 505. https://doi.org/10.3390/bioengineering9100505
Kanasugi K, Arimura K, Alanazi A, Ohgoe Y, Manome Y, Hiratsuka M, Hirakuri K. UV Sterilization Effects and Osteoblast Proliferation on Amorphous Carbon Films Classified Based on Optical Constants. Bioengineering. 2022; 9(10):505. https://doi.org/10.3390/bioengineering9100505
Chicago/Turabian StyleKanasugi, Kazuya, Keita Arimura, Ali Alanazi, Yasuharu Ohgoe, Yoshinobu Manome, Masanori Hiratsuka, and Kenji Hirakuri. 2022. "UV Sterilization Effects and Osteoblast Proliferation on Amorphous Carbon Films Classified Based on Optical Constants" Bioengineering 9, no. 10: 505. https://doi.org/10.3390/bioengineering9100505
APA StyleKanasugi, K., Arimura, K., Alanazi, A., Ohgoe, Y., Manome, Y., Hiratsuka, M., & Hirakuri, K. (2022). UV Sterilization Effects and Osteoblast Proliferation on Amorphous Carbon Films Classified Based on Optical Constants. Bioengineering, 9(10), 505. https://doi.org/10.3390/bioengineering9100505