Unravelling Alveolar Bone Regeneration Ability of Platelet-Rich Plasma: A Systematic Review with Meta-Analysis
Abstract
:1. Introduction
2. Materials and Methods
2.1. Protocol Registration and Reporting Format
2.2. Focus Question
2.3. PICO Strategy
- -
- (P) Population: we included patients without a severe underlying disease requiring tooth extraction.
- -
- (I) Interventions: we considered all interventions employing PRP alone for socket filling.
- -
- (C) Comparison: natural healing or blood clot
- -
- (O) Outcome: our primary outcome was new bone formation, whereas we considered bone density as a secondary outcome.
2.4. Eligibility Criteria
2.5. Data Sources and Search Strategy
2.6. Data Collection and Management
2.7. Data Extraction
2.8. Risk of Bias in Individual Research Studies
2.9. Outcomes
2.10. Statistical Analysis
2.11. Certainty of Evidence
3. Results
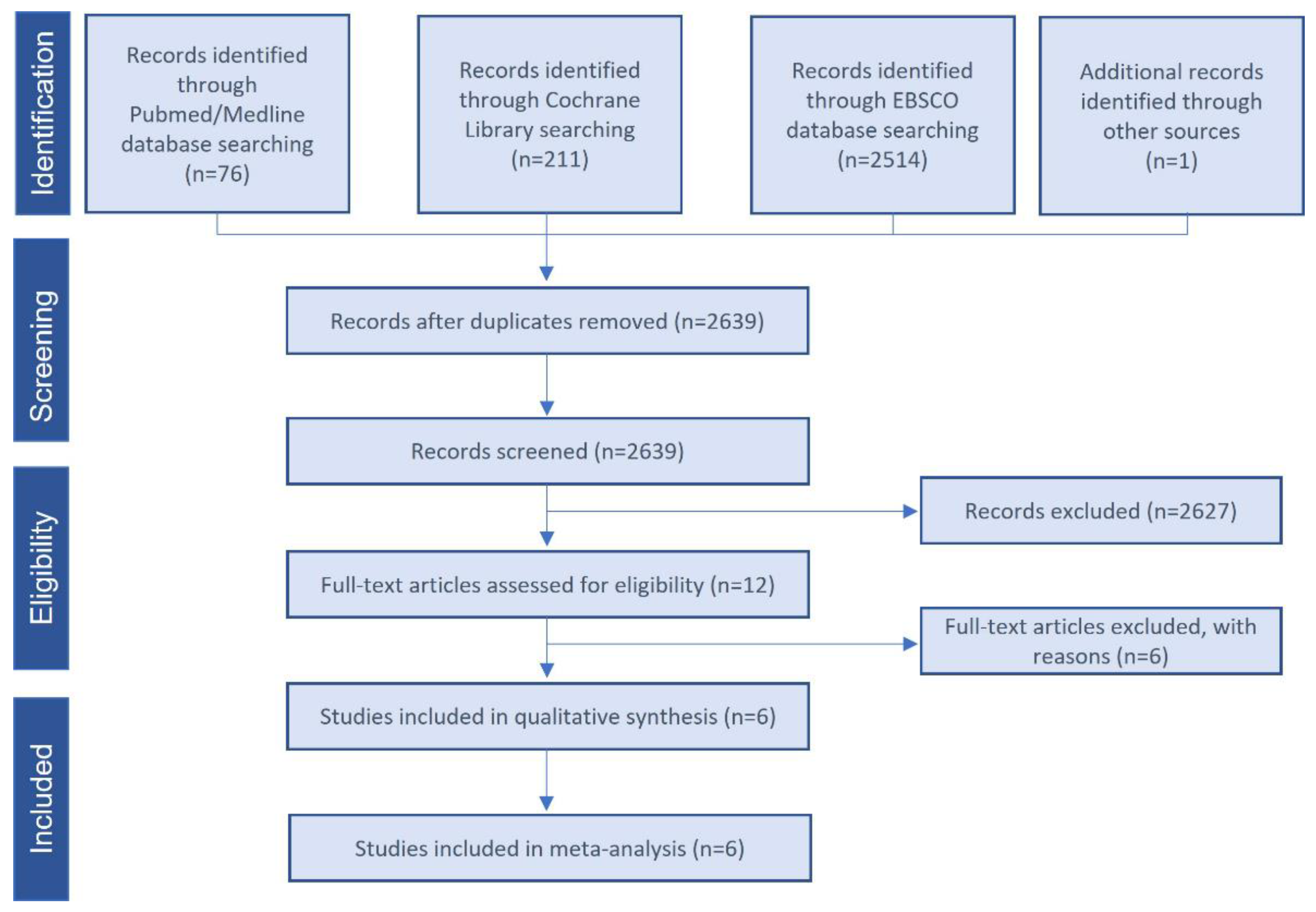
3.1. Study Selection and Characteristics
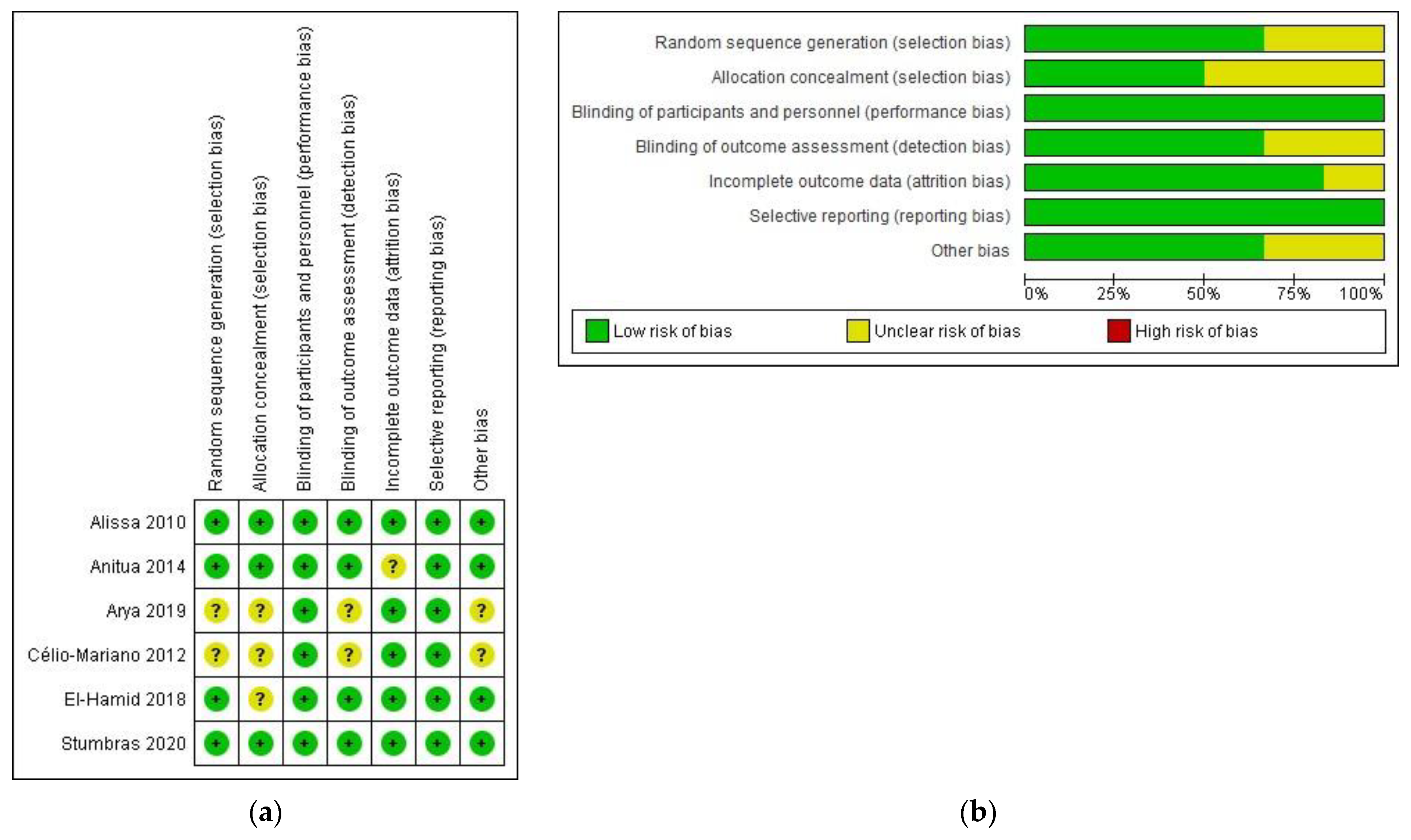
3.2. Risk of Bias of Included Trials
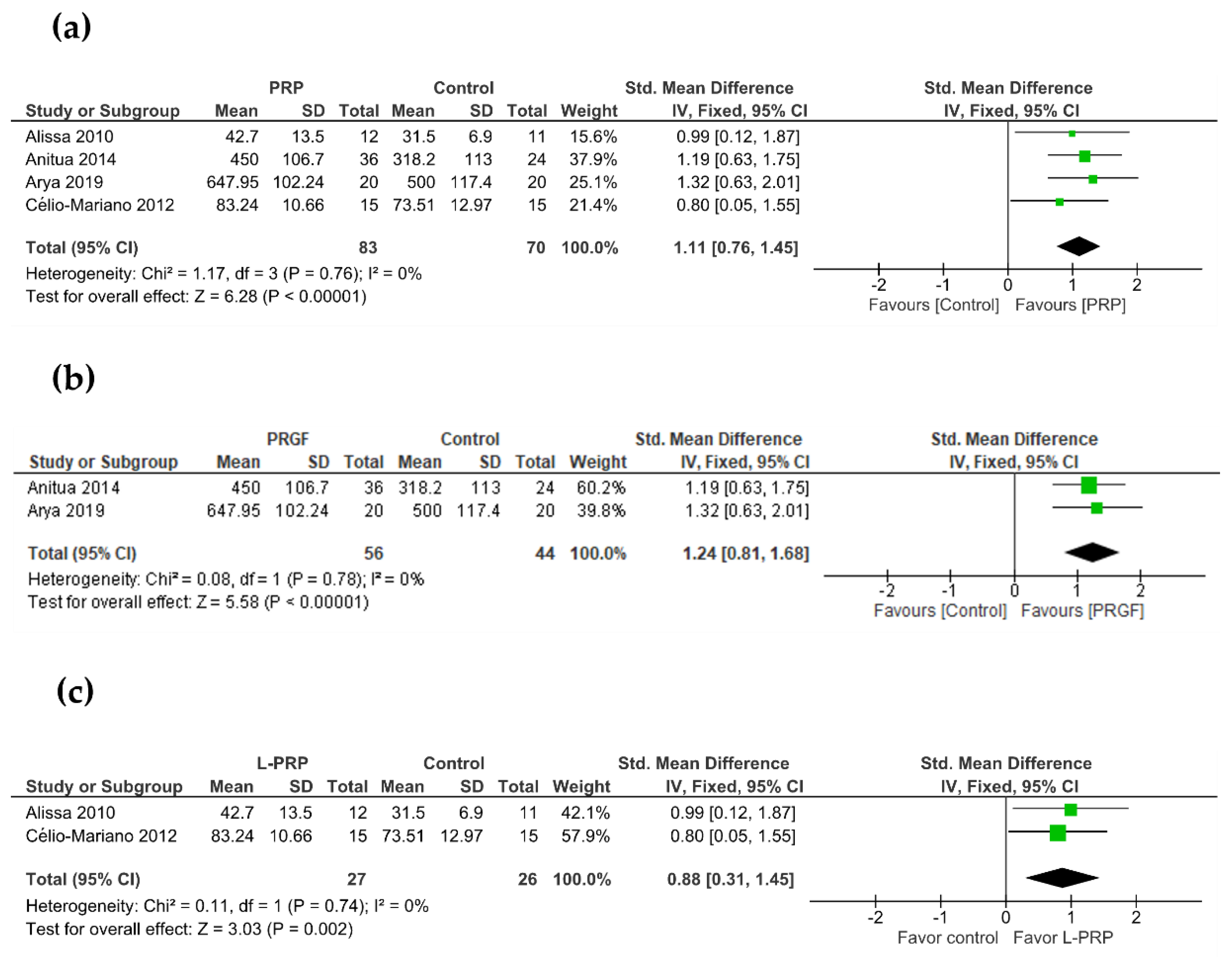
3.3. Primary Outcome: New Bone Formation
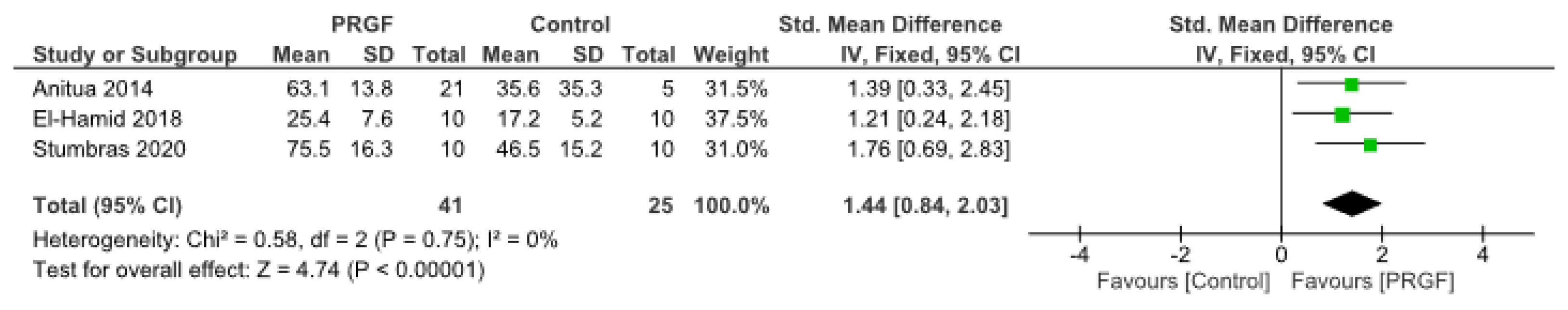
3.4. Secondary Outcome: Bone Density
3.5. Quality of Evidence
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Schropp, L.; Wenzel, A.; Kostopoulos, L.; Karring, T. Bone Healing and Soft Tissue Contour Changes Following Single-Tooth Extraction: A Clinical and Radiographic 12-Month Prospective Study. Int. J Periodontics Restor. Dent. 2003, 23, 313–323. [Google Scholar]
- Jahangiri, L.; Devlin, H.; Ting, K.; Nishimura, I. Current Perspectives in Residual Ridge Remodeling and Its Clinical Implications: A Review. J. Prosthet. Dent. 1998, 80, 224–237. [Google Scholar] [CrossRef]
- Chappuis, V.; Engel, O.; Reyes, M.; Shahim, K.; Nolte, L.-P.; Buser, D. Ridge Alterations Post-Extraction in the Esthetic Zone. J. Dent. Res. 2013, 92, 195S–201S. [Google Scholar] [CrossRef] [PubMed]
- Tan, W.L.; Wong, T.L.T.; Wong, M.C.M.; Lang, N.P. A Systematic Review of Post-Extractional Alveolar Hard and Soft Tissue Dimensional Changes in Humans. Clin. Oral Implants Res. 2012, 23, 1–21. [Google Scholar] [CrossRef] [PubMed]
- Cardaropoli, G.; Araújo, M.; Lindhe, J. Dynamics of Bone Tissue Formation in Tooth Extraction Sites: An Experimental Study in Dogs. J. Clin. Periodontol. 2003, 30, 809–818. [Google Scholar] [CrossRef]
- Iasella, J.M.; Greenwell, H.; Miller, R.L.; Hill, M.; Drisko, C.; Bohra, A.A.; Scheetz, J.P. Ridge Preservation with Freeze-Dried Bone Allograft and a Collagen Membrane Compared to Extraction Alone for Implant Site Development: A Clinical and Histologic Study in Humans. J. Periodontol. 2003, 74, 990–999. [Google Scholar] [CrossRef]
- Barone, A.; Aldini, N.N.; Fini, M.; Giardino, R.; Calvo Guirado, J.L.; Covani, U. Xenograft Versus Extraction Alone for Ridge Preservation After Tooth Removal: A Clinical and Histomorphometric Study. J. Periodontol. 2008, 79, 1370–1377. [Google Scholar] [CrossRef]
- Milani, S.; Dal Pozzo, L.; Rasperini, G.; Sforza, C.; Dellavia, C. Deproteinized Bovine Bone Remodeling Pattern in Alveolar Socket: A Clinical Immunohistological Evaluation. Clin. Oral Implants Res. 2016, 27, 295–302. [Google Scholar] [CrossRef] [PubMed]
- Machtei, E.E.; Mayer, Y.; Horwitz, J.; Zigdon-Giladi, H. Prospective Randomized Controlled Clinical Trial to Compare Hard Tissue Changes Following Socket Preservation Using Alloplasts, Xenografts vs. No Grafting: Clinical and Histological Findings. Clin. Implant Dent. Relat. Res. 2019, 21, 14–20. [Google Scholar] [CrossRef] [PubMed]
- Nart, J.; Barallat, L.; Jimenez, D.; Mestres, J.; Gómez, A.; Carrasco, M.A.; Violant, D.; Ruíz-Magaz, V. Radiographic and Histological Evaluation of Deproteinized Bovine Bone Mineral vs. Deproteinized Bovine Bone Mineral with 10% Collagen in Ridge Preservation. A Randomized Controlled Clinical Trial. Clin. Oral Implants Res. 2017, 28, 840–848. [Google Scholar] [CrossRef]
- Corning, P.J.; Mealey, B.L. Ridge Preservation Following Tooth Extraction Using Mineralized Freeze-Dried Bone Allograft Compared to Mineralized Solvent-Dehydrated Bone Allograft: A Randomized Controlled Clinical Trial. J. Periodontol. 2019, 90, 126–133. [Google Scholar] [CrossRef]
- Sadeghi, R.; Babaei, M.; Miremadi, S.A.; Abbas, F.M. A Randomized Controlled Evaluation of Alveolar Ridge Preservation Following Tooth Extraction Using Deproteinized Bovine Bone Mineral and Demineralized Freeze-Dried Bone Allograft. Dent. Res. J. (Isfahan) 2016, 13, 151–159. [Google Scholar] [CrossRef]
- Shim, J.Y.; Lee, Y.; Lim, J.H.; Jin, M.U.; Lee, J.M.; Suh, J.Y.; Kim, Y.G. Comparative Evaluation of Recombinant Human Bone Morphogenetic Protein-2/Hydroxyapatite and Bovine Bone for New Bone Formation in Alveolar Ridge Preservation. Implant Dent. 2018, 27, 623–629. [Google Scholar] [CrossRef]
- Susin, C.; Wikesjö, U.M.E. Regenerative Periodontal Therapy: 30 Years of Lessons Learned and Unlearned. Periodontology 2013, 62, 232–242. [Google Scholar] [CrossRef]
- Keskin, D.; Gündoǧdu, C.; Atac, A.C. Experimental Comparison of Bovine-Derived Xenograft, Xenograft-Autologous Bone Marrow and Autogenous Bone Graft for the Treatment of Bony Defects in the Rabbit Ulna. Med. Princ. Pract. 2007, 16, 299–305. [Google Scholar] [CrossRef]
- Dimitriou, R.; Jones, E.; McGonagle, D.; Giannoudis, P.V. Bone Regeneration: Current Concepts and Future Directions. BMC Med. 2011, 9, 66. [Google Scholar] [CrossRef]
- Suárez-López Del Amo, F.; Monje, A.; Padial-Molina, M.; Tang, Z.H.; Wang, H.L. Biologic Agents for Periodontal Regeneration and Implant Site Development. Biomed. Res. Int. 2015, 2015, 957518. [Google Scholar] [CrossRef]
- Anitua, E.; Murias-Freijo, A.; Alkhraisat, M.H.; Orive, G. Clinical, Radiographical, and Histological Outcomes of Plasma Rich in Growth Factors in Extraction Socket: A Randomized Controlled Clinical Trial. Clin. Oral Investig. 2015, 19, 589–600. [Google Scholar] [CrossRef]
- Canellas, J.V.D.S.; Medeiros, P.J.D.; Figueredo, C.M.D.S.; Fischer, R.G.; Ritto, F.G. Platelet-Rich Fibrin in Oral Surgical Procedures: A Systematic Review and Meta-Analysis. Int. J. Oral Maxillofac. Surg. 2019, 48, 395–414. [Google Scholar] [CrossRef]
- Anitua, E. Plasma Rich in Growth Factors: Preliminary Results of Use in the Preparation of Future Sites for Implants. Int. J. Oral Maxillofac. Implants 1999, 14, 529–535. [Google Scholar]
- Anitua, E.; Zalduendo, M.; Troya, M.; Tierno, R.; Alkhraisat, M.H. The Inclusion of Leukocytes into Platelet Rich Plasma Reduces Scaffold Stability and Hinders Extracellular Matrix Remodelling. Ann. Anat. 2022, 240, 151853. [Google Scholar] [CrossRef] [PubMed]
- Anitua, E.; Fernández-de-Retana, S.; Alkhraisat, M.H. Platelet Rich Plasma in Oral and Maxillofacial Surgery from the Perspective of Composition. Platelets 2021, 32, 174–182. [Google Scholar] [CrossRef] [PubMed]
- Mozzati, M.; Martinasso, G.; Pol, R.; Polastri, C.; Cristiano, A.; Muzio, G.; Canuto, R. The Impact of Plasma Rich in Growth Factors on Clinical and Biological Factors Involved in Healing Processes after Third Molar Extraction. J. Biomed. Mater. Res. Part A 2010, 95A, 741–746. [Google Scholar] [CrossRef] [PubMed]
- Mozzati, M.; Gallesio, G.; di Romana, S.; Bergamasco, L.; Pol, R. Efficacy of Plasma-Rich Growth Factor in the Healing of Postextraction Sockets in Patients Affected by Insulin-Dependent Diabetes Mellitus. J. Oral Maxillofac. Surg. 2014, 72, 456–462. [Google Scholar] [CrossRef] [PubMed]
- Haraji, A.; Lassemi, E.; Motamedi, M.; Alavi, M.; Adibnejad, S. Effect of Plasma Rich in Growth Factors on Alveolar Osteitis. Natl. J. Maxillofac. Surg. 2012, 3, 38–41. [Google Scholar] [CrossRef]
- Ogundipe, O.; Ugboko, V.; Owotade, F. Can Autologous Platelet-Rich Plasma Gel Enhance Healing after Surgical Extraction of Mandibular Third Molars? J. Oral Maxillofac. Surg. 2011, 69, 2305–2310. [Google Scholar] [CrossRef]
- Arya, V.; Malhotra, V.L.; Rao, J.D.; Kirti, S.; Malhotra, S.; Sharma, R.S. Reduction in Post Extraction Waiting Period for Dental Implant Patients Using Plasma Rich in Growth Factors: An in Vivo Study Using Cone-Beam Computed Tomography. J. Korean Assoc. Oral Maxillofac. Surg. 2019, 45, 285. [Google Scholar] [CrossRef]
- Farina, R.; Bressan, E.; Taut, A.; Cucchi, A.; Trombelli, L. Plasma Rich in Growth Factors in Human Extraction Sockets: A Radiographic and Histomorphometric Study on Early Bone Deposition. Clin. Oral Implants Res. 2013, 24, 1360–1368. [Google Scholar] [CrossRef]
- Alissa, R.; Esposito, M.; Horner, K.; Oliver, R. The Influence of Platelet-Rich Plasma on the Healing of Extraction Sockets: An Explorative Randomised Clinical Trial. Eur. J. Oral Implantol. 2010, 3, 121–134. [Google Scholar]
- de Antonello, G.; Torres Do Couto, R.; Giongo, C.; Corrêa, M.; Chagas, O.J.; Lemes, C. Evaluation of the Effects of the Use of Platelet-Rich Plasma (PRP) on Alveolar Bone Repair Following Extraction of Impacted Third Molars: Prospective Study. J. Cranio-Maxillofacial Surg. 2013, 41, e70–e75. [Google Scholar] [CrossRef]
- Rutkowski, J.; Johnson, D.; Radio, N.; Fennell, J. Platelet Rich Plasma to Facilitate Wound Healing Following Tooth Extraction. J. Oral Implantol. 2010, 36, 11–23. [Google Scholar] [CrossRef]
- Canellas, J.V.D.S.; Ritto, F.G.; Figueredo, C.M.D.S.; Fischer, R.G.; de Oliveira, G.P.; Thole, A.A.; Medeiros, P.J.D. Histomorphometric Evaluation of Different Grafting Materials Used for Alveolar Ridge Preservation: A Systematic Review and Network Meta-Analysis. Int. J. Oral Maxillofac. Surg. 2020, 49, 797–810. [Google Scholar] [CrossRef] [PubMed]
- Pan, J.; Xu, Q.; Hou, J.; Wu, Y.; Liu, Y.; Li, R.; Pan, Y.; Zhang, D. Effect of Platelet-Rich Fibrin on Alveolar Ridge Preservation: A Systematic Review. J. Am. Dent. Assoc. 2019, 1500, 766–778. [Google Scholar] [CrossRef] [PubMed]
- Del Fabbro, M.; Bucchi, C.; Lolato, A.; Corbella, S.; Testori, T.; Taschieri, S. Healing of Postextraction Sockets Preserved with Autologous Platelet Concentrates. A Systematic Review and Meta-Analysis. J. Oral Maxillofac. Surg. 2017, 75, 1601–1615. [Google Scholar] [CrossRef]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 Statement: An Updated Guideline for Reporting Systematic Reviews. PLoS Med. 2021, 18, e1003583. [Google Scholar] [CrossRef]
- Higgins, J.P.T.; Green, S. (Eds.) Cochrane Handbook for Systematic Reviews of Interventions, Version 5.1.0; The Cochrane Collaboration, 2011. Available online: www.handbook.cochrane.org (accessed on 20 July 2022).
- Higgins, J.P.T.; Thompson, S.G.; Deeks, J.J.; Altman, D.G. Measuring Inconsistency in Meta-Analyses. BMJ 2003, 327, 557. [Google Scholar] [CrossRef]
- Schünemann, H.; Brożek, J.; Guyatt, G.; Oxman, A. GRADE Handbook for Grading Quality of Evidence and Strength of Recommendations, The GRADE Working Group. 2013. Available online: https://gdt.gradepro.org/app/handbook/handbook.html (accessed on 20 July 2022).
- Stumbras, A.; Januzis, G.; Gervickas, A.; Kubilius, R.; Juodzbalys, G. Randomized and Controlled Clinical Trial of Bone Healing after Alveolar Ridge Preservation Using Xenografts and Allografts versus Plasma Rich in Growth Factors. J. Oral Implantol. 2020, 46, 515–525. [Google Scholar] [CrossRef]
- El-Hamid, I.S.A.; Abdel–Ghaffar, K.A.; Ezzatt, O.M.; El Demerdash, F.H.; ELZalabany, N.N.; Antar, M.F. Socket Preservation Using Atorvastatin Loaded in Growth Factor Plasma Rich Fibrin Scaffold: A Randomized Clinical Trial. Nat. Sci. (East Lansing) 2018, 16, 82–91. [Google Scholar]
- Célio-Mariano, R.; de Melo, W.M.; Carneiro-Avelino, C. Comparative Radiographic Evaluation of Alveolar Bone Healing Associated with Autologous Platelet-Rich Plasma After Impacted Mandibular Third Molar Surgery. J. Oral Maxillofac. Surg. 2012, 70, 19–24. [Google Scholar] [CrossRef] [PubMed]
- Oyama, T.; Nishimoto, S.; Tsugawa, T.; Shimizu, F. Efficacy of Platelet-Rich Plasma in Alveolar Bone Grafting. J. Oral Maxillofac. Surg. 2004, 62, 555–558. [Google Scholar] [CrossRef]
- Saleem, M.; Pisani, F.; Zahid, F.M.; Georgakopoulos, I.; Pustina-Krasniqi, T.; Xhajanka, E.; Almasri, M. Adjunctive Platelet-Rich Plasma (PRP) in Infrabony Regenerative Treatment: A Systematic Review and RCT’s Meta-Analysis. Stem Cells Int. 2018, 2018, 9594235. [Google Scholar] [CrossRef]
- Dohan Ehrenfest, D.M.; Rasmusson, L.; Albrektsson, T. Classification of Platelet Concentrates: From Pure Platelet-Rich Plasma (P-PRP) to Leucocyte- and Platelet-Rich Fibrin (L-PRF). Trends Biotechnol. 2009, 27, 158–167. [Google Scholar] [CrossRef] [PubMed]
- Mishra, A.; Woodall, J.; Vieira, A. Treatment of Tendon and Muscle Using Platelet-Rich Plasma. Clin. Sports Med. 2009, 28, 113–125. [Google Scholar] [CrossRef]
- McAleer, J.P.; Sharma, S.; Kaplan, E.M.; Persich, G. Use of Autologous Platelet Concentrate in a Nonhealing Lower Extremity Wound. Adv. Ski. Wound Care 2006, 19, 354–363. [Google Scholar] [CrossRef]
- Knighton, D.R.; Ciresi, K.F.; Fiegel, V.D.; Austin, L.L.; Butler, E.L. Classification and Treatment of Chronic Nonhealing Wounds: Successful Treatment with Autologous Platelet-Derived Wound Healing Factors (PDWHF). Ann. Surg. 1986, 204, 322–330. [Google Scholar] [CrossRef]
- Ghoncheh, Z.; Kaviani, H.; Ghadiri Harvani, H.; Goodarzipoor, D.; Shamshiri, A.R.; Shams, P. Assessment of the Capability of Bone Density Contrast Dissociation in Cone Beam Computed Tomography Compared to Digital Periapical Radiography by Using a Phantom. J. Dent. (Shiraz Univ. Med. Sci.) 2019, 20, 203–209. [Google Scholar] [CrossRef]
- Kotsakis, G.; Javed, F.; Hinrichs, J.; Karoussis, I.; Romanos, G. Impact of Cigarette Smoking on Clinical Outcomes of Periodontal Flap Surgical Procedures: A Systematic Review and Meta-Analysis. J. Periodontol. 2015, 86, 254–263. [Google Scholar] [CrossRef] [PubMed]
- Saldanha, J.; Casati, M.; Neto, F.; Sallum, E.; Nociti, F. Smoking May Affect the Alveolar Process Dimensions and Radiographic Bone Density in Maxillary Extraction Sites: A Prospective Study in Humans. J. Oral Maxillofac. Surg. 2006, 64, 1359–1365. [Google Scholar] [CrossRef]
| Study | Design | Patients (Teeth) | Sex Male/Female | Age Years | Site Characteristics | Follow-Up | PRP Preparation Protocol | Intervention | |
|---|---|---|---|---|---|---|---|---|---|
| Control | Test | ||||||||
| Stumbras et al., 2020 [39] | RCT 1 | 40 | 14/26 | Anterior maxilla | 12 weeks | 580 g; 8 min | Natural healing | PRGF | |
| Alissa et al., 2010 [29] | RCT | 23 (29) | 8/7 | 20–52 | Mandible or maxilla | 12 weeks | 3200 rpm; 12 min | Non-PRP | L-PRP |
| Arya et al., 2019 [27] | Split mouth RCT | 20 (40) | 13/7 | 15–30 | Mandible | 13 weeks | 580 g; 8 min | Empty socket | PRGF |
| Célio-Mariano et al., 2012 [40] | Split mouth RCT | 15 (30) | 7/8 | 18–22 | 3ºmolars | 6 months | 160 g; 20 min 400 g; 15 min | Blood clot | L-PRP |
| Anitua et al., 2015 [18] | RCT | 60 | 29/31 | 18–74 | Molar extraction in the mandible | 12 weeks | 580 g; 8 min | Blood clot | PRGF |
| El-Hamid et al., 2018 [41] | RCT | 30 | 6/24 | C:30.1 ± 7.5 T: 29.2 ± 4.4 | Premolars | 2 months | 580 g; 8 min | Natural healing | PRGF |
| Study | Time of Measurement | Sample Size | Staining | Histomorphometric Analysis | |
|---|---|---|---|---|---|
| C/T | Control | Test | |||
| Stumbras et al., 2020 [39] | 12 weeks | 10/10 | May Grünwald-Giemsa | New formed mineral tissue (%) 46.5 ± 15.2 | New formed mineral tissue (%) 75.5 ± 16.3 |
| Anitua et al., 2015 [18] | 10–12 weeks | 5/21 | HE and MGG | New bone regeneration (%) 35.6 ± 35.3 | New bone regeneration (%) 63.1 ± 13.8 |
| El-Hamid et al., 2018 [41] | 8 weeks | 10/10 | Masson’s Trichrome | Mineralized tissues (%) 17.2 ± 5.2 | Mineralized tissues (%) 25.4 ± 7.6 |
| Study | Time of Measurement | Sample Size | Method | Bone Density | |
|---|---|---|---|---|---|
| C/T | Control | Test | |||
| Alissa et al., 2010 [29] | 12 weeks | 8/8 | Periapical radiographs | Trabecular bone volume (%) 31.5 ± 6.9 | Trabecular bone volume (%) 42.7 ± 13.5 |
| Arya et al., 2019 [27] | 13 weeks | 20/20 | CBCT | Mean bone density (HU) 500.05 ± 117.40 | Mean bone density (HU) 647.95 ± 102.24 |
| Célio-Mariano et al., 2012 [40] | 3 months | 15/15 | Periapical radiographs | Mean bone density (%) 73.51 | Mean bone density (%) 83.24 |
| Anitua et al., 2015 [18] | 10–12 weeks | 22/30 | CBCT | Mean bone density (HU) 318.2 ± 113.0 | Mean bone density (HU) 450.0 ± 106.7 |
| Certainty Assessment | № of Patients | Effect | Certainty | Importance | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| No of Studies | Study Design | Risk of Bias | Inconsistency | Indirectness | Imprecision | Other Considerations | [Intervention] | [Comparison] | Relative (95% CI) | Absolute (95% CI) | ||
| New bone formation (assessed with: histomorphometry) | ||||||||||||
| 3 | Randomized trials | Not serious | Not serious | Not serious | Serious a | Publication bias strongly suspected b Strong association c | 41 | 25 | - | SMD 1.44 SD higher (0.27 higher to 2.6 higher) | ⨁⨁⨁◯ Moderate | CRITICAL |
| Bone density (assessed with: CBCT) | ||||||||||||
| 2 | Randomized trials | Not serious | Not serious | Not serious | Serious a | Publication bias strongly suspected b Strong association c | 50 | 42 | - | SMD 1.24 SD higher (0.39 higher to 2.09 higher) | ⨁⨁⨁◯ Moderate | CRITICAL |
| Certainty Assessment | № of Patients | Effect | Certainty | Importance | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| No of Studies | Study Design | Risk of Bias | Inconsistency | Indirectness | Imprecision | Other Considerations | [Intervention] | [Comparison] | Relative (95% CI) | Absolute (95% CI) | ||
| Bone density (assessed with: periapical radiography) | ||||||||||||
| 2 | RCT | Not serious | Not serious | Not serious | Serious a | Publication bias strongly suspected b | 23 | 23 | - | SMD 0.88 SD higher (0.24 lower to 2 higher) | ⨁⨁◯◯ Low | CRITICAL |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Anitua, E.; Allende, M.; Alkhraisat, M.H. Unravelling Alveolar Bone Regeneration Ability of Platelet-Rich Plasma: A Systematic Review with Meta-Analysis. Bioengineering 2022, 9, 506. https://doi.org/10.3390/bioengineering9100506
Anitua E, Allende M, Alkhraisat MH. Unravelling Alveolar Bone Regeneration Ability of Platelet-Rich Plasma: A Systematic Review with Meta-Analysis. Bioengineering. 2022; 9(10):506. https://doi.org/10.3390/bioengineering9100506
Chicago/Turabian StyleAnitua, Eduardo, Mikel Allende, and Mohammad Hamdan Alkhraisat. 2022. "Unravelling Alveolar Bone Regeneration Ability of Platelet-Rich Plasma: A Systematic Review with Meta-Analysis" Bioengineering 9, no. 10: 506. https://doi.org/10.3390/bioengineering9100506
APA StyleAnitua, E., Allende, M., & Alkhraisat, M. H. (2022). Unravelling Alveolar Bone Regeneration Ability of Platelet-Rich Plasma: A Systematic Review with Meta-Analysis. Bioengineering, 9(10), 506. https://doi.org/10.3390/bioengineering9100506







