Comparative Effects of Basic Fibroblast Growth Factor Delivery or Voluntary Exercise on Muscle Regeneration after Volumetric Muscle Loss
Abstract
:1. Introduction
2. Materials and Methods
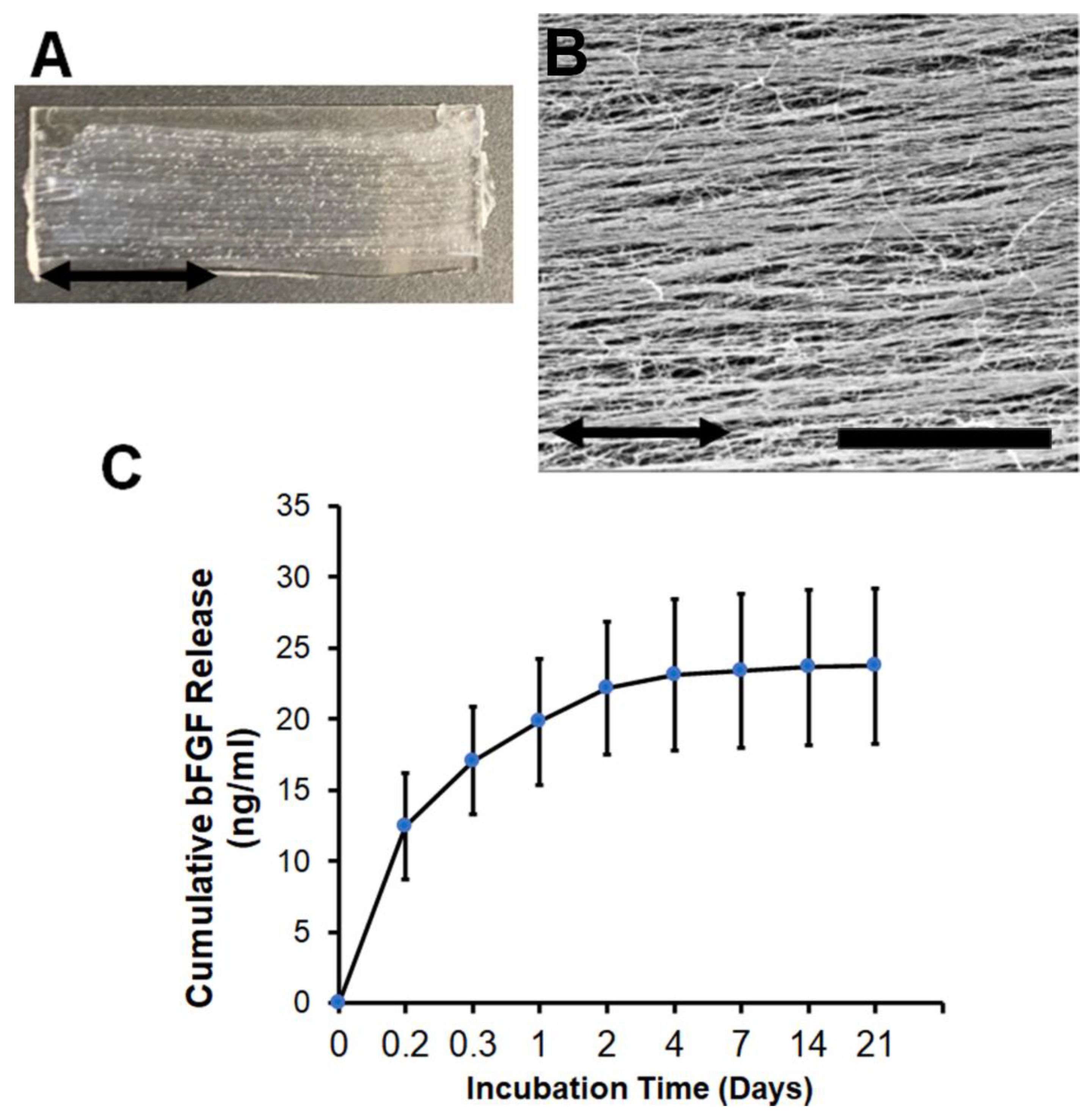
2.1. Fabrication of Aligned Nanofibrillar Collagen Scaffolds
2.2. Quantification of bFGF Release
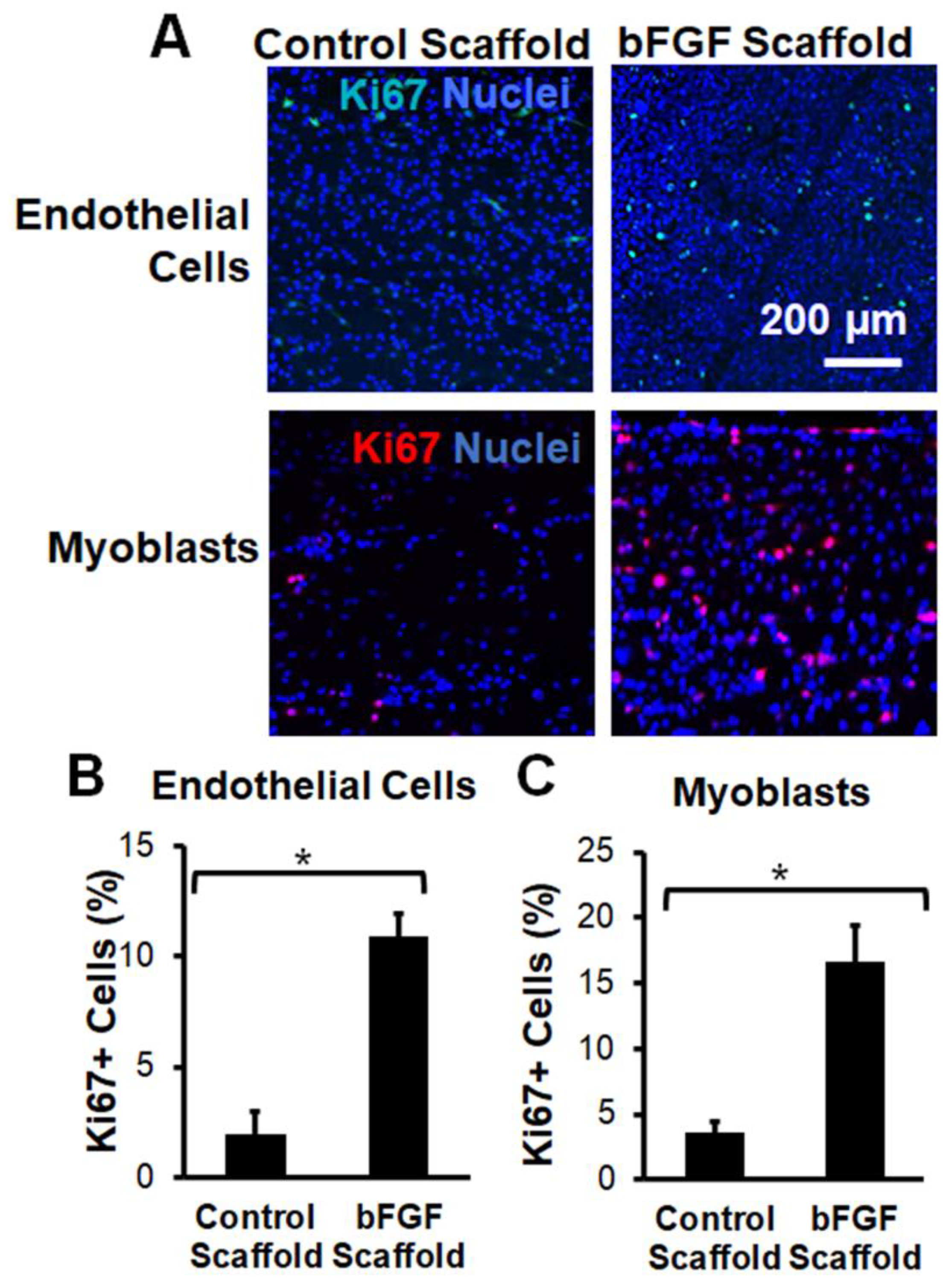
2.3. Bioactivity of bFGF-Laden Scaffolds
2.4. bFGF-Laden Scaffolds and Exercise in a Mouse Full-Thickness VML Model
2.5. Optimization of the Delayed Exercise Regimen
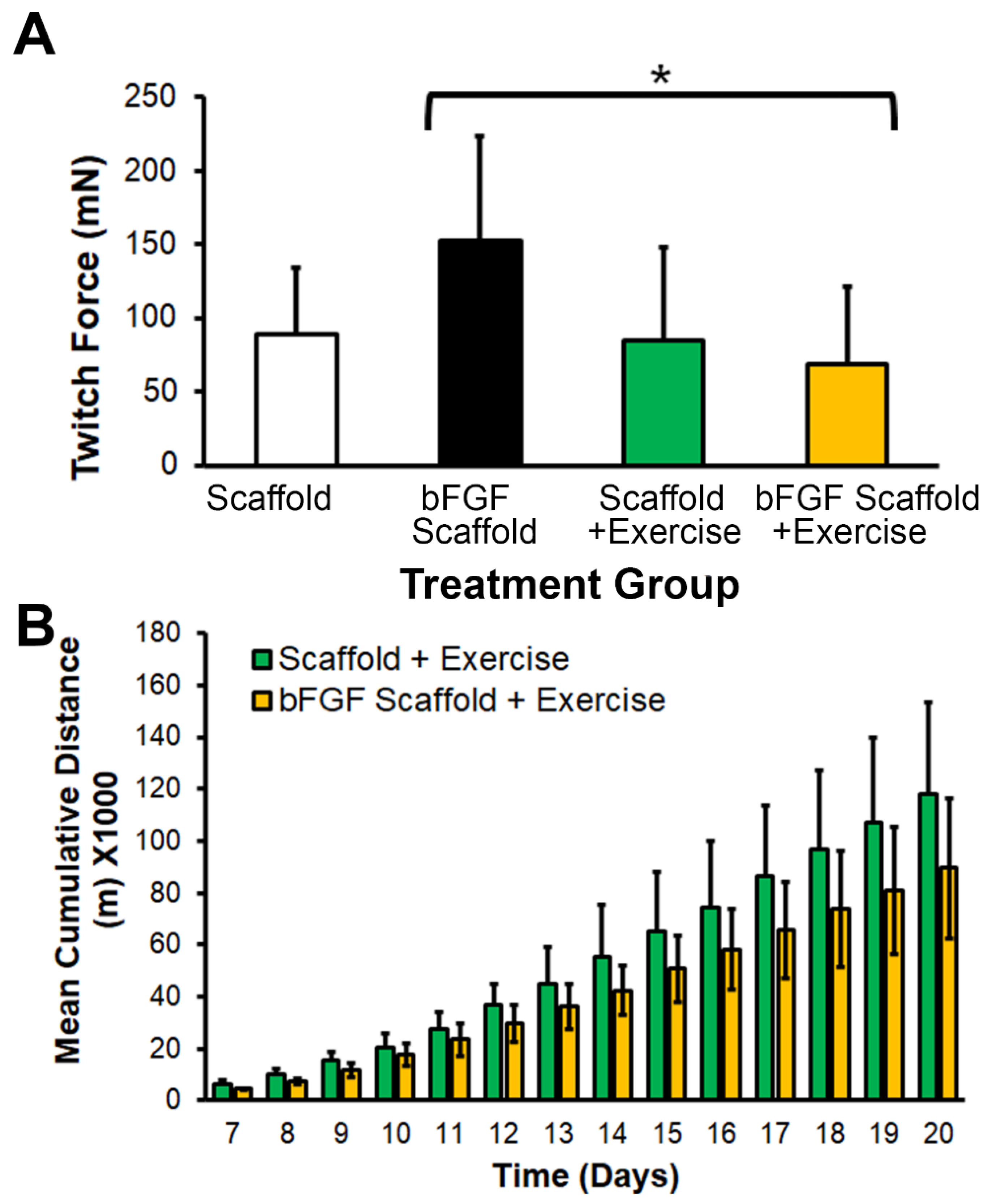
2.6. Muscle Physiology
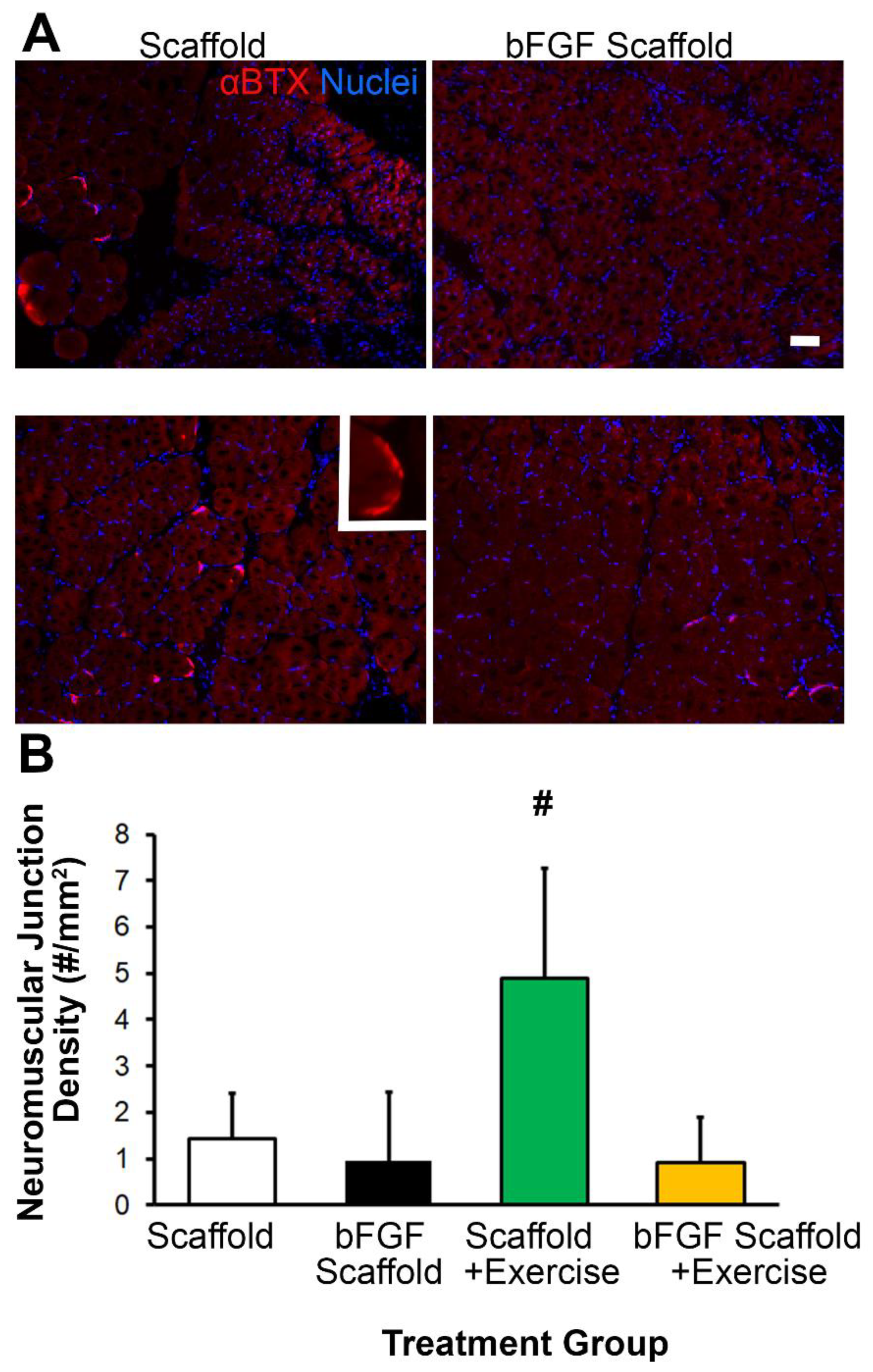
2.7. Immunofluorescence Staining and Quantification of Tissue Cross Sections
2.8. Statistical Analysis
3. Results
3.1. Characterization of bFGF-Laden Nanofibrillar Scaffolds
3.2. Implantation of Aligned Nanofibrillar Scaffolds into the Site of VML
3.3. bFGF-Laden Scaffolds Induced Muscle Function Improvement
3.4. Scaffold Implantation with Exercise Promotes Re-Innervation
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Sicari, B.M.; Rubin, J.P.; Dearth, C.L.; Wolf, M.T.; Ambrosio, F.; Boninger, M.; Turner, N.J.; Weber, D.J.; Simpson, T.W.; Wyse, A.; et al. An acellular biologic scaffold promotes skeletal muscle formation in mice and humans with volumetric muscle loss. Sci. Transl. Med. 2014, 6, 234ra58. [Google Scholar] [CrossRef] [Green Version]
- Garg, K.; Ward, C.L.; Hurtgen, B.J.; Wilken, J.M.; Stinner, D.J.; Wenke, J.C.; Owens, J.G.; Corona, B.T. Volumetric muscle loss: Persistent functional deficits beyond frank loss of tissue. J. Orthop. Res. 2015, 33, 40–46. [Google Scholar] [CrossRef]
- Lin, C.H.; Lin, Y.T.; Yeh, J.T.; Chen, C.T. Free functioning muscle transfer for lower extremity posttraumatic composite structure and functional defect. Plast. Reconstr. Surg. 2007, 119, 2118–2126. [Google Scholar] [CrossRef]
- Lin, S.H.; Chuang, D.C.; Hattori, Y.; Chen, H.C. Traumatic major muscle loss in the upper extremity: Reconstruction using functioning free muscle transplantation. J. Reconstr. Microsurg. 2004, 20, 227–235. [Google Scholar] [PubMed]
- Klinkenberg, M.; Fischer, S.; Kremer, T.; Hernekamp, F.; Lehnhardt, M.; Daigeler, A. Comparison of anterolateral thigh, lateral arm, and parascapular free flaps with regard to donor-site morbidity and aesthetic and functional outcomes. Plast. Reconstr. Surg. 2013, 131, 293–302. [Google Scholar] [CrossRef]
- Kannan, R.Y.; Salacinski, H.J.; Sales, K.; Butler, P.; Seifalian, A.M. The roles of tissue engineering and vascularisation in the development of micro-vascular networks: A review. Biomaterials 2005, 26, 1857–1875. [Google Scholar] [CrossRef] [PubMed]
- Mehiri, S.N.; Barreiro, E.; Hayot, M.; Voyer, M.; Comtois, A.S.; Grassino, A.E.; Czaika, G. Time-based gene expression programme following diaphragm injury in a rat model. Eur. Respir. J. 2005, 25, 422–430. [Google Scholar] [CrossRef] [Green Version]
- Xiao, W.; Liu, Y.; Luo, B.; Zhao, L.; Liu, X.; Zeng, Z.; Chen, P. Time-dependent gene expression analysis after mouse skeletal muscle contusion. J. Sport Health Sci. 2016, 5, 101–108. [Google Scholar] [CrossRef] [Green Version]
- Shayan, M.; Huang, N.F. Pre-clinical cell therapeutic approaches for repair of volumetric muscle loss. Bioengineering 2020, 7, 97. [Google Scholar] [CrossRef] [PubMed]
- Corona, B.T.; Machingal, M.A.; Criswell, T.; Vadhavkar, M.; Dannahower, A.C.; Bergman, C.; Zhao, W.; Christ, G.J. Further development of a tissue engineered muscle repair construct in vitro for enhanced functional recovery following implantation in vivo in a murine model of volumetric muscle loss injury. Tissue Eng. Part A 2012, 18, 1213–1228. [Google Scholar] [CrossRef] [Green Version]
- Corona, B.T.; Ward, C.L.; Baker, H.B.; Walters, T.J.; Christ, G.J. Implantation of in vitro tissue engineered muscle repair constructs and bladder acellular matrices partially restore in vivo skeletal muscle function in a rat model of volumetric muscle loss injury. Tissue Eng. Part A 2014, 20, 705–715. [Google Scholar] [PubMed] [Green Version]
- Dziki, J.; Badylak, S.; Yabroudi, M.; Sicari, B.; Ambrosio, F.; Stearns, K.; Turner, N.; Wyse, A.; Boninger, M.L.; Brown, E.H.P.; et al. An acellular biologic scaffold treatment for volumetric muscle loss: Results of a 13-patient cohort study. NPJ Regen. Med. 2016, 1, 16008. [Google Scholar] [CrossRef] [PubMed]
- Nakayama, K.H.; Alcazar, C.; Yang, G.; Quarta, M.; Paine, P.; Doan, L.; Davies, A.; Rando, T.A.; Huang, N.F. Rehabilitative exercise and spatially patterned nanofibrillar scaffolds enhance vascularization and innervation following volumetric muscle loss. NPJ Regen. Med. 2018, 3, 16. [Google Scholar] [CrossRef] [PubMed]
- Panayi, A.C.; Smit, L.; Hays, N.; Udeh, K.; Endo, Y.; Li, B.; Sakthivel, D.; Tamayol, A.; Neppl, R.L.; Orgill, D.P.; et al. A porous collagen-gag scaffold promotes muscle Regeneration following volumetric muscle loss injury. Wound Repair Regen. 2020, 28, 61–74. [Google Scholar] [CrossRef] [PubMed]
- Matthias, N.; Hunt, S.D.; Wu, J.; Lo, J.; Smith Callahan, L.A.; Li, Y.; Huard, J.; Darabi, R. Volumetric muscle loss injury repair using in situ fibrin gel cast seeded with muscle-derived stem cells (mdscs). Stem Cell Res. 2018, 27, 65–73. [Google Scholar] [CrossRef]
- Nakayama, K.H.; Quarta, M.; Paine, P.; Alcazar, C.; Karakikes, I.; Garcia, V.; Abilez, O.J.; Calvo, N.S.; Simmons, C.S.; Rando, T.A.; et al. Treatment of volumetric muscle loss in mice using nanofibrillar scaffolds enhances vascular organization and integration. Commun. Biol. 2019, 2, 170. [Google Scholar] [CrossRef]
- Huang, N.F.; Okogbaa, J.; Lee, J.C.; Jha, A.; Zaitseva, T.S.; Paukshto, M.V.; Sun, J.S.; Punjya, N.; Fuller, G.G.; Cooke, J.P. The modulation of endothelial cell morphology, function, and survival using anisotropic nanofibrillar collagen scaffolds. Biomaterials 2013, 34, 4038–4047. [Google Scholar] [CrossRef] [Green Version]
- Huang, N.F.; Li, S. Mesenchymal stem cells for vascular regeneration. Regen. Med. 2008, 3, 877–892. [Google Scholar] [CrossRef] [Green Version]
- Nakayama, K.H.; Hong, G.; Lee, J.C.; Patel, J.; Edwards, B.; Zaitseva, T.S.; Paukshto, M.V.; Dai, H.; Cooke, J.P.; Woo, Y.J.; et al. Aligned-braided nanofibrillar scaffold with endothelial cells enhances arteriogenesis. ACS Nano 2015, 9, 6900–6908. [Google Scholar] [CrossRef] [Green Version]
- Nakayama, K.H.; Surya, V.N.; Gole, M.; Walker, T.W.; Yang, W.; Lai, E.S.; Ostrowski, M.A.; Fuller, G.G.; Dunn, A.R.; Huang, N.F. Nanoscale patterning of extracellular matrix alters endothelial function under shear stress. Nano Lett. 2016, 16, 410–419. [Google Scholar] [CrossRef] [Green Version]
- Huang, N.F.; Patel, S.; Thakar, R.G.; Wu, J.; Hsiao, B.S.; Chu, B.; Lee, R.J.; Li, S. Myotube assembly on nanofibrous and micropatterned polymers. Nano Lett. 2006, 6, 537–542. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; McNamara, L.E.; Gadegaard, N.; Alakpa, E.V.; Burgess, K.V.; Meek, R.M.; Dalby, M.J. Nanotopographical induction of osteogenesis through adhesion, bone morphogenic protein cosignaling, and regulation of micrornas. ACS Nano 2014, 8, 9941–9953. [Google Scholar] [CrossRef]
- McBeath, R.; Pirone, D.M.; Nelson, C.M.; Bhadriraju, K.; Chen, C.S. Cell shape, cytoskeletal tension, and rhoa regulate stem cell lineage commitment. Dev. Cell 2004, 6, 483–495. [Google Scholar] [CrossRef] [Green Version]
- Bursac, N.; Parker, K.K.; Iravanian, S.; Tung, L. Cardiomyocyte cultures with controlled macroscopic anisotropy: A model for functional electrophysiological studies of cardiac muscle. Circ. Res. 2002, 91, e45–e54. [Google Scholar] [CrossRef]
- Aurora, A.; Garg, K.; Corona, B.T.; Walters, T.J. Physical rehabilitation improves muscle function following volumetric muscle loss injury. BMC Sports Sci. Med. Rehabil. 2014, 6, 41. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Quarta, M.; Cromie, M.; Chacon, R.; Blonigan, J.; Garcia, V.; Akimenko, I.; Hamer, M.; Paine, P.; Stok, M.; Shrager, J.B.; et al. Bioengineered constructs combined with exercise enhance stem cell-mediated treatment of volumetric muscle loss. Nat. Commun. 2017, 8, 15613. [Google Scholar] [CrossRef]
- Gentile, N.E.; Stearns, K.M.; Brown, E.H.; Rubin, J.P.; Boninger, M.L.; Dearth, C.L.; Ambrosio, F.; Badylak, S.F. Targeted rehabilitation after extracellular matrix scaffold transplantation for the treatment of volumetric muscle loss. Am. J. Phys. Med. Rehabil. 2014, 93, S79–S87. [Google Scholar] [CrossRef]
- Alcazar, C.A.; Hu, C.; Rando, T.A.; Huang, N.F.; Nakayama, K.H. Transplantation of insulin-like growth factor-1 laden scaffolds combined with exercise promotes neuroregeneration and angiogenesis in a preclinical muscle injury model. Biomater. Sci. 2020, 8, 5376–5389. [Google Scholar] [CrossRef]
- Lai, E.S.; Huang, N.F.; Cooke, J.P.; Fuller, G.G. Aligned nanofibrillar collagen regulates endothelial organization and migration. Regen. Med. 2012, 7, 649–661. [Google Scholar] [CrossRef] [Green Version]
- Nakayama, K.H.; Joshi, P.A.; Lai, E.S.; Gujar, P.; Joubert, L.M.; Chen, B.; Huang, N.F. Bilayered vascular graft derived from human induced pluripotent stem cells with biomimetic structure and function. Regen. Med. 2015, 10, 745–755. [Google Scholar] [CrossRef]
- Huang, N.F.; Fleissner, F.; Sun, J.; Cooke, J.P. Role of nitric oxide signaling in endothelial differentiation of embryonic stem cells. Stem Cells Dev. 2010, 19, 1617–1626. [Google Scholar] [CrossRef]
- Wanjare, M.; Hou, L.; Nakayama, K.H.; Kim, J.J.; Mezak, N.P.; Abilez, O.J.; Tzatzalos, E.; Wu, J.C.; Huang, N.F. Anisotropic microfibrous scaffolds enhance the organization and function of cardiomyocytes derived from induced pluripotent stem cells. Biomater. Sci. 2017, 5, 1567–1578. [Google Scholar] [CrossRef]
- Wanjare, M.; Kawamura, M.; Hu, C.; Alcazar, C.; Wang, H.; Woo, Y.J.; Huang, N.F. Vascularization of engineered spatially patterned myocardial tissue derived from human pluripotent stem cells in vivo. Front. Bioeng. Biotechnol. 2019, 7, 208. [Google Scholar] [CrossRef]
- Fu, X.; Shen, Z.; Chen, Y. Basic fibroblast growth factor (bfgf) and wound healing: A multi-centers and controlled clinical trial in 1024 cases. Zhongguo Xiu Fu Chong Jian Wai Ke Za Zhi 1998, 12, 209–211. [Google Scholar]
- Lazarous, D.F.; Unger, E.F.; Epstein, S.E.; Stine, A.; Arevalo, J.L.; Chew, E.Y.; Quyyumi, A.A. Basic fibroblast growth factor in patients with intermittent claudication: Results of a phase i trial. J. Am. Coll. Cardiol. 2000, 36, 1239–1244. [Google Scholar] [CrossRef] [Green Version]
- Izadi, M.R.; Habibi, A.; Khodabandeh, Z.; Nikbakht, M. Synergistic effect of high-intensity interval training and stem cell transplantation with amniotic membrane scaffold on repair and rehabilitation after volumetric muscle loss injury. Cell Tissue Res. 2021, 383, 765–779. [Google Scholar] [CrossRef]
- Megeney, L.A.; Perry, R.L.; LeCouter, J.E.; Rudnicki, M.A. Bfgf and lif signaling activates stat3 in proliferating myoblasts. Dev. Genet. 1996, 19, 139–145. [Google Scholar] [CrossRef]
- Trenerry, M.K.; Carey, K.A.; Ward, A.C.; Cameron-Smith, D. Stat3 signaling is activated in human skeletal muscle following acute resistance exercise. J. Appl. Physiol. 2007, 102, 1483–1489. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Guadagnin, E.; Mazala, D.; Chen, Y.W. Stat3 in skeletal muscle function and disorders. Int. J. Mol. Sci. 2018, 19, 2265. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tsujinaka, T.; Fujita, J.; Ebisui, C.; Yano, M.; Kominami, E.; Suzuki, K.; Tanaka, K.; Katsume, A.; Ohsugi, Y.; Shiozaki, H.; et al. Interleukin 6 receptor antibody inhibits muscle atrophy and modulates proteolytic systems in interleukin 6 transgenic mice. J. Clin. Investig. 1996, 97, 244–249. [Google Scholar] [CrossRef] [Green Version]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hu, C.; Ayan, B.; Chiang, G.; Chan, A.H.P.; Rando, T.A.; Huang, N.F. Comparative Effects of Basic Fibroblast Growth Factor Delivery or Voluntary Exercise on Muscle Regeneration after Volumetric Muscle Loss. Bioengineering 2022, 9, 37. https://doi.org/10.3390/bioengineering9010037
Hu C, Ayan B, Chiang G, Chan AHP, Rando TA, Huang NF. Comparative Effects of Basic Fibroblast Growth Factor Delivery or Voluntary Exercise on Muscle Regeneration after Volumetric Muscle Loss. Bioengineering. 2022; 9(1):37. https://doi.org/10.3390/bioengineering9010037
Chicago/Turabian StyleHu, Caroline, Bugra Ayan, Gladys Chiang, Alex H. P. Chan, Thomas A. Rando, and Ngan F. Huang. 2022. "Comparative Effects of Basic Fibroblast Growth Factor Delivery or Voluntary Exercise on Muscle Regeneration after Volumetric Muscle Loss" Bioengineering 9, no. 1: 37. https://doi.org/10.3390/bioengineering9010037
APA StyleHu, C., Ayan, B., Chiang, G., Chan, A. H. P., Rando, T. A., & Huang, N. F. (2022). Comparative Effects of Basic Fibroblast Growth Factor Delivery or Voluntary Exercise on Muscle Regeneration after Volumetric Muscle Loss. Bioengineering, 9(1), 37. https://doi.org/10.3390/bioengineering9010037








