Heat-Inactivation of Fetal and Newborn Sera Did Not Impair the Expansion and Scaffold Engineering Potentials of Fibroblasts
Abstract
:1. Introduction
2. Materials and Methods
2.1. Ethics Statement
2.2. Cell Culture
2.3. Serum Inactivation
2.4. Adhesion and Proliferation of Fibroblasts
2.5. Metabolism Evaluation of Fibroblast Cultures
2.6. Stroma Reconstruction Using the Self-Assembly Approach
2.7. Histology and Thickness Measurement
2.8. Mechanical Testings
2.9. Statistic
3. Results
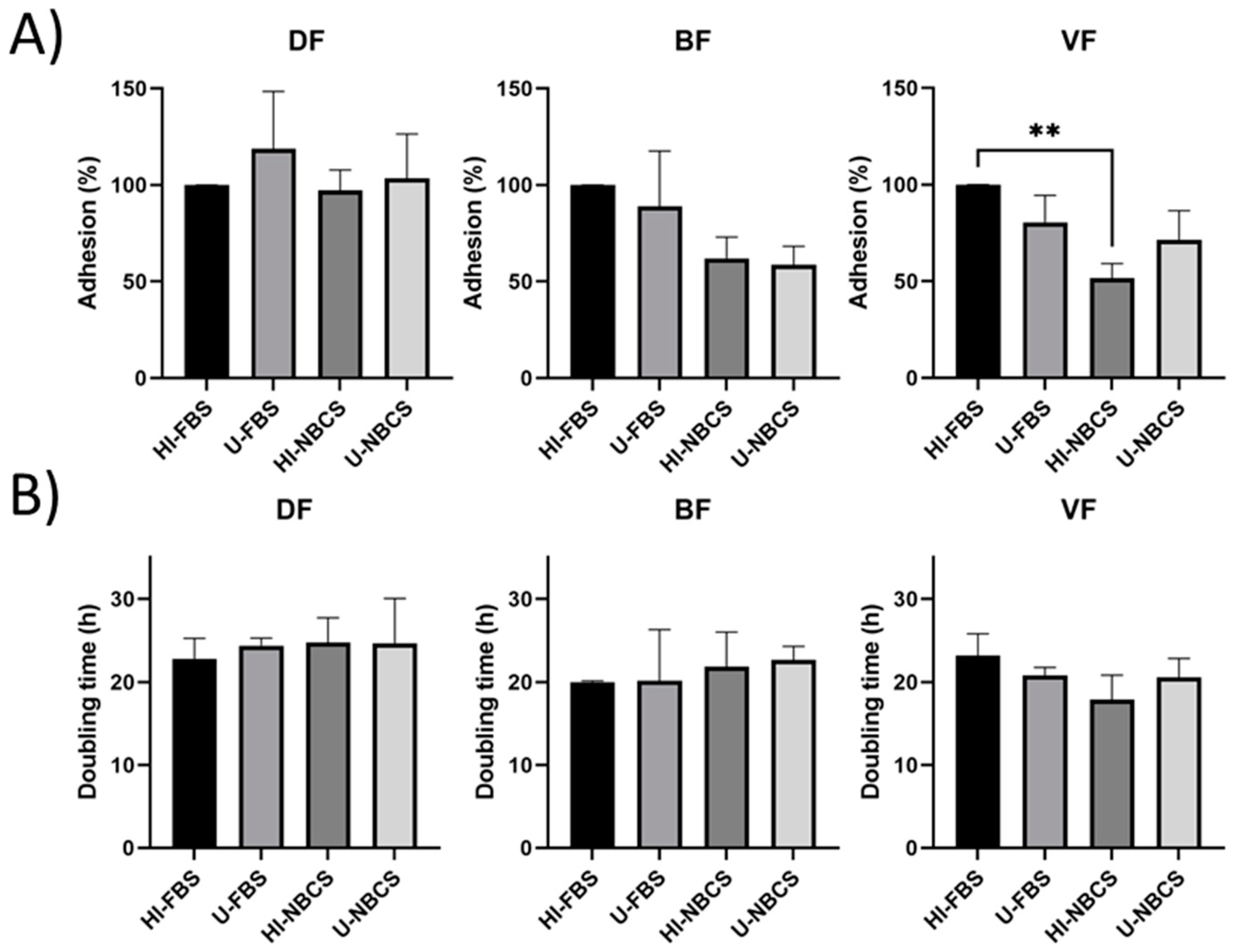
3.1. Adhesion in 2D Cultures
3.2. Proliferation in 2D Cultures
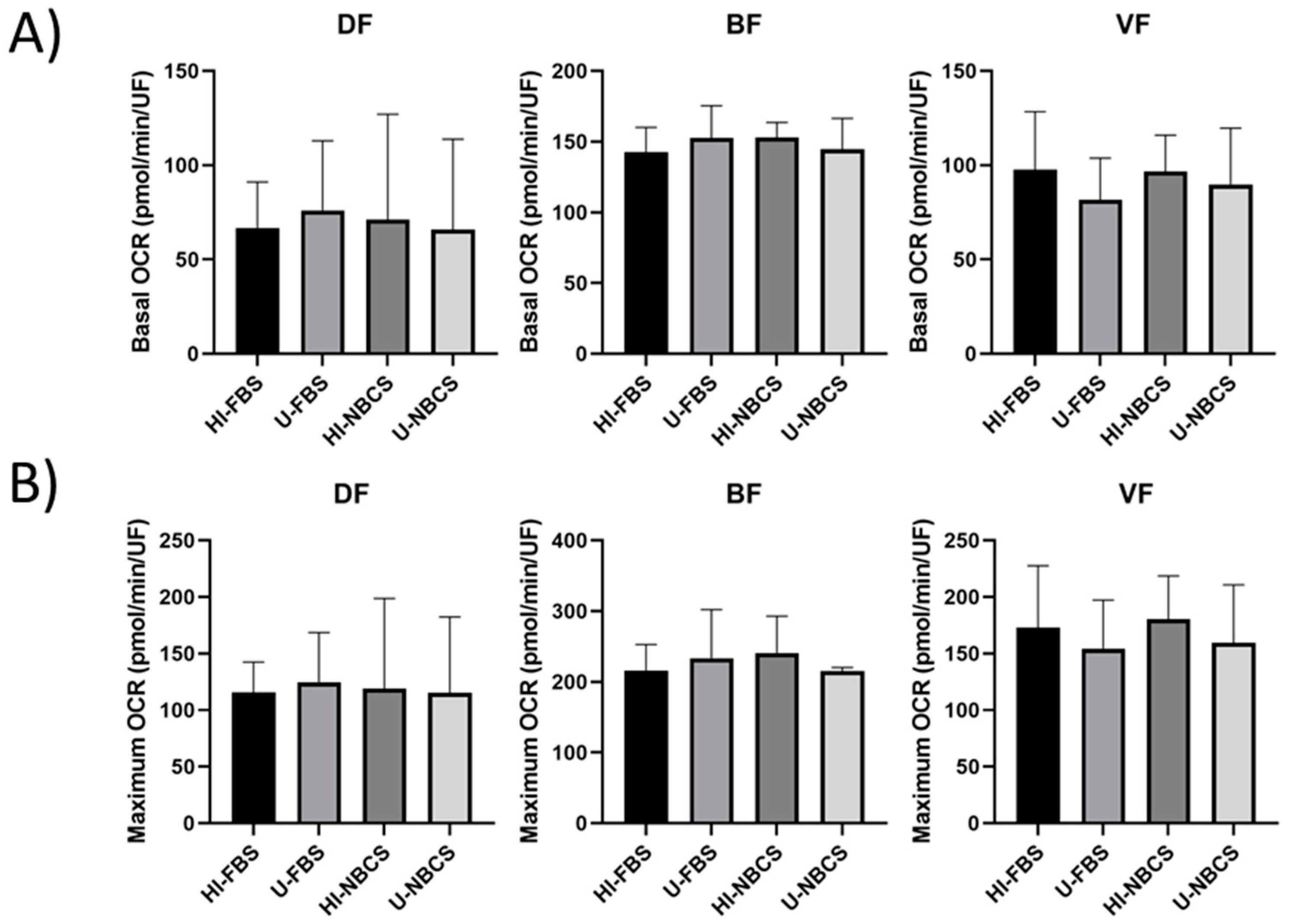
3.3. Mitochondrial Respiration in 2D Cultures
3.4. Glycolysis in 2D Cultures
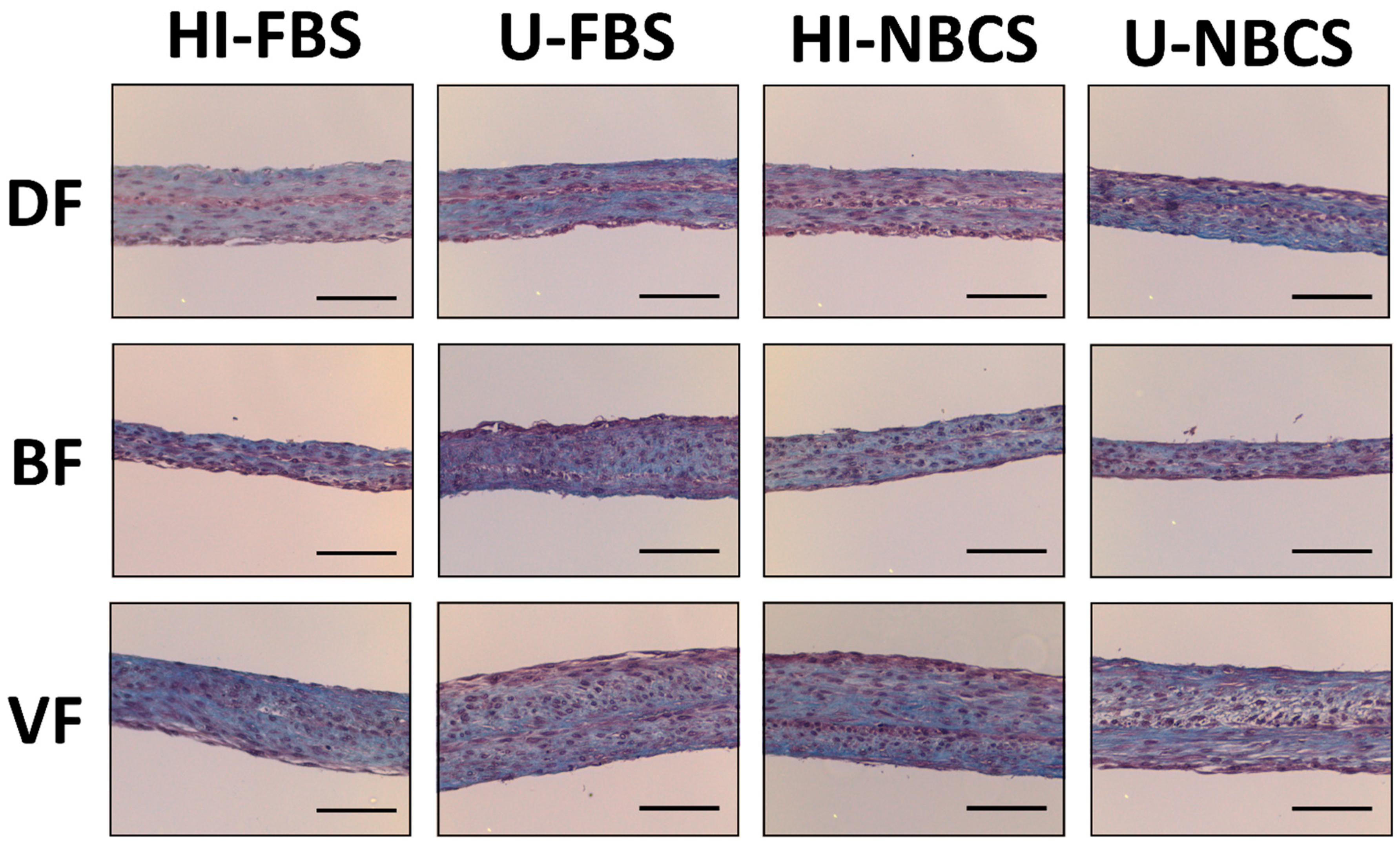
3.5. Histology of 3D Reconstructed Tissues
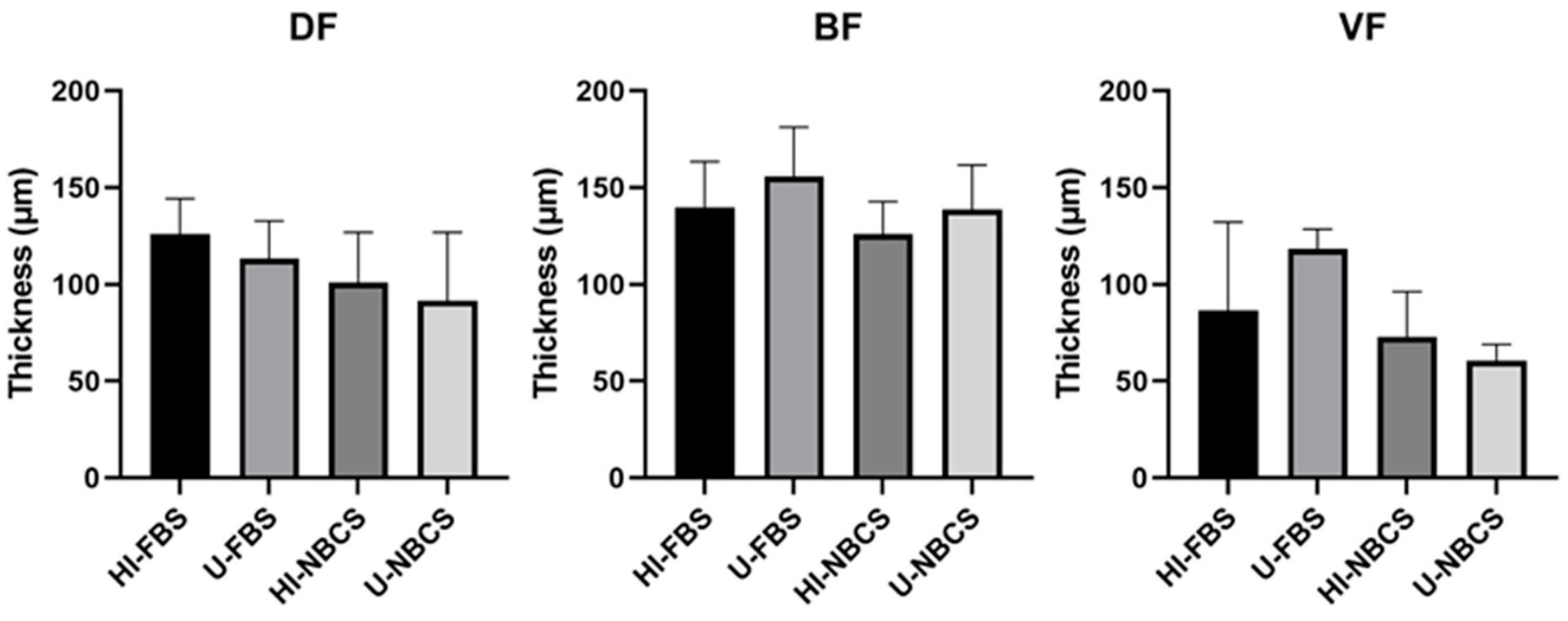
3.6. Thickness of 3D Reconstructed Tissues
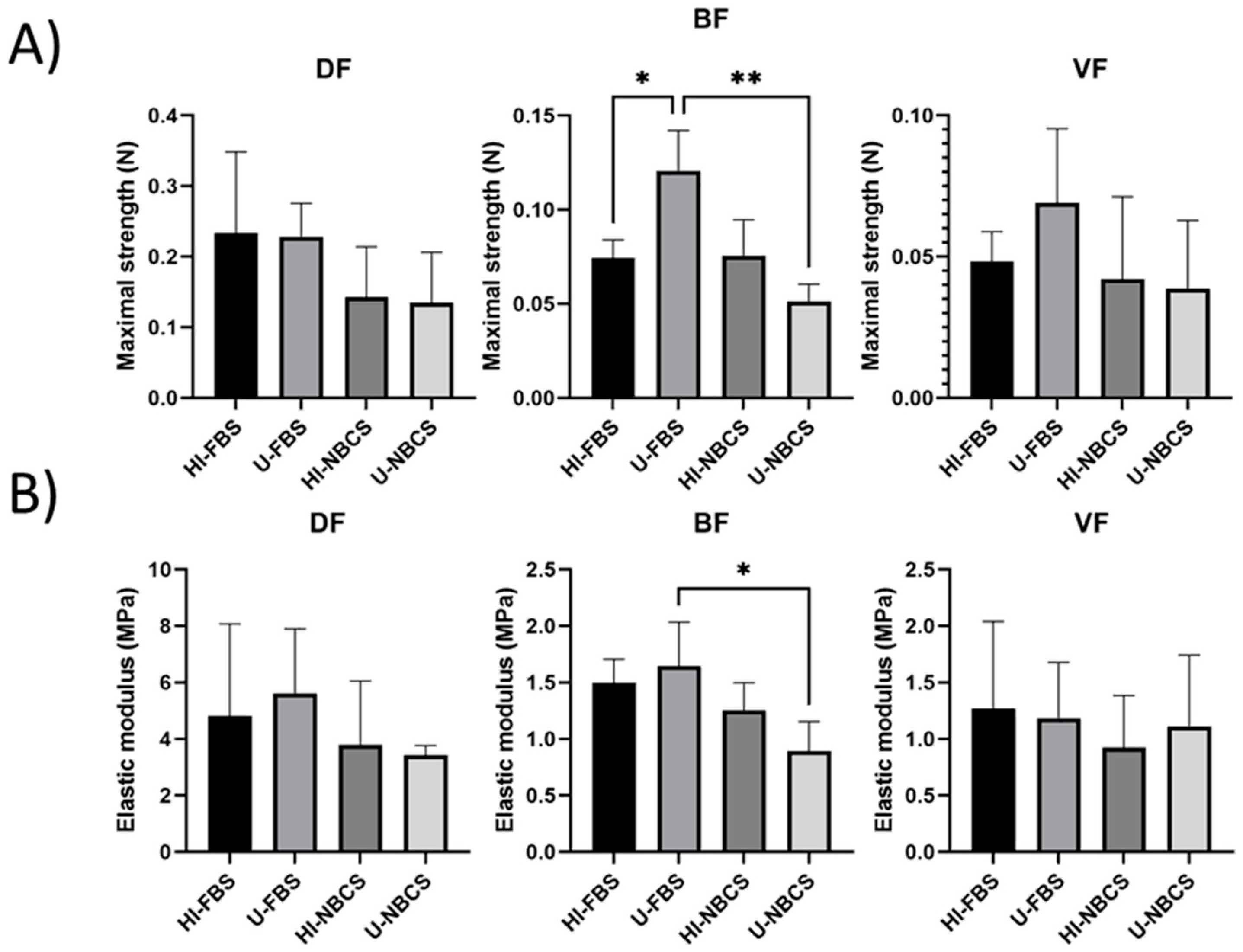
3.7. Maximal Strength of 3D Reconstructed Tissues
3.8. Elastic Modulus of 3D Reconstructed Tissues
3.9. Ultimate Tensile Strength of 3D Reconstructed Tissues
3.10. Failure Strain of 3D Reconstructed Tissues
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Nims, R.W.; Harbell, J.W. Best practices for the use and evaluation of animal serum as a component of cell culture medium. In Vitro Cell. Dev. Biol. Anim. 2017, 53, 682–690. [Google Scholar] [CrossRef] [PubMed]
- Bédard, P.; Gauvin, S.; Ferland, K.; Caneparo, C.; Pellerin, È.; Chabaud, S.; Bolduc, S. Innovative Human Three-Dimensional Tissue-Engineered Models as an Alternative to Animal Testing. Bioengineering 2020, 7, 115. [Google Scholar] [CrossRef] [PubMed]
- Triglia, R.P.; Linscott, W.D. Titers of nine complement components, conglutinin and C3b-inactivator in adult and fetal bovine sera. Mol. Immunol. 1980, 17, 741–748. [Google Scholar] [CrossRef]
- Soltis, R.D.; Hasz, D.; Morris, M.J.; Wilson, I.D. The effect of heat inactivation of serum on aggregation of immunoglobulins. Immunology 1979, 36, 37–45. [Google Scholar]
- Linscott, W.D.; Triglia, R.P. The bovine complement system. Adv. Exp. Med. Biol. 1981, 137, 413–430. [Google Scholar]
- Fante, M.A.; Decking, S.M.; Bruss, C.; Schreml, S.; Siska, P.J.; Kreutz, M.; Renner, K. Heat-Inactivation of Human Serum Destroys C1 Inhibitor, Pro-motes Immune Complex Formation, and Improves Human T Cell Function. Int. J. Mol. Sci. 2021, 22, 2646. [Google Scholar] [CrossRef] [PubMed]
- Leshem, B.; Yogev, D.; Fiorentini, D. Heat inactivation of fetal calf serum is not required for in vitro measurement of lymphocyte functions. J. Immunol. Methods 1999, 223, 249–254. [Google Scholar] [CrossRef]
- Giard, D.J. Routine heat inactivation of serum reduces its capacity to promote cell attachment. In Vitro Cell. Dev. Biol. 1987, 23, 691–697. [Google Scholar] [CrossRef]
- Bird, M.; Owen, A. Neurite outgrowth-regulating properties of GABA and the effect of serum on mouse spinal cord neurons in culture. J. Anat. 1998, 193 Pt 4, 503–508. [Google Scholar] [CrossRef] [PubMed]
- Tonarova, P.; Lochovska, K.; Pytlik, R.; Hubalek Kalbacova, M. The Impact of Various Culture Conditions on Human Mesenchymal Stromal Cells Metabolism. Stem Cells Int. 2021, 2021, 6659244. [Google Scholar] [CrossRef]
- Pinyopummintr, T.; Bavister, B.D. Development of bovine embryos in a cell-free culture medium: Effects of type of serum, timing of its inclusion and heat inactivation. Theriogenology 1994, 41, 1241–1249. [Google Scholar] [CrossRef]
- Namekar, M.; Kumar, M.; O’Connell, M.; Nerurkar, V.R. Effect of serum heat-inactivation and dilution on detection of anti-WNV antibodies in mice by West Nile virus E-protein microsphere immunoassay. PLoS ONE 2012, 7, e45851. [Google Scholar] [CrossRef]
- Jungkind, D.L.; DiRenzo, S.A.; Young, S.J. Effect of using heat-inactivated serum with the Abbott human T-cell lymphotropic virus type III antibody test. J. Clin. Microbiol. 1986, 23, 381–382. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hu, X.; An, T.; Situ, B.; Hu, Y.; Ou, Z.; Li, Q.; He, X.; Zhang, Y.; Tian, P.; Sun, D.; et al. Heat inactivation of serum interferes with the immunoanalysis of antibodies to SARS-CoV-2. J. Clin. Lab. Anal. 2020, 34, e23411. [Google Scholar] [CrossRef] [PubMed]
- Langer, R.; Vacanti, J.P. Tissue engineering. Science 1993, 260, 920–926. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Switzer, B.R.; Summer, G.K. Collagen synthesis in human skin fibroblasts: Effects of ascorbate, -ketoglutarate and ferrous ion on proline hydroxylation. J. Nutr. 1972, 102, 721–728. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hata, R.; Senoo, H. L-ascorbic acid 2-phosphate stimulates collagen accumulation, cell proliferation, and formation of a three-dimensional tissue-like substance by skin fibroblasts. J. Cell. Physiol. 1989, 138, 8–16. [Google Scholar] [CrossRef] [PubMed]
- L’Heureux, N.; Pâquet, S.; Labbé, R.; Germain, L.; Auger, F.A. A completely biological tissue-engineered human blood vessel. FASEB J. 1998, 12, 47–56. [Google Scholar] [CrossRef]
- Saba, I.; Jakubowska, W.; Bolduc, S.; Chabaud, S. Engineering Tissues without the Use of a Synthetic Scaffold: A Twenty-Year History of the Self-Assembly Method. Biomed. Res. Int. 2018, 2018, 5684679. [Google Scholar] [CrossRef] [Green Version]
- Kelly, C.; Wallace, D.; Moulin, V.; Germain, L.; Zuccaro, J.; Galdyn, I.; Fish, J.S. Surviving an Extensive Burn Injury Using Advanced Skin Replacement Technologies. J. Burn Care Res. 2021, irab146. [Google Scholar] [CrossRef] [PubMed]
- Roy, V.; Magne, B.; Vaillancourt-Audet, M.; Blais, M.; Chabaud, S.; Grammond, E.; Piquet, L.; Fradette, J.; Laverdière, I.; Moulin, V.J.; et al. Human Organ-Specific 3D Cancer Models Produced by the Stromal Self-Assembly Method of Tissue Engineering for the Study of Solid Tumors. BioMed Res. Int. 2020, 2020, 6051210. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Caneparo, C.; Brownell, D.; Chabaud, S.; Bolduc, S. Genitourinary Tissue Engineering: Reconstruction and Research Models. Bioengineering 2021, 8, 99. [Google Scholar] [CrossRef] [PubMed]
- Pellerin, È.; Chabaud, S.; Pouliot, F.; Pelletier, M.; Bolduc, S. Bisphenol A Alters the Energy Metabolism of Stromal Cells and Could Promote Bladder Cancer Progression. Cancers 2021, 13, 5461. [Google Scholar] [CrossRef]
- Chabaud, S.; Simard, M.; Gendreau, I.; Pouliot, R.; Bolduc, S. Origin of Serum Affects Quality of Engineered Tissues Produced by the Self-Assembly Approach. Scientifica (Cairo) 2016, 2016, 3825645. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chabaud, S.; Saba, I.; Baratange, C.; Boiroux, B.; Leclerc, M.; Rousseau, A.; Bouhout, S.; Bolduc, S. Urothelial cell expansion and differentiation are improved by exposure to hypoxia. J. Tissue Eng. Regen. Med. 2017, 11, 3090–3099. [Google Scholar] [CrossRef] [PubMed]
- Jakubowska, W.; Chabaud, S.; Saba, I.; Galbraith, T.; Berthod, F.; Bolduc, S. Prevascularized Tissue-Engineered Human Vaginal Mucosa: In Vitro Optimization and In Vivo Validation. Tissue Eng. Part A 2020, 26, 811–822. [Google Scholar] [CrossRef] [PubMed]
- Proulx, S.; d’Arc Uwamaliya, J.; Carrier, P.; Deschambeault, A.; Audet, C.; Giasson, C.J.; Guérin, S.L.; Auger, F.A.; Germain, L. Reconstruction of a human cornea by the self-assembly approach of tissue engineering using the three native cell types. Mol. Vis. 2010, 16, 2192–2201. [Google Scholar] [PubMed]
- Vermette, M.; Trottier, V.; Ménard, V.; Saint-Pierre, L.; Roy, A.; Fradette, J. Production of a new tissue-engineered adipose substitute from human adipose-derived stromal cells. Biomaterials 2007, 28, 2850–2860. [Google Scholar] [CrossRef]
- Bouhout, S.; Chabaud, S.; Bolduc, S. Organ-specific matrix self-assembled by mesenchymal cells improves the normal urothelial differentiation in vitro. World J. Urol. 2016, 34, 121–130. [Google Scholar] [CrossRef]
- Cao, M.; Tasian, G.; Wang, M.H.; Liu, B.; Cunha, G.; Baskin, L. Urothelium-derived Sonic hedgehog promotes mesenchymal proliferation and induces bladder smooth muscle differentiation. Differentiation 2010, 79, 244–250. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mendelsohn, C. Going in circles: Conserved mechanisms control radial patterning in the urinary and digestive tracts. J. Clin. Investig. 2006, 116, 635–637. [Google Scholar] [CrossRef] [Green Version]
- Cable, D.G.; Hisamochi, K.; Schaff, H.V. Complement mediates attenuation of endothelium-dependent relaxations in canine coronary arteries after porcine serum exposure: A mechanism for vascular thrombosis in xenograft hyperacute rejection. Circulation 1997, 96 (Suppl. 9), II-58–63. discussion II-63–64. [Google Scholar]
- Hamelmann, W.; Gray, D.W.; Cairns, T.D.; Ozasa, T.; Ferguson, D.J.; Cahill, A.; Welsh, K.I.; Morris, P.J. Immediate destruction of xenogeneic islets in a primate model. Transplantation 1994, 58, 1109–1114. [Google Scholar] [CrossRef] [PubMed]
- Valdivia, L.A.; Fung, J.J.; Demetris, A.J.; Celli, S.; Pan, F.; Tsugita, M.; Starzl, T.E. Donor species complement after liver xenotransplantation. The mechanism of protection from hyperacute rejection. Transplantation 1994, 57, 918–922. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rioux, G.; Simard, M.; Morin, S.; Lorthois, I.; Guérin, S.L.; Pouliot, R. Development of a 3D psoriatic skin model optimized for infiltration of IL-17A producing T cells: Focus on the crosstalk between T cells and psoriatic keratinocytes. Acta Biomater. 2021. [Google Scholar] [CrossRef] [PubMed]
- Lorthois, I.; Simard, M.; Morin, S.; Pouliot, R. Infiltration of T Cells into a Three-Dimensional Psoriatic Skin Model Mimics Pathological Key Features. Int. J. Mol. Sci. 2019, 20, 1670. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Corriveau, M.P.; Boufaied, I.; Lessard, J.; Chabaud, S.; Senécal, J.L.; Grodzicky, T.; Chartier, S.; Raymond, Y.; Moulin, V.J. The fibrotic phenotype of systemic sclerosis fibroblasts varies with disease duration and severity of skin involvement: Reconstitution of skin fibrosis development using a tissue engineering approach. J. Pathol. 2009, 217, 534–542. [Google Scholar] [CrossRef] [PubMed]
- Simon, F.; Bergeron, D.; Larochelle, S.; Lopez-Vallé, C.A.; Genest, H.; Armour, A.; Moulin, V.J. Enhanced secretion of TIMP-1 by human hypertrophic scar keratinocytes could contribute to fibrosis. Burns 2012, 38, 421–427. [Google Scholar] [CrossRef]
- Ringuette Goulet, C.; Bernard, G.; Chabaud, S.; Couture, A.; Langlois, A.; Neveu, B.; Pouliot, F.; Bolduc, S. Tissue-engineered human 3D model of bladder cancer for invasion study and drug discovery. Biomaterials 2017, 145, 233–241. [Google Scholar] [CrossRef]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pellerin, F.-A.; Caneparo, C.; Pellerin, È.; Chabaud, S.; Pelletier, M.; Bolduc, S. Heat-Inactivation of Fetal and Newborn Sera Did Not Impair the Expansion and Scaffold Engineering Potentials of Fibroblasts. Bioengineering 2021, 8, 184. https://doi.org/10.3390/bioengineering8110184
Pellerin F-A, Caneparo C, Pellerin È, Chabaud S, Pelletier M, Bolduc S. Heat-Inactivation of Fetal and Newborn Sera Did Not Impair the Expansion and Scaffold Engineering Potentials of Fibroblasts. Bioengineering. 2021; 8(11):184. https://doi.org/10.3390/bioengineering8110184
Chicago/Turabian StylePellerin, Félix-Antoine, Christophe Caneparo, Ève Pellerin, Stéphane Chabaud, Martin Pelletier, and Stéphane Bolduc. 2021. "Heat-Inactivation of Fetal and Newborn Sera Did Not Impair the Expansion and Scaffold Engineering Potentials of Fibroblasts" Bioengineering 8, no. 11: 184. https://doi.org/10.3390/bioengineering8110184
APA StylePellerin, F.-A., Caneparo, C., Pellerin, È., Chabaud, S., Pelletier, M., & Bolduc, S. (2021). Heat-Inactivation of Fetal and Newborn Sera Did Not Impair the Expansion and Scaffold Engineering Potentials of Fibroblasts. Bioengineering, 8(11), 184. https://doi.org/10.3390/bioengineering8110184






