Design and Analysis of a Biodegradable Polycaprolactone Flow Diverting Stent for Brain Aneurysms
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals
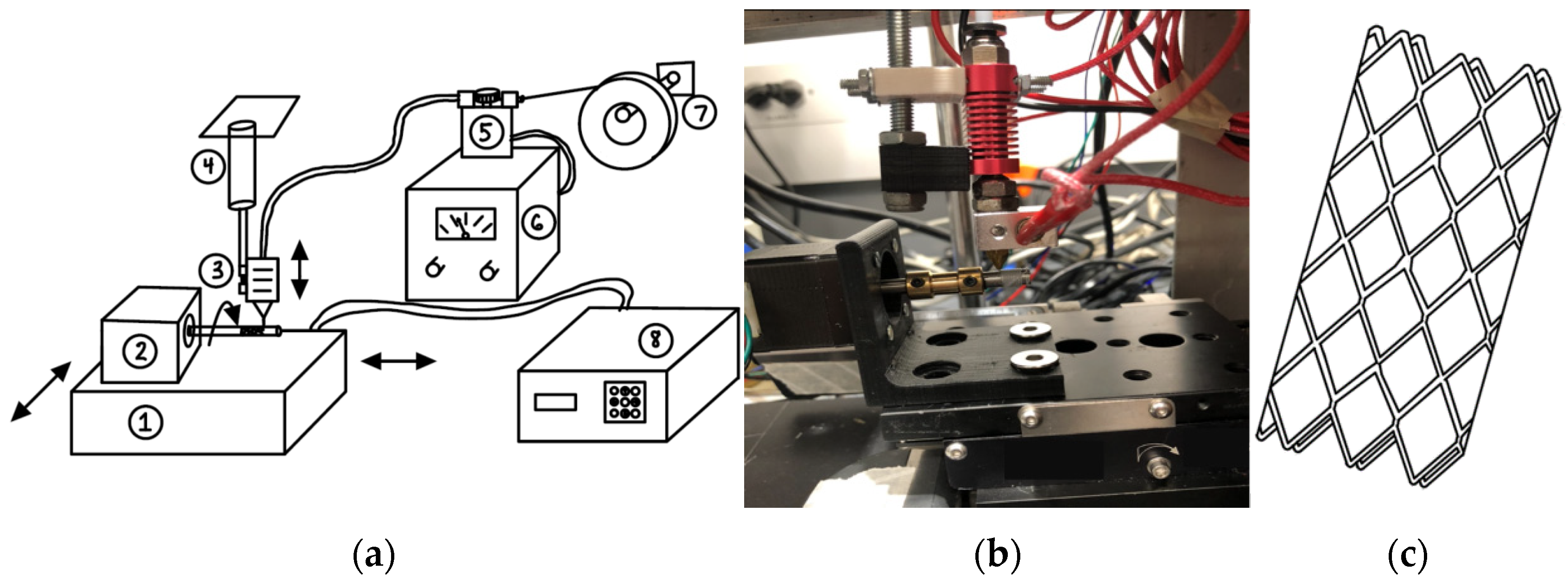
2.2. Design of Fabrication Unit and Flow Diverting Stent
2.3. Surface Characterization and Mechanical Testing
2.4. BaSO4 Coating for X-ray Image Analysis
2.5. HUVEC Cytotoxicity and NO Production Studies
2.6. Proliferation, Adhesion, and Cell Morphology Analysis
2.7. Statistical Analysis
3. Results
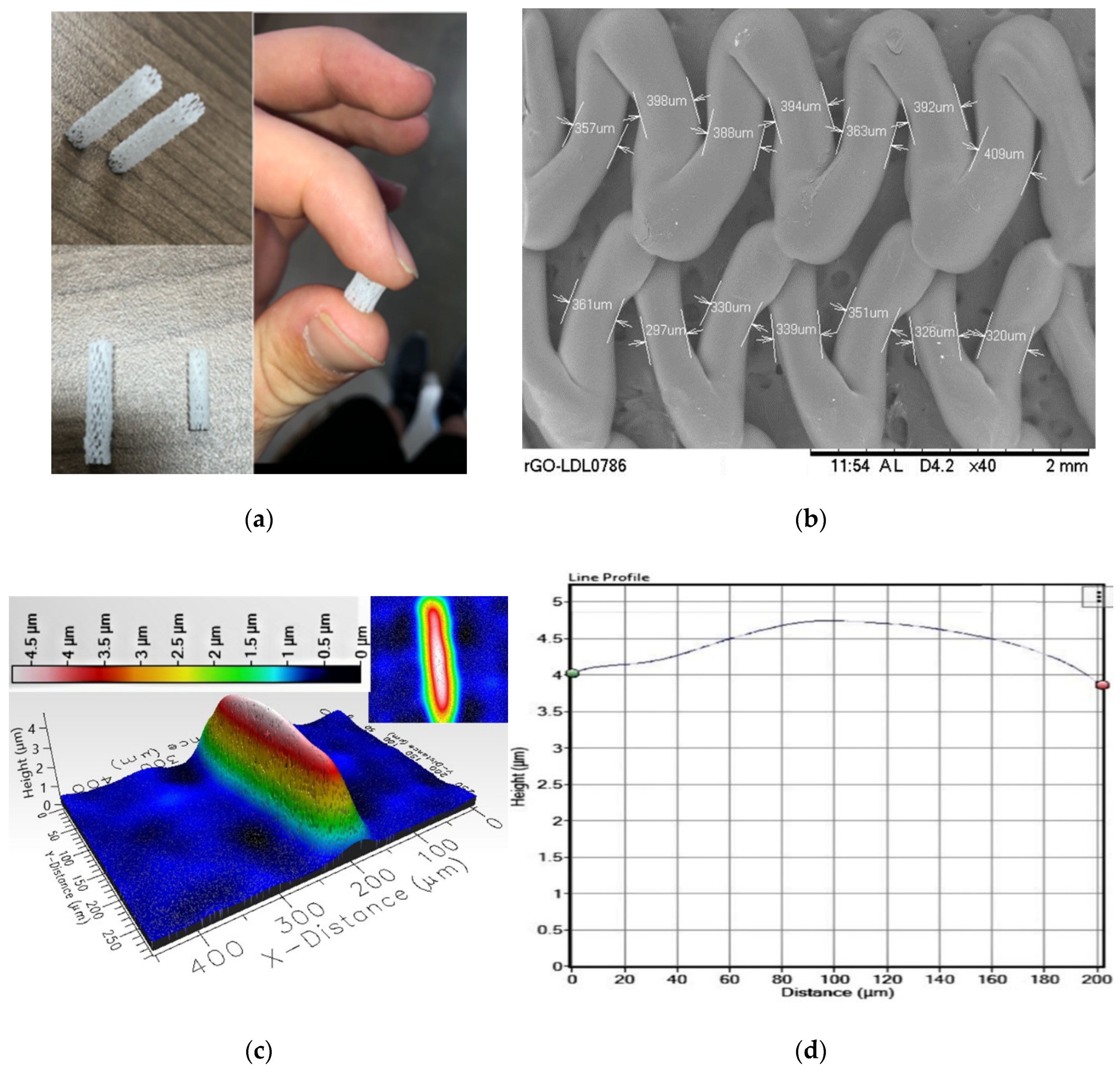
3.1. Surface Characterization and Mechanical Testing
3.2. BaSO4 Coating for X-ray Image Analysis
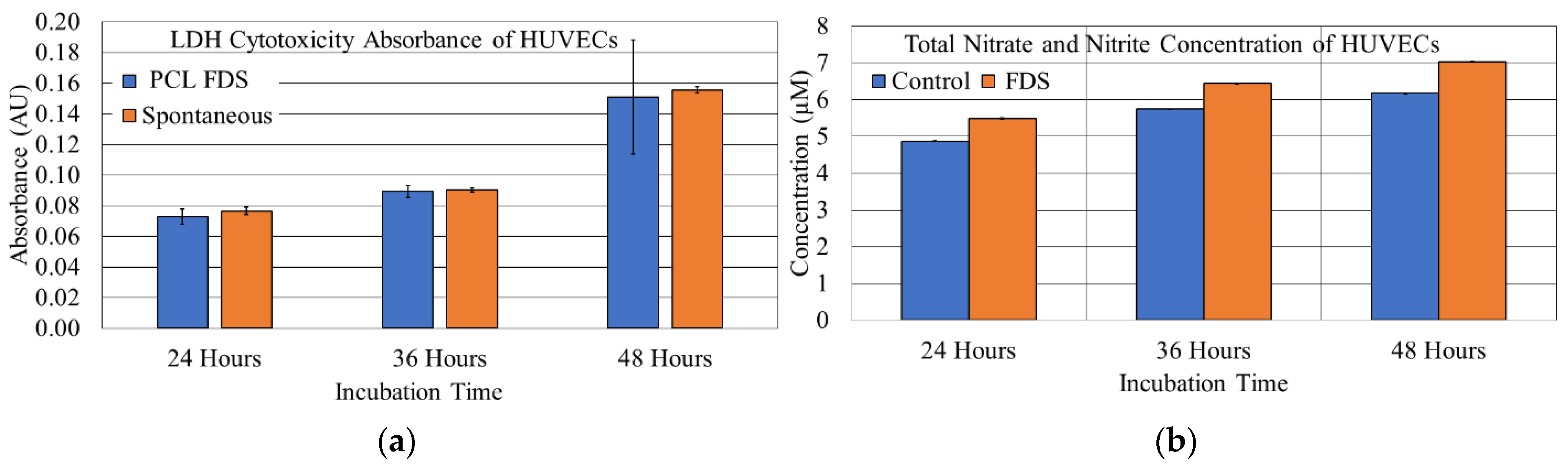
3.3. HUVEC Cytotoxicity and NO Production Studies
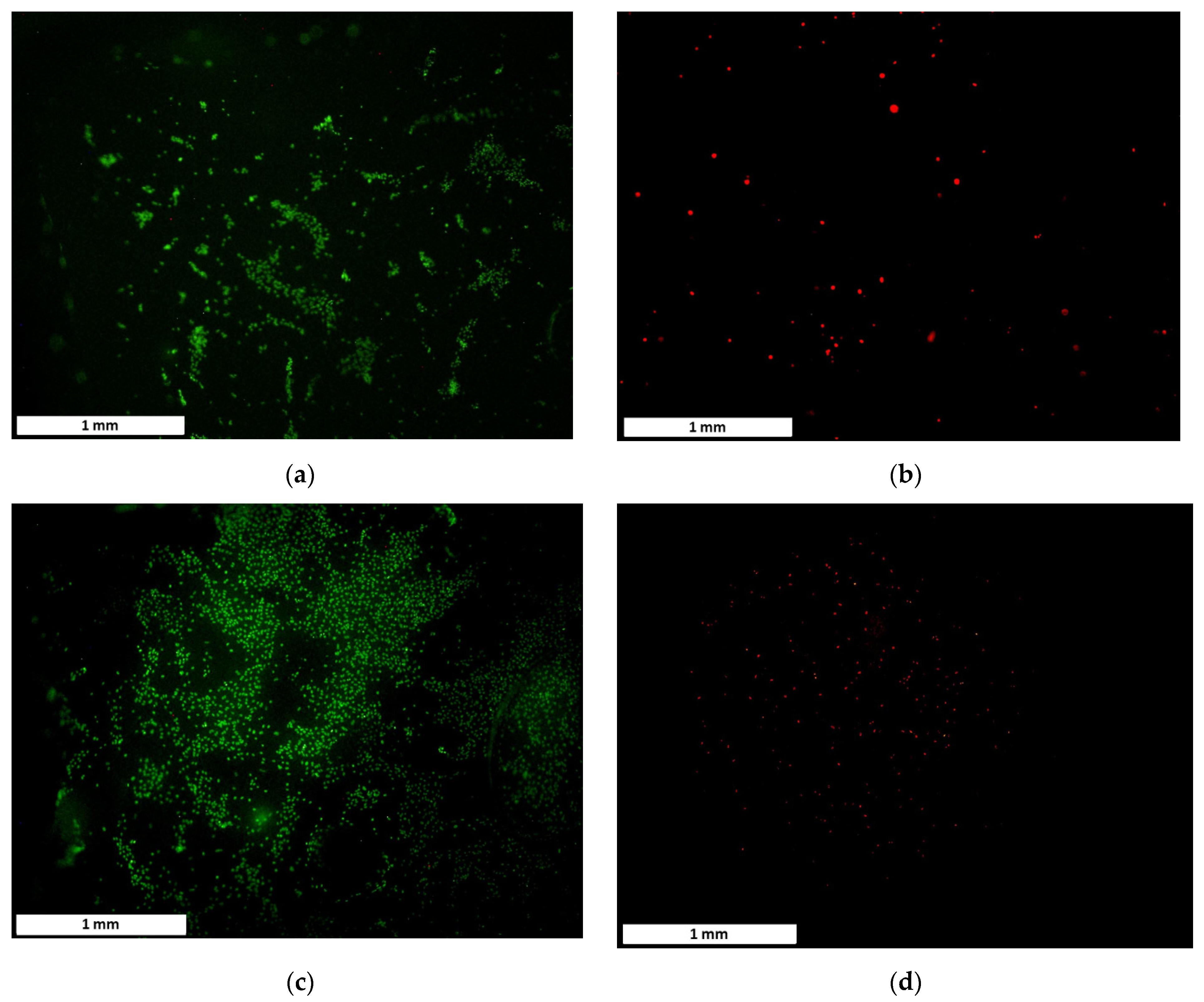
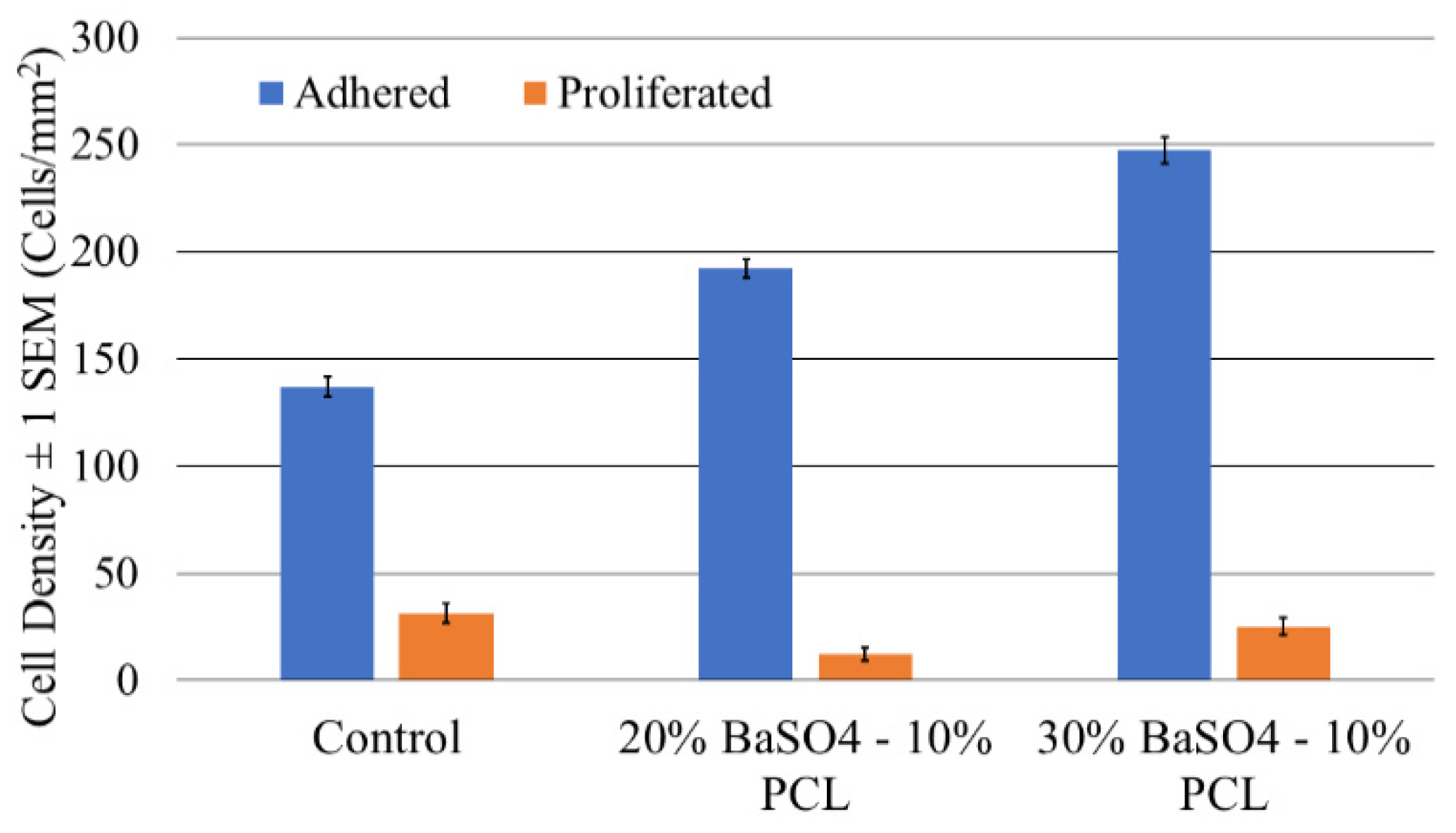
3.4. Proliferation and Adhesion Analysis
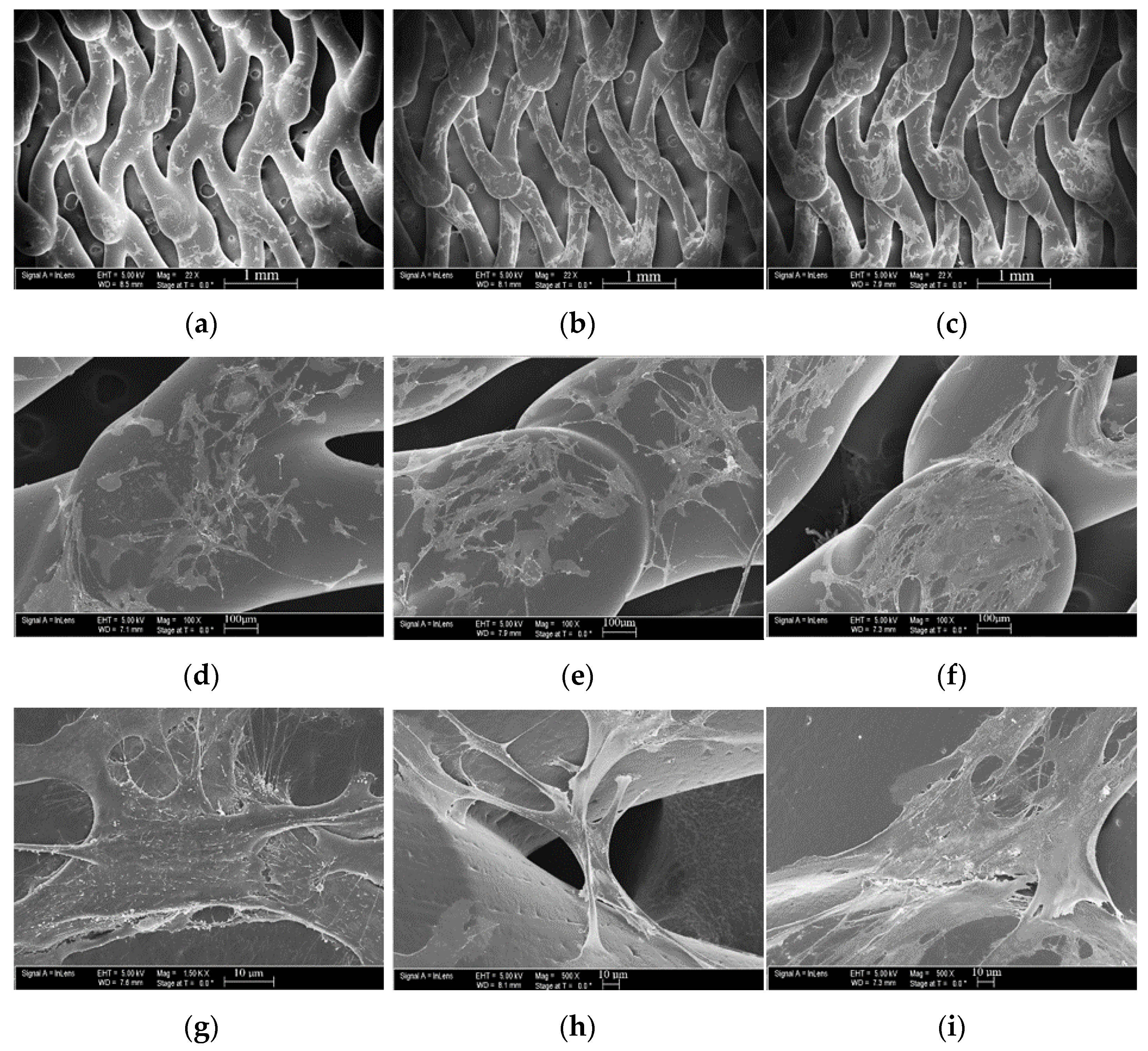
3.5. Cell Morphology
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Shin, D.S.; Carroll, C.P.; Elghareeb, M.; Hoh, B.L.; Kim, B.T. The evolution of flow-diverting stents for cerebral aneurysms; historical review, modern application, complications, and future direction. J. Korean Neurosurg. Soc. 2020, 63, 137. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chong, W.; Zhang, Y.; Qian, Y.; Lai, L.; Parker, G.; Mitchell, K. Computational hemodynamics analysis of intracranial aneurysms treated with flow diverters: Correlation with clinical outcomes. Am. J. Neuroradiol. 2014, 35, 136–142. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Arrese, I.; Sarabia, R.; Pintado, R.; Delgado-Rodriguez, M. Flow-diverter devices for intracranial aneurysms: Systematic review and meta-analysis. Neurosurgery 2013, 73, 193–200. [Google Scholar] [CrossRef] [PubMed]
- Briganti, F.; Leone, G.; Marseglia, M.; Mariniello, G.; Caranci, F.; Brunetti, A.; Maiuri, F. Endovascular treatment of cerebral aneurysms using flow-diverter devices: A systematic review. Neuroradiol. J. 2015, 28, 365–375. [Google Scholar] [CrossRef] [Green Version]
- Brinjikji, W.; Murad, M.H.; Lanzino, G.; Cloft, H.J.; Kallmes, D.F. Endovascular treatment of intracranial aneurysms with flow diverters: A meta-analysis. Stroke 2013, 44, 442–447. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Korkmazer, B.; Kocak, B.; Islak, C.; Kocer, N.; Kizilkilic, O. Long-term results of flow diversion in the treatment of intracranial aneurysms: A retrospective data analysis of a single center. Acta Neurochir. 2019, 161, 1165–1173. [Google Scholar] [CrossRef] [PubMed]
- Hofma, S.H.; Whelan, D.M.C.; Van Beusekom, H.M.M.; Verdouw, P.D.; Van der Giessen, W.J. Increasing arterial wall injury after long-term implantation of two types of stent in a porcine coronary model. Eur. Heart J. 1998, 19, 601–609. [Google Scholar] [CrossRef] [Green Version]
- Peschillo, S.; Caporlingua, A.; Resta, M.C.; Peluso, J.P.P.; Burdi, N.; Sourour, N.; Diana, F.; Guidetti, G.; Clarençon, F.; Bloemsma, G.C.; et al. Endovascular treatment of large and giant carotid aneurysms with flow-diverter stents alone or in combination with coils: A multicenter experience and long-term follow-up. Oper. Neurosurg. 2017, 13, 492–502. [Google Scholar] [CrossRef] [PubMed]
- Petr, O.; Brinjikji, W.; Cloft, H.; Kallmes, D.F.; Lanzino, G. Current trends and results of endovascular treatment of unruptured intracranial aneurysms at a single institution in the flow-diverter era. Am. J. Neuroradiol. 2016, 37, 1106–1113. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Brancati, M.F.; Burzotta, F.; Trani, C.; Leonzi, O.; Cuccia, C.; Crea, F. Coronary stents and vascular response to implantation: Literature review. Pragmat. Obs. Res. 2017, 8, 137. [Google Scholar] [CrossRef] [Green Version]
- Crimi, G.; Gritti, V.; Galiffa, V.A.; Scotti, V.; Leonardi, S.; Ferrario, M.; Ferlini, M.; De Ferrari, G.M.; Oltrona Visconti, L.; Klersy, C. Drug eluting stents are superior to bare metal stents to reduce clinical outcome and stent-related complications in CKD patients, a systematic review, meta-analysis and network meta-analysis. J. Interv. Cardiol. 2018, 31, 319–329. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kuramitsu, S.; Jinnouchi, H.; Shinozaki, T.; Hiromasa, T.; Matsumura, Y.; Yamaji, Y.; Miura, M.; Matsuda, H.; Masuda, H.; Domei, T.; et al. Incidence and long-term clinical impact of late-acquired stent fracture after sirolimus-eluting stent implantation in narrowed coronary arteries. Am. J. Cardiol. 2017, 120, 55–62. [Google Scholar] [CrossRef] [PubMed]
- Egbuche, O.; Mezue, K.N.; Nwokike, S.I.; Abe, T.; Olanipekun, T.; Onuorah, I.; Tharpe, C. Left main stenting with stent dislodgement and entrapment in the common femoral artery: A successful transcatheter stent retrieval. Am. J. Cardiovasc. Dis. 2021, 11, 421. [Google Scholar] [PubMed]
- Hu, T.; Yang, C.; Lin, S.; Yu, Q.; Wang, G. Biodegradable stents for coronary artery disease treatment: Recent advances and future perspectives. Mater. Sci. Eng. C 2018, 91, 163–178. [Google Scholar] [CrossRef]
- Onuma, Y.; Piazza, N.; Ormiston, J.A.; Serruys, P.W. Everolimus-eluting bioabsorbable Stent-Abbot vascular programme. EuroIntervention 2009, 5, F98–F102. [Google Scholar] [CrossRef] [PubMed]
- Tenekecioğlu, E.; Bourantas, C.; AbdelGhani, M.; Zeng, Y.; Silva, R.C.; Tateishi, H.; Sotomi, Y.; Onuma, Y.; Yılmaz, M.; Serruys, P.W. From drug eluting stents to bioresorbable scaffolds; to new horizons in PCI. Expert Rev. Med. Devices 2016, 13, 271–286. [Google Scholar] [CrossRef] [PubMed]
- Sotomi, Y.; Onuma, Y.; Collet, C.; Tenekecioglu, E.; Virmani, R.; Kleiman, N.S.; Serruys, P.W. Bioresorbable scaffold: The emerging reality and future directions. Circ. Res. 2017, 120, 1341–1352. [Google Scholar] [CrossRef] [PubMed]
- Waksman, R. Biodegradable stents: They do their job and disappear. J. Invasive Cardiol. 2006, 18, 70. [Google Scholar] [PubMed]
- Simard, T.; Hibbert, B.; Ramirez, F.D.; Froeschl, M.; Chen, Y.X.; O’Brien, E.R. The evolution of coronary stents: A brief review. Can. J. Cardiol. 2014, 30, 35–45. [Google Scholar] [CrossRef] [PubMed]
- Hytönen, J.P.; Taavitsainen, J.; Tarvainen, S.; Ylä-Herttuala, S. Biodegradable coronary scaffolds: Their future and clinical and technological challenges. Cardiovasc. Res. 2018, 114, 1063–1072. [Google Scholar] [CrossRef] [PubMed]
- Pauck, R.G.; Reddy, B.D. Computational analysis of the radial mechanical performance of PLLA coronary artery stents. Med. Eng. Phys. 2015, 37, 7–12. [Google Scholar] [CrossRef]
- Qiu, T.; Zhao, L. Research into biodegradable polymeric stents: A review of experimental and modelling work. Vessel. Plus 2018, 2, 12. [Google Scholar] [CrossRef]
- Ormiston, J.A.; Serruys, P.W. Bioabsorbable coronary stents. Circ. Cardiovasc. Interv. 2009, 2, 255–260. [Google Scholar] [CrossRef] [Green Version]
- Schmidt, W.; Behrens, P.; Brandt-Wunderlich, C.; Siewert, S.; Grabow, N.; Schmitz, K.P. In vitro performance investigation of bioresorbable scaffolds–standard tests for vascular stents and beyond. Cardiovasc. Revasculariz. Med. 2016, 17, 375–383. [Google Scholar] [CrossRef] [PubMed]
- Welch, T.R.; Eberhart, R.C.; Reisch, J.; Chuong, C.J. Influence of thermal annealing on the mechanical properties of PLLA coiled stents. Cardiovasc. Eng. Technol. 2014, 5, 270–280. [Google Scholar] [CrossRef]
- Yang, G.; Xie, H.; Huang, Y.; Lv, Y.; Zhang, M.; Shang, Y.; Zhou, J.; Wang, L.; Wang, J.-Y.; Chen, F. Immersed multilayer biodegradable ureteral stent with reformed biodegradation: An in vitro experiment. J. Biomater. Appl. 2017, 31, 1235–1244. [Google Scholar] [CrossRef]
- Qiu, T.; He, R.; Abunassar, C.; Hossainy, S.; Zhao, L.G. Effect of two-year degradation on mechanical interaction between a bioresorbable scaffold and blood vessel. J. Mech. Behav. Biomed. Mater. 2018, 78, 254–265. [Google Scholar] [CrossRef] [Green Version]
- Liu, R.; Xu, S.; Luo, X.; Liu, Z. Theoretical and numerical analysis of mechanical behaviors of a metamaterial-based shape memory polymer stent. Polymers 2020, 12, 1784. [Google Scholar] [CrossRef]
- Hutmacher, D.W.; Schantz, T.; Zein, I.; Ng, K.W.; Teoh, S.H.; Tan, K.C. Mechanical properties and cell cultural response of polycaprolactone scaffolds designed and fabricated via fused deposition modeling. J. Biomed. Mater. Res. Off. J. Soc. Biomater. Jpn. Soc. Biomater. Aust. Soc. Biomater. Korean Soc. Biomater. 2001, 55, 203–216. [Google Scholar] [CrossRef]
- Semba, T.; Kitagawa, K.; Ishiaku, U.S.; Hamada, H. The effect of crosslinking on the mechanical properties of polylactic acid/polycaprolactone blends. J. Appl. Polym. Sci. 2006, 101, 1816–1825. [Google Scholar] [CrossRef]
- Woodruff, M.A.; Hutmacher, D.W. The return of a forgotten polymer—Polycaprolactone in the 21st century. Prog. Polym. Sci. 2010, 35, 1217–1256. [Google Scholar] [CrossRef] [Green Version]
- Ang, H.Y.; Bulluck, H.; Wong, P.; Venkatraman, S.S.; Huang, Y.; Foin, N. Foin Bioresorbable stents: Current and upcoming bioresorbable technologies. Int. J. Cardiol. 2017, 228, 931–939. [Google Scholar] [CrossRef]
- Estellés, J.M.; Vidaurre, A.; Duenas, J.M.M.; Cortázar, I.C. Physical characterization of polycaprolactone scaffolds. J. Mat. Sci. Mat. Med. 2008, 19, 189–195. [Google Scholar] [CrossRef]
- Bastioli, C. Handbook of Biodegradable Polymers; Walter de Gruyter GmbH & Co. KG: Berlin, Germany, 2020. [Google Scholar]
- Khandaker, M.; Riahinezhad, S.; Jamadagni, H.G.; Morris, T.L.; Coles, A.V.; Vaughan, M.B. Use of polycaprolactone electrospun nanofibers as a coating for poly(methyl methacrylate) bone cement. Nanomaterials 2017, 7, 175. [Google Scholar] [CrossRef] [Green Version]
- Park, S.H.; Park, S.A.; Kang, Y.G.; Shin, J.W.; Park, Y.S.; Gu, S.R.; Wu, Y.R.; Wei, J.; Shin, J.W. PCL/β-TCP composite scaffolds exhibit positive osteogenic differentiation with mechanical stimulation. Tissue Eng. Regen. Med. 2017, 14, 349–358. [Google Scholar] [CrossRef] [PubMed]
- East, B.; Plencner, M.; Kralovic, M.; Rampichova, M.; Sovkova, V.; Vocetkova, K.; Otahal, M.; Tonar, Z.; Kolinko, Y.; Amler, E.; et al. A polypropylene mesh modified with poly-ε-caprolactone nanofibers in hernia repair: Large animal experiment. Int. J. Nanomed. 2018, 13, 3129. [Google Scholar] [CrossRef] [Green Version]
- Oh, S.H.; Park, I.K.; Kim, J.M.; Lee, J.H. In vitro and in vivo characteristics of PCL scaffolds with pore size gradient fabricated by a centrifugation method. Biomaterials 2007, 28, 1664–1671. [Google Scholar] [CrossRef] [PubMed]
- Sayyar, S.; Murray, E.; Thompson, B.C.; Gambhir, S.; Officer, D.L.; Wallace, G.G. Wallace Covalently linked biocompatible graphene/polycaprolactone composites for tissue engineering. Carbon 2013, 52, 296–304. [Google Scholar] [CrossRef] [Green Version]
- Zhu, Y.; Gao, C.; Liu, X.; Shen, J. Surface modification of polycaprolactone membrane via aminolysis and biomacromolecule immobilization for promoting cytocompatibility of human endothelial cells. Biomacromolecules 2002, 3, 1312–1319. [Google Scholar] [CrossRef]
- Guerra, A.J.; Ciurana, J. Design 3D-printed bioabsordable polycaprolactone stent: The effect of process parameters on its physical features. Mater. Des. 2018, 137, 430–437. [Google Scholar] [CrossRef]
- Qiu, T.; Jiang, W.; Yan, P.; Jiao, L.; Wang, X. Development of 3D-printed sulfated chitosan modified bioresorbable stents for coronary artery disease. Front. Bioeng. Biotechnol. 2020, 8, 462. [Google Scholar] [CrossRef] [PubMed]
- Coombes, A.G.A.; Rizzi, S.C.; Williamson, M.; Barralet, J.E.; Downes, S.; Wallace, W.A. Wallace Precipitation casting of polycaprolactone for applications in tissue engineering and drug delivery. Biomaterials 2004, 25, 315–325. [Google Scholar] [CrossRef]
- Yokoo, T.; Shimizu, I.; Wada, A.; Takaki, A.; Okada, S.; Hatakeyama, M.; Yamashita, S. Development of Test Methods for Mechanical Property Evaluation of Balloon-Expandable CoCr Alloy Stent. J. Jpn. Soc. Exp. Mech. 2014, 14, s285–s290. [Google Scholar]
- McGrath, D.; O’Brien, B.; Bruzzi, M.; Kelly, N.; Clauser, J.; Steinseifer, U.; McHugh, P. Evaluation of cover effects on bare stent mechanical response. J. Mech. Behav. Biomed. Mater. 2016, 61, 567–580. [Google Scholar] [CrossRef]
- Ravindran, K.; Casabella, A.M.; Cebral, J.; Brinjikji, W.; Kallmes, D.F.; Kadirvel, R. Mechanism of action and biology of flow diverters in the treatment of intracranial aneurysms. Neurosurgery 2020, 86 (Suppl. S1), S13–S19. [Google Scholar] [CrossRef]
- Taufik, M.; Jain, P.K. Part surface quality improvement studies in fused deposition modelling process: A review. Aust. J. Mech. Eng. 2020, 1–25. [Google Scholar] [CrossRef]
- Dholakia, R.; Sadasivan, C.; Fiorella, D.J.; Woo, H.H.; Lieber, B.B. Hemodynamics of flow diverters. J. Biomech. Eng. 2017, 139, 21002. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stöckel, D. Nitinol-A material with unusual properties. Endovasc. Update 1998, 1, 1–8. [Google Scholar]
- Boyer, C.J.; Boktor, M.; Samant, H.; White, L.A.; Wang, Y.; Ballard, D.H.; Huebert, R.C.; Woerner, J.E.; Ghali, G.E.; Alexander, J.S. 3D printing for bio-synthetic biliary stents. Bioengineering 2019, 6, 16. [Google Scholar] [CrossRef] [Green Version]
- Lämsä, T.; Jin, H.; Mikkonen, J.; Laukkarinen, J.; Sand, J.; Nordback, I. Biocompatibility of a New Bioabsorbable Radiopaque Stent Material (BaSO4 Containing Poly-L, D-Lactide) in the Rat Pancreas. Pancreatology 2006, 6, 301–305. [Google Scholar] [CrossRef] [PubMed]
- Ang, H.Y.; Toong, D.; Chow, W.S.; Seisilya, W.; Wu, W.; Wong, P.; Venkatraman, S.S.; Foin, N.; Huang, Y. Radiopaque fully degradable nanocomposites for coronary stents. Sci. Rep. 2018, 8, 1–14. [Google Scholar]
- Murray, P.E.; Lumley, P.J.; Ross, H.F.; Smith, A.J. Tooth slice organ culture for cytotoxicity assessment of dental materials. Biomaterials 2000, 21, 1711–1721. [Google Scholar] [CrossRef]
- Garrett, P.R.; Meshkov, S.L.; Perlmutter, G.S. Oral contrast agents in CT of the abdomen. Radiology 1984, 153, 545–546. [Google Scholar] [CrossRef] [PubMed]
- Uscátegui, Y.L.; Arévalo, F.R.; Díaz, L.E.; Cobo, M.I.; Valero, M.F. Microbial degradation, cytotoxicity and antibacterial activity of polyurethanes based on modified castor oil and polycaprolactone. J. Biomat. Sci. Polym. Ed. 2016, 27, 1860–1879. [Google Scholar] [CrossRef] [PubMed]
- Forstermann, U.; Munzel, T. Endothelial nitric oxide synthase in vascular disease: From marvel to menace. Circulation 2006, 113, 1708–1714. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Serrano, M.C.; Pagani, R.; Vallet-Regí, M.; Peña, J.; Comas, J.V.; Portolés, M.T. Nitric oxide production by endothelial cells derived from blood progenitors cultured on NaOH-treated polycaprolactone films: A biofunctionality study. Acta Biomater. 2009, 5, 2045–2053. [Google Scholar] [CrossRef]
- Gérard, C.; Goldbeter, A. The balance between cell cycle arrest and cell proliferation: Control by the extracellular matrix and by contact inhibition. Interface Focus 2014, 4, 20130075. [Google Scholar] [CrossRef] [Green Version]




Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tidwell, K.; Harriet, S.; Barot, V.; Bauer, A.; Vaughan, M.B.; Hossan, M.R. Design and Analysis of a Biodegradable Polycaprolactone Flow Diverting Stent for Brain Aneurysms. Bioengineering 2021, 8, 183. https://doi.org/10.3390/bioengineering8110183
Tidwell K, Harriet S, Barot V, Bauer A, Vaughan MB, Hossan MR. Design and Analysis of a Biodegradable Polycaprolactone Flow Diverting Stent for Brain Aneurysms. Bioengineering. 2021; 8(11):183. https://doi.org/10.3390/bioengineering8110183
Chicago/Turabian StyleTidwell, Kaitlyn, Seth Harriet, Vishal Barot, Andrew Bauer, Melville B. Vaughan, and Mohammad R. Hossan. 2021. "Design and Analysis of a Biodegradable Polycaprolactone Flow Diverting Stent for Brain Aneurysms" Bioengineering 8, no. 11: 183. https://doi.org/10.3390/bioengineering8110183
APA StyleTidwell, K., Harriet, S., Barot, V., Bauer, A., Vaughan, M. B., & Hossan, M. R. (2021). Design and Analysis of a Biodegradable Polycaprolactone Flow Diverting Stent for Brain Aneurysms. Bioengineering, 8(11), 183. https://doi.org/10.3390/bioengineering8110183








