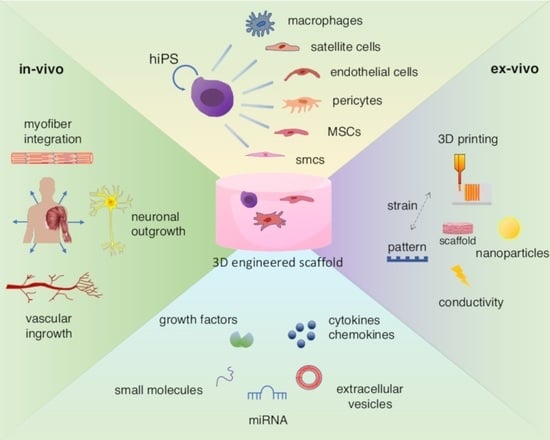
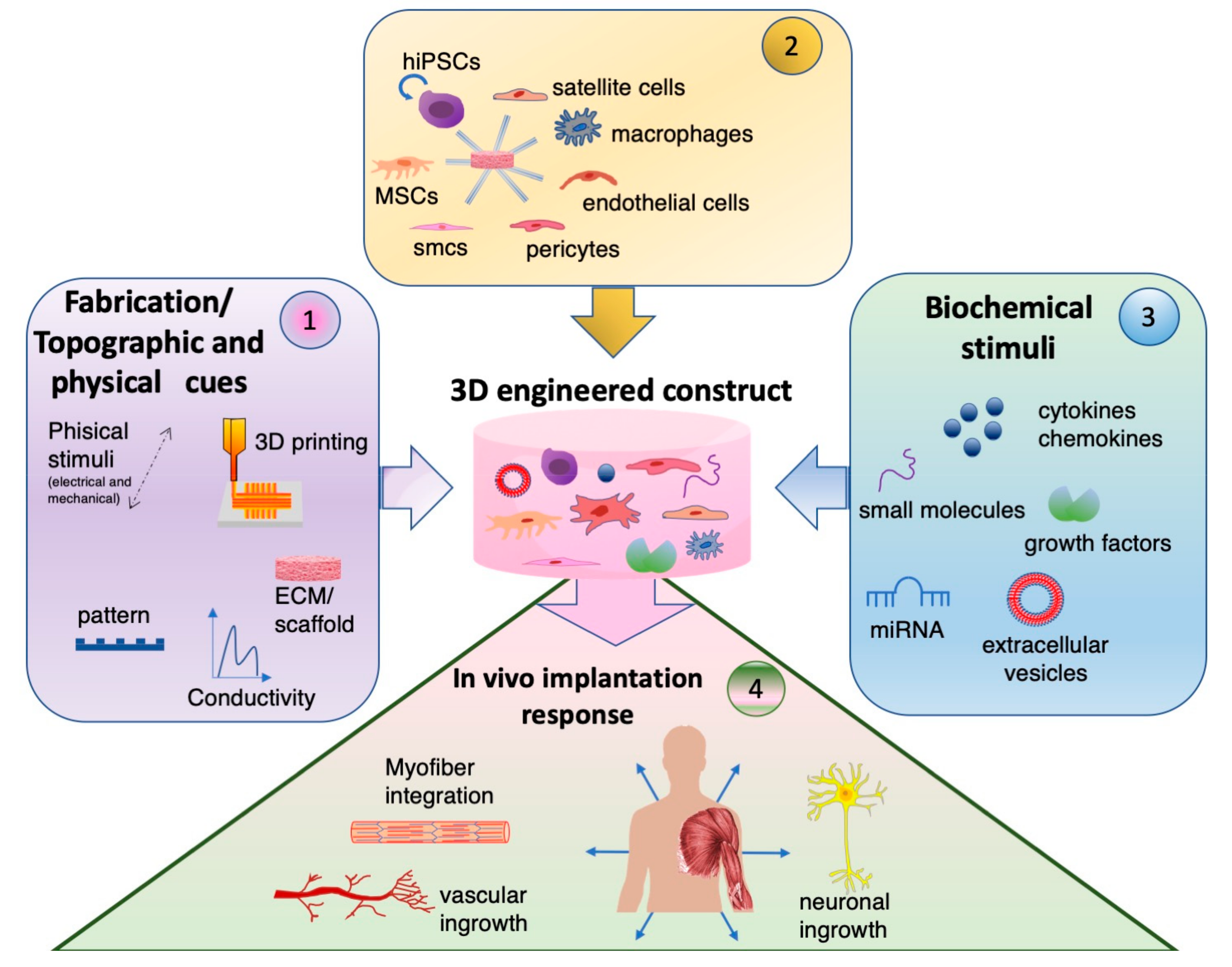
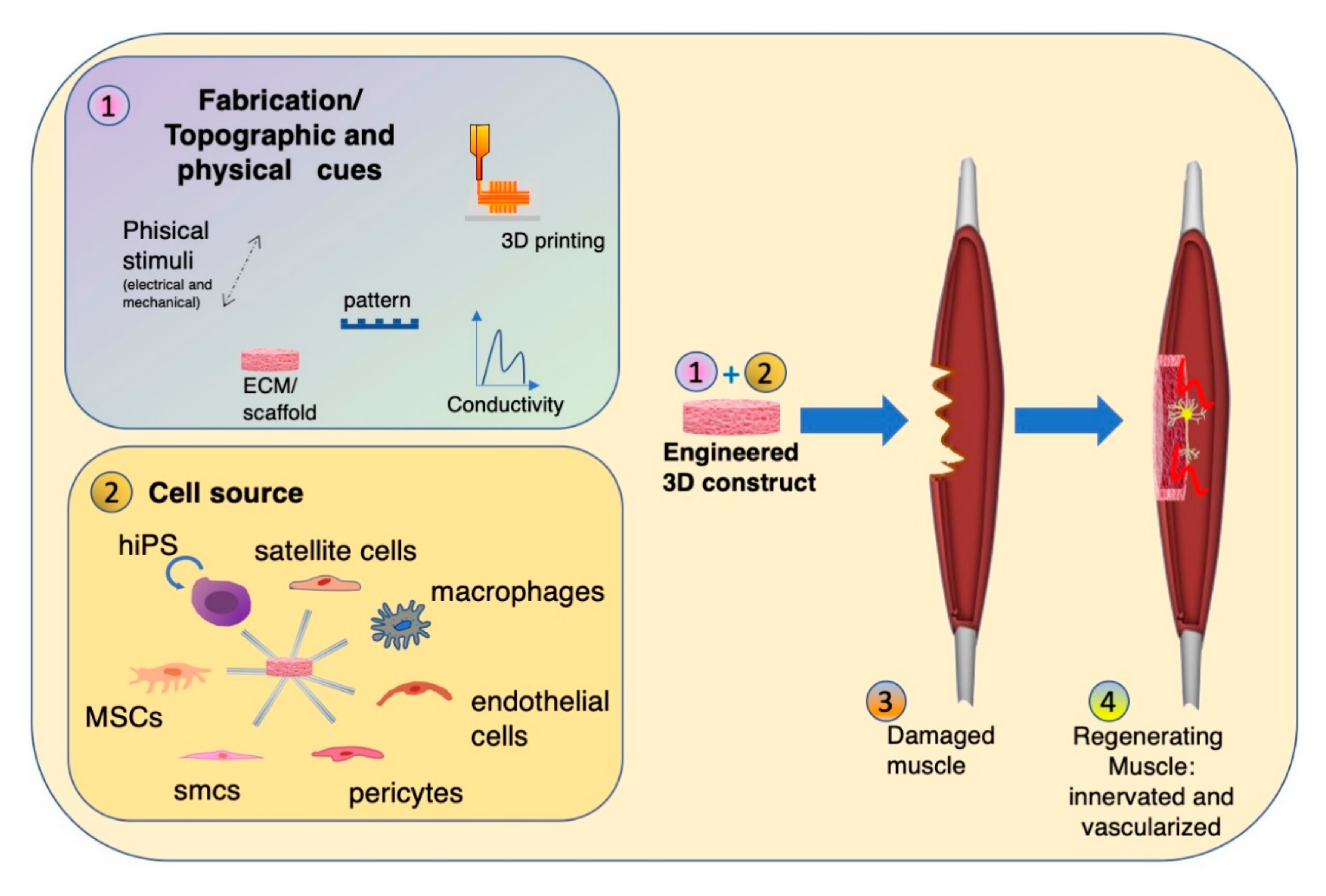
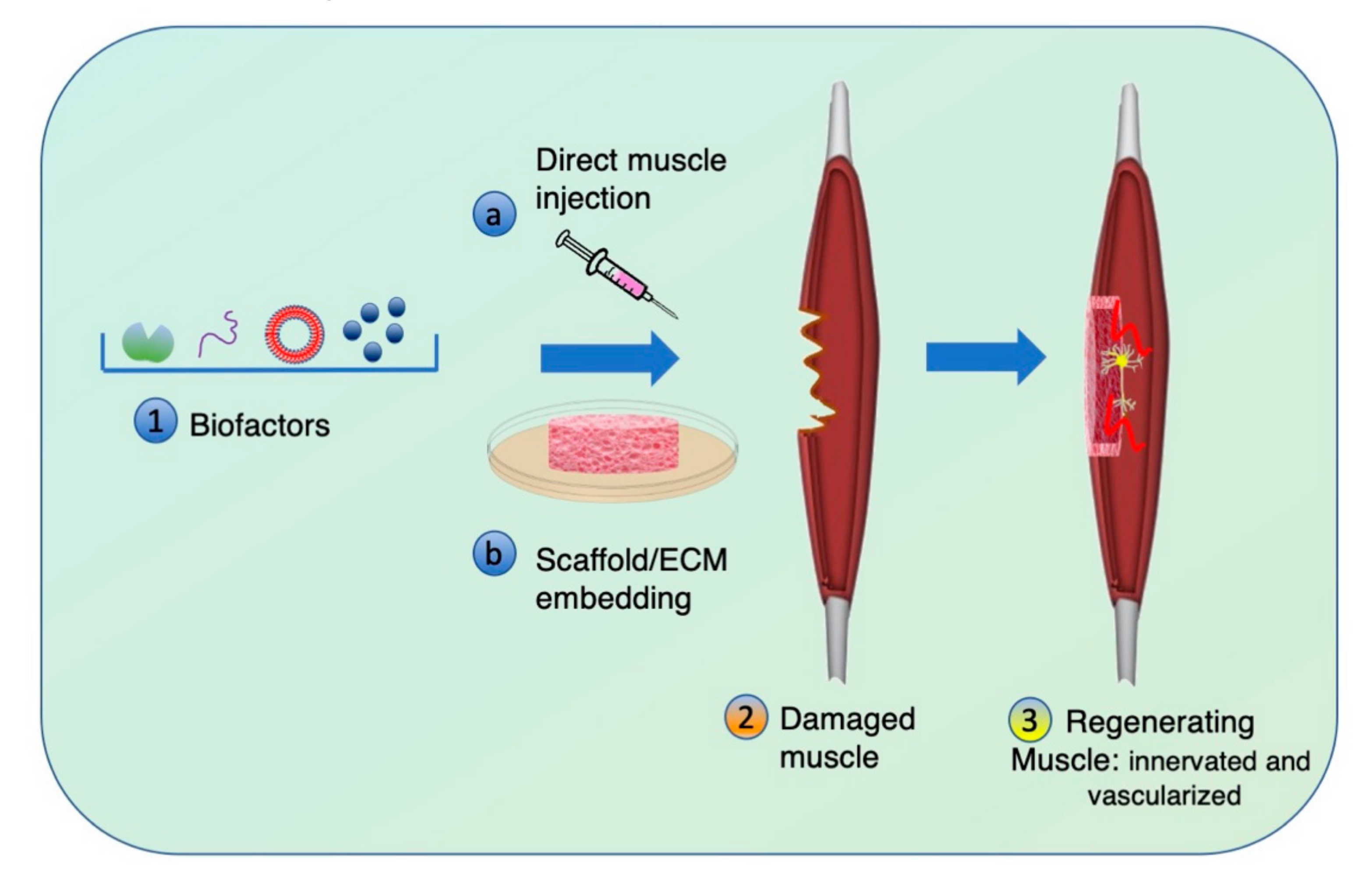
Next Stage Approach to Tissue Engineering Skeletal Muscle
Abstract
1. Introduction
2. Scaffold Composition, Topography and Fabrication
2.1. Scaffold Composition
2.2. Tunable Factors
Topography
2.3. Fabrication Methods
2.3.1. 3D-Bioprinting
2.3.2. Electrospinning
3. Multicellular Environment for Muscle Tissue Engineering
3.1. Stem Cells
3.2. Co-Culture with Adipose Tissue-Derived Stem Cells
3.3. Co-Culture with Vascular Cells (Pre-Vascularization Approach)
3.4. Nerve Transfer Approach and Co-Culture with Neural Cells
3.5. Immune Cell–Satellite Cell Interaction
4. Biochemical Stimuli to Adjuvate Muscle Regeneration
4.1. Soluble Factors: Growth Factors and Small Molecules
4.2. Extracellular Vesicles
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Carlson, B.M. Muscle Regeneration in Animal Models. In Skeletal Muscle Repair and Regeneration; Springer: Dordrecht, The Netherlands, 2008; pp. 163–180. ISBN 978-1-4020-6768-6. [Google Scholar]
- Anderson, S.E.; Han, W.M.; Srinivasa, V.; Mohiuddin, M.; Ruehle, M.A.; Moon, J.Y.; Shin, E.; San Emeterio, C.L.; Ogle, M.E.; Botchwey, E.A.; et al. Determination of a critical size threshold for volumetric muscle loss in the mouse quadriceps. Tissue Eng. Part C Methods 2019, 25, 59–70. [Google Scholar] [CrossRef] [PubMed]
- Vega-Soto, E.E.; Rodriguez, B.L.; Armstrong, R.E.; Larkin, L.M. A 30% Volumetric Muscle Loss Does Not Result in Sustained Functional Deficits After a 90-Day Recovery in Rats. Regen. Eng. Transl. Med. 2019, 6, 62–68. [Google Scholar] [CrossRef] [PubMed]
- Shansky, J.; Chromiak, J.; Del Tatto, M.; Vandenburgh, H. A simplified method for tissue engineering skeletal muscle organoids in vitro. Vitr. Cell. Dev. Biol. Anim. 1997, 33, 659–661. [Google Scholar] [CrossRef]
- Okano, T.; Satoh, S.; Oka, T.; Matsuda, T. Tissue engineering of skeletal muscle. Highly dense, highly oriented hybrid muscular tissues biomimicking native tissues. ASAIO J. 1997, 43, M749–M753. [Google Scholar] [CrossRef] [PubMed]
- Aurora, A.; Roe, J.L.; Corona, B.T.; Walters, T.J. An acellular biologic scaffold does not regenerate appreciable de novo muscle tissue in rat models of volumetric muscle loss injury. Biomaterials 2015, 67, 393–407. [Google Scholar] [CrossRef] [PubMed]
- Dziki, J.; Badylak, S.; Yabroudi, M.; Sicari, B.; Ambrosio, F.; Stearns, K.; Turner, N.; Wyse, A.; Boninger, M.L.; Brown, E.H.P.; et al. An acellular biologic scaffold treatment for volumetric muscle loss: results of a 13-patient cohort study. NPJ Regen. Med. 2016, 1, 1–12. [Google Scholar] [CrossRef]
- Iberite, F.; Gerges, I.; Vannozzi, L.; Marino, A.; Piazzoni, M.; Santaniello, T.; Lenardi, C.; Ricotti, L. Combined Effects of Electrical Stimulation and Protein Coatings on Myotube Formation in a Soft Porous Scaffold. Ann. Biomed. Eng. 2020, 48, 734–746. [Google Scholar] [CrossRef]
- Raj, R.; Sobhan, P.K.; Pratheesh, K.V.; Anilkumar, T.V. A cholecystic extracellular matrix-based hybrid hydrogel for skeletal muscle tissue engineering. J. Biomed. Mater. Res. Part A 2020, 108, 1922–1933. [Google Scholar] [CrossRef]
- Chen, H.; Zhong, J.; Wang, J.; Huang, R.; Qiao, X.; Wang, H.; Tan, Z. Enhanced growth and differentiation of myoblast cells grown on E-jet 3D printed platforms. Int. J. Nanomedicine 2019, 14, 937–950. [Google Scholar] [CrossRef]
- Apsite, I.; Uribe, J.M.; Posada, A.F.; Rosenfeldt, S.; Salehi, S.; Ionov, L. 4D biofabrication of skeletal muscle microtissues. Biofabrication 2020, 12. [Google Scholar] [CrossRef]
- Yang, G.H.; Lee, J.U.; Kim, G.H. The fabrication of uniaxially aligned micro-textured polycaprolactone struts and application for skeletal muscle tissue regeneration. Biofabrication 2019, 11. [Google Scholar] [CrossRef] [PubMed]
- Mei, C.; Chao, C.W.; Lin, C.W.; Li, S.T.; Wu, K.H.; Yang, K.C.; Yu, J. Three-dimensional spherical gelatin bubble-based scaffold improves the myotube formation of H9c2 myoblasts. Biotechnol. Bioeng. 2019, 116, 1190–1200. [Google Scholar] [CrossRef] [PubMed]
- Osaki, T.; Sivathanu, V.; Kamm, R.D. Crosstalk between developing vasculature and optogenetically engineered skeletal muscle improves muscle contraction and angiogenesis. Biomaterials 2018, 156, 65–76. [Google Scholar] [CrossRef]
- Lee, J.; Jun, I.; Park, H.J.; Kang, T.J.; Shin, H.; Cho, S.W. Genetically engineered myoblast sheet for therapeutic angiogenesis. Biomacromolecules 2014, 15, 361–372. [Google Scholar] [CrossRef]
- Aguilar-Agon, K.W.; Capel, A.J.; Martin, N.R.W.; Player, D.J.; Lewis, M.P. Mechanical loading stimulates hypertrophy in tissue-engineered skeletal muscle: Molecular and phenotypic responses. J. Cell. Physiol. 2019, 234, 23547–23558. [Google Scholar] [CrossRef] [PubMed]
- Arifuzzaman, M.; Ito, A.; Ikeda, K.; Kawabe, Y.; Kamihira, M. Fabricating Muscle-Neuron Constructs with Improved Contractile Force Generation. Tissue Eng. Part A 2019, 25, 563–574. [Google Scholar] [CrossRef]
- Bakooshli, M.A.; Lippmann, E.S.; Mulcahy, B.; Iyer, N.; Nguyen, C.T.; Tung, K.; Stewart, B.A.; Van Den Dorpel, H.; Fuehrmann, T.; Shoichet, M.; et al. A 3d culture model of innervated human skeletal muscle enables studies of the adult neuromuscular junction. Elife 2019, 8, 1–29. [Google Scholar] [CrossRef]
- Das, S.; Browne, K.D.; Laimo, F.A.; Maggiore, J.C.; Hilman, M.C.; Kaisaier, H.; Aguilar, C.A.; Ali, Z.S.; Mourkioti, F.; Cullen, D.K. Pre-innervated tissue-engineered muscle promotes a pro-regenerative microenvironment following volumetric muscle loss. Commun. Biol. 2020, 3, 1–14. [Google Scholar] [CrossRef]
- Kim, J.H.; Kim, I.; Seol, Y.J.; Ko, I.K.; Yoo, J.J.; Atala, A.; Lee, S.J. Neural cell integration into 3D bioprinted skeletal muscle constructs accelerates restoration of muscle function. Nat. Commun. 2020, 11, 1–12. [Google Scholar] [CrossRef]
- Novakova, S.S.; Rodriguez, B.L.; Vega-Soto, E.E.; Nutter, G.P.; Armstrong, R.E.; Macpherson, P.C.D.; Larkin, L.M. Repairing Volumetric Muscle Loss in the Ovine Peroneus Tertius Following a 3-Month Recovery. Tissue Eng. Part A 2020, 1–38. [Google Scholar] [CrossRef]
- Panayi, A.C.; Smit, L.; Hays, N.; Udeh, K.; Endo, Y.; Li, B.; Sakthivel, D.; Tamayol, A.; Neppl, R.L.; Orgill, D.P.; et al. A porous collagen-GAG scaffold promotes muscle regeneration following volumetric muscle loss injury. Wound Repair Regen. 2020, 28, 61–74. [Google Scholar] [CrossRef] [PubMed]
- Guo, B.; Qu, J.; Zhao, X.; Zhang, M. Degradable conductive self-healing hydrogels based on dextran-graft-tetraaniline and N-carboxyethyl chitosan as injectable carriers for myoblast cell therapy and muscle regeneration. Acta Biomater. 2019, 84, 180–193. [Google Scholar] [CrossRef] [PubMed]
- Choi, Y.J.; Jun, Y.J.; Kim, D.Y.; Yi, H.G.; Chae, S.H.; Kang, J.; Lee, J.; Gao, G.; Kong, J.S.; Jang, J.; et al. A 3D cell printed muscle construct with tissue-derived bioink for the treatment of volumetric muscle loss. Biomaterials 2019, 206, 160–169. [Google Scholar] [CrossRef]
- Kim, J.; Kasukonis, B.; Roberts, K.; Dunlap, G.; Brown, L.; Washington, T.; Wolchok, J. Graft alignment impacts the regenerative response of skeletal muscle after volumetric muscle loss in a rat model. Acta Biomater. 2020, 105, 191–202. [Google Scholar] [CrossRef]
- Zhu, M.; Li, W.; Dong, X.; Yuan, X.; Midgley, A.C.; Chang, H.; Wang, Y.; Wang, H.; Wang, K.; Ma, P.X.; et al. In vivo engineered extracellular matrix scaffolds with instructive niches for oriented tissue regeneration. Nat. Commun. 2019, 10. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.H.; Seol, Y.J.; Ko, I.K.; Kang, H.W.; Lee, Y.K.; Yoo, J.J.; Atala, A.; Lee, S.J. 3D Bioprinted Human Skeletal Muscle Constructs for Muscle Function Restoration. Sci. Rep. 2018, 8, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Gholobova, D.; Terrie, L.; Mackova, K.; Desender, L.; Carpentier, G.; Gerard, M.; Hympanova, L.; Deprest, J.; Thorrez, L. Functional evaluation of prevascularization in one-stage versus two-stage tissue engineering approach of human bio-artificial muscle. Biofabrication 2020, 12, 35021. [Google Scholar] [CrossRef] [PubMed]
- Perry, L.; Merdler, U.; Elishaev, M.; Levenberg, S. Enhanced Host Neovascularization of Prevascularized Engineered Muscle Following Transplantation into Immunocompetent versus Immunocompromised Mice. Cells 2019, 8, 1472. [Google Scholar] [CrossRef]
- Juhas, M.; Abutaleb, N.; Wang, J.T.; Ye, J.; Shaikh, Z.; Sriworarat, C.; Qian, Y.; Bursac, N. Incorporation of macrophages into engineered skeletal muscle enables enhanced muscle regeneration. Nat. Biomed. Eng. 2018, 2, 942–954. [Google Scholar] [CrossRef]
- Smoak, M.M.; Han, A.; Watson, E.; Kishan, A.; Grande-Allen, K.J.; Cosgriff-Hernandez, E.; Mikos, A.G. Fabrication and characterization of electrospun decellularized muscle-derived scaffolds. Tissue Eng. Part C Methods 2019, 25, 276–287. [Google Scholar] [CrossRef]
- Edri, R.; Gal, I.; Noor, N.; Harel, T.; Fleischer, S.; Adadi, N.; Green, O.; Shabat, D.; Heller, L.; Shapira, A.; et al. Personalized Hydrogels for Engineering Diverse Fully Autologous Tissue Implants. Adv. Mater. 2019, 31, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Jenkins, T.L.; Little, D. Synthetic scaffolds for musculoskeletal tissue engineering: cellular responses to fiber parameters. NPJ Regen. Med. 2019, 4, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Kwee, B.J.; Mooney, D.J.; Opin, C.; Author, B. Biomaterials for Skeletal Muscle Tissue Engineering Graphical Abstract HHS Public Access Author manuscript. Curr. Opin. Biotechnol. 2017, 47, 16–22. [Google Scholar] [CrossRef] [PubMed]
- Bartolacci, J.; Dziki, J.; Badylak, S.F. Scaffolds for Skeletal Muscle Tissue Engineering; Elsevier Ltd: Amsterdam, The Netherlands, 2019; ISBN 9780081025635. [Google Scholar]
- Nakayama, K.H.; Shayan, M.; Huang, N.F. Engineering Biomimetic Materials for Skeletal Muscle Repair and Regeneration. Adv. Healthc. Mater. 2019, 8, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Rossi, C.A.; Flaibani, M.; Blaauw, B.; Pozzobon, M.; Figallo, E.; Reggiani, C.; Vitiello, L.; Elvassore, N.; De Coppi, P. In vivo tissue engineering of functional skeletal muscle by freshly isolated satellite cells embedded in a photopolymerizable hydrogel. FASEB J. 2011, 25, 2296–2304. [Google Scholar] [CrossRef]
- Monge, C.; Ren, K.; Berton, K.; Guillot, R.; Peyrade, D.; Picart, C. Engineering muscle tissues on microstructured polyelectrolyte multilayer films. Tissue Eng. Part A 2012, 18, 1664–1676. [Google Scholar] [CrossRef]
- Yang, H.S.; Ieronimakis, N.; Tsui, J.H.; Kim, H.N.; Suh, K.Y.; Reyes, M.; Kim, D.H. Nanopatterned muscle cell patches for enhanced myogenesis and dystrophin expression in a mouse model of muscular dystrophy. Biomaterials 2014, 35, 1478–1486. [Google Scholar] [CrossRef]
- Chaturvedi, V.; Naskar, D.; Kinnear, B.F.; Grenik, E.; Dye, D.E.; Grounds, M.D.; Kundu, S.C.; Coombe, D.R. Silk fibroin scaffolds with muscle-like elasticity support in vitro differentiation of human skeletal muscle cells. J. Tissue Eng. Regen. Med. 2017, 11, 3178–3192. [Google Scholar] [CrossRef]
- Hajiabbas, M.; Mashayekhan, S.; Nazaripouya, A.; Naji, M.; Hunkeler, D.; Rajabi Zeleti, S.; Sharifiaghdas, F. Chitosan-gelatin sheets as scaffolds for muscle tissue engineering. Artif. Cells Nanomed. Biotechnol. 2015, 43, 124–132. [Google Scholar] [CrossRef]
- Flaibani, M.; Boldrin, L.; Cimetta, E.; Piccoli, M.; De Coppi, P.; Elvassore, N. Muscle differentiation and myotubes alignment is influenced by micropatterned surfaces and exogenous electrical stimulation. Tissue Eng. Part A 2009, 15, 2447–2457. [Google Scholar] [CrossRef]
- Choi, J.S.; Lee, S.J.; Christ, G.J.; Atala, A.; Yoo, J.J. The influence of electrospun aligned poly(ε-caprolactone)/collagen nanofiber meshes on the formation of self-aligned skeletal muscle myotubes. Biomaterials 2008, 29, 2899–2906. [Google Scholar] [CrossRef] [PubMed]
- Cavanaugh, M.; Silantyeva, E.; Koh, G.P.; Malekzadeh, E.; Lanzinger, W.D.; Willits, R.K.; Becker, M.L. RGD-modified nanofibers enhance outcomes in rats after sciatic nerve injury. J. Funct. Biomater. 2019, 10, 24. [Google Scholar] [CrossRef] [PubMed]
- Seyedhassantehrani, N.; Li, Y.; Yao, L. Dynamic behaviors of astrocytes in chemically modified fibrin and collagen hydrogels. Integr. Biol. 2016, 8, 624–634. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Zheng, K.; Niu, L.; Zhang, Y.; Liu, Y.; Wang, C.; Chu, F. Highly mechanical properties nanocomposite hydrogels with biorenewable lignin nanoparticles. Int. J. Biol. Macromol. 2019, 128, 414–420. [Google Scholar] [CrossRef] [PubMed]
- Ding, Y.; Zhao, A.S.; Liu, T.; Wang, Y.N.; Gao, Y.; Li, J.A.; Yang, P. An Injectable Nanocomposite Hydrogel for Potential Application of Vascularization and Tissue Repair. Ann. Biomed. Eng. 2020, 48, 1511–1523. [Google Scholar] [CrossRef] [PubMed]
- Marcinczyk, M.; Dunn, A.; Haas, G.; Madsen, J.; Scheidt, R.; Patel, K.; Talovic, M.; Garg, K. The Effect of Laminin-111 Hydrogels on Muscle Regeneration in a Murine Model of Injury. Tissue Eng. Part A 2019, 25, 1001–1012. [Google Scholar] [CrossRef]
- Tian, B.; Ding, X.; Song, Y.; Chen, W.; Liang, J.; Yang, L.; Fan, Y.; Li, S.; Zhou, Y. Matrix stiffness regulates SMC functions via TGF-β signaling pathway. Biomaterials 2019, 221, 119407. [Google Scholar] [CrossRef]
- Sultankulov, B.; Berillo, D.; Sultankulova, K.; Tokay, T.; Saparov, A. Progress in the development of chitosan-based biomaterials for tissue engineering and regenerative medicine. Biomolecules 2019, 9, 470. [Google Scholar] [CrossRef]
- Mokhames, Z.; Rezaie, Z.; Ardeshirylajimi, A.; Basiri, A.; Taheri, M.; Omrani, M.D. Efficient smooth muscle cell differentiation of iPS cells on curcumin-incorporated chitosan/collagen/polyvinyl-alcohol nanofibers. Vitr. Cell. Dev. Biol. Anim. 2020, 56, 313–321. [Google Scholar] [CrossRef]
- Bushkalova, R.; Farno, M.; Tenailleau, C.; Duployer, B.; Cussac, D.; Parini, A.; Sallerin, B.; Girod Fullana, S. Alginate-chitosan PEC scaffolds: A useful tool for soft tissues cell therapy. Int. J. Pharm. 2019, 571, 118692. [Google Scholar] [CrossRef]
- Wang, X.; Wang, L.; Wu, Q.; Bao, F.; Yang, H.; Qiu, X.; Chang, J. Chitosan/Calcium Silicate Cardiac Patch Stimulates Cardiomyocyte Activity and Myocardial Performance after Infarction by Synergistic Effect of Bioactive Ions and Aligned Nanostructure. ACS Appl. Mater. Interfaces 2019, 11, 1449–1468. [Google Scholar] [CrossRef] [PubMed]
- Mombini, S.; Mohammadnejad, J.; Bakhshandeh, B.; Narmani, A.; Nourmohammadi, J.; Vahdat, S.; Zirak, S. Chitosan-PVA-CNT nanofibers as electrically conductive scaffolds for cardiovascular tissue engineering. Int. J. Biol. Macromol. 2019, 140, 278–287. [Google Scholar] [CrossRef] [PubMed]
- Gniesmer, S.; Brehm, R.; Hoffmann, A.; de Cassan, D.; Menzel, H.; Hoheisel, A.L.; Glasmacher, B.; Willbold, E.; Reifenrath, J.; Ludwig, N.; et al. Vascularization and biocompatibility of poly(ε-caprolactone) fiber mats for rotator cuff tear repair. PLoS ONE 2020, 15, 1–18. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez-Vázquez, M.; Vega-Ruiz, B.; Ramos-Zúñiga, R.; Saldaña-Koppel, D.A.; Quiñones-Olvera, L.F. Chitosan and Its Potential Use as a Scaffold for Tissue Engineering in Regenerative Medicine. Biomed Res. Int. 2015, 2015. [Google Scholar] [CrossRef]
- Quarta, M.; Cromie, M.; Chacon, R.; Blonigan, J.; Garcia, V.; Akimenko, I.; Hamer, M.; Paine, P.; Stok, M.; Shrager, J.B.; et al. Bioengineered constructs combined with exercise enhance stem cell-mediated treatment of volumetric muscle loss. Nat. Commun. 2017, 8, 1–17. [Google Scholar] [CrossRef]
- Fallas, M.E.A.; Piccoli, M.; Franzin, C.; Sgrò, A.; Dedja, A.; Urbani, L.; Bertin, E.; Trevisan, C.; Gamba, P.; Burns, A.J.; et al. Decellularized diaphragmatic muscle drives a constructive angiogenic response in vivo. Int. J. Mol. Sci. 2018, 19, 1319. [Google Scholar] [CrossRef]
- Sicari, B.M.; Peter Rubin, J.; Dearth, C.L.; Wolf, M.T.; Ambrosio, F.; Boninger, M.; Turner, N.J.; Weber, D.J.; Simpson, T.W.; Wyse, A.; et al. An acellular biologic scaffold promotes skeletal muscle formation in mice and humans with volumetric muscle loss. Sci. Transl. Med. 2014, 6, 1–12. [Google Scholar] [CrossRef]
- Ferrigno, B.; Bordett, R.; Duraisamy, N.; Moskow, J.; Arul, M.R.; Rudraiah, S.; Nukavarapu, S.P.; Vella, A.T.; Kumbar, S.G. Bioactive polymeric materials and electrical stimulation strategies for musculoskeletal tissue repair and regeneration. Bioact. Mater. 2020, 5, 468–485. [Google Scholar] [CrossRef]
- Bansai, S.; Morikura, T.; Onoe, H.; Miyata, S. Effect of cyclic stretch on tissue maturation in myoblast-laden hydrogel fibers. Micromachines 2019, 10, 399. [Google Scholar] [CrossRef]
- Khodabukus, A.; Madden, L.; Prabhu, N.K.; Koves, T.R.; Jackman, C.P.; Muoio, D.M.; Bursac, N. Electrical stimulation increases hypertrophy and metabolic flux in tissue-engineered human skeletal muscle. Biomaterials 2019, 198, 259–269. [Google Scholar] [CrossRef]
- Chen, J.; Hu, H.; Feng, L.; Zhu, Q.; Hancharou, A.; Liu, B.; Yan, C.; Xu, Y.; Guo, R. Preparation and characterization of 3D porous conductive scaffolds with magnetic resonance enhancement in tissue engineering. Biomed. Mater. 2019, 14. [Google Scholar] [CrossRef] [PubMed]
- Peña, B.; Maldonado, M.; Bonham, A.J.; Aguado, B.A.; Dominguez-Alfaro, A.; Laughter, M.; Rowland, T.J.; Bardill, J.; Farnsworth, N.L.; Alegret Ramon, N.; et al. Gold Nanoparticle-Functionalized Reverse Thermal Gel for Tissue Engineering Applications. ACS Appl. Mater. Interfaces 2019, 11, 18671–18680. [Google Scholar] [CrossRef] [PubMed]
- Somers, S.M.; Zhang, N.Y.; Morrissette-McAlmon, J.B.F.; Tran, K.; Mao, H.Q.; Grayson, W.L. Myoblast maturity on aligned microfiber bundles at the onset of strain application impacts myogenic outcomes. Acta Biomater. 2019, 94, 232–242. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Guo, F.; Hao, Y.; Gupta, S.K.; Hu, J.; Wang, Y.; Wang, N.; Zhao, Y.; Guo, M. Helical nanofiber yarn enabling highly stretchable engineered microtissue. Proc. Natl. Acad. Sci. USA 2019, 116, 9245–9250. [Google Scholar] [CrossRef]
- Vajanthri, K.Y.; Sidu, R.K.; Poddar, S.; Singh, A.K.; Mahto, S.K. Combined substrate micropatterning and FFT analysis reveals myotube size control and alignment by contact guidance. Cytoskeleton 2019, 76, 269–285. [Google Scholar] [CrossRef]
- Almonacid Suarez, A.M.; Zhou, Q.; van Rijn, P.; Harmsen, M.C. Directional topography gradients drive optimum alignment and differentiation of human myoblasts. J. Tissue Eng. Regen. Med. 2019, 13, 2234–2245. [Google Scholar] [CrossRef]
- Takahashi, H.; Shimizu, T.; Okano, T. Engineered Human Contractile Myofiber Sheets as a Platform for Studies of Skeletal Muscle Physiology. Sci. Rep. 2018, 8, 1–11. [Google Scholar] [CrossRef]
- Nagayama, K.; Uchida, K.; Sato, A. A novel micro-grooved collagen substrate for inducing vascular smooth muscle differentiation through cell tissue arrangement and nucleus remodeling. J. Mech. Behav. Biomed. Mater. 2019, 90, 295–305. [Google Scholar] [CrossRef]
- Williams, N.P.; Rhodehamel, M.; Yan, C.; Smith, A.S.T.; Jiao, A.; Murry, C.E.; Scatena, M.; Kim, D.H. Engineering anisotropic 3D tubular tissues with flexible thermoresponsive nanofabricated substrates. Biomaterials 2020, 240, 119856. [Google Scholar] [CrossRef]
- Nakayama, K.H.; Quarta, M.; Paine, P.; Alcazar, C.; Karakikes, I.; Garcia, V.; Abilez, O.J.; Calvo, N.S.; Simmons, C.S.; Rando, T.A.; et al. Treatment of volumetric muscle loss in mice using nanofibrillar scaffolds enhances vascular organization and integration. Commun. Biol. 2019, 2. [Google Scholar] [CrossRef]
- Carnes, M.E.; Pins, G.D. Etching anisotropic surface topography onto fibrin microthread scaffolds for guiding myoblast alignment. J. Biomed. Mater. Res. Part B Appl. Biomater. 2020, 108, 2308–2319. [Google Scholar] [CrossRef] [PubMed]
- Keijdener, H.; Konrad, J.; Hoffmann, B.; Gerardo-Nava, J.; Rütten, S.; Merkel, R.; Vázquez-Jiménez, J.; Brook, G.A.; Jockenhoevel, S.; Mela, P. A bench-top molding method for the production of cell-laden fibrin micro-fibers with longitudinal topography. J. Biomed. Mater. Res. Part B Appl. Biomater. 2020, 108, 1198–1212. [Google Scholar] [CrossRef] [PubMed]
- Kankala, R.K.; Zhao, J.; Liu, C.G.; Song, X.J.; Yang, D.Y.; Zhu, K.; Wang, S.B.; Zhang, Y.S.; Chen, A.Z. Highly Porous Microcarriers for Minimally Invasive In Situ Skeletal Muscle Cell Delivery. Small 2019, 15, 1–15. [Google Scholar] [CrossRef]
- Arrigoni, C.; Petta, D.; Bersini, S.; Mironov, V.; Candrian, C.; Moretti, M. Engineering complex muscle-tissue interfaces through microfabrication. Biofabrication 2019, 11. [Google Scholar] [CrossRef]
- Fan, D.; Li, Y.; Wang, X.; Zhu, T.; Wang, Q.; Cai, H.; Li, W.; Tian, Y.; Liu, Z. Progressive 3D Printing Technology and Its Application in Medical Materials. Front. Pharmacol. 2020, 11, 1–12. [Google Scholar] [CrossRef]
- Capel, A.J.; Rimington, R.P.; Fleming, J.W.; Player, D.J.; Baker, L.A.; Turner, M.C.; Jones, J.M.; Martin, N.R.W.; Ferguson, R.A.; Mudera, V.C.; et al. Scalable 3D printed molds for human tissue engineered skeletal muscle. Front. Bioeng. Biotechnol. 2019, 7, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Ostrovidov, S.; Salehi, S.; Costantini, M.; Suthiwanich, K.; Ebrahimi, M.; Sadeghian, R.B.; Fujie, T.; Shi, X.; Cannata, S.; Gargioli, C.; et al. 3D Bioprinting in Skeletal Muscle Tissue Engineering. Small 2019, 15, 1–14. [Google Scholar] [CrossRef]
- Yu, C.; Ma, X.; Zhu, W.; Wang, P.; Miller, K.L.; Stupin, J.; Koroleva-Maharajh, A.; Hairabedian, A.; Chen, S. Scanningless and continuous 3D bioprinting of human tissues with decellularized extracellular matrix. Biomaterials 2019, 194, 1–13. [Google Scholar] [CrossRef]
- Abudupataer, M.; Chen, N.; Yan, S.; Alam, F.; Shi, Y.; Wang, L.; Lai, H.; Li, J.; Zhu, K.; Wang, C. Bioprinting a 3D vascular construct for engineering a vessel-on-a-chip. Biomed. Microdevices 2020, 22, 1–10. [Google Scholar] [CrossRef]
- Dickman, C.T.D.; Russo, V.; Thain, K.; Pan, S.; Beyer, S.T.; Walus, K.; Getsios, S.; Mohamed, T.; Wadsworth, S.J. Functional characterization of 3D contractile smooth muscle tissues generated using a unique microfluidic 3D bioprinting technology. FASEB J. 2020, 34, 1652–1664. [Google Scholar] [CrossRef]
- Seyedmahmoud, R.; Çelebi-Saltik, B.; Barros, N.; Nasiri, R.; Banton, E.; Shamloo, A.; Ashammakhi, N.; Dokmeci, M.R.; Ahadian, S. Three-dimensional bioprinting of functional skeletal muscle tissue using gelatin methacryloyl-alginate bioinks. Micromachines 2019, 10, 679. [Google Scholar] [CrossRef] [PubMed]
- Maiullari, F.; Costantini, M.; Milan, M.; Pace, V.; Chirivì, M.; Maiullari, S.; Rainer, A.; Baci, D.; Marei, H.E.S.; Seliktar, D.; et al. A multi-cellular 3D bioprinting approach for vascularized heart tissue engineering based on HUVECs and iPSC-derived cardiomyocytes. Sci. Rep. 2018, 8, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Evans, E.B.; Brady, S.W.; Tripathi, A.; Hoffman-Kim, D. Schwann cell durotaxis can be guided by physiologically relevant stiffness gradients. Biomater. Res. 2018, 22, 1–13. [Google Scholar] [CrossRef]
- Parandakh, A.; Anbarlou, A.; Tafazzoli-Shadpour, M.; Ardeshirylajimi, A.; Khani, M.M. Substrate topography interacts with substrate stiffness and culture time to regulate mechanical properties and smooth muscle differentiation of mesenchymal stem cells. Colloids Surf. B Biointerfaces 2019, 173, 194–201. [Google Scholar] [CrossRef] [PubMed]
- Dalton, P.D.; Vaquette, C.; Farrugia, B.L.; Dargaville, T.R.; Brown, T.D.; Hutmacher, D.W. Electrospinning and additive manufacturing: Converging technologies. Biomater. Sci. 2013, 1, 171–185. [Google Scholar] [CrossRef]
- Arumugam, R.; Srinadhu, E.S.; Subramanian, B.; Nallani, S. β-PVDF based electrospun nanofibers – A promising material for developing cardiac patches. Med. Hypotheses 2019, 122, 31–34. [Google Scholar] [CrossRef]
- Hong, J.; Yeo, M.; Yang, G.H.; Kim, G. Cell-electrospinning and its application for tissue engineering. Int. J. Mol. Sci. 2019, 20, 6208. [Google Scholar] [CrossRef]
- Xue, J.; Wu, T.; Xia, Y. Perspective: Aligned arrays of electrospun nanofibers for directing cell migration. APL Mater. 2018, 6. [Google Scholar] [CrossRef]
- Kaiser, N.J.; Bellows, J.A.; Kant, R.J.; Coulombe, K.L.K. Digital Design and Automated Fabrication of Bespoke Collagen Microfiber Scaffolds. Tissue Eng. Part C Methods 2019, 25, 687–700. [Google Scholar] [CrossRef]
- Liu, J.; Qin, Y.; Wu, Y.; Sun, Z.; Li, B.; Jing, H.; Zhang, C.; Li, C.; Leng, X.; Wang, Z.; et al. The surrounding tissue contributes to smooth muscle cells’ regeneration and vascularization of small diameter vascular grafts. Biomater. Sci. 2019, 7, 914–925. [Google Scholar] [CrossRef]
- Rao, F.; Yuan, Z.; Li, M.; Yu, F.; Fang, X.; Jiang, B.; Wen, Y.; Zhang, P. Expanded 3D nanofibre sponge scaffolds by gas-foaming technique enhance peripheral nerve regeneration. Artif. Cells Nanomed. Biotechnol. 2019, 47, 491–500. [Google Scholar] [CrossRef] [PubMed]
- Sarhane, K.A.; Ibrahim, Z.; Martin, R.; Krick, K.; Cashman, C.R.; Tuffaha, S.H.; Broyles, J.M.; Prasad, N.; Yao, Z.C.; Cooney, D.S.; et al. Macroporous nanofiber wraps promote axonal regeneration and functional recovery in nerve repair by limiting fibrosis. Acta Biomater. 2019, 88, 332–345. [Google Scholar] [CrossRef] [PubMed]
- Xue, J.; Pisignano, D.; Xia, Y. Maneuvering the Migration and Differentiation of Stem Cells with Electrospun Nanofibers. Adv. Sci. 2020, 7, 1–17. [Google Scholar] [CrossRef]
- Lee, H.; Kim, W.J.; Lee, J.U.; Yoo, J.J.; Kim, G.H.; Lee, S.J. Effect of Hierarchical Scaffold Consisting of Aligned dECM Nanofibers and Poly(lactide- co-glycolide) Struts on the Orientation and Maturation of Human Muscle Progenitor Cells. ACS Appl. Mater. Interfaces 2019, 11, 39449–39458. [Google Scholar] [CrossRef] [PubMed]
- Patel, K.H.; Dunn, A.J.; Talovic, M.; Haas, G.J.; Marcinczyk, M.; Elmashhady, H.; Kalaf, E.G.; Sell, S.A.; Garg, K. Aligned nanofibers of decellularized muscle ECM support myogenic activity in primary satellite cells in vitro. Biomed. Mater. 2019, 14. [Google Scholar] [CrossRef]
- Rezaei, H.; Rezaie, Z.; Seifati, S.M.; Ardeshirylajimi, A.; Basiri, A.; Taheri, M.; Omrani, M.D. Poly-phosphate increases SMC differentiation of mesenchymal stem cells on PLGA–polyurethane nanofibrous scaffold. Cell Tissue Bank. 2020, 1. [Google Scholar] [CrossRef]
- Ezhilarasu, H.; Sadiq, A.; Ratheesh, G.; Sridhar, S.; Ramakrishna, S.; Mohd, M.H.; Yusoff, M.M.; Jose, R.; Reddy, V.J. Functionalized core/shell nanofibers for the differentiation of mesenchymal stem cells for vascular tissue engineering. Nanomedicine 2019, 14, 201–214. [Google Scholar] [CrossRef]
- Miao, S.; Nowicki, M.; Cui, H.; Lee, S.J.; Zhou, X.; Mills, D.K.; Zhang, L.G. 4D anisotropic skeletal muscle tissue constructs fabricated by staircase effect strategy. Biofabrication 2019, 11. [Google Scholar] [CrossRef]
- Damanik, F.F.R.; Spadolini, G.; Rotmans, J.; Farè, S.; Moroni, L. Biological activity of human mesenchymal stromal cells on polymeric electrospun scaffolds. Biomater. Sci. 2019, 7, 1088–1100. [Google Scholar] [CrossRef]
- Yeo, M.; Kim, G.H. Anisotropically Aligned Cell-Laden Nanofibrous Bundle Fabricated via Cell Electrospinning to Regenerate Skeletal Muscle Tissue. Small 2018, 14, 1–13. [Google Scholar] [CrossRef]
- Nosoudi, N.; Jacob, A.O.; Stultz, S.; Jordan, M.; Aldabel, S.; Hohne, C.; Mosser, J.; Archacki, B.; Turner, A.; Turner, P. Electrospinning live cells using Gelatin and Pullulan. Bioengineering 2020, 7, 21. [Google Scholar] [CrossRef] [PubMed]
- Guo, Y.; Gilbert-Honick, J.; Somers, S.M.; Mao, H.Q.; Grayson, W.L. Modified cell-electrospinning for 3D myogenesis of C2C12s in aligned fibrin microfiber bundles. Biochem. Biophys. Res. Commun. 2019, 516, 558–564. [Google Scholar] [CrossRef] [PubMed]
- Gilbert-Honick, J.; Iyer, S.R.; Somers, S.M.; Lovering, R.M.; Wagner, K.; Mao, H.Q.; Grayson, W.L. Engineering functional and histological regeneration of vascularized skeletal muscle. Biomaterials 2018, 164, 70–79. [Google Scholar] [CrossRef] [PubMed]
- Gilbert-Honick, J.; Iyer, S.R.; Somers, S.M.; Takasuka, H.; Lovering, R.M.; Wagner, K.R.; Mao, H.Q.; Grayson, W.L. Engineering 3D skeletal muscle primed for neuromuscular regeneration following volumetric muscle loss. Biomaterials 2020, 255, 120154. [Google Scholar] [CrossRef] [PubMed]
- Willson, K.; Ke, D.; Kengla, C.; Atala, A.; Murphy, S. V Extrusion-Based Bioprinting: Current Standards and Relevancy for Human-Sized Tissue Fabrication. In 3D Bioprinting: Principles and Protocols; Crook, J.M., Ed.; Springer US: New York, NY, USA, 2020; pp. 65–92. ISBN 978-1-0716-0520-2. [Google Scholar]
- Yeo, M.; Kim, G.H. Nano/microscale topographically designed alginate/PCL scaffolds for inducing myoblast alignment and myogenic differentiation. Carbohydr. Polym. 2019, 223, 115041. [Google Scholar] [CrossRef]
- Liu, Y.X.; Wu, B.B.; Gong, L.; An, C.R.; Lin, J.X.; Li, Q.K.; Jiang, D.M.; Jin, K.X.; Mechakra, A.; Bunpetch, V.; et al. Dissecting cell diversity and connectivity in skeletal muscle for myogenesis. Cell Death Dis. 2019, 10. [Google Scholar] [CrossRef]
- Zhang, D.; Yan, K.; Zhou, J.; Xu, T.; Xu, M.; Lin, J.; Bai, J.; Ge, G.; Hu, D.; Si, W.; et al. Myogenic differentiation of human amniotic mesenchymal cells and its tissue repair capacity on volumetric muscle loss. J. Tissue Eng. 2019, 10. [Google Scholar] [CrossRef]
- Colunga, T.; Hayworth, M.; Kreß, S.; Reynolds, D.M.; Chen, L.; Nazor, K.L.; Baur, J.; Singh, A.M.; Loring, J.F.; Metzger, M.; et al. Human Pluripotent Stem Cell-Derived Multipotent Vascular Progenitors of the Mesothelium Lineage Have Utility in Tissue Engineering and Repair. Cell Rep. 2019, 26, 2566–2579.e10. [Google Scholar] [CrossRef]
- Mazini, L.; Rochette, L.; Amine, M.; Malka, G. Regenerative capacity of adipose derived stem cells (ADSCs), comparison with mesenchymal stem cells (MSCs). Int. J. Mol. Sci. 2019, 20, 2523. [Google Scholar] [CrossRef]
- Moyle, L.A.; Tedesco, F.S.; Benedetti, S. Pericytes in Muscular Dystrophies; Springer: Cham, Switzerland, 2019; Volume 1147, ISBN 9783030169077. [Google Scholar]
- Gerli, M.F.M.; Moyle, L.A.; Benedetti, S.; Ferrari, G.; Ucuncu, E.; Ragazzi, M.; Constantinou, C.; Louca, I.; Sakai, H.; Ala, P.; et al. Combined Notch and PDGF Signaling Enhances Migration and Expression of Stem Cell Markers while Inducing Perivascular Cell Features in Muscle Satellite Cells. Stem Cell Reports 2019, 12, 461–473. [Google Scholar] [CrossRef]
- del Carmen Ortuño-Costela, M.; García-López, M.; Cerrada, V.; Gallardo, M.E. iPSCs: A powerful tool for skeletal muscle tissue engineering. J. Cell. Mol. Med. 2019, 23, 3784–3794. [Google Scholar] [CrossRef] [PubMed]
- Rao, L.; Qian, Y.; Khodabukus, A.; Ribar, T.; Bursac, N. Engineering human pluripotent stem cells into a functional skeletal muscle tissue. Nat. Commun. 2018, 9, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Chal, J.; Al Tanoury, Z.; Hestin, M.; Gobert, B.; Aivio, S.; Hick, A.; Cherrier, T.; Nesmith, A.P.; Parker, K.K.; Pourquié, O. Generation of human muscle fibers and satellite-like cells from human pluripotent stem cells in vitro. Nat. Protoc. 2016, 11, 1833–1850. [Google Scholar] [CrossRef] [PubMed]
- Kong, A.M.; Yap, K.K.; Lim, S.Y.; Marre, D.; Pébay, A.; Gerrand, Y.W.; Lees, J.G.; Palmer, J.A.; Morrison, W.A.; Mitchell, G.M. Bio-engineering a tissue flap utilizing a porous scaffold incorporating a human induced pluripotent stem cell-derived endothelial cell capillary network connected to a vascular pedicle. Acta Biomater. 2019, 94, 281–294. [Google Scholar] [CrossRef]
- Besse, L.; Sheeba, C.J.; Holt, M.; Labuhn, M.; Wilde, S.; Feneck, E.; Bell, D.; Kucharska, A.; Logan, M.P.O. Individual Limb Muscle Bundles Are Formed through Progressive Steps Orchestrated by Adjacent Connective Tissue Cells during Primary Myogenesis. Cell Rep. 2020, 30, 3552–3565.e6. [Google Scholar] [CrossRef] [PubMed]
- Boldyreva, M.A.; Shevchenko, E.K.; Molokotina, Y.D.; Makarevich, P.I.; Beloglazova, I.B.; Zubkova, E.S.; Dergilev, K.V.; Tsokolaeva, Z.I.; Penkov, D.; Hsu, M.N.; et al. Transplantation of adipose stromal cell sheet producing hepatocyte growth factor induces pleiotropic effect in ischemic skeletal muscle. Int. J. Mol. Sci. 2019, 20, 3088. [Google Scholar] [CrossRef] [PubMed]
- Kang, Y.; Liu, Y.; Liu, Z.; Ren, S.; Xiong, H.; Chen, J.; Duscher, D.; Machens, H.G.; Liu, W.; Guo, G.; et al. Differentiated human adipose-derived stromal cells exhibit the phenotypic and functional characteristics of mature Schwann cells through a modified approach. Cytotherapy 2019, 21, 987–1003. [Google Scholar] [CrossRef]
- Choi, Y.S.; Vincent, L.G.; Lee, A.R.; Dobke, M.K.; Engler, A.J. Mechanical derivation of functional myotubes from adipose-derived stem cells. Biomaterials 2012, 33, 2482–2491. [Google Scholar] [CrossRef]
- Ergene, E.; Sezlev Bilecen, D.; Kaya, B.; Yilgor Huri, P.; Hasirci, V. 3D cellular alignment and biomimetic mechanical stimulation enhance human adipose-derived stem cell myogenesis. Biomed. Mater. 2020, 15, 55017. [Google Scholar] [CrossRef]
- Gilbert-Honick, J.; Ginn, B.; Zhang, Y.; Salehi, S.; Wagner, K.R.; Mao, H.Q.; Grayson, W.L. Adipose-derived Stem/Stromal Cells on Electrospun Fibrin Microfiber Bundles Enable Moderate Muscle Reconstruction in a Volumetric Muscle Loss Model. Cell Transplant. 2018, 27, 1644–1656. [Google Scholar] [CrossRef]
- Richards, D.; Swift, J.; Wong, L.S.; Richardson, S.M. Photoresponsive Hydrogels with Photoswitchable Stiffness: Emerging Platforms to Study Temporal Aspects of Mesenchymal Stem Cell Responses to Extracellular Stiffness Regulation. Adv. Exp. Med. Biol. 2019, 1144, 53–69. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Xu, R.; Tu, X.; Janairo, R.R.R.; Kwong, G.; Wang, D.; Zhu, Y.; Li, S. Differentiation of Neural Crest Stem Cells in Response to Matrix Stiffness and TGF-β1 in Vascular Regeneration. Stem Cells Dev. 2020, 29, 249–256. [Google Scholar] [CrossRef] [PubMed]
- Choi, Y.S.; Vincent, L.G.; Lee, A.R.; Kretchmer, K.C.; Chirasatitsin, S.; Dobke, M.K.; Engler, A.J. The alignment and fusion assembly of adipose-derived stem cells on mechanically patterned matrices. Biomaterials 2012, 33, 6943–6951. [Google Scholar] [CrossRef]
- An, Y.; Reimers, K.; Allmeling, C.; Liu, J.; Lazaridis, A.; Strauss, S.; Vogt, P.M. Large-Volume Vascularized Muscle Grafts Engineered From Groin Adipose Tissue in Perfusion Bioreactor Culture. J. Craniofac. Surg. 2020, 31, 588–593. [Google Scholar] [CrossRef]
- Rivera-Izquierdo, M.; Cabeza, L.; Láinez-Ramos-Bossini, A.; Quesada, R.; Perazzoli, G.; Alvarez, P.; Prados, J.; Melguizo, C. An updated review of adipose derived-mesenchymal stem cells and their applications in musculoskeletal disorders. Expert Opin. Biol. Ther. 2019, 19, 233–248. [Google Scholar] [CrossRef] [PubMed]
- Klar, A.S.; Zimoch, J.; Biedermann, T. Skin Tissue Engineering: Application of Adipose-Derived Stem Cells. Biomed Res. Int. 2017, 2017. [Google Scholar] [CrossRef]
- Moon, K.C.; Chung, H.Y.; Han, S.K.; Jeong, S.H.; Dhong, E.S. Possibility of injecting adipose-derived stromal vascular fraction cells to accelerate microcirculation in ischemic diabetic feet: A pilot study. Int. J. Stem Cells 2019, 12, 107–113. [Google Scholar] [CrossRef]
- Cerino, G.; Gaudiello, E.; Grussenmeyer, T.; Melly, L.; Massai, D.; Banfi, A.; Martin, I.; Eckstein, F.; Grapow, M.; Marsano, A. Three dimensional multi-cellular muscle-like tissue engineering in perfusion-based bioreactors. Biotechnol. Bioeng. 2016, 113, 226–236. [Google Scholar] [CrossRef]
- Costa, M.; Cerqueira, M.T.; Santos, T.C.; Sampaio-Marques, B.; Ludovico, P.; Marques, A.P.; Pirraco, R.P.; Reis, R.L. Cell sheet engineering using the stromal vascular fraction of adipose tissue as a vascularization strategy. Acta Biomater. 2017, 55, 131–143. [Google Scholar] [CrossRef]
- Vezzani, B.; Shaw, I.; Lesme, H.; Yong, L.; Khan, N.; Tremolada, C.; Péault, B. Higher Pericyte Content and Secretory Activity of Microfragmented Human Adipose Tissue Compared to Enzymatically Derived Stromal Vascular Fraction. Stem Cells Transl. Med. 2018, 7, 876–886. [Google Scholar] [CrossRef]
- Fuoco, C.; Sangalli, E.; Vono, R.; Testa, S.; Sacchetti, B.; Latronico, M.V.G.; Bernardini, S.; Madeddu, P.; Cesareni, G.; Seliktar, D.; et al. 3D hydrogel environment rejuvenates aged pericytes for skeletal muscle tissue engineering. Front. Physiol. 2014, 5, 1–8. [Google Scholar] [CrossRef]
- Wang, Q.; Pei, S.; Lu, X.L.; Wang, L.; Wu, Q. On the characterization of interstitial fluid flow in the skeletal muscle endomysium. J. Mech. Behav. Biomed. Mater. 2020, 102, 103504. [Google Scholar] [CrossRef] [PubMed]
- Nudel, I.; Hadas, O.; deBotton, G. Experimental study of muscle permeability under various loading conditions. Biomech. Model. Mechanobiol. 2019, 18, 1189–1195. [Google Scholar] [CrossRef] [PubMed]
- Kusters, Y.H.A.M.; Barrett, E.J. Muscle microvasculature’s structural and functional specializations facilitate muscle metabolism. Am. J. Physiol. Endocrinol. Metab. 2016, 310, E379–E387. [Google Scholar] [CrossRef] [PubMed]
- Hirai, D.M.; Colburn, T.D.; Craig, J.C.; Hotta, K.; Kano, Y.; Musch, T.I.; Poole, D.C. Skeletal muscle interstitial O2 pressures: bridging the gap between the capillary and myocyte. Microcirculation 2019, 26, 1–12. [Google Scholar] [CrossRef]
- Skylar-Scott, M.A.; Uzel, S.G.M.; Nam, L.L.; Ahrens, J.H.; Truby, R.L.; Damaraju, S.; Lewis, J.A. Biomanufacturing of organ-specific tissues with high cellular density and embedded vascular channels. Sci. Adv. 2019, 5. [Google Scholar] [CrossRef]
- Sarker, M.D.; Naghieh, S.; Sharma, N.K.; Ning, L.; Chen, X. Bioprinting of vascularized tissue scaffolds: Influence of biopolymer, cells, growth factors, and gene delivery. J. Healthc. Eng. 2019, 2019. [Google Scholar] [CrossRef]
- Wimmer, R.A.; Leopoldi, A.; Aichinger, M.; Kerjaschki, D.; Penninger, J.M. Generation of blood vessel organoids from human pluripotent stem cells. Nat. Protoc. 2019, 14, 3082–3100. [Google Scholar] [CrossRef]
- Generali, M.; Casanova, E.A.; Kehl, D.; Wanner, D.; Hoerstrup, S.P.; Cinelli, P.; Weber, B. Autologous endothelialized small-caliber vascular grafts engineered from blood-derived induced pluripotent stem cells. Acta Biomater. 2019, 97, 333–343. [Google Scholar] [CrossRef]
- Wong, H.K.; Ivan Lam, C.R.; Wen, F.; Mark Chong, S.K.; Tan, N.S.; Jerry, C.; Pal, M.; Tan, L.P. Novel method to improve vascularization of tissue engineered constructs with biodegradable fibers. Biofabrication 2016, 8. [Google Scholar] [CrossRef]
- Kirkton, R.D.; Santiago-Maysonet, M.; Lawson, J.H.; Tente, W.E.; Dahl, S.L.M.; Niklason, L.E.; Prichard, H.L. Erratum: Bioengineered human acellular vessels recellularize and evolve into living blood vessels after human implantation. Sci. Transl. Med. 2019, 11, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Slater, C.R. The structure of human neuromuscular junctions: Some unanswered molecular questions. Int. J. Mol. Sci. 2017, 18, 2183. [Google Scholar] [CrossRef] [PubMed]
- Yin, X.; Yu, T.; Chen, B.; Xu, J.; Chen, W.; Qi, Y.; Zhang, P.; Li, Y.; Kou, Y.; Ma, Y.; et al. Spatial distribution of motor endplates and its adaptive change in skeletal muscle. Theranostics 2019, 9, 734–746. [Google Scholar] [CrossRef]
- Yoshioka, K.; Ito, A.; Kawabe, Y.; Kamihira, M. Novel neuromuscular junction model in 2D and 3D myotubes co-cultured with induced pluripotent stem cell-derived motor neurons. J. Biosci. Bioeng. 2020, 129, 486–493. [Google Scholar] [CrossRef] [PubMed]
- Kaufman, T.; Kaplan, B.; Perry, L.; Shandalov, Y.; Landau, S.; Srugo, I.; Ad-El, D.; Levenberg, S. Innervation of an engineered muscle graft for reconstruction of muscle defects. Am. J. Transplant. 2019, 19, 37–47. [Google Scholar] [CrossRef]
- Moon, H.Y.; Javadi, S.; Stremlau, M.; Yoon, K.J.; Becker, B.; Kang, S.U.; Zhao, X.; van Praag, H. Conditioned media from AICAR-treated skeletal muscle cells increases neuronal differentiation of adult neural progenitor cells. Neuropharmacology 2019, 145, 123–130. [Google Scholar] [CrossRef]
- Tiburcy, M.; Markov, A.; Kraemer, L.K.; Christalla, P.; Rave-Fraenk, M.; Fischer, H.J.; Reichardt, H.M.; Zimmermann, W. Regeneration competent satellite cell niches in rat engineered skeletal muscle. FASEB BioAdvances 2019, 1, 731–746. [Google Scholar] [CrossRef]
- Han, W.M.; Mohiuddin, M.; Anderson, S.E.; García, A.J.; Jang, Y.C. Co-delivery of Wnt7a and muscle stem cells using synthetic bioadhesive hydrogel enhances murine muscle regeneration and cell migration during engraftment. Acta Biomater. 2019, 94, 243–252. [Google Scholar] [CrossRef]
- Zhang, X.; Simmons, C.A.; Paul Santerre, J. Paracrine signalling from monocytes enables desirable extracellular matrix accumulation and temporally appropriate phenotype of vascular smooth muscle cell-like cells derived from adipose stromal cells. Acta Biomater. 2020, 103, 129–141. [Google Scholar] [CrossRef]
- Später, T.; Menger, M.M.; Nickels, R.M.; Menger, M.D.; Laschke, M.W. Macrophages promote network formation and maturation of transplanted adipose tissue–derived microvascular fragments. J. Tissue Eng. 2020, 11. [Google Scholar] [CrossRef]
- Carleton, M.M.; Sefton, M.V. Injectable and degradable methacrylic acid hydrogel alters macrophage response in skeletal muscle. Biomaterials 2019, 223, 119477. [Google Scholar] [CrossRef] [PubMed]
- Cho, J.; Kuswanto, W.; Benoist, C.; Mathis, D. T cell receptor specificity drives accumulation of a reparative population of regulatory T cells within acutely injured skeletal muscle. Proc. Natl. Acad. Sci. USA 2019, 116, 26727–26733. [Google Scholar] [CrossRef] [PubMed]
- Somers, S.M.; Spector, A.A.; Digirolamo, D.J.; Grayson, W.L. Biophysical stimulation for engineering functional skeletal muscle. Tissue Eng. Part B Rev. 2017, 23, 362–372. [Google Scholar] [CrossRef] [PubMed]
- Pedersen, B.K.; Febbraio, M.A. Muscle as an endocrine organ: Focus on muscle-derived interleukin-6. Physiol. Rev. 2008, 88, 1379–1406. [Google Scholar] [CrossRef]
- Giudice, J.; Taylor, J.M. Muscle as a paracrine and endocrine organ. Curr. Opin. Pharmacol. 2017, 34, 49–55. [Google Scholar] [CrossRef]
- Muñoz-Cánoves, P.; Scheele, C.; Pedersen, B.K.; Serrano, A.L. Interleukin-6 myokine signaling in skeletal muscle: A double-edged sword? FEBS J. 2013, 280, 4131–4148. [Google Scholar] [CrossRef]
- Tanaka, T.; Narazaki, M.; Kishimoto, T. IL-6 in Inflammation, Immunity, and Disease. Cold Spring Harb. Perspect. Biol. 2020, 6, a016295. [Google Scholar] [CrossRef]
- Piñol-Jurado, P.; Gallardo, E.; de Luna, N.; Suárez-Calvet, X.; Sánchez-Riera, C.; Fernández-Simón, E.; Gomis, C.; Illa, I.; Díaz-Manera, J. Platelet-Derived Growth Factor BB Influences Muscle Regeneration in Duchenne Muscle Dystrophy. Am. J. Pathol. 2017, 187, 1814–1827. [Google Scholar] [CrossRef]
- Sheehan, S.M.; Tatsumi, R.; Temm-Grove, C.J.; Allen, R.E. HGF is an autocrine growth factor for skeletal muscle satellite cells in vitro. Muscle Nerve 2000, 23, 239–245. [Google Scholar] [CrossRef]
- Floss, T.; Arnold, H.H.; Braun, T. A role for FGF-6 in skeletal muscle regeneration. Genes Dev. 1997, 11, 2040–2051. [Google Scholar] [CrossRef]
- Borselli, C.; Storrie, H.; Benesch-Lee, F.; Shvartsman, D.; Cezar, C.; Lichtman, J.W.; Vandenburgh, H.H.; Mooney, D.J. Functional muscle regeneration with combined delivery of angiogenesis and myogenesis factors. Proc. Natl. Acad. Sci. USA 2010, 107, 3287–3292. [Google Scholar] [CrossRef] [PubMed]
- Setayesh, K.; Villarreal, A.; Gottschalk, A.; Tokish, J.M.; Choate, W.S. Treatment of Muscle Injuries with Platelet-Rich Plasma: A Review of the Literature. Curr. Rev. Musculoskelet. Med. 2018, 11, 635–642. [Google Scholar] [CrossRef] [PubMed]
- Rossi, L.A.; Molina Rómoli, A.R.; Bertona Altieri, B.A.; Burgos Flor, J.A.; Scordo, W.E.; Elizondo, C.M. Does platelet-rich plasma decrease time to return to sports in acute muscle tear? A randomized controlled trial. Knee Surg. Sport. Traumatol. Arthrosc. 2017, 25, 3319–3325. [Google Scholar] [CrossRef] [PubMed]
- Engebretsen, L.; Steffen, K.; Alsousou, J.; Anitua, E.; Bachl, N.; Devilee, R.; Everts, P.; Hamilton, B.; Huard, J.; Jenoure, P.; et al. IOC consensus paper on the use of platelet-rich plasma in sports medicine. Br. J. Sports Med. 2010, 44, 1072–1081. [Google Scholar] [CrossRef]
- Kjaer, M.; Bayer, M. The use of platelet-rich plasma in sports medicine: A quick fix or medical doctors on shaky ethical ground? Scand. J. Med. Sci. Sport. 2011, 21, 493–495. [Google Scholar] [CrossRef]
- Li, H.; Hicks, J.J.; Wang, L.; Oyster, N.; Philippon, M.J.; Hurwitz, S.; Hogan, M.C.V.; Huard, J. Customized platelet-rich plasma with transforming growth factor β1 neutralization antibody to reduce fibrosis in skeletal muscle. Biomaterials 2016, 87, 147–156. [Google Scholar] [CrossRef]
- Reurink, G.; Goudswaard, G.J.; Moen, M.H.; Weir, A.; Verhaar, J.A.N.; Bierma-Zeinstra, S.M.A.; Maas, M.; Tol, J.L. Platelet-rich plasma injections in acute muscle injury. N. Engl. J. Med. 2014, 370, 2546–2547. [Google Scholar] [CrossRef]
- Latalski, M.; Walczyk, A.; Fatyga, M.; Rutz, E.; Szponder, T.; Bielecki, T.; Danielewicz, A. Allergic reaction to platelet-rich plasma (PRP). Medicine 2019, 98, e14702. [Google Scholar] [CrossRef]
- Ye, F.; Mathur, S.; Liu, M.; Borst, S.E.; Walter, G.A.; Sweeney, H.L.; Vandenborne, K. Overexpression of insulin-like growth factor-1 attenuates skeletal muscle damage and accelerates muscle regeneration and functional recovery after disuse. Exp. Physiol. 2013, 98, 1038–1052. [Google Scholar] [CrossRef]
- Bonadio, J.; Smiley, E.; Patil, P.; Goldstein, S. Localized, direct plasmid gene delivery in vivo: Prolonged therapy results in reproducible tissue regeneration. Nat. Med. 1999, 5, 753–759. [Google Scholar] [CrossRef]
- Falco, E.E.; Wang, M.O.; Thompson, J.A.; Chetta, J.M.; Yoon, D.M.; Li, E.Z.; Kulkami, M.M.; Shah, S.; Pandit, A.; Roth, J.S.; et al. Porous EH and EH-PEG scaffolds as gene delivery vehicles to skeletal muscle. Pharm. Res. 2011, 28, 1306–1316. [Google Scholar] [CrossRef] [PubMed]
- Doukas, J.; Blease, K.; Craig, D.; Ma, C.; Chandler, L.A.; Sosnowski, B.A.; Pierce, G.F. Delivery of FGF genes to wound repair cells enhances arteriogenesis and myogenesis in skeletal muscle. Mol. Ther. 2002, 5, 517–527. [Google Scholar] [CrossRef] [PubMed]
- Stilhano, R.S.; Madrigal, J.L.; Wong, K.; Williams, P.A.; Martin, P.K.M.; Yamaguchi, F.S.M.; Samoto, V.Y.; Han, S.W.; Silva, E.A. Injectable alginate hydrogel for enhanced spatiotemporal control of lentivector delivery in murine skeletal muscle. J. Control. Release 2016, 237, 42–49. [Google Scholar] [CrossRef] [PubMed]
- Caballero Aguilar, L.M.; Silva, S.M.; Moulton, S.E. Growth factor delivery: Defining the next generation platforms for tissue engineering. J. Control. Release 2019, 306, 40–58. [Google Scholar] [CrossRef] [PubMed]
- Yi, H.; Forsythe, S.; He, Y.; Liu, Q.; Xiong, G.; Wei, S.; Li, G.; Atala, A.; Skardal, A.; Zhang, Y. Tissue-specific extracellular matrix promotes myogenic differentiation of human muscle progenitor cells on gelatin and heparin conjugated alginate hydrogels. Acta Biomater. 2017, 62, 222–233. [Google Scholar] [CrossRef] [PubMed]
- Ehrbar, M.; Metters, A.; Zammaretti, P.; Hubbell, J.A.; Zisch, A.H. Endothelial cell proliferation and progenitor maturation by fibrin-bound VEGF variants with differential susceptibilities to local cellular activity. J. Control. Release 2005, 101, 93–109. [Google Scholar] [CrossRef]
- Zisch, A.H.; Schenk, U.; Schense, J.C.; Sakiyama-Elbert, S.E.; Hubbell, J.A. Covalently conjugated VEGF-fibrin matrices for endothelialization. J. Control. Release 2001, 72, 101–113. [Google Scholar] [CrossRef]
- Raimondo, T.M.; Li, H.; Kwee, B.J.; Kinsley, S.; Budina, E.; Anderson, E.M.; Doherty, E.J.; Talbot, S.G.; Mooney, D.J. Combined delivery of VEGF and IGF-1 promotes functional innervation in mice and improves muscle transplantation in rabbits. Biomaterials 2019, 216, 119246. [Google Scholar] [CrossRef]
- Bin, Z.; Zhihu, Z.; Jianxiong, M.; Xinlong, M. Repairing peripheral nerve defects with revascularized tissue-engineered nerve based on a vascular endothelial growth factor-heparin sustained release system. J. Tissue Eng. Regen. Med. 2020, 14, 819–828. [Google Scholar] [CrossRef]
- Rybalko, V.Y.; Pham, C.B.; Hsieh, P.L.; Hammers, D.W.; Merscham-Banda, M.; Suggs, L.J.; Farrar, R.P. Controlled delivery of SDF-1α and IGF-1: CXCR4+ cell recruitment and functional skeletal muscle recovery. Biomater. Sci. 2015, 3, 1475–1486. [Google Scholar] [CrossRef]
- Blaich, G.; Janssen, B.; Roth, G.; Salfeld, J. Overview: Differentiating Issues in the Development of Macromolecules Compared with Small Molecules. Handb. Pharm. Biotechnol. 2006, 89–123. [Google Scholar] [CrossRef]
- Lo, K.W.-H.; Ashe, K.M.; Kan, H.M.; Laurencin, C.T. The role of small molecules in musculoskeletal regeneration. Regen. Med. 2012, 7, 535–549. [Google Scholar] [CrossRef] [PubMed]
- Bernacchioni, C.; Cencetti, F.; Blescia, S.; Donati, C.; Bruni, P. Sphingosine kinase/sphingosine 1-phosphate axis: a new player for insulin-like growth factor-1-induced myoblast differentiation. Skelet. Muscle 2012, 2, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Danieli-Betto, D.; Peron, S.; Germinario, E.; Zanin, M.; Sorci, G.; Franzoso, S.; Sandonà, D.; Betto, R. Sphingosine 1-phosphate signaling is involved in skeletal muscle regeneration. Am. J. Physiol. Cell Physiol. 2010, 298, 550–558. [Google Scholar] [CrossRef] [PubMed]
- Kim, W.H.; Jung, D.W.; Kim, J.; Im, S.H.; Hwang, S.Y.; Williams, D.R. Small molecules that recapitulate the early steps of urodele amphibian limb regeneration and confer multipotency. ACS Chem. Biol. 2012, 7, 732–743. [Google Scholar] [CrossRef] [PubMed]
- El Haddad, M.; Notarnicola, C.; Evano, B.; El Khatib, N.; Blaquière, M.; Bonnieu, A.; Tajbakhsh, S.; Hugon, G.; Vernus, B.; Mercier, J.; et al. Retinoic acid maintains human skeletal muscle progenitor cells in an immature state. Cell. Mol. Life Sci. 2017, 74, 1923–1936. [Google Scholar] [CrossRef] [PubMed]
- Raposo, G.; Stoorvogel, W. Extracellular vesicles: Exosomes, microvesicles, and friends. J. Cell Biol. 2013, 200, 373–383. [Google Scholar] [CrossRef] [PubMed]
- Robbins, P.D.; Morelli, A.E. Regulation of immune responses by extracellular vesicles. Nat. Rev. Immunol. 2014, 14, 195–208. [Google Scholar] [CrossRef] [PubMed]
- Romancino, D.P.; Paterniti, G.; Campos, Y.; De Luca, A.; Di Felice, V.; D’Azzo, A.; Bongiovanni, A. Identification and characterization of the nano-sized vesicles released by muscle cells. FEBS Lett. 2013, 587, 1379–1384. [Google Scholar] [CrossRef]
- Guescini, M.; Guidolin, D.; Vallorani, L.; Casadei, L.; Gioacchini, A.M.; Tibollo, P.; Battistelli, M.; Falcieri, E.; Battistin, L.; Agnati, L.F.; et al. C2C12 myoblasts release micro-vesicles containing mtDNA and proteins involved in signal transduction. Exp. Cell Res. 2010, 316, 1977–1984. [Google Scholar] [CrossRef]
- Forterre, A.; Jalabert, A.; Chikh, K.; Pesenti, S.; Euthine, V.; Granjon, A.; Errazuriz, E.; Lefai, E.; Vidal, H.; Rome, S. Myotube-derived exosomal miRNAs downregulate Sirtuin1 in myoblasts during muscle cell differentiation. Cell Cycle 2014, 13, 78–89. [Google Scholar] [CrossRef] [PubMed]
- Bittel, D.C.; Jaiswal, J.K. Contribution of extracellular vesicles in rebuilding injured muscles. Front. Physiol. 2019, 10. [Google Scholar] [CrossRef] [PubMed]
- Qazi, T.H.; Mooney, D.J.; Duda, G.N.; Geissler, S. Biomaterials that promote cell-cell interactions enhance the paracrine function of MSCs. Biomaterials 2017, 140, 103–114. [Google Scholar] [CrossRef] [PubMed]
- Choi, J.S.; Yoon, H.I.; Lee, K.S.; Choi, Y.C.; Yang, S.H.; Kim, I.S.; Cho, Y.W. Exosomes from differentiating human skeletal muscle cells trigger myogenesis of stem cells and provide biochemical cues for skeletal muscle regeneration. J. Control. Release 2016, 222, 107–115. [Google Scholar] [CrossRef] [PubMed]
- Balbi, C.; Piccoli, M.; Barile, L.; Papait, A.; Armirotti, A.; Principi, E.; Reverberi, D.; Pascucci, L.; Becherini, P.; Varesio, L.; et al. First characterization of human amniotic fluid stem cell extracellular vesicles as a powerful paracrine tool endowed with regenerative potential. Stem Cells Transl. Med. 2017, 6, 1340–1355. [Google Scholar] [CrossRef]
- Lo Sicco, C.; Reverberi, D.; Balbi, C.; Ulivi, V.; Principi, E.; Pascucci, L.; Becherini, P.; Bosco, M.C.; Varesio, L.; Franzin, C.; et al. Mesenchymal Stem Cell-Derived Extracellular Vesicles as Mediators of Anti-Inflammatory Effects: Endorsement of Macrophage Polarization. Stem Cells Transl. Med. 2017, 6, 1018–1028. [Google Scholar] [CrossRef]
- Madison, R.D.; McGee, C.; Rawson, R.; Robinson, G.A. Extracellular vesicles from a muscle cell line (C2C12) enhance cell survival and neurite outgrowth of a motor neuron cell line (NSC-34). J. Extracell. Vesicles 2014, 3, 1–9. [Google Scholar] [CrossRef]
- Madison, R.D.; Robinson, G.A. Muscle-Derived Extracellular Vesicles Influence Motor Neuron Regeneration Accuracy. Neuroscience 2019, 419, 46–59. [Google Scholar] [CrossRef]
- Valadi, H.; Ekström, K.; Bossios, A.; Sjöstrand, M.; Lee, J.J.; Lötvall, J.O. Exosome-mediated transfer of mRNAs and microRNAs is a novel mechanism of genetic exchange between cells. Nat. Cell Biol. 2007, 9, 654–659. [Google Scholar] [CrossRef]
- Kosaka, N.; Iguchi, H.; Yoshioka, Y.; Takeshita, F.; Matsuki, Y.; Ochiya, T. Secretory mechanisms and intercellular transfer of microRNAs in living cells. J. Biol. Chem. 2010, 285, 17442–17452. [Google Scholar] [CrossRef]
- Nakasa, T.; Ishikawa, M.; Shi, M.; Shibuya, H.; Adachi, N.; Ochi, M. Acceleration of muscle regeneration by local injection of muscle-specific microRNAs in rat skeletal muscle injury model. J. Cell. Mol. Med. 2010, 14, 2495–2505. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Zhang, X.; Ren, X.P.; Chen, J.; Liu, H.; Yang, J.; Medvedovic, M.; Hu, Z.; Fan, G.C. MicroRNA-494 targeting both proapoptotic and antiapoptotic proteins protects against ischemia/reperfusion-induced cardiac injury. Circulation 2010, 122, 1308–1318. [Google Scholar] [CrossRef] [PubMed]
- Lai, R.C.; Yeo, R.W.Y.; Tan, K.H.; Lim, S.K. Exosomes for drug delivery - A novel application for the mesenchymal stem cell. Biotechnol. Adv. 2013, 31, 543–551. [Google Scholar] [CrossRef] [PubMed]
- Tian, Y.; Li, S.; Song, J.; Ji, T.; Zhu, M.; Anderson, G.J.; Wei, J.; Nie, G. A doxorubicin delivery platform using engineered natural membrane vesicle exosomes for targeted tumor therapy. Biomaterials 2014, 35, 2383–2390. [Google Scholar] [CrossRef]
- Alvarez-Erviti, L.; Seow, Y.; Yin, H.; Betts, C.; Lakhal, S.; Wood, M.J.A. Delivery of siRNA to the mouse brain by systemic injection of targeted exosomes. Nat. Biotechnol. 2011, 29, 341–345. [Google Scholar] [CrossRef]
| In Vitro | |||
|---|---|---|---|
| Scaffold | Model | Main Findings | References |
| Muscle Compartment | |||
| PU | PU and dynamically perfused C2C12 | 3D Polyurethane-based soft porous scaffold functionalized with laminin and fibronectin coating allow better differentiation of C2C12. | Iberite et al. (2020) [8] |
| PEGDA, colecistic ECM hydrogel. | Hydrogel and C2C12 | PEGDA conjugated with porcine cholecystic derived ECM, formed biocompatible hydrogel suitable for growth and maturation of C2C12. | Raj et al. (2020) [9] |
| PLGA bioprinting | C2C12 in 3D printed scaffold. | PLGA 3D printed scaffold with C2C12 promote myogenesis and upregulate the expressions of myogenic genes (MyHC and MyOG). | Chen et al. (2019) [10] |
| AA-MA, PLC bilayer scaffold; electrospinning | Self fold bilayer scaffold and C2C12 | A bilayer scaffold of AA-MA and aligned PLC seeded with C2C12 form aligned myotubes that contract under electrical stimuli | Apsite et al. (2020) [11] |
| PCL bioprinting | Stretched 3D printed scaffold and C2C12 | 3D PCL scaffold used to culture C2C12 lead to better myotube formation when the scaffold is stretched. | Yang et al. (2019) [12] |
| Gelatin | 3D gelatin scaffold and H9c2 cells | Rat H9c2 myotube formation is improved by 3D spherical gelatin bubble-based scaffold compared to 2D gelatin plating. | Mei et al. (2019) [13] |
| Vascular Compartment | |||
| Muscle bundles | Channelrhodopsin-2 C2C12 muscle fiber bundles and collagen HUVEC vascular structures. | Muscle fiber bundle modulate the endothelial cell sprouting. In turn myogenesis was also upregulated by interaction with the vascular cells, improving muscle contraction via angiopoetin-1/neuregulin-1 signaling. Optical stimulation of muscle tissue induces significantly more angiogenic sprouting. | Osaki et al. (2017) [14] |
| Tetronic-tyramine hydrogel RGD | C2C12-VEGF cell sheets | The conditioned medium of VEGF-transfected C2C12 increases HUVEC sprouting in capillary formation assay. | Lee et al. (2014) [15] |
| Neural Compartment | |||
| Collagen hydrogel | Collagen with differentiated C2C12. | Scaffold with myotubes shows hypertrophy and increased contractile strength after mechanical loading. | Aguilar-Agon et al. (2019) [16] |
| Collagen and matrigel scaffold. | C2C12 and PC12 in petri dish and 3D Matrigel | Co-culture of muscular and neural cells in a 3D model improve sarcomere formation and contractile activity of differentiated C2C12 in comparison to 2D model. | Arifuzzaman et al. (2019) [17] |
| Fibrin/Geltrex Hydrogel | Hydrogel with hMPCs and hESC derived motoneuron. | hMPC and human motoneuron co-cultured only when cultured in hydrogel and not in petri dish, show after 3 weeks an increase in myofiber diameter and neuromuscular junction functionality. Calcium imaging proved functional connectivity between motor neuron endplates and muscle fibers. | Bakooshli et al. (2019) [18] |
| PCL mold | Aligned PCL with C2C12 and E16 Sprague Dawley motor neuron | Co-culture of C2C12 and E16 ameliorate myogenic index of C2C12 myotubes. | Das et al. (2020) [19] |
| PCL bioprinting | hMPCs co-cultured with hNSCs in PLC contruct | 3D construct of hMPCs and hNSCs shows good cell survival, muscle differentiation and NMJs formation. | Kim et al. (2020) [20] |
| In Vivo | |||
|---|---|---|---|
| Scaffold | Model | Main Findings | References |
| Neuro-Muscular Compartment | |||
| SMUs and ENC | VML in sheep | 3 months post implant, sheep treated with SMU recovered muscle mass and tetanic force production. | Novakova et al. (2020) [21] |
| Collagen-GAG scaffold | VML in mouse | Increased hypertrophy in treated mouse 2 and 6 weeks post implant. increased running speed on a treadmill after 6 weeks compared to sham mice. | Panayi et al. (2019) [22] |
| Dex-AT and CECS hydrogel. | VML in rat, injectable hydrogel with C2C12 and HUVEC-GFP. | 1 and 4 weeks post treatment, cells were proportionally released over time. Higher myofiber density was present in animals treated with hydrogel and cells when compared with animals treated with hydrogel alone. | Guo et al. (2019) [23] |
| Porcine muscle ECM sponge and bioink. Descending aorta ECM bioink for bioprinting. | VML in rat and ECM with hSKMs and HUVEC. | 3 scaffolds were compared: (1) muscle ECM sponge with hSKMs (2) ECM hydrogel used as bioink with hSKMs (3) gelatin granules mold for muscle and aorta ECM seeded with hSKMs and HUVEC. 4-weeks after implant, scaffold number 3 produced better cell viability, myotube formation, angiogenesis and muscle strength recovery in respect to the other scaffolds. | Choi et al. (2019) [24] |
| Muscle tissue plug | Rat VML. | 3 different alignment (0°,45°,90°) of muscle defect plug were implanted. The best tissue regeneration was achieved with aligned muscle plug (0°): increased expression of myogenic genes 2 weeks after implant; a peak of tetanic torque force and reduced collagen deposition after 12 weeks. | Kim et al. (2020) [25] |
| Subcutaneous implant of PCL fibers | Rat VML, sciatic nerve and abdominal artery defects. | In vivo implant of depleted PCL ECM allows: (1) muscle tissue regeneration with reduction of collagen deposition; (2) good axon diameter, thickness of myelin sheets; (3) vascular regeneration with good morphology of vascular microchannels | Zhu et al. (2019) [26] |
| Fibrinogen, gelatin, hyaluronic acid and glycerol bioink in PCL pillar | VML in rat and bioink with hMPCs | 8-weeks post implant, muscle strength, vascularization and number of NMJs were higher in comparison with rats treated with bioink without cells (printed and non-printed). | Kim et al. (2018) [27] |
| PCL bioprinting | VML in rat and scaffold with hMPCs co-cultured with hNSCs. | Analysis were performed at 4 and 8 weeks. Pre-innervated scaffold ameliorated functional muscle regeneration, NMJs formation and reduce fibrotic tissue deposition compared to rat treated with scaffold seeded with hMPCs alone. | Kim et al. (2020) [20] |
| Vascular Compartment | |||
| BAM in fibrin hydrogel | Subcutaneous injection on the fascia of the latissimus dorsi muscle of hydrogel and BAM with and without HUVEC-GFP | (1) BAM alone, (2) BAM co-cultured with HUVEC-GFP and (3) BAM with HUVEC in fibrin hydrogel (two-stage approach). 14 days post treatment, myotube length and area, vessel length and branching were evaluated. The number 3 construct gave better results. | Gholobova et al. (2020) [28] |
| PLLA and PLGA scaffold | Abdominal wall defect in mouse and PLLA/PLGA with satellite and lung microvascular cells. | Scaffolds with satellite cells alone and scaffolds with both cell types were implanted in immunocompetent and immunocompromised mice. 18 days post implantation, the pre-vascularized scaffold inserted in immunocompromised mice showed better neovascularization and myogenesis in respect to the immunocompetent. | Perry et al. (2019) [29] |
| Fibrinogen hydrogel | VML in rat and hydrogel with BMDMs. | After 15 days, implantation of gel with muscle cells+BMDMs shows increased vascularization, muscle area and muscle strength compared with implantation of gel containing only muscle cells. | Juhas et al. (2018) [30] |
| Tetronic-tyramine hydrogel RGD | C2C12-VEGF cell sheets | Ischemic model with myoblasts sheets: promoted the formation of capillaries and arterioles in ischemic muscles, attenuated the muscle necrosis and fibrosis progressed by ischemia, and prevented ischemic limb loss. | Lee et al. (2014) [15] |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Reid, G.; Magarotto, F.; Marsano, A.; Pozzobon, M. Next Stage Approach to Tissue Engineering Skeletal Muscle. Bioengineering 2020, 7, 118. https://doi.org/10.3390/bioengineering7040118
Reid G, Magarotto F, Marsano A, Pozzobon M. Next Stage Approach to Tissue Engineering Skeletal Muscle. Bioengineering. 2020; 7(4):118. https://doi.org/10.3390/bioengineering7040118
Chicago/Turabian StyleReid, Gregory, Fabio Magarotto, Anna Marsano, and Michela Pozzobon. 2020. "Next Stage Approach to Tissue Engineering Skeletal Muscle" Bioengineering 7, no. 4: 118. https://doi.org/10.3390/bioengineering7040118
APA StyleReid, G., Magarotto, F., Marsano, A., & Pozzobon, M. (2020). Next Stage Approach to Tissue Engineering Skeletal Muscle. Bioengineering, 7(4), 118. https://doi.org/10.3390/bioengineering7040118