Cell Scaffolds for Bone Tissue Engineering
Abstract
1. Introduction
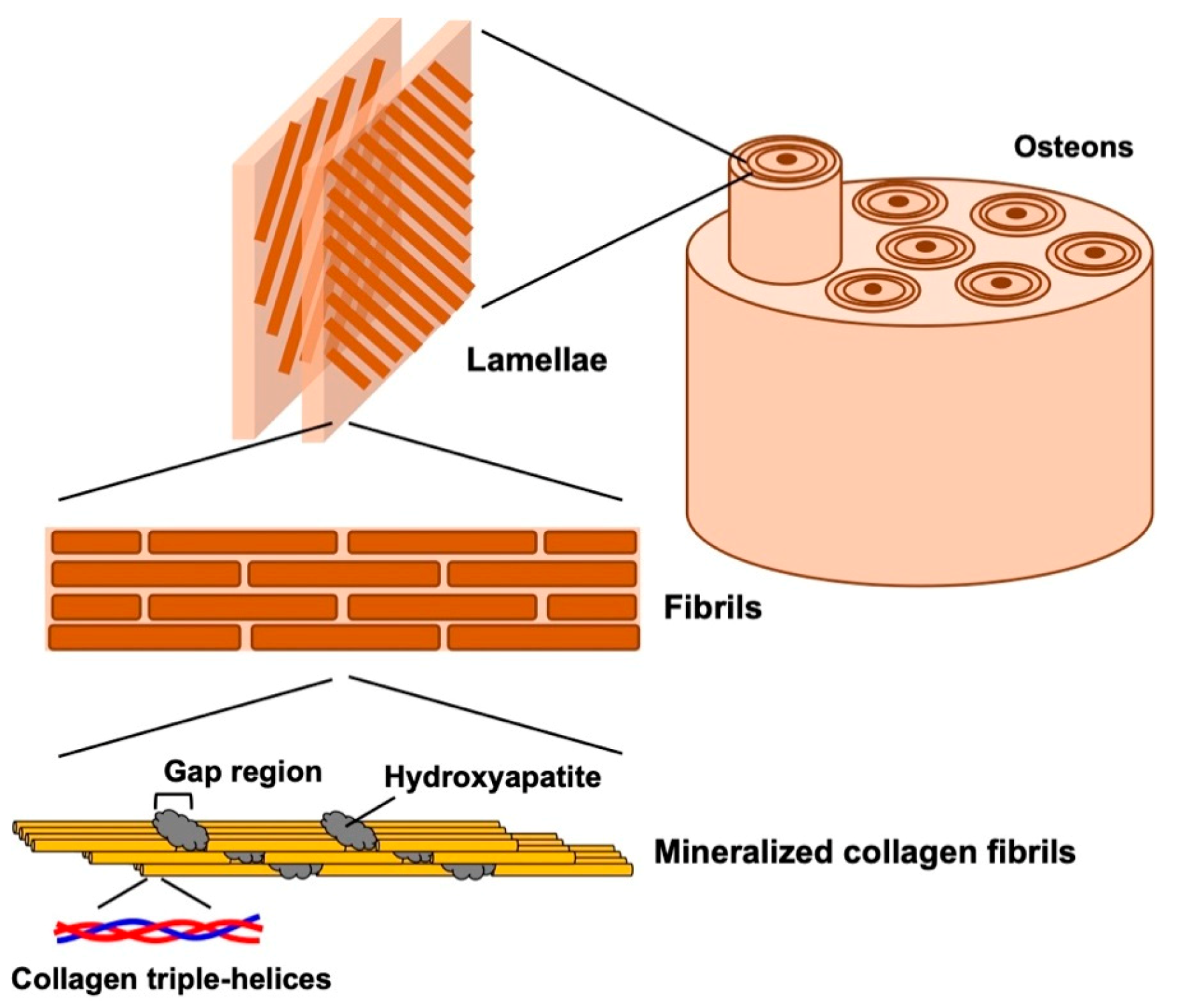
2. Structure of Natural Bone
3. Treatment of Bone Defects
4. Bone Tissue Regeneration Using Mesenchymal Stem Cells (MSCs)
5. Cell Scaffolds for Bone Tissue Regeneration Using MSCs
5.1. Ceramics-Based Scaffolds
5.2. Synthetic Polymers-Based Scaffolds
5.3. Collagen and Its Derivatives-Based Scaffolds
5.4. Inorganic/Organic Composites Scaffolds
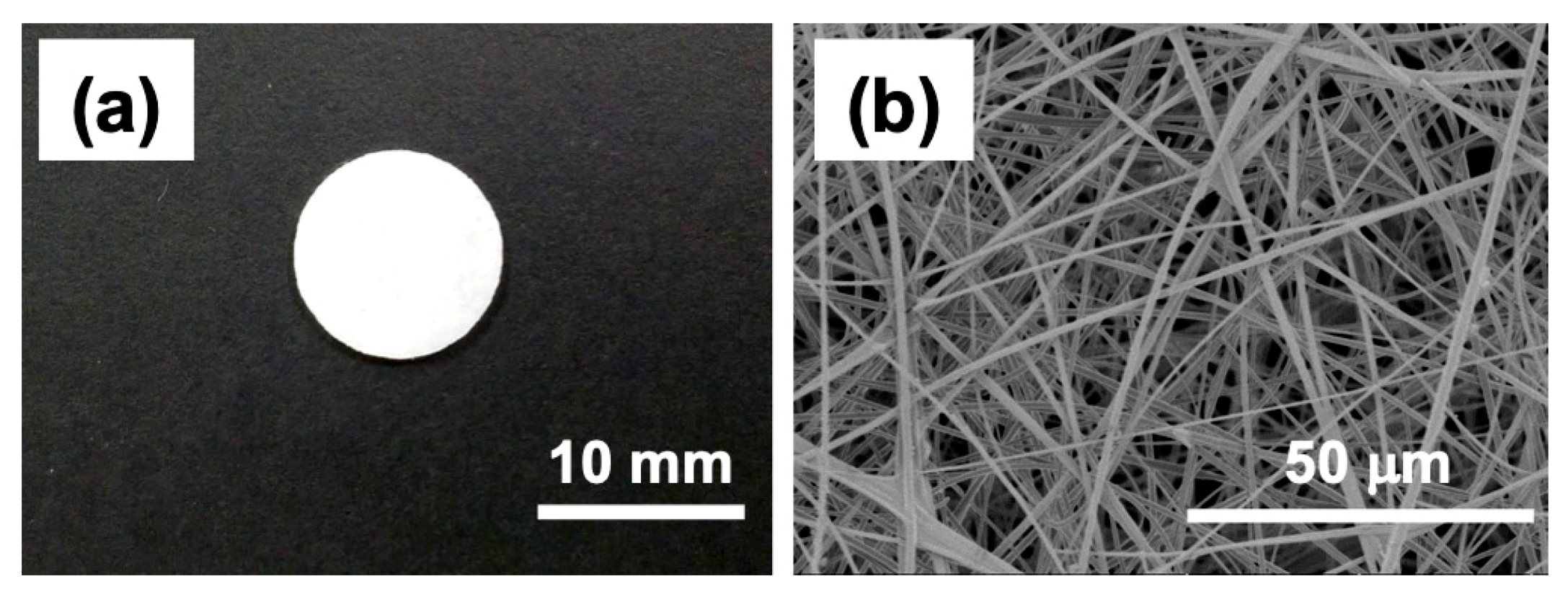
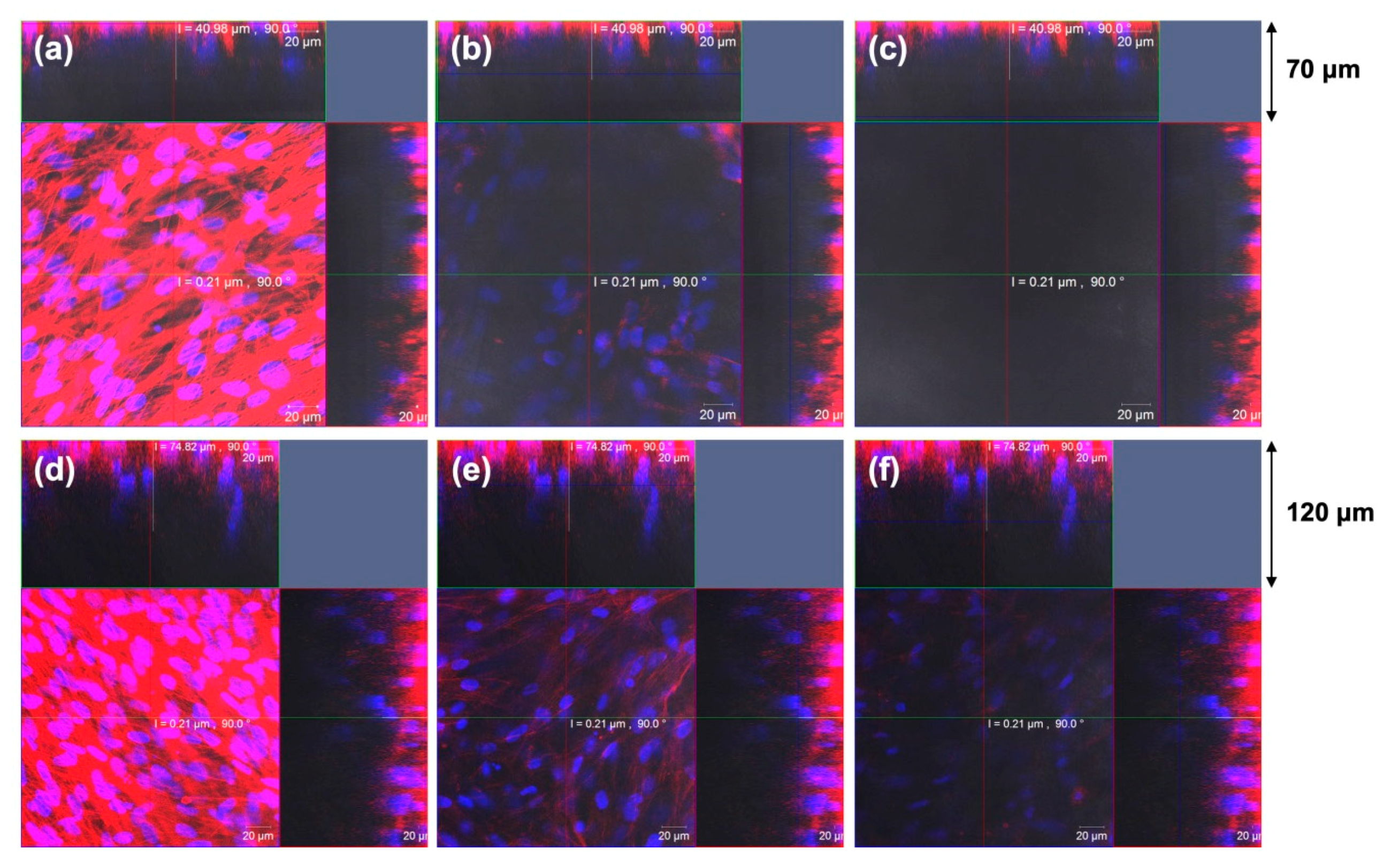
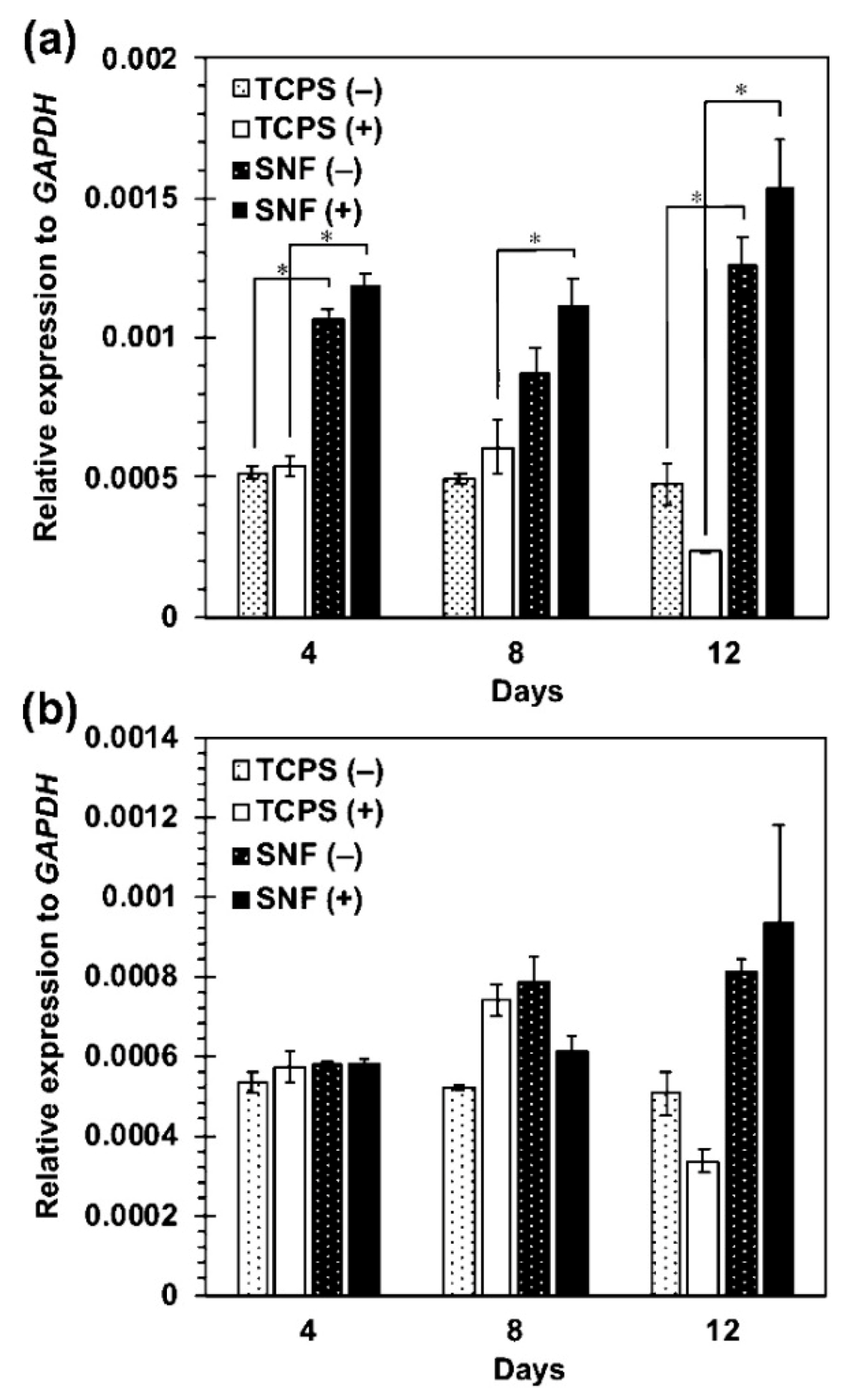
5.5. Electrospun Silica Nonwoven Fabrics
6. Future Prospects
Author Contributions
Funding
Conflicts of Interest
References
- Kretlow, J.D.; Mikos, A.G. Review: Mineralization of synthetic polymer scaffolds for bone tissue engineering. Tissue Eng. 2007, 13, 927–938. [Google Scholar] [CrossRef]
- Johnell, O.; Kanis, J.A. An estimate of the worldwide prevalence and disability associated with osteoporotic fractures. Osteoporos. Int. 2006, 17, 1726–1733. [Google Scholar] [CrossRef]
- Weiner, S.; Wagner, H.D. The material bone: Structure mechanical function relations. Annu. Rev. Mater. Sci. 1998, 28, 271–298. [Google Scholar] [CrossRef]
- Fratzl, P.; Gupta, H.S.; Paschalis, E.P.; Roschger, P. Structure and mechanical quality of the collagen-mineral nano-composite in bone. J. Mater. Chem. 2004, 14, 2115–2123. [Google Scholar] [CrossRef]
- Landis, W.J.; Hodgens, K.J.; Arena, J.; Song, M.J.; McEwen, B.F. Structural relations between collagen and mineral in none as determined by high voltage electron microscopic tomography. Microsc. Res. Tech. 1996, 33, 192–202. [Google Scholar] [CrossRef]
- Olszta, M.J.; Cheng, X.G.; Jee, S.S.; Kumar, R.; Kim, Y.Y.; Kaufman, M.J.; Douglas, E.P.; Gower, L.B. Bone structure and formation: A new perspective. Mater. Sci. Eng. 2007, 58, 77–116. [Google Scholar] [CrossRef]
- Cantaert, B.; Beniash, E.; Meldrum, F.C. Nanoscale confinement controls the crystallization of calcium phosphate: Relevance to bone formation. Chem. Eur. J. 2013, 19, 14918–14924. [Google Scholar] [CrossRef]
- Jakoi, A.M.; Iorio, J.A.; Cahill, P.J. Autologous bone graft harvesting: A review of grafts and surgical techniques. Musculoskelet. Surg. 2015, 99, 171–178. [Google Scholar] [CrossRef]
- Shegarfi, H.; Reikeras, O. Bone transplantation and immune response. J. Orthop. Surg. 2009, 17, 206–211. [Google Scholar] [CrossRef]
- Meyer, S.; Floerkemeier, T.; Windhagen, H. Histological osseointegration of Tutobone®: Wrst results in human. Arch. Orthop. Trauma Surg. 2008, 128, 539–544. [Google Scholar] [CrossRef] [PubMed]
- Fernandez de Grado, G.; Keller, L.; Idoux-Gillet, Y.; Wagner, Q.; Musset, A.M.; Benkirane-Jessel, N.; Bornert, F.; Offner, D. Bone substitutes: A review of their characteristics, clinical use, and perspectives for large bone defects management. J. Tissue Eng. 2018, 9, 2041731418776819. [Google Scholar] [CrossRef] [PubMed]
- Calori, G.M.; Colombo, M.; Mazza, E.L.; Mazzola, S.; Malagoli, E.; Mineo, G.V. Incidence of donor site morbidity following harvesting from iliac crest or RIA graft. Injury 2014, 45, S116–S120. [Google Scholar] [CrossRef]
- Lei, P.; Sun, R.; Wang, L.; Zhou, J.; Wan, L.; Zhou, T.; Hu, Y. A new method for xenogeneic bone graft deproteinization: Comparative study of radius defects in a rabbit model. PLoS ONE 2015, 10, e0146005. [Google Scholar] [CrossRef]
- Smith, L. Ceramic-plastic material as a bone substitute. Arch. Surg. 1963, 87, 653–661. [Google Scholar] [CrossRef]
- Kattimani, V.S.; Kondaka, S.; Lingamaneni, K.P. Hydroxyapatite–past, present, and future in bone regeneration. Bone Tissue Regen. Insights 2016, 7, 9–19. [Google Scholar] [CrossRef]
- Horowitz, R.A.; Mazor, Z.; Foitzik, C.; Prasad, H.; Rohrer, M.; Palti, A. β-Tricalcium phosphate as bone substitute material: Properties and clinical applications. J. Osseointegration 2010, 2, 61–68. [Google Scholar] [CrossRef]
- Ishikawa, K. Carbonate apatite bone replacement: Learn from the bone. J. Ceram. Soc. Jpn. 2019, 127, 595–601. [Google Scholar] [CrossRef]
- Sepulveda, P.; Jones, J.R.; Hench, L.L. In vitro dissolution of melt-derived 45S5 and sol-gel derived 58S bioactive glasses. J. Biomed. Mater. Res. 2002, 61, 301–311. [Google Scholar] [CrossRef]
- Jones, J.R.; Ehrenfried, L.M.; Hench, L.L. Optimising bioactive glass scaffolds for bone tissue engineering. Biomaterials 2006, 27, 964–973. [Google Scholar] [CrossRef]
- van Gestel, N.A.; Geurts, J.; Hulsen, D.J.; van Rietbergen, B.; Hofmann, S.; Arts, J.J. Clinical applications of S53P4 bioactive glass in bone healing and osteomyelitic treatment: A literature review. Biomed. Res. Int. 2015, 2015, 684826. [Google Scholar] [CrossRef]
- Ardeshirylajimi, A.; Farhadian, S.; Jamshidi Adegani, F.; Mirzaei, S.; Soufi Zomorrod, M.; Langroudi, L.; Doostmohammadi, A.; Seyedjafari, E.; Soleimani, M. Enhanced osteoconductivity of polyethersulphone nanofibres loaded with bioactive glass nanoparticles in in vitro and in vivo models. Cell Prolif. 2015, 48, 455–464. [Google Scholar] [CrossRef] [PubMed]
- Knight, M.N.; Hankenson, K.D. Mesenchymal stem cells in bone regeneration. Adv. Wound Care 2013, 2, 306–316. [Google Scholar] [CrossRef] [PubMed]
- Charbord, P. Bone marrow mesenchymal stem cells: Historical overview and concepts. Hum. Gene Ther. 2010, 21, 1045–1056. [Google Scholar] [CrossRef] [PubMed]
- Minteer, D.; Marra, K.G.; Rubin, J.P. Adipose-derived mesenchymal stem cells: Biology and potential applications. Adv. Biochem. Eng. Biotechnol. 2013, 129, 59–71. [Google Scholar] [CrossRef]
- Nagamura-Inoue, T.; He, H. Umbilical cord-derived mesenchymal stem cells: Their advantages and potential clinical utility. World J. Stem Cells 2014, 6, 195–202. [Google Scholar] [CrossRef] [PubMed]
- Ledesma-Martínez, E.; Mendoza-Núñez, V.M.; Santiago-Osorio, E. Mesenchymal stem cells derived from dental pulp: A review. Stem Cells Int. 2016, 2016, 4709572. [Google Scholar] [CrossRef]
- Yousefi, A.-M.; James, P.F.; Akbarzadeh, R.; Subramanian, A.; Flavin, C.; Oudadesse, H. Prospect of stem cells in bone tissue engineering: A review. Stem Cells Int. 2016, 2016, 6180487. [Google Scholar] [CrossRef]
- Krampera, M.; Franchini, M.; Pizzolo, G.; Aprili, G. Mesenchymal stem cells: From biology to clinical use. Blood Transfus. 2007, 5, 120–129. [Google Scholar] [CrossRef]
- Katagiri, W.; Kawai, T.; Osugi, M.; Sugimura-Wakayama, Y.; Sakaguchi, K.; Kojima, T.; Kobayashi, T. Angiogenesis in newly regenerated bone by secretomes of human mesenchymal stem cells. Maxillofac. Plast. Reconstr. Surg. 2017, 39, 8. [Google Scholar] [CrossRef]
- Sugimoto, T.; Yamazaki, Y.; Kumazawa, K.; Sone, Y.; Takeda, A.; Uchinuma, E. The significance of performing osteogenic differentiation in human bone tissue-derived mesenchymal stromal cells. J. Oral. Tissue Engin. 2013, 11, 103–112. [Google Scholar] [CrossRef]
- Calabrese, G.; Giuffrida, R.; Forte, S.; Fabbi, C.; Figallo, E.; Salvatorelli, L.; Memeo, L.; Parenti, R.; Gulisano, M.; Gulino, R. Human adipose-derived mesenchymal stem cells seeded into a collagen-hydroxyapatite scaffold promote bone augmentation after implantation in the mouse. Sci. Rep. 2017, 7, 7110. [Google Scholar] [CrossRef] [PubMed]
- Sándor, G.K.; Numminen, J.; Wolff, J.; Thesleff, T.; Miettinen, A.; Tuovinen, V.J.; Mannerström, B.; Patrikoski, M.; Seppänen, R.; Miettinen, S.; et al. Adipose stem cells used to reconstruct 13 cases with cranio-maxillofacial hard-tissue defects. Stem Cells Transl. Med. 2014, 3, 530–540. [Google Scholar] [CrossRef] [PubMed]
- Anselme, K.; Noël, B.; Flautre, B.; Blary, M.C.; Delecourt, C.; Descamps, M.; Hardouin, P. Association of porous hydroxyapatite and bone marrow cells for bone regeneration. Bone 1999, 25, 51S–54S. [Google Scholar] [CrossRef]
- Lobo, S.E.; Arinzeh, T.L. Biphasic calcium phosphate ceramics for bone regeneration and tissue engineering applications. Materials 2010, 3, 815–826. [Google Scholar] [CrossRef]
- Ohgushi, H.; Dohi, Y.; Yoshikawa, T.; Tamai, S.; Tabata, S.; Okunaga, K.; Shibuya, T. Osteogenic differentiation of cultured marrow stromal stem cells on the surface of bioactive glass ceramics. J. Biomed. Mater. Res. 1996, 32, 341–348. [Google Scholar] [CrossRef]
- Iijima, K.; Ishikawa, S.; Sasaki, K.; Hashizume, M.; Kawabe, M.; Otsuka, H. Osteogenic differentiation of bone marrow-derived mesenchymal stem cells in electrospun silica nonwoven fabrics. ACS Omega 2018, 3, 10180–10187. [Google Scholar] [CrossRef]
- Casagrande, S.; Tiribuzi, R.; Cassetti, E.; Selmin, F.; Gervasi, G.L.; Barberini, L.; Freddolini, M.; Ricci, M.; Schoubben, A.; Cerulli, G.G.; et al. Biodegradable composite porous poly(dl-lactide-co-glycolide) scaffold supports mesenchymal stem cell differentiation and calcium phosphate deposition. Artif. Cell. Nanomed. B. 2018, 46, 219–229. [Google Scholar] [CrossRef]
- Xue, R.; Qian, Y.; Li, L.; Yao, G.; Yang, L.; Sun, Y. Polycaprolactone nanofiber scaffold enhances the osteogenic differentiation potency of various human tissue-derived mesenchymal stem cells. Stem Cell Res. 2017, 8, 148. [Google Scholar] [CrossRef]
- Kutikov, A.B.; Song, J. Biodegradable PEG-based amphiphilic block copolymers for tissue engineering applications. ACS Biomater. Sci. Eng. 2015, 1, 463–480. [Google Scholar] [CrossRef]
- Donzelli, E.; Salvadè, A.; Mimo, P.; Viganò, M.; Morrone, M.; Papagna, R.; Carini, F.; Zaopo, A.; Miloso, M.; Baldoni, M.; et al. Mesenchymal stem cells cultured on a collagen scaffold: In vitro osteogenic differentiation. Arch. Oral Biol. 2007, 52, 64–73. [Google Scholar] [CrossRef]
- Schneider, R.K.; Puellen, A.; Kramann, R.; Raupach, K.; Bornemann, J.; Knuechel, R.; Pérez-Bouza, A.; Neuss, S. The osteogenic differentiation of adult bone marrow and perinatal umbilical mesenchymal stem cells and matrix remodelling in three-dimensional collagen scaffolds. Biomaterials 2010, 31, 467–480. [Google Scholar] [CrossRef] [PubMed]
- Dong, Z.; Yuan, Q.; Huang, K.; Xu, W.; Liu, G.; Gu, Z. Gelatin methacryloyl (GelMA)-based biomaterials for bone regeneration. RSC Adv. 2019, 9, 17737–17744. [Google Scholar] [CrossRef]
- Melke, J.; Midha, S.; Ghosh, S.; Ito, K.; Hofmann, S. Silk fibroin as biomaterial for bone tissue engineering. Acta Biomater. 2016, 31, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Klemm, D.; Heublein, B.; Fink, H.; Bohn, A. Cellulose: Fascinating biopolymer and sustainable raw material. Angew. Chem. Int. Ed. 2005, 44, 3358–3393. [Google Scholar] [CrossRef]
- Saravanan, S.; Leena, R.S.; Selvamurugan, N. Chitosan based biocomposite scaffolds for bone tissue engineering. Int. J. Biol. Macromol. 2016, 93B, 1354–1365. [Google Scholar] [CrossRef] [PubMed]
- Andrews, S.; Cheng, A.; Stevens, H.; Logun, M.T.; Webb, R.; Jordan, E.; Xia, B.; Karumbaiah, L.; Guldberg, R.E.; Stice, S. Chondroitin sulfate glycosaminoglycan scaffolds for cell and recombinant protein-based bone regeneration. Stem Cells Transl. Med. 2019, 8, 575–585. [Google Scholar] [CrossRef] [PubMed]
- Cowan, C.M.; Shi, Y.Y.; Aalami, O.O.; Chou, Y.F.; Mari, C.; Thomas, R.; Quarto, N.; Contag, C.H.; Wu, B.; Longaker, M.T. Adipose-derived adult stromal cells heal critical-size mouse calvarial defects. Nat. Biotechnol. 2004, 22, 560–567. [Google Scholar] [CrossRef]
- Zhang, X.; Meng, S.; Huang, Y.; Xu, M.; He, Y.; Lin, H.; Han, J.; Chai, Y.; Wei, Y.; Deng, X. Electrospun gelatin/β-TCP composite nanofibers enhance osteogenic differentiation of BMSCs and in vivo bone formation by activating Ca2+-sensing receptor signaling. Stem Cells Int. 2015, 2015, 507154. [Google Scholar] [CrossRef]
- Liu, L.; Li, C.; Jiao, Y.; Jiang, G.; Mao, J.; Wang, F.; Wang, L. Homogeneous organic/inorganic hybrid scaffolds with high osteoinductive activity for bone tissue engineering. Polym. Test. 2020, 91, 106798. [Google Scholar] [CrossRef]
- Machado, C.B.; Ventura, J.M.G.; Lemos, A.F.; Ferreira, J.M.F.; Leite, M.F.; Goes, A.M. 3D chitosan–gelatin–chondroitin porous scaffold improves osteogenic differentiation of mesenchymal stem cells. Biomed. Mater. 2007, 2, 124–131. [Google Scholar] [CrossRef]
- Iijima, K.; Suzuki, R.; Iizuka, A.; Ueno-Yokohata, H.; Kiyokawa, N.; Hashizume, M. Surface Functionalization of Tissue Culture Polystyrene Plates with Hydroxyapatite Under Body Fluid Conditions and Its Effect on Differentiation Behaviors of Mesenchymal Stem Cells. Colloids Surf. B 2016, 147, 351–359. [Google Scholar] [CrossRef] [PubMed]
- Li, W.-J.; Tuli, R.; Huang, X.; Laquerriere, P.; Tuan, R.S. Multilineage Differentiation of Human Mesenchymal Stem Cells in a Three-dimensional Nanofibrous Scaffold. Biomaterials 2005, 26, 5158–5166. [Google Scholar] [CrossRef] [PubMed]
- Cao, Y.; Li, D.; Shang, C.; Yang, S.T.; Wang, J.; Wang, X. Three-Dimensional Culture of Human Mesenchymal Stem Cells in a Polyethylene Terephthalate Matrix. Biomed. Mater. 2010, 5, 065013. [Google Scholar] [CrossRef] [PubMed]
- Ardeshirylajimi, A.; Mossahebi-Mohammadi, M.; Vakilian, S.; Langroudi, L.; Seyedjafari, E.; Atashi, A.; Soleimani, M. Comparison of Osteogenic Differentiation Potential of Human Adult Stem Cells Loaded on Bioceramic-Coated Electrospun Poly (L-lactide) Nanofibres. Cell Prolif. 2015, 48, 47–58. [Google Scholar] [CrossRef] [PubMed]
- Pournaqi, F.; Ghiaee, A.; Vakilian, S.; Ardeshirylajimi, A. Improved Proliferation and Osteogenic Differentiation of Mesenchymal Stem Cells on Polyaniline Composited by Polyethersulfone Nanofibers. Biologicals 2017, 45, 78–84. [Google Scholar] [CrossRef]
- Yardimci, A.I.; Baskan, O.; Yilmaz, S.; Mese, G.; Ozcivici, E.; Selamet, Y. Osteogenic differentiation of mesenchymal stem cells on random and aligned PAN/PPy nanofibrous scaffolds. J Biomater. Appl. 2019, 34, 640–650. [Google Scholar] [CrossRef]
- Yamaguchi, T.; Sakai, S.; Kawakami, K. Application of silicate electrospun nanofibers for cell culture. J. Sol-Gel Sci. Technol. 2008, 48, 350–355. [Google Scholar] [CrossRef]
- Yamaguchi, T.; Sakai, S.; Watanabe, R.; Tarao, T.; Kawakami, K. Heat treatment of electrospun silicate fiber substrates enhances cellular adhesion and proliferation. J. Biosci. Bioengin. 2010, 109, 304–306. [Google Scholar] [CrossRef]
- Otsuka, H.; Sasaki, K.; Okimura, S.; Nagamura, M.; Watanabe, R.; Kawabe, M. Contribution of fibroblasts cultured on 3D silica nonwoven fabrics to cocultured hepatocytes function. Chem. Lett. 2014, 43, 343–345. [Google Scholar] [CrossRef]
- Ishikawa, S.; Iijima, K.; Sasaki, K.; Kawabe, M.; Osawa, S.; Otsuka, H. Silica-based nonwoven fiber fabricated by electrospinning to promote fibroblast functions. Bull. Chem. Soc. Jpn. 2020, 93, 477–481. [Google Scholar] [CrossRef]
- Ishikawa, S.; Iijima, K.; Sasaki, K.; Hashizume, M.; Kawabe, M.; Otsuka, H. Cartilage differentiation of bone marrow-derived mesenchymal stem cells in three-dimensional silica nonwoven fabrics. Appl. Sci. 2018, 8, 1398. [Google Scholar] [CrossRef]


| Cell Source | Advantage | Disadvantage |
|---|---|---|
| Bone marrow-derived mesenchymal stem cells (BM-MSCs) | (i) High osteogenic potential | (i) Low abundance |
| (ii) Studied extensively | (ii) Highly invasive | |
| Adipose-derived stem cells (ASCs) | (i) High abundant | More studies are needed to test their use in bone repair |
| (ii) Easy to harvest surgically | ||
| Umbilical cord mesenchymal stem cells (UC-MSCs) | Lowly invasive | (i) More studies are needed to test their use in bone repair |
| (ii) Limited time to harvest | ||
| Dental pulp stem cells (DPSCs) | Easy to harvest | More studies are needed to test their use in bone repair |
| Type | Materials | References |
|---|---|---|
| Ceramics | β-Tricalcium phosphate (β-TCP) | [32] |
| Hydroxyapatite (HAp) | [33] | |
| Biphasic calcium phosphate (BCP) | [34] | |
| Bioactive glass | [35] | |
| Silica nonwoven fabrics (SNF) | [36] | |
| Synthetic Polymers | Poly(lactide-co-glycolide) (PLGA) | [37] |
| Poly(caprolactone) (PCL) | [38] | |
| PEG-based amphiphilic block copolymers | [39] | |
| Biopolymers | Type I collagen | [40,41] |
| Gelatin methacryloyl (GelMA) | [42] | |
| Silk fibroin | [43] | |
| Cellulose | [44] | |
| Chitosan | [45] | |
| Chondroitin sulfate | [46] | |
| Composites | HAp/PLGA | [47] |
| HAp/Type I collagen | [31] | |
| β-TCP/Gelatin | [48] | |
| β-TCP/PCL | [49] | |
| Chitosan–Gelatin–Chondroitin | [50] |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Iijima, K.; Otsuka, H. Cell Scaffolds for Bone Tissue Engineering. Bioengineering 2020, 7, 119. https://doi.org/10.3390/bioengineering7040119
Iijima K, Otsuka H. Cell Scaffolds for Bone Tissue Engineering. Bioengineering. 2020; 7(4):119. https://doi.org/10.3390/bioengineering7040119
Chicago/Turabian StyleIijima, Kazutoshi, and Hidenori Otsuka. 2020. "Cell Scaffolds for Bone Tissue Engineering" Bioengineering 7, no. 4: 119. https://doi.org/10.3390/bioengineering7040119
APA StyleIijima, K., & Otsuka, H. (2020). Cell Scaffolds for Bone Tissue Engineering. Bioengineering, 7(4), 119. https://doi.org/10.3390/bioengineering7040119






