Sonodelivery in Skeletal Muscle: Current Approaches and Future Potential
Abstract
1. Introduction
2. Methods for Cellular and Intramuscular Gene Delivery
2.1. Direct Injection of Naked DNA
2.2. Polymers and Nanoparticles
2.3. Viral Vectors
2.4. Electroporation
3. Ultrasound-Mediated Gene Delivery in Skeletal Muscle
3.1. Benefits of Ultrasound
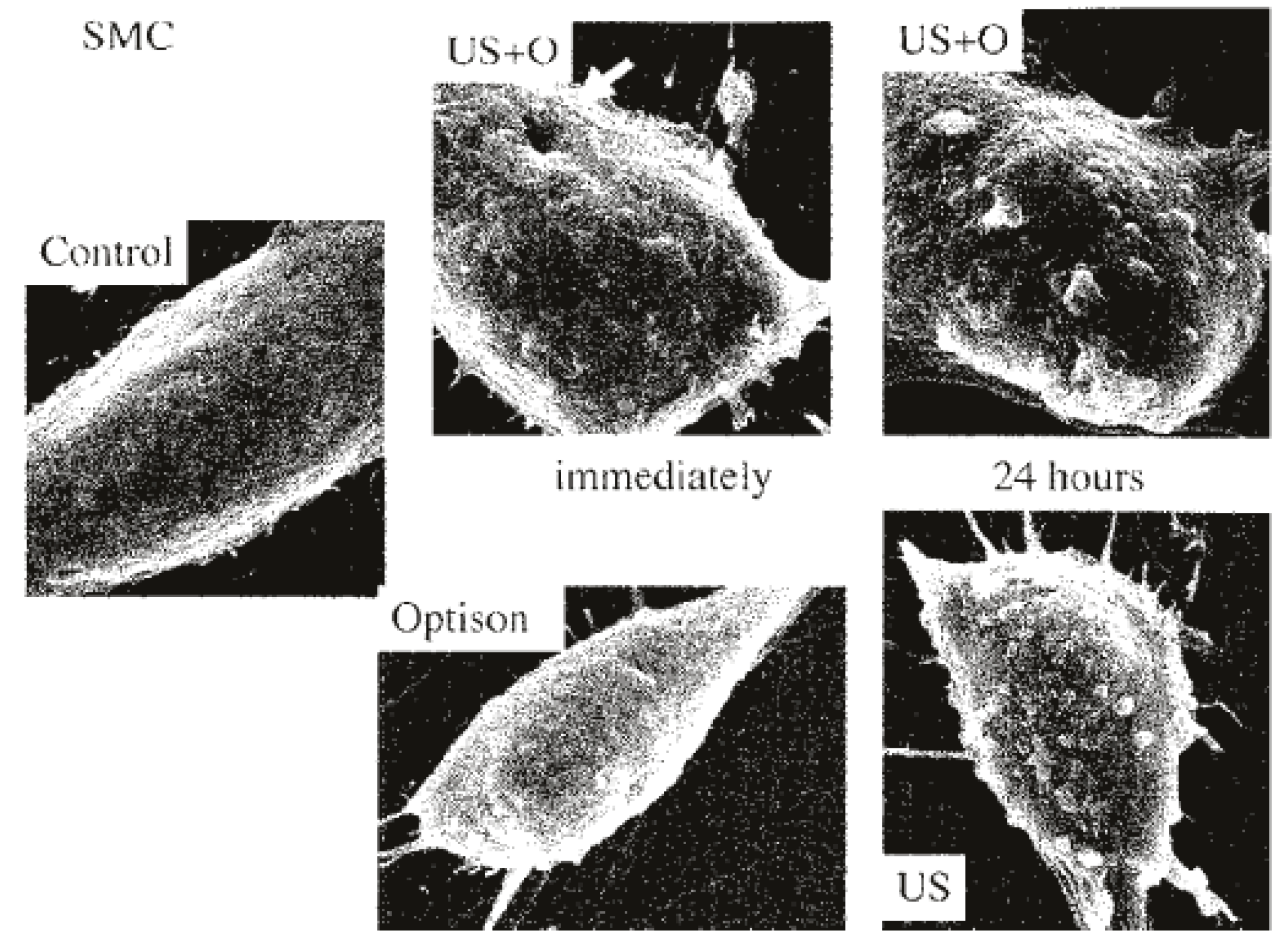
3.2. Sonoporation In Vitro
3.3. Skeletal Muscle as a Therapeutic “Factory”
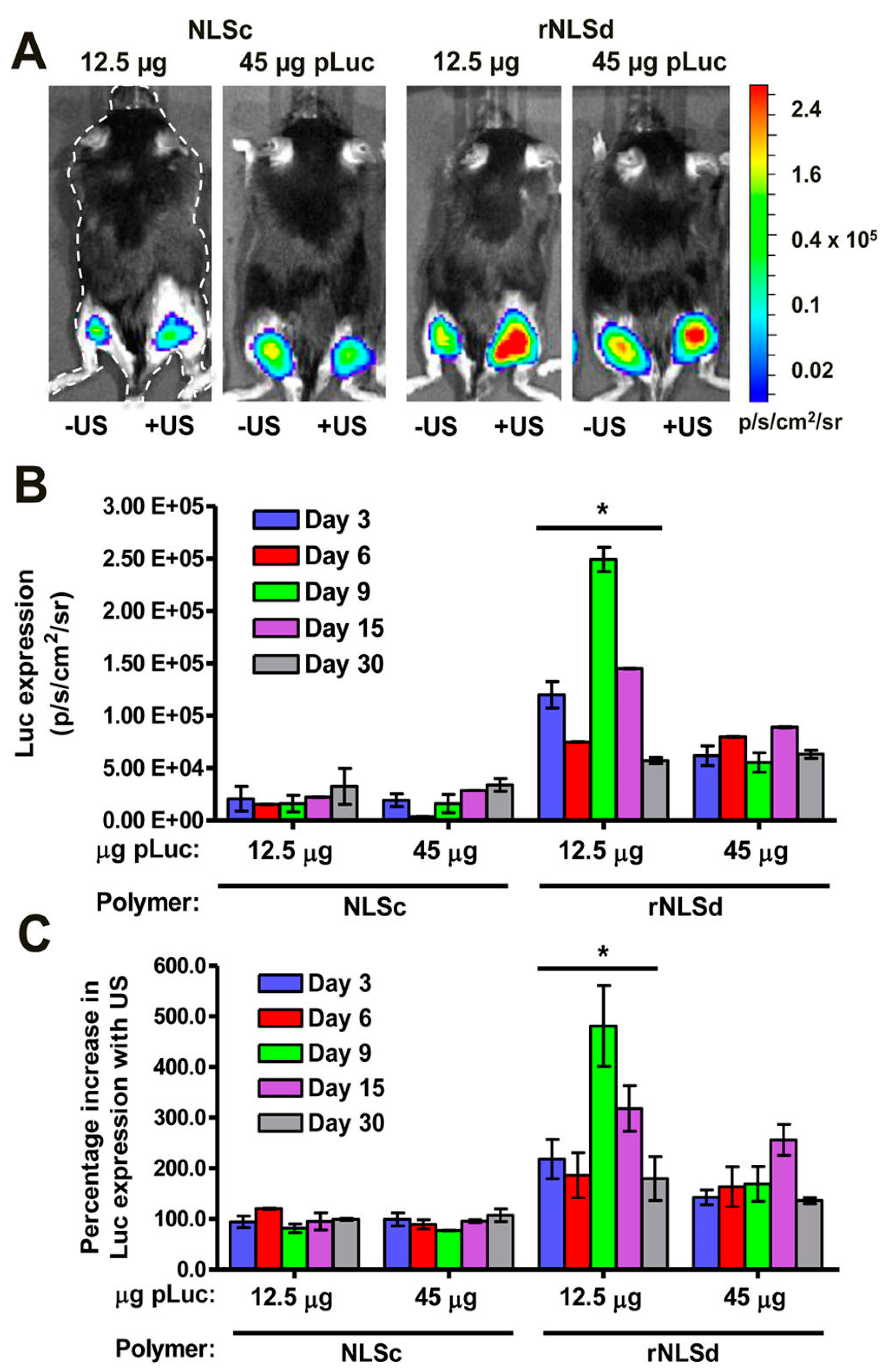
3.4. Sonoporation in Skeletal Muscle
3.5. Effects of Sonoporation on Skeletal Muscle
3.6. Variability in Sonoporation Conditions
4. Future Directions
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Misra, S. Human gene therapy: A brief overview of the genetic revolution. J. Assoc. Phys. India 2013, 61, 127–133. [Google Scholar]
- Miller, D.L.; Pislaru, S.V.; Greenleaf, J.F. Sonoporation: Mechanical DNA Delivery by Ultrasonic Cavitation. Somat. Cell Mol. Genet. 2002, 27, 115–134. [Google Scholar] [CrossRef] [PubMed]
- Newman, C.M.H.; Bettinger, T. Gene therapy progress and prospects: Ultrasound for gene transfer. Gene Ther. 2007, 14, 465–475. [Google Scholar] [CrossRef] [PubMed]
- Wolff, J.A.; Budker, V. The Mechanism of Naked DNA Uptake and Expression. Epigenetics Cancer Part A 2005, 54, 1–20. [Google Scholar] [CrossRef]
- Schratzberger, P.; Krainin, J.G.; Schratzberger, G.; Silver, M.; Ma, H.; Kearney, M.; Zuk, R.F.; Brisken, A.F.; Losordo, D.W.; Isner, J.M. Transcutaneous Ultrasound Augments Naked DNA Transfection of Skeletal Muscle. Mol. Ther. 2002, 6, 576–583. [Google Scholar] [CrossRef]
- Mumper, R.J.; Duguid, J.G.; Anwer, K.; Barron, M.K.; Nitta, H.; Rolland, A.P.; Barren, M.K. Polyvinyl Derivatives as Novel Interactive Polymers for Controlled Gene Delivery to Muscle. Pharm. Res. 1996, 13, 701–709. [Google Scholar] [CrossRef]
- Davis, H.L.; Demeneix, B.A.; Quantin, B.; Coulombe, J.; Whalen, R.G. Plasmid DNA is Superior to Viral Vectors for Direct Gene Transfer into Adult Mouse Skeletal Muscle. Hum. Gene Ther. 1993, 4, 733–740. [Google Scholar] [CrossRef]
- Wells, D.J.; Wells, K.E. Gene transfer studies in animals: What do they really tell us about the prospects for gene therapy in DMD? Neuromuscul Disord. 2002, 12, S11–S22. [Google Scholar] [CrossRef]
- Wolff, J.; Malone, R.; Williams, P.; Chong, W.; Acsadi, G.; Jani, A.; Felgner, P. Direct gene transfer into mouse muscle in vivo. Science 1990, 247, 1465–1468. [Google Scholar] [CrossRef]
- Shapiro, G.; Wong, A.W.; Bez, M.; Yang, F.; Tam, S.; Even, L.; Sheyn, D.; Ben-David, S.; Tawackoli, W.; Pelled, G.; et al. Multiparameter evaluation of in vivo gene delivery using ultrasound-guided, microbubble-enhanced sonoporation. J. Control. Release 2016, 223, 157–164. [Google Scholar] [CrossRef]
- Westhoff, B.; Seller, K.; Wild, A.; Jaeger, M.; Krauspe, R. Ultrasound-guided botulinum toxin injection technique for the iliopsoas muscle. Dev. Med. Child Neurol. 2007, 45, 829–832. [Google Scholar] [CrossRef]
- Bubnov, R. Ultrasound guided injections of Platelets Rich Plasma for muscle injury in professional athletes. Comparative study. Med. Ultrason. 2013, 15, 101–105. [Google Scholar] [CrossRef] [PubMed]
- Bez, M.; Foiret, J.; Shapiro, G.; Pelled, G.; Ferrara, K.W.; Gazit, D. Nonviral ultrasound-mediated gene delivery in small and large animal models. Nat. Protoc. 2019, 14, 1015–1026. [Google Scholar] [CrossRef]
- Jiao, S.; Williams, P.; Berg, R.K.; Hodgeman, B.A.; Liu, L.; Repetto, G.M.; Wolff, J.A. Direct Gene Transfer into Nonhuman Primate Myofibers In Vivo. Hum. Gene Ther. 1992, 3, 21–33. [Google Scholar] [CrossRef] [PubMed]
- Rai, R.; Alwani, S.; Badea, I. Polymeric Nanoparticles in Gene Therapy: New Avenues of Design and Optimization for Delivery Applications. Polymers 2019, 11, 745. [Google Scholar] [CrossRef] [PubMed]
- Hosseinkhani, H.; Abedini, F.; Ou, K.-L.; Domb, A. Polymers in gene therapy technology. Polym. Adv. Technol. 2014, 26, 198–211. [Google Scholar] [CrossRef]
- Parelkar, S.S.; Letteri, R.A.; Chan-Seng, D.; Zolochevska, O.; Ellis, J.; Figueiredo, M.; Emrick, T. Polymer–Peptide Delivery Platforms: Effect of Oligopeptide Orientation on Polymer-Based DNA Delivery. Biomacromolecules 2014, 15, 1328–1336. [Google Scholar] [CrossRef]
- Chen, Y.-C.; Jiang, L.-P.; Liu, N.-X.; Wang, Z.-H.; Hong, K.; Zhang, Q.-P. P85, Optison microbubbles and ultrasound cooperate in mediating plasmid DNA transfection in mouse skeletal muscles in vivo. Ultrason. Sonochem. 2011, 18, 513–519. [Google Scholar] [CrossRef]
- Lu, Q.L.; Liang, H.-D.; Partridge, T.; Blomley, M.J.K. Microbubble ultrasound improves the efficiency of gene transduction in skeletal muscle in vivo with reduced tissue damage. Gene Ther. 2003, 10, 396–405. [Google Scholar] [CrossRef]
- Singh, R.P.; RamaRao, P. Accumulated Polymer Degradation Products as Effector Molecules in Cytotoxicity of Polymeric Nanoparticles. Toxicol. Sci. 2013, 136, 131–143. [Google Scholar] [CrossRef]
- Lazzari, S.; Moscatelli, D.; Codari, F.; Salmona, M.; Morbidelli, M.; Diomede, L. Colloidal stability of polymeric nanoparticles in biological fluids. J. Nanopart. Res. 2012, 14, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Owens, D.; Peppas, N.A. Opsonization, biodistribution, and pharmacokinetics of polymeric nanoparticles. Int. J. Pharm. 2006, 307, 93–102. [Google Scholar] [CrossRef] [PubMed]
- Lundstrom, K. Viral Vectors in Gene Therapy. Diseases 2018, 6, 42. [Google Scholar] [CrossRef]
- Wang, D.; Zhong, L.; Abu Nahid, M.; Gao, G. The potential of adeno-associated viral vectors for gene delivery to muscle tissue. Expert Opin. Drug Deliv. 2014, 11, 345–364. [Google Scholar] [CrossRef] [PubMed]
- Maroun, J.; Muñoz-Alía, M.; Ammayappan, A.; Schulze, A.; Peng, K.-W.; Russell, S. Designing and building oncolytic viruses. Futur. Virol. 2017, 12, 193–213. [Google Scholar] [CrossRef]
- Suerth, J.D.; Labenski, V.; Schambach, A. Alpharetroviral Vectors: From a Cancer-Causing Agent to a Useful Tool for Human Gene Therapy. Viruses 2014, 6, 4811–4838. [Google Scholar] [CrossRef]
- Shi, W.; Arnold, G.S.; Bartlett, J.S. Insertional Mutagenesis of the Adeno-Associated Virus Type 2 (AAV2) Capsid Gene and Generation of AAV2 Vectors Targeted to Alternative Cell-Surface Receptors. Hum. Gene Ther. 2001, 12, 1697–1711. [Google Scholar] [CrossRef]
- Potter, H.; Heller, R. Transfection by Electroporation. Curr. Protoc. Mol. Boil. 2003, 62, 931–936. [Google Scholar] [CrossRef]
- Sokolowska, E.; Blachnio-Zabielska, A.U. A Critical Review of Electroporation as A Plasmid Delivery System in Mouse Skeletal Muscle. Int. J. Mol. Sci. 2019, 20, 2776. [Google Scholar] [CrossRef]
- Chang, D.C.; Reese, T.S. Changes in membrane structure induced by electroporation as revealed by rapid-freezing electron microscopy. Biophys. J. 1990, 58, 1–12. [Google Scholar] [CrossRef]
- Nishi, T.; Yoshizato, K.; Yamashiro, S.; Takeshima, H.; Sato, K.; Hamada, K.; Kitamura, I.; Yoshimura, T.; Saya, H.; Kuratsu, J.-I.; et al. High-efficiency in vivo gene transfer using intraarterial plasmid DNA injection following in vivo electroporation. Cancer Res. 1996, 56, 1050–1055. [Google Scholar] [PubMed]
- Stacey, K.J.; Ross, I.L.; Hume, D.A. Electroporation and DNA-dependent cell death in murine macrophages. Immunol. Cell Boil. 1993, 71, 75–85. [Google Scholar] [CrossRef] [PubMed]
- Sadiq, A.A.; Zaltum, M.A.M.; Mamman, H.B.; Adon, M.N.; Othman, N.B.; Dalimin, M.; Jamil, M.M.B.A. An overview: Investigation of electroporation and sonoporation techniques. In Proceedings of the 2015 2nd International Conference on Biomedical Engineering (ICoBE), Penang, Malaysia, 30–31 March 2015; Institute of Electrical and Electronics Engineers (IEEE): Piscataway, NJ, USA, 2015; pp. 1–6. [Google Scholar]
- Feril, L.B. Ultrasound-Mediated Gene Transfection. In Gene Therapy of Cancer: Methods and Protocols; Walther, W., Stein, U.S., Eds.; Humana Press: Totowa, NJ, USA, 2009; pp. 179–194. [Google Scholar]
- Nakamura, H. Electroporation and Sonoporation in Developmental Biology; Springer Science Business Media: Berlin, Germany, 2009. [Google Scholar]
- Campbell, S. A Short History of Sonography in Obstetrics and Gynaecology. Facts Views Vis. ObGyn 2013, 5, 213–229. [Google Scholar] [PubMed]
- Pepe, J.; Rincón, M.; Wu, J. Experimental comparison of sonoporation and electroporation in cell transfection applications. Acoust. Res. Lett. Online 2004, 5, 62–67. [Google Scholar] [CrossRef]
- Wang, X.; Liang, H.-D.; Dong, B.; Lu, Q.-L.; Blomley, M.J.K. Gene Transfer with Microbubble Ultrasound and Plasmid DNA into Skeletal Muscle of Mice: Comparison between Commercially Available Microbubble Contrast Agents 1. Radiology 2005, 237, 224–229. [Google Scholar] [CrossRef] [PubMed]
- Pislaru, S.V.; Pislaru, C.; Kinnick, R.R.; Singh, R.; Gulati, R.; Greenleaf, J.F.; Simari, R.D. Optimization of ultrasound-mediated gene transfer: Comparison of contrast agents and ultrasound modalities. Eur. Hear. J. 2003, 24, 1690–1698. [Google Scholar] [CrossRef]
- Mittelstein, D.R.; Ye, J.; Schibber, E.F.; Roychoudhury, A.; Martinez, L.T.; Fekrazad, M.H.; Ortiz, M.; Lee, P.P.; Shapiro, M.G.; Gharib, M. Selective ablation of cancer cells with low intensity pulsed ultrasound. Appl. Phys. Lett. 2020, 116, 013701. [Google Scholar] [CrossRef]
- Nozaki, T.; Ogawa, R.; Feril, L.B.; Kagiya, G.; Fuse, H.; Kondo, T. Enhancement of ultrasound-mediated gene transfection by membrane modification. J. Gene Med. 2003, 5, 1046–1055. [Google Scholar] [CrossRef]
- Li, Y.S.; Davidson, E.; Reid, C.N.; McHale, A.P. Optimising ultrasound-mediated gene transfer (sonoporation) in vitro and prolonged expression of a transgene in vivo: Potential applications for gene therapy of cancer. Cancer Lett. 2009, 273, 62–69. [Google Scholar] [CrossRef]
- Taniyama, Y.; Tachibana, K.; Hiraoka, K.; Aoki, M.; Yamamoto, S.; Matsumoto, K.; Nakamura, T.; Ogihara, T.; Kaneda, Y.; Morishita, R. Development of safe and efficient novel nonviral gene transfer using ultrasound: Enhancement of transfection efficiency of naked plasmid DNA in skeletal muscle. Gene Ther. 2002, 9, 372–380. [Google Scholar] [CrossRef]
- Suzuki, R.; Oda, Y.; Namai, E.; Takizawa, T.; Negishi, Y.; Utoguchi, N.; Tachibana, K.; Maruyama, K. Development of site specific gene delivery system with sonoporation. YAKUGAKU ZASSHI 2008, 128, 187–192. [Google Scholar] [CrossRef] [PubMed]
- Mehier-Humbert, S.; Bettinger, T.; Yan, F.; Guy, R.H. Ultrasound-mediated gene delivery: Kinetics of plasmid internalization and gene expression. J. Control. Release 2005, 104, 203–211. [Google Scholar] [CrossRef] [PubMed]
- Liang, H.-D.; Lu, Q.L.; Xue, S.-A.; Halliwell, M.; Kodama, T.; Cosgrove, D.O.; Stauss, H.J.; Partridge, T.A.; Blomley, M.J. Optimisation of ultrasound-mediated gene transfer (sonoporation) in skeletal muscle cells. Ultrasound Med. Boil. 2004, 30, 1523–1529. [Google Scholar] [CrossRef] [PubMed]
- Duvshani-Eshet, M.; Machluf, M. Therapeutic ultrasound optimization for gene delivery: A key factor achieving nuclear DNA localization. J. Control. Release 2005, 108, 513–528. [Google Scholar] [CrossRef] [PubMed]
- Unger, E.C.; Hersh, E.; Vannan, M.; Matsunaga, T.O.; McCreery, T. Local drug and gene delivery through microbubbles. Prog. Cardiovasc. Dis. 2001, 44, 45–54. [Google Scholar] [CrossRef] [PubMed]
- Sloand, J.N.; Nguyen, T.T.; Zinck, S.A.; Cook, E.C.; Zimudzi, T.J.; Showalter, S.A.; Glick, A.B.; Simon, J.C.; Medina, S.H. Ultrasound-Guided Cytosolic Protein Delivery via Transient Fluorous Masks. ACS Nano 2020, 14, 4061–4073. [Google Scholar] [CrossRef]
- De Carlo, F.; Thomas, L.; Brooke, B.; Varney, E.T.; Nande, R.; Boskovic, O.; Marshall, G.D.; Claudio, P.P.; Howard, C.M. Microbubble-mediated delivery of human adenoviruses does not elicit innate and adaptive immunity response in an immunocompetent mouse model of prostate cancer. J. Transl. Med. 2019, 17, 19. [Google Scholar] [CrossRef]
- Negishi, Y.; Endo, Y.; Fukuyama, T.; Suzuki, R.; Takizawa, T.; Omata, D.; Maruyama, K.; Aramaki, Y. Delivery of siRNA into the cytoplasm by liposomal bubbles and ultrasound. J. Control. Release 2008, 132, 124–130. [Google Scholar] [CrossRef]
- Sarisozen, C.; Salzano, G.; Torchilin, V. Recent advances in siRNA delivery. Biomol. Concepts 2015, 6, 321–341. [Google Scholar] [CrossRef]
- Morgan, J.E.; Partridge, T.A. Muscle satellite cells. Int. J. Biochem. Cell Boil. 2003, 35, 1151–1156. [Google Scholar] [CrossRef]
- Yin, H.; Price, F.; Rudnicki, M.A. Satellite Cells and the Muscle Stem Cell Niche. Physiol. Rev. 2013, 93, 23–67. [Google Scholar] [CrossRef] [PubMed]
- Liao, Z.-K.; Tsai, K.-C.; Wang, H.-T.; Tseng, S.-H.; Deng, W.-P.; Chen, W.-S.; Hwang, L.-H. Sonoporation-mediated anti-angiogenic gene transfer into muscle effectively regresses distant orthotopic tumors. Cancer Gene Ther. 2011, 19, 171–180. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Lu, Q.L.; Bou-Gharios, G.; Partridge, T.A. Non-viral gene delivery in skeletal muscle: A protein factory. Gene Ther. 2003, 10, 131–142. [Google Scholar] [CrossRef] [PubMed]
- McMahon, J.; Wells, D. Electroporation for Gene Transfer to Skeletal Muscles. BioDrugs 2004, 18, 155–165. [Google Scholar] [CrossRef] [PubMed]
- Yamashita, Y.-I.; Shimada, M.; Tachibana, K.; Harimoto, N.; Tsujita, E.; Shirabe, K.; Miyazaki, J.; Sugimachi, K. In Vivo Gene Transfer into Muscle via Electro-Sonoporation. Hum. Gene Ther. 2002, 13, 2079–2084. [Google Scholar] [CrossRef]
- Figueiredo Neto, M.; Letteri, R.A.; Chan-Seng, D.; Emrick, T.; Figueiredo, M.L. Sonodelivery Facilitates Sustained Luciferase Expression from an Episomal Vector in Skeletal Muscle. Materials 2015, 8, 4608–4617. [Google Scholar] [CrossRef]
- Figueiredo, M.L.; Figueiredo Neto, M.; Salameh, J.W.; Decker, R.E.; Letteri, R.; Chan-Seng, D.; Emrick, T. Ligand-Mediated Targeting of Cytokine Interleukin-27 Enhances Its Bioactivity In Vivo. Mol. Ther. Methods Clin. Dev. 2020, 17, 739–751. [Google Scholar] [CrossRef]
- Burke, C.W.; Suk, J.S.; Kim, A.J.; Hsiang, Y.-H.J.; Klibanov, A.L.; Hanes, J.; Price, R.J. Markedly enhanced skeletal muscle transfection achieved by the ultrasound-targeted delivery of non-viral gene nanocarriers with microbubbles. J. Control. Release 2012, 162, 414–421. [Google Scholar] [CrossRef][Green Version]
- Zolochevska, O.; Xia, X.; Williams, B.J.; Ramsay, A.; Li, S.; Figueiredo, M.L. Sonoporation Delivery of Interleukin-27 Gene Therapy Efficiently Reduces Prostate Tumor Cell Growth In Vivo. Hum. Gene Ther. 2011, 22, 1537–1550. [Google Scholar] [CrossRef]
- Upadhyay, A.; Dalvi, S.V. Microbubble Formulations: Synthesis, Stability, Modeling and Biomedical Applications. Ultrasound Med. Boil. 2019, 45, 301–343. [Google Scholar] [CrossRef]
- Unga, J.; Hashida, M. Ultrasound induced cancer immunotherapy. Adv. Drug Deliv. Rev. 2014, 72, 144–153. [Google Scholar] [CrossRef] [PubMed]
- Frinking, P.J.; Bouakaz, A.; Kirkhorn, J.; Cate, F.J.T.; De Jong, N. Ultrasound contrast imaging: Current and new potential methods. Ultrasound Med. Boil. 2000, 26, 965–975. [Google Scholar] [CrossRef]
- Moran, C.M. CHAPTER 6—Ultrasonic contrast agents. In Clinical Ultrasound (Third Edition); Allan, P.L., Baxter, G.M., Weston, M.J., Eds.; Churchill Livingstone: Edinburgh, UK, 2011; pp. 77–89. [Google Scholar]
- Deng, C.X.; Sieling, F.; Pan, H.; Cui, J. Ultrasound-induced cell membrane porosity. Ultrasound Med. Boil. 2004, 30, 519–526. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.; Xi, X. Eit (Electrical Impedance Tomography) Guided Sonoporation, Ultrasound Tissue Ablation and Their Use. Thereof. Patent WO2010127369A1, 4 November 2010. [Google Scholar]
- Shih, C.-P.; Chen, H.-C.; Lin, Y.-C.; Chen, H.-K.; Wang, H.; Kuo, C.-Y.; Lin, Y.-Y.; Wang, C.-H. Middle-ear dexamethasone delivery via ultrasound microbubbles attenuates noise-induced hearing loss. Laryngoscope 2018, 129, 1907–1914. [Google Scholar] [CrossRef]
- Tlaxca, J.L.; Rychak, J.J.; Ernst, P.B.; Konkalmatt, P.R.; Shevchenko, T.I.; Pizzaro, T.T.; Rivera-Nieves, J.; Klibanov, A.L.; Lawrence, M.B. Ultrasound-based molecular imaging and specific gene delivery to mesenteric vasculature by endothelial adhesion molecule targeted microbubbles in a mouse model of Crohn’s disease. J. Control. Release 2012, 165, 216–225. [Google Scholar] [CrossRef]
- Tomizawa, M.; Shinozaki, F.; Motoyoshi, Y.; Sugiyama, T.; Yamamoto, S.; Sueishi, M. Sonoporation: Gene transfer using ultrasound. World J. Methodol. 2013, 3, 39–44. [Google Scholar] [CrossRef]
- Xu, W.; Zhang, X.; Hu, X.; Zhiyi, C.; Huang, P. Translational Prospects of ultrasound-mediated tumor immunotherapy: Preclinical advances and safety considerations. Cancer Lett. 2019, 460, 86–95. [Google Scholar] [CrossRef]
- Li, Y.; Wang, J.; Liu, J.; Liu, F. A novel system for in vivo neprilysin gene delivery using a syringe electrode. J. Neurosci. Methods 2010, 193, 226–231. [Google Scholar] [CrossRef]
- Watanabe, Y.; Horie, S.; Funaki, Y.; Kikuchi, Y.; Yamazaki, H.; Ishii, K.; Mori, S.; Vassaux, G.; Kodama, T. Delivery of Na/I Symporter Gene into Skeletal Muscle Using Nanobubbles and Ultrasound: Visualization of Gene Expression by PET. J. Nucl. Med. 2010, 51, 951–958. [Google Scholar] [CrossRef]
- Feichtinger, G.A.; Hofmann, A.T.; Slezak, P.; Schützenberger, S.; Kaipel, M.; Schwartz, E.; Neef, A.; Nomikou, N.; Nau, T.; Van Griensven, M.; et al. Sonoporation Increases Therapeutic Efficacy of Inducible and Constitutive BMP2/7 In Vivo Gene Delivery. Hum. Gene Ther. Methods 2014, 25, 57–71. [Google Scholar] [CrossRef]
- Zolochevska, O.; Ellis, J.; Parelkar, S.; Chan-Seng, D.; Emrick, T.; Wei, J.; Patrikeev, I.; Motamedi, M.; Figueiredo, M.L. Interleukin-27 Gene Delivery for Modifying Malignant Interactions Between Prostate Tumor and Bone. Hum. Gene Ther. 2013, 24, 970–981. [Google Scholar] [CrossRef]
- Tsai, K.-C.; Liao, Z.-K.; Yang, S.-J.; Lin, W.-L.; Shieh, M.-J.; Hwang, L.-H.; Chen, W.-S. Differences in gene expression between sonoporation in tumor and in muscle. J. Gene Med. 2009, 11, 933–940. [Google Scholar] [CrossRef]
- Sheyn, D.; Kimelman-Bleich, N.; Pelled, G.; Zilberman, Y.; Gazit, D.; Gazit, Z. Ultrasound-based nonviral gene delivery induces bone formation in vivo. Gene Ther. 2007, 15, 257–266. [Google Scholar] [CrossRef] [PubMed]
- Osawa, K.; Okubo, Y.; Nakao, K.; Koyama, N.; Bessho, K. Osteoinduction by microbubble-enhanced transcutaneous sonoporation of human bone morphogenetic protein-2. J. Gene Med. 2009, 11, 633–641. [Google Scholar] [CrossRef] [PubMed]
- Danialou, G.; Comtois, A.S.; Dudley, R.W.; Nalbantoglu, J.; Gilbert, R.; Karpati, G.; Jones, D.H.; Petrof, B.J. Ultrasound Increases Plasmid-Mediated Gene Transfer to Dystrophic Muscles without Collateral Damage. Mol. Ther. 2002, 6, 687–693. [Google Scholar] [CrossRef]
- Meschersky, M.E.; Kaaba, S.I.; Sokolovsky, A.A.; Moskovtsev, A.A.; Blokhin, D.Y.; Andriyanov, Y.V.; Kubatiev, A.A. A new method of combined electroporation and sonoporation with a reduced stress-inducing effect on cells. Pathogenesis 2012, 10, 59–67. [Google Scholar]
- Longsine-Parker, W.; Wang, H.; Koo, C.; Kim, J.; Kim, B.J.; Jayaraman, A.; Han, A. Microfluidic electro-sonoporation: A multi-modal cell poration methodology through simultaneous application of electric field and ultrasonic wave. Lab a Chip 2013, 13, 2144. [Google Scholar] [CrossRef]
- Piekarowicz, K.; Bertrand, A.T.; Azibani, F.; Beuvin, M.; Julien, L.; Machowska, M.; Bonne, G.; Rzepecki, R. A Muscle Hybrid Promoter as a Novel Tool for Gene Therapy. Mol. Ther. Methods Clin. Dev. 2019, 15, 157–169. [Google Scholar] [CrossRef]





| Sonoporator | Frequency (MHz) | Burst (W/cm2) | PRF (Hz) | Duty Cycle (%) | Duration (s) | Cell Type | Reference |
|---|---|---|---|---|---|---|---|
| KTAC-4000 | 1.015 | 0.7 | - | 50 | 360 | C2C12 | Our Group * |
| KTAC-4000 | 2 | 2.5 | 2 | 50 | 10 | COS-7 | [44] |
| Sonidel SP100 | 1 | 2 | 100 | 60 | 450 | HeLa | [42] |
| Sonidel SP100 | 1 | 4 | - | 60 | 450 | C2C12 | Our Group * |
| Panametrics | 2.25 | - | 100 | 20 | 10 | MAT B III | [45] |
| ES-1 Ultrasonic Generator | 1 | 3.6 | - | - | 20 | PC3 | [41] |
| “Dedicated Continuous Wave System” | 1 | 0.75 | - | - | 30 | VSMC, HUVEC | [39] |
| Mark 3, EMS Limited ** | 1 | 0.8 | 100 | 20 | 20 | H2K Myoblast | [46] |
| UltraMax ** | 1 | 2 | 100 | 30 | 1800 | BHK, LNCaP, BCE | [47] |
| Sonoporator | Frequency (MHz) | Burst (W/cm2) | PRF (Hz) | Duty Cycle (%) | Duration (s) | Reference |
|---|---|---|---|---|---|---|
| Dedicated Sonoporators | ||||||
| BFC Applications Probe + WF1946A Frequency Synthesizer | 1 | 3 | 1000 | 20 | 60 | [74] |
| KTAC-4000 | 1.015 | 3 | - | 20 | 60 | Our Group * |
| Sonidel SP 100 | 1 | 2 | - | 50 | 180 | Our Group * |
| Sonidel SP 100 | 1 | 1.9 | - | 25 | 360 | [42] |
| Sonidel SP 100 | 1 | 2 | - | 25 | 180 | [75] |
| Sonigene | 1 | 3 | - | 20 | 60 | [76] |
| Sonigene | 1 | 2 | - | 20 | 60 | [17] |
| Sonitron 2000 | 1 | 0.4 | 200 | 20 | 1200 | [55,77] |
| Sonitron 2000 | 1 | 5 | - | 50 | 600 | [78] |
| Sonitron 2000 | 1 | 4 | - | 50 | 300 (60 * 5) | [79] |
| Sonopore 3000 | 2 | 2.5 | 2 | 50 | 60 | [51] |
| Therapeutic Instruments | ||||||
| Mark 3, EMS Limited | 1 | 1 | - | 20 | 120 | [18] |
| Mark 3, EMS Limited+ | 1 | 3 | 100 | 20 | 60 | [19] |
| Mark 3, EMS Limited | 1 | 2 | 100 | 20 | 30 | [38] |
| Modified Siemens Antares | 1.4 | - | 540 | - | 120 | [10] |
| Ultax UX-301+ | 1 | 2.5 | - | - | 60 | [43] |
| UltraMax | 1 | 1.5 | - | 30 | 120 | [80] |
| System V, GE Vingmed | 1.7 | Mechanical Index = 1.7 | 180 | [39] | ||
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Decker, R.E.; Lamantia, Z.E.; Emrick, T.S.; Figueiredo, M.L. Sonodelivery in Skeletal Muscle: Current Approaches and Future Potential. Bioengineering 2020, 7, 107. https://doi.org/10.3390/bioengineering7030107
Decker RE, Lamantia ZE, Emrick TS, Figueiredo ML. Sonodelivery in Skeletal Muscle: Current Approaches and Future Potential. Bioengineering. 2020; 7(3):107. https://doi.org/10.3390/bioengineering7030107
Chicago/Turabian StyleDecker, Richard E., Zachary E. Lamantia, Todd S. Emrick, and Marxa L. Figueiredo. 2020. "Sonodelivery in Skeletal Muscle: Current Approaches and Future Potential" Bioengineering 7, no. 3: 107. https://doi.org/10.3390/bioengineering7030107
APA StyleDecker, R. E., Lamantia, Z. E., Emrick, T. S., & Figueiredo, M. L. (2020). Sonodelivery in Skeletal Muscle: Current Approaches and Future Potential. Bioengineering, 7(3), 107. https://doi.org/10.3390/bioengineering7030107






