Prospects and Challenges of Translational Corneal Bioprinting
Abstract
1. Corneal Transplantation and Tissue Engineering
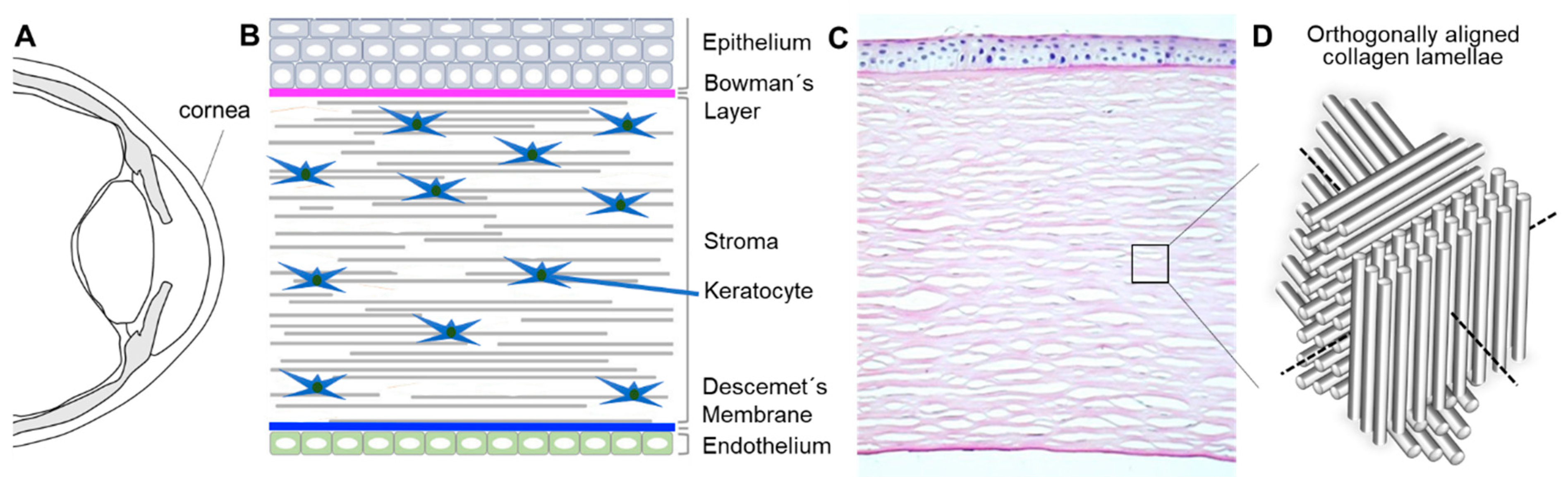
1.1. Bioarchitecture and Physiology of the Human Cornea
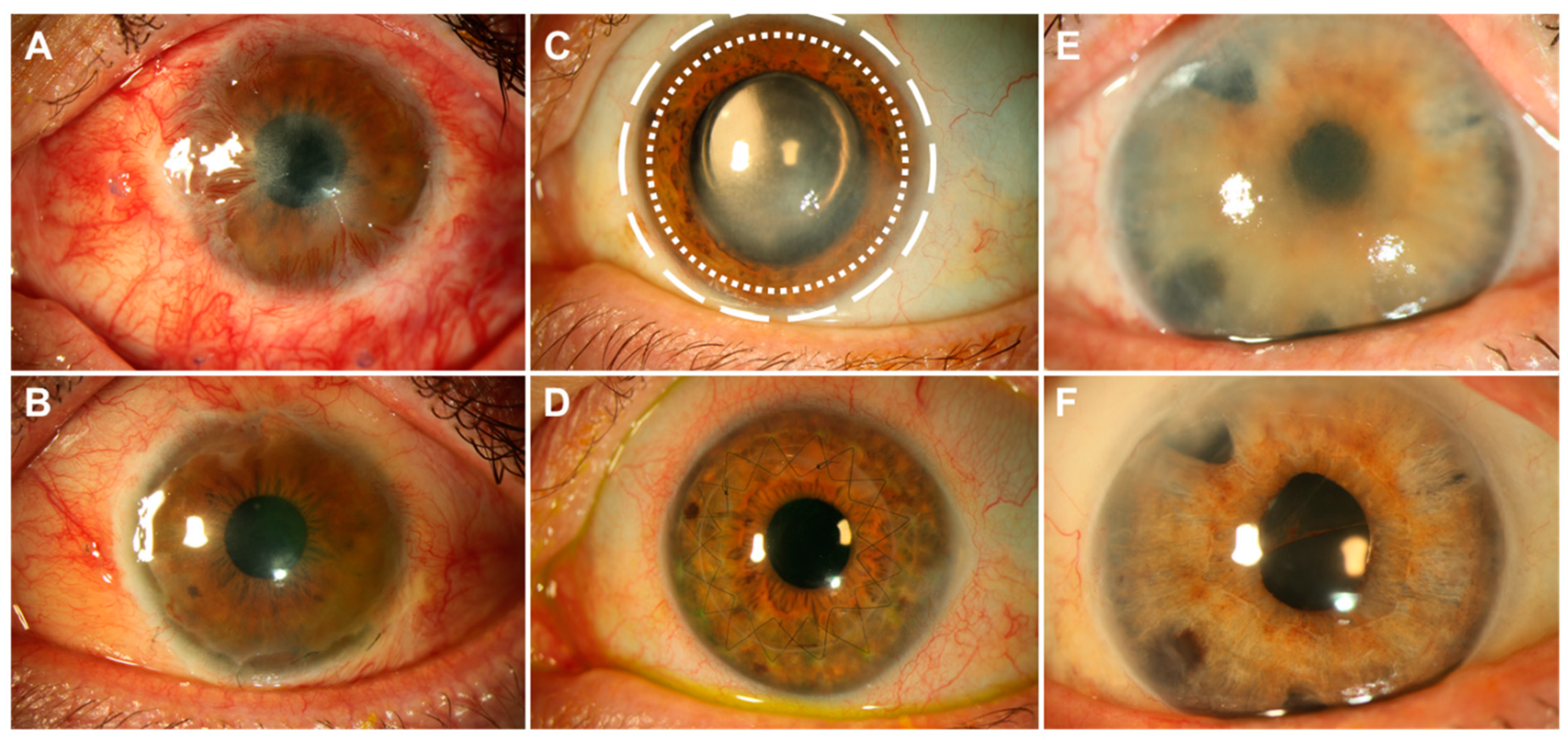
1.2. State-of-the-Art Corneal Transplantation
1.3. State-of-the-Art Corneal Tissue Engineering Strategies
1.4. Drawbacks of State-of-the-Art Approaches and Future Directions
- (a)
- Biological function: CSKs should show typical dendritic shapes, forming networks, and not differentiate into scar-inducing SF and Myo-SF phenotypes [59];
- (b)
- Transparency: the human corneal light-transmittance rises rapidly from 300 nm, reaching 80% at 380 nm and more than 90% between 500 and 1300 nm [60];
- (c)
- Mechanical properties: The biomechanical characteristics of the human cornea and, hence, the natural habitat of CSKs are complex. In short, different grades of stiffness can be found in the human cornea, depending on age, strain, and position. Ex vivo destructive testing has successfully confirmed the following biomechanical principles:
- (i)
- The cornea exhibits a non-linear stress versus strain response with progressive stiffening at high strains [61].
- (ii)
- The cornea shows regional in-plane variation in strain and deformation, meaning that the paracentral and peripheral cornea is stiffer than the central cornea due to the differing orientation and number of collagen fibrils [62].
- (iii)
- Corneal elastic strength is a function of depth with decreasing strength from the anterior to the posterior stroma [63]. Young’s modulus of elasticity for the anterior human cornea (first 50 µm including Bowman’s lamella) was measured by indentation at 245 ± 209 kPa (range: 82–530 kPa), and for the posterior stroma at 100 ± 61 kPa (28–162 kPa) [64].
- (iv)
- (d)
- Curvature: The cornea has the highest refractive power of the human eye (approximately 43 diopters). The average radius of the anterior corneal surface measured by Scheimpflug imaging was 7.7 ± 0.2 mm, and the average radius of the posterior corneal surface was 6.5 ± 0.2 mm [67].
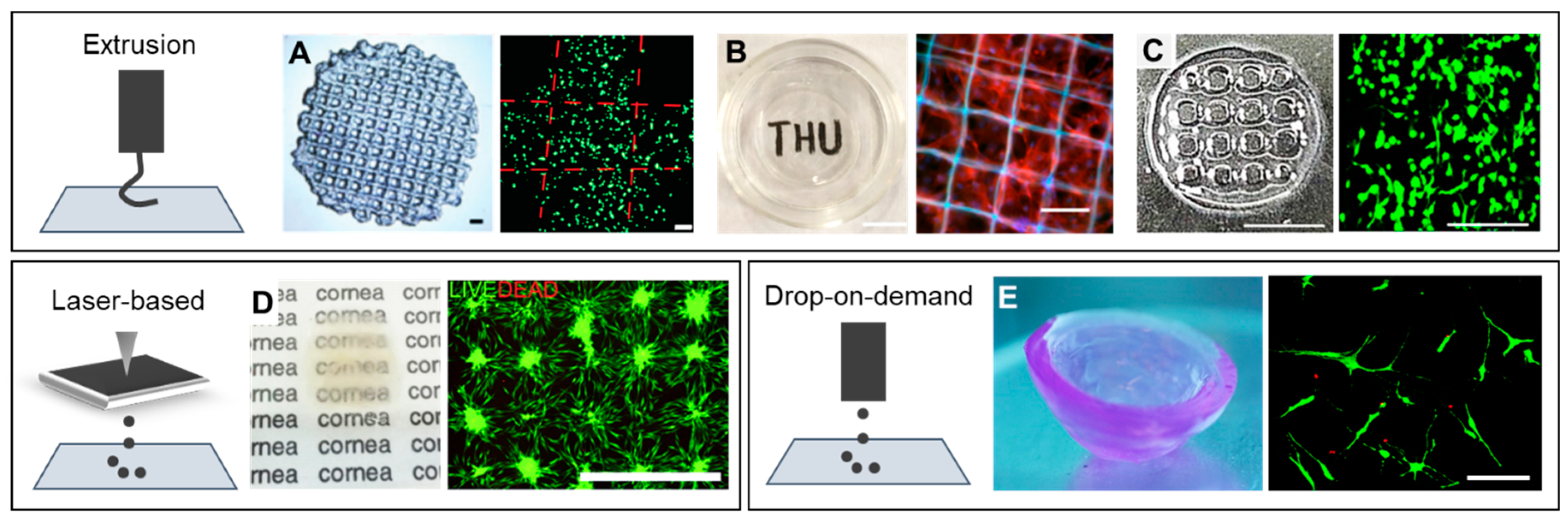
2. Bioprinting Methods for Tissue Engineering
3. Corneal Bioprinting: Focus on Stroma
4. Future Directions of Corneal Bioprinting: Full-Thickness Human Corneas
Funding
Conflicts of Interest
References
- DelMonte, D.W.; Kim, T. Anatomy and physiology of the cornea. J. Cataract Refract. Surg. 2011, 37, 588–598. [Google Scholar] [CrossRef] [PubMed]
- Nakamura, T.; Inatomi, T.; Sotozono, C.; Koizumi, N.; Kinoshita, S. Ocular surface reconstruction using stem cell and tissue engineering. Prog. Retin. Eye Res. 2016, 51, 187–207. [Google Scholar] [CrossRef] [PubMed]
- O’Callaghan, A.R.; Daniels, J.T. Concise Review: Limbal Epithelial Stem Cell Therapy: Controversies and Challenges. Stem Cells 2011, 29, 1923–1932. [Google Scholar] [CrossRef] [PubMed]
- Yam, G.H.F.; Riau, A.K.; Funderburgh, M.L.; Mehta, J.S.; Jhanji, V. Keratocyte biology. Exp. Eye Res. 2020, 196, 108062. [Google Scholar] [CrossRef]
- Doughty, M.J.; Zaman, M.L. Human corneal thickness and its impact on intraocular pressure measures: A review and meta-analysis approach. Surv. Ophthalmol. 2000, 44, 367–408. [Google Scholar] [CrossRef]
- Bergmanson, J.P.G. Anatomy and Physiology of the Cornea and Related Structures. In Contact Lenses; Elsevier: Amsterdam, The Netherlands, 2019; ISBN 9780702071683. [Google Scholar]
- Pascolini, D.; Mariotti, S.P. Global estimates of visual impairment: 2010. Br. J. Ophthalmol. 2012, 96, 614–618. [Google Scholar] [CrossRef]
- Rocher, M.; Robert, P.Y.; Desmoulière, A. The myofibroblast, biological activities and roles in eye repair and fibrosis. A focus on healing mechanisms in avascular cornea. Eye 2020, 34, 232–240. [Google Scholar] [CrossRef]
- Du, Y.; Funderburgh, M.L.; Mann, M.M.; SundarRaj, N.; Funderburgh, J.L. Multipotent Stem Cells in Human Corneal Stroma. Stem Cells 2005, 23, 1266–1275. [Google Scholar] [CrossRef]
- Bonanno, J.A. Molecular mechanisms underlying the corneal endothelial pump. Exp. Eye Res. 2012, 95, 2–7. [Google Scholar] [CrossRef]
- Yan Yu, W.; Sheridan, C.; Grierson, I.; Mason, S.; Kearns, V.; Cheuk Yin Lo, A.; Wong, D. Progenitors for the Corneal Endothelium and Trabecular Meshwork: A Potential Source for Personalized Stem Cell Therapy in Corneal Endothelial Diseases and Glaucoma. J. Biomed. Biotechnol. 2011, 2011, 13. [Google Scholar] [CrossRef]
- Yam, G.H.F.; Seah, X.; Yusoff, N.Z.B.M.; Setiawan, M.; Wahlig, S.; Htoon, H.M.; Peh, G.S.L.; Kocaba, V.; Mehta, J.S. Mehta Characterization of Human Transition Zone Reveals a Putative Progenitor-Enriched Niche of Corneal Endothelium. Cells 2019, 8, 1244. [Google Scholar] [CrossRef] [PubMed]
- Tan, D.T.H.; Dart, J.K.G.; Holland, E.J.; Kinoshita, S. Corneal transplantation. Lancet 2012, 379, 1749–1761. [Google Scholar] [CrossRef]
- de By, T.M. Shortage in the face of plenty: Improving the allocation of corneas for transplantation. Dev. Ophthalmol. 2003, 36, 56–61. [Google Scholar] [PubMed]
- Van Meter, M.D.; Spears, W.; Sheth, P.H. Potential Adverse Effects on the Cornea Donor Pool in 2031. Int. J. Eye Bank. 2013, 1, 1–9. [Google Scholar] [CrossRef]
- Taylor, A.W. Ocular Immune Privilege and Transplantation. Front. Immunol. 2016, 7, 37. [Google Scholar] [CrossRef]
- Fuest, M.; Yam, G.H.-F.; Peh, G.S.-L.; Mehta, J.S. Advances in corneal cell therapy. Regen. Med. 2016, 11, 601–615. [Google Scholar] [CrossRef]
- Feng, Y.; Borrelli, M.; Reichl, S.; Schrader, S.; Geerling, G. Review of alternative carrier materials for ocular surface reconstruction. Curr. Eye Res. 2014, 39, 541–552. [Google Scholar] [CrossRef]
- Zhang, T.; Yam, G.H.F.; Riau, A.K.; Poh, R.; Allen, J.C.; Peh, G.S.; Beuerman, R.W.; Tan, D.T.; Mehta, J.S. The effect of amniotic membrane de-epithelialization method on its biological properties and ability to promote limbal epithelial cell culture. Investig. Ophthalmol. Vis. Sci. 2013, 54, 3072–3081. [Google Scholar] [CrossRef]
- Levis, H.; Kureshi, A.; Massie, I.; Morgan, L.; Vernon, A.; Daniels, J. Tissue Engineering the Cornea: The Evolution of RAFT. J. Funct. Biomater. 2015, 6, 50–65. [Google Scholar] [CrossRef]
- Jirsova, K.; Jones, G.L.A. Amniotic membrane in ophthalmology: Properties, preparation, storage and indications for grafting—A review. Cell Tissue Bank. 2017, 18, 193–204. [Google Scholar] [CrossRef]
- Wagner, M.; Walter, P.; Salla, S.; Johnen, S.; Plange, N.; Rütten, S.; Goecke, T.W.; Fuest, M. Cryopreservation of amniotic membrane with and without glycerol additive. Graefe’s Arch. Clin. Exp. Ophthalmol. 2018, 256, 1117–1126. [Google Scholar] [CrossRef] [PubMed]
- Hopkinson, A.; McIntosh, R.S.; Tighe, P.J.; James, D.K.; Dua, H.S. Amniotic membrane for ocular surface reconstruction: Donor variations and the effect of handling on TGF-β content. Investig. Ophthalmol. Vis. Sci. 2006, 47, 4316–4322. [Google Scholar] [CrossRef] [PubMed]
- Marsit, N.M.; Sidney, L.E.; Britchford, E.R.; McIntosh, O.D.; Allen, C.L.; Ashraf, W.; Bayston, R.; Hopkinson, A. Validation and assessment of an antibiotic-based, aseptic decontamination manufacturing protocol for therapeutic, vacuum-dried human amniotic membrane. Sci. Rep. 2019, 9, 1–11. [Google Scholar] [CrossRef]
- Rama, P.; Bonini, S.; Lambiase, A.; Golisano, O.; Paterna, P.; De Luca, M.; Pellegrini, G. Autologous fibrin-cultured limbal stem cells permanently restore the corneal surface of patients with total limbal stem cell deficiency. Transplantation 2001, 72, 1478–1485. [Google Scholar] [CrossRef] [PubMed]
- Rama, P.; Matuska, S.; Paganoni, G.; Spinelli, A.; De Luca, M.; Pellegrini, G. Limbal Stem-Cell Therapy and Long-Term Corneal Regeneration. N. Engl. J. Med. 2010, 363, 147–155. [Google Scholar] [CrossRef] [PubMed]
- Aghaei-Ghareh-Bolagh, B.; Guan, J.; Wang, Y.; Martin, A.D.; Dawson, R.; Mithieux, S.M.; Weiss, A.S. Optically robust, highly permeable and elastic protein films that support dual cornea cell types. Biomaterials 2019, 188, 50–62. [Google Scholar] [CrossRef]
- Galal, A.; Perez-Santonja, J.J.; Rodriguez-Prats, J.L.; Abad, M.; Alio, J. Human anterior lens capsule as a biologic substrate for the ex vivo expansion of limbal stem cells in ocular surface reconstruction. Cornea 2007, 26, 473–478. [Google Scholar] [CrossRef]
- Borrelli, M.; Reichl, S.; Feng, Y.; Schargus, M.; Schrader, S.; Geerling, G. In vitro characterization and ex vivo surgical evaluation of human hair keratin films in ocular surface reconstruction after sterilization processing. J. Mater. Sci. Mater. Med. 2013, 24, 221–230. [Google Scholar] [CrossRef]
- De La Mata, A.; Nieto-Miguel, T.; López-Paniagua, M.; Galindo, S.; Aguilar, M.R.; García-Fernández, L.; Gonzalo, S.; Vázquez, B.; Román, J.S.; Corrales, R.M.; et al. Chitosan-gelatin biopolymers as carrier substrata for limbal epithelial stem cells. J. Mater. Sci. Mater. Med. 2013, 24, 2819–2829. [Google Scholar] [CrossRef]
- Bentley, E.; Murphy, C.J.; Li, F.; Carlsson, D.J.; Griffith, M. Biosynthetic corneal substitute implantation in dogs. Cornea 2010, 29, 910–916. [Google Scholar] [CrossRef]
- Oelker, A.M.; Grinstaff, M.W. Synthesis, characterization, and in vitro evaluation of a hydrogel-based corneal onlay. IEEE Trans. Nanobiosci. 2012, 11, 37–45. [Google Scholar] [CrossRef]
- Tan, X.W.; Hartman, L.; Tan, K.P.; Poh, R.; Myung, D.; Zheng, L.L.; Waters, D.; Noolandi, J.; Beuerman, R.W.; Frank, C.W.; et al. In vivo biocompatibility of two PEG/PAA interpenetrating polymer networks as corneal inlays following deep stromal pocket implantation. J. Mater. Sci. Mater. Med. 2013, 24, 967–977. [Google Scholar] [CrossRef]
- Hartmann, L.; Watanabe, K.; Zheng, L.L.; Kim, C.-Y.; Beck, S.E.; Huie, P.; Noolandi, J.; Cochran, J.R.; Ta, C.N.; Frank, C.W. Toward the development of an artificial cornea: Improved stability of interpenetrating polymer networks. J. Biomed. Mater. Res. Part B Appl. Biomater. 2011, 98, 8–17. [Google Scholar] [CrossRef]
- Fagerholm, P.; Lagali, N.S.; Merrett, K.; Jackson, W.B.; Munger, R.; Liu, Y.; Polarek, J.W.; Soderqvist, M.; Griffith, M. A Biosynthetic Alternative to Human Donor Tissue for Inducing Corneal Regeneration: 24-Month Follow-Up of a Phase 1 Clinical Study. Sci. Transl. Med. 2010, 2, 46ra61-46ra61. [Google Scholar] [CrossRef] [PubMed]
- Ambrose, W.M.I.; Salahuddin, A.; So, S.; Ng, S.; Márquez, S.P.; Takezawa, T.; Schein, O.; Elisseeff, J. Collagen vitrigel membranes for the in vitro reconstruction of separate corneal epithelial, stromal, and endothelial cell layers. J. Biomed. Mater. Res.—Part B Appl. Biomater. 2009, 90, 818–831. [Google Scholar] [CrossRef] [PubMed]
- Lai, J.Y.; Li, Y.T.; Cho, C.H.; Yu, T.C. Nanoscale modification of porous gelatin scaffolds with chondroitin sulfate for corneal stromal tissue engineering. Int. J. Nanomedicine 2012, 7, 1101–1114. [Google Scholar] [CrossRef] [PubMed]
- Ma, X.-Y.; Bao, H.-J.; Cui, L.; Zou, J. The Graft of Autologous Adipose-Derived Stem Cells in the Corneal Stromal after Mechanic Damage. PLoS ONE 2013, 8, e76103. [Google Scholar] [CrossRef] [PubMed]
- Islam, M.M.; Buznyk, O.; Reddy, J.C.; Pasyechnikova, N.; Alarcon, E.I.; Hayes, S.; Lewis, P.; Fagerholm, P.; He, C.; Iakymenko, S.; et al. Biomaterials-enabled cornea regeneration in patients at high risk for rejection of donor tissue transplantation. npj Regen. Med. 2018, 3, 1–10. [Google Scholar] [CrossRef]
- Gouveia, R.M.; Koudouna, E.; Jester, J.; Figueiredo, F.; Connon, C.J. Template Curvature Influences Cell Alignment to Create Improved Human Corneal Tissue Equivalents. Adv. Biosyst. 2017, 1, 1700135. [Google Scholar] [CrossRef]
- Gouveia, R.M.; González-Andrades, E.; Cardona, J.C.; González-Gallardo, C.; Ionescu, A.M.; Garzon, I.; Alaminos, M.; González-Andrades, M.; Connon, C.J. Controlling the 3D architecture of Self-Lifting Auto-generated Tissue Equivalents (SLATEs) for optimized corneal graft composition and stability. Biomaterials 2017, 121, 205–219. [Google Scholar] [CrossRef]
- Miotto, M.; Gouveia, R.M.; Ionescu, A.M.; Figueiredo, F.; Hamley, I.W.; Connon, C.J. 4D Corneal Tissue Engineering: Achieving Time-Dependent Tissue Self-Curvature through Localized Control of Cell Actuators. Adv. Funct. Mater. 2019, 29, 1807334. [Google Scholar] [CrossRef]
- Ghezzi, C.E.; Rnjak-Kovacina, J.; Kaplan, D.L. Corneal Tissue Engineering: Recent Advances and Future Perspectives. Tissue Eng. Part B Rev. 2015, 21, 278–287. [Google Scholar] [CrossRef] [PubMed]
- Wilson, S.L.; Sidney, L.E.; Dunphy, S.E.; Dua, H.S.; Hopkinson, A. Corneal Decellularization: A Method of Recycling Unsuitable Donor Tissue for Clinical Translation? Curr. Eye Res. 2016, 41, 769–782. [Google Scholar] [CrossRef]
- Porzionato, A.; Stocco, E.; Barbon, S.; Grandi, F.; Macchi, V.; De Caro, R. Tissue-engineered grafts from human decellularized extracellular matrices: A systematic review and future perspectives. Int. J. Mol. Sci. 2018, 19, 4117. [Google Scholar] [CrossRef]
- González-Andrades, M.; Carriel, V.; Rivera-Izquierdo, M.; Garzón, I.; González-Andrades, E.; Medialdea, S.; Alaminos, M.; Campos, A. Effects of Detergent-Based Protocols on Decellularization of Corneas With Sclerocorneal Limbus. Evaluation of Regional Differences. Transl. Vis. Sci. Technol. 2015, 4, 13. [Google Scholar] [CrossRef] [PubMed]
- Wilson, S.; Sidney, L.; Dunphy, S.; Rose, J.; Hopkinson, A. Keeping an Eye on Decellularized Corneas: A Review of Methods, Characterization and Applications. J. Funct. Biomater. 2013, 4, 114–161. [Google Scholar] [CrossRef]
- Fernández-Pérez, J.; Ahearne, M. Decellularization and recellularization of cornea: Progress towards a donor alternative. Methods 2020, 171, 86–96. [Google Scholar] [CrossRef] [PubMed]
- Kinoshita, S.; Koizumi, N.; Ueno, M.; Okumura, N.; Imai, K.; Tanaka, H.; Yamamoto, Y.; Nakamura, T.; Inatomi, T.; Bush, J.; et al. Injection of Cultured Cells with a ROCK Inhibitor for Bullous Keratopathy. N. Engl. J. Med. 2018, 378, 995–1003. [Google Scholar] [CrossRef]
- Rizwan, M.; Peh, G.S.L.; Ang, H.P.; Lwin, N.C.; Adnan, K.; Mehta, J.S.; Tan, W.S.; Yim, E.K.F. Sequentially-crosslinked bioactive hydrogels as nano-patterned substrates with customizable stiffness and degradation for corneal tissue engineering applications. Biomaterials 2017, 120, 139–154. [Google Scholar] [CrossRef]
- Koizumi, N.; Sakamoto, Y.; Okumura, N.; Tsuchiya, H.; Torii, R.; Cooper, L.J.; Ban, Y.; Tanioka, H.; Kinoshita, S. Cultivated corneal endothelial transplantation in a primate: Possible future clinical application in corneal endothelial regenerative medicine. Cornea 2008, 27, S48–S55. [Google Scholar] [CrossRef]
- Bayyoud, T.; Thaler, S.; Hofmann, J.; Maurus, C.; Spitzer, M.S.; Bartz-Schmidt, K.U.; Szurman, P.; Yoeruek, E. Decellularized bovine corneal posterior lamellae as carrier matrix for cultivated human corneal endothelial cells. Curr. Eye Res. 2012, 37, 179–186. [Google Scholar] [CrossRef]
- Yoshida, J.; Oshikata-Miyazaki, A.; Yokoo, S.; Yamagami, S.; Takezawa, T.; Amano, S. Development and evaluation of porcine atelocollagen vitrigel membrane with a spherical curve and transplantable artificial corneal endothelial grafts. Investig. Ophthalmol. Vis. Sci. 2014, 55, 4975–4981. [Google Scholar] [CrossRef] [PubMed]
- Fan, T.; Ma, X.; Zhao, J.; Wen, Q.; Hu, X.; Yu, H.; Shi, W. Transplantation of tissue-engineered human corneal endothelium in cat models. Mol. Vis. 2013, 19, 400–407. [Google Scholar] [CrossRef] [PubMed]
- Yoeruek, E.; Bayyoud, T.; Maurus, C.; Hofmann, J.; Spitzer, M.S.; Bartz-Schmidt, K.U.; Szurman, P. Decellularization of porcine corneas and repopulation with human corneal cells for tissue-engineered xenografts. Acta Ophthalmol. 2012, 90, e125–e131. [Google Scholar] [CrossRef]
- Ozcelik, B.; Brown, K.D.; Blencowe, A.; Ladewig, K.; Stevens, G.W.; Scheerlinck, J.P.Y.; Abberton, K.; Daniell, M.; Qiao, G.G. Biodegradable and biocompatible poly(ethylene glycol)-based hydrogel films for the regeneration of corneal endothelium. Adv. Healthc. Mater. 2014, 3, 1496–1507. [Google Scholar] [CrossRef] [PubMed]
- Gao, X.; Liu, W.; Han, B.; Wei, X.; Yang, C. Preparation and properties of a chitosan-based carrier of corneal endothelial cells. J. Mater. Sci. Mater. Med. 2008, 19, 3611–3619. [Google Scholar] [CrossRef]
- Arnalich-Montiel, F.; Moratilla, A.; Fuentes-Julián, S.; Aparicio, V.; Cadenas Martin, M.; Peh, G.; Mehta, J.S.; Adnan, K.; Porrua, L.; Pérez-Sarriegui, A.; et al. Treatment of corneal endothelial damage in a rabbit model with a bioengineered graft using human decellularized corneal lamina and cultured human corneal endothelium. PLoS ONE 2019, 14, e0225480. [Google Scholar] [CrossRef]
- Yam, G.H.-F.; Yusoff, N.Z.B.M.; Kadaba, A.; Tian, D.; Myint, H.H.; Beuerman, R.W.; Zhou, L.; Mehta, J.S. Ex Vivo Propagation of Human Corneal Stromal “Activated Keratocytes” for Tissue Engineering. Cell Transplant. 2015, 24, 1845–1861. [Google Scholar] [CrossRef]
- Meek, K.M.; Knupp, C. Corneal structure and transparency. Prog. Retin. Eye Res. 2015, 49, 1–16. [Google Scholar] [CrossRef]
- Shin, T.J.; Vito, R.P.; Johnson, L.W.; McCarey, B.E. The distribution of strain in the human cornea. J. Biomech. 1997, 30, 497–503. [Google Scholar] [CrossRef]
- Hjortdal, J. Regional elastic performance of the human cornea. J. Biomech. 1996, 29, 931–942. [Google Scholar] [CrossRef]
- Randleman, J.B.; Dawson, D.G.; Grossniklaus, H.E.; McCarey, B.E.; Edelhauser, H.F. Depth-dependent Cohesive Tensile Strength in Human Donor Corneas: Implications for Refractive Surgery. J. Refract. Surg. 2008, 24, S85–S89. [Google Scholar] [CrossRef] [PubMed]
- Dias, J.; Diakonis, V.F.; Kankariya, V.P.; Yoo, S.H.; Ziebarth, N.M. Anterior and posterior corneal stroma elasticity after corneal collagen crosslinking treatment. Exp. Eye Res. 2013, 116, 58–62. [Google Scholar] [CrossRef] [PubMed]
- Elsheikh, A.; Wang, D.; Brown, M.; Rama, P.; Campanelli, M.; Pye, D. Assessment of corneal biomechanical properties and their variation with age. Curr. Eye Res. 2007, 32, 11–19. [Google Scholar] [CrossRef] [PubMed]
- Cartwright, N.E.K.; Tyrer, J.R.; Marshall, J. Age-related differences in the elasticity of the human cornea. Investig. Ophthalmol. Vis. Sci. 2011, 52, 4324–4329. [Google Scholar] [CrossRef]
- Dubbelman, M.; Sicam, V.A.D.P.; Van Der Heijde, G.L. The shape of the anterior and posterior surface of the aging human cornea. Vision Res. 2006, 46, 993–1001. [Google Scholar] [CrossRef] [PubMed]
- Murphy, S.V.; Atala, A. 3D bioprinting of tissues and organs. Nat. Biotechnol. 2014, 32, 773–785. [Google Scholar] [CrossRef]
- Leberfinger, A.N.; Dinda, S.; Wu, Y.; Koduru, S.V.; Ozbolat, V.; Ravnic, D.J.; Ozbolat, I.T. Bioprinting functional tissues. Acta Biomater. 2019, 95, 32–49. [Google Scholar] [CrossRef]
- Dubbin, K.; Tabet, A.; Heilshorn, S.C. Quantitative criteria to benchmark new and existing bio-inks for cell compatibility. Biofabrication 2017, 9, 044102. [Google Scholar] [CrossRef]
- Cidonio, G.; Glinka, M.; Dawson, J.I.; Oreffo, R.O.C. The cell in the ink: Improving biofabrication by printing stem cells for skeletal regenerative medicine. Biomaterials 2019, 209, 10–24. [Google Scholar] [CrossRef]
- Liu, W.; Heinrich, M.A.; Zhou, Y.; Akpek, A.; Hu, N.; Liu, X.; Guan, X.; Zhong, Z.; Jin, X.; Khademhosseini, A.; et al. Extrusion Bioprinting of Shear-Thinning Gelatin Methacryloyl Bioinks. Adv. Healthc. Mater. 2017, 6, 1601451. [Google Scholar] [CrossRef] [PubMed]
- Omidinia-Anarkoli, A.; Boesveld, S.; Tuvshindorj, U.; Rose, J.C.; Haraszti, T.; De Laporte, L. An Injectable Hybrid Hydrogel with Oriented Short Fibers Induces Unidirectional Growth of Functional Nerve Cells. Small 2017, 13, 1702207. [Google Scholar] [CrossRef] [PubMed]
- Shin, M.; Galarraga, J.H.; Kwon, M.Y.; Lee, H.; Burdick, J.A. Gallol-derived ECM-mimetic adhesive bioinks exhibiting temporal shear-thinning and stabilization behavior. Acta Biomater. 2019, 95, 165–175. [Google Scholar] [CrossRef]
- Foster, A.A.; Marquardt, L.M.; Heilshorn, S.C. The diverse roles of hydrogel mechanics in injectable stem cell transplantation. Curr. Opin. Chem. Eng. 2017, 15, 15–23. [Google Scholar] [CrossRef]
- Lindsay, C.D.; Roth, J.G.; Lesavage, B.L.; Heilshorn, S.C. Bioprinting of stem cell expansion lattices. Acta Biomater. 2019, 95, 225–235. [Google Scholar] [CrossRef]
- Phamduy, T.B.; Sweat, R.S.; Azimi, M.S.; Burow, M.E.; Murfee, W.L.; Chrisey, D.B. Printing cancer cells into intact microvascular networks: A model for investigating cancer cell dynamics during angiogenesis. Integr. Biol. (United Kingdom) 2015, 7, 1068–1078. [Google Scholar] [CrossRef]
- Betsch, M.; Cristian, C.; Lin, Y.-Y.; Blaeser, A.; Schöneberg, J.; Vogt, M.; Buhl, E.M.; Fischer, H.; Duarte Campos, D.F. Incorporating 4D into Bioprinting: Real-Time Magnetically Directed Collagen Fiber Alignment for Generating Complex Multilayered Tissues. Adv. Healthc. Mater. 2018, 7, 1800894. [Google Scholar] [CrossRef]
- Bernal, P.N.; Delrot, P.; Loterie, D.; Li, Y.; Malda, J.; Moser, C.; Levato, R. Volumetric Bioprinting of Complex Living-Tissue Constructs within Seconds. Adv. Mater. 2019, 31, 1904209. [Google Scholar] [CrossRef] [PubMed]
- Grigoryan, B.; Paulsen, S.J.; Corbett, D.C.; Sazer, D.W.; Fortin, C.L.; Zaita, A.J.; Greenfield, P.T.; Calafat, N.J.; Gounley, J.P.; Ta, A.H.; et al. Multivascular networks and functional intravascular topologies within biocompatible hydrogels. Science 2019, 364, 458–464. [Google Scholar] [CrossRef] [PubMed]
- Skylar-Scott, M.A.; Uzel, S.G.M.; Nam, L.L.; Ahrens, J.H.; Truby, R.L.; Damaraju, S.; Lewis, J.A. Biomanufacturing of organ-specific tissues with high cellular density and embedded vascular channels. Sci. Adv. 2019, 5, eaaw2459. [Google Scholar] [CrossRef]
- Lee, A.; Hudson, A.R.; Shiwarski, D.J.; Tashman, J.W.; Hinton, T.J.; Yerneni, S.; Bliley, J.M.; Campbell, P.G.; Feinberg, A.W. 3D bioprinting of collagen to rebuild components of the human heart. Science 2019, 365, 482–487. [Google Scholar] [CrossRef] [PubMed]
- Gouveia, R.M.; Lepert, G.; Gupta, S.; Mohan, R.R.; Paterson, C.; Connon, C.J. Assessment of corneal substrate biomechanics and its effect on epithelial stem cell maintenance and differentiation. Nat. Commun. 2019, 10, 1–17. [Google Scholar] [CrossRef] [PubMed]
- Sorkio, A.; Koch, L.; Koivusalo, L.; Deiwick, A.; Miettinen, S.; Chichkov, B.; Skottman, H. Human stem cell based corneal tissue mimicking structures using laser-assisted 3D bioprinting and functional bioinks. Biomaterials 2018, 171, 57–71. [Google Scholar] [CrossRef] [PubMed]
- Isaacson, A.; Swioklo, S.; Connon, C.J. 3D bioprinting of a corneal stroma equivalent. Exp. Eye Res. 2018, 173, 188–193. [Google Scholar] [CrossRef] [PubMed]
- Duarte Campos, D.F.; Rohde, M.; Ross, M.; Anvari, P.; Blaeser, A.; Vogt, M.; Panfil, C.; Yam, G.H.; Mehta, J.S.; Fischer, H.; et al. Corneal bioprinting utilizing collagen-based bioinks and primary human keratocytes. J. Biomed. Mater. Res. Part A 2019, 107, 1945–1953. [Google Scholar] [CrossRef]
- Kong, B.; Chen, Y.; Liu, R.; Liu, X.; Liu, C.; Shao, Z.; Xiong, L.; Liu, X.; Sun, W.; Mi, S. Fiber reinforced GelMA hydrogel to induce the regeneration of corneal stroma. Nat. Commun. 2020, 11, 1–12. [Google Scholar] [CrossRef]
- Kilic Bektas, C.; Hasirci, V. Cell loaded 3D bioprinted GelMA hydrogels for corneal stroma engineering. Biomater. Sci. 2020, 8, 438–449. [Google Scholar] [CrossRef]
- Kim, H.; Park, M.-N.; Kim, J.; Jang, J.; Kim, H.-K.; Cho, D.-W. Characterization of cornea-specific bioink: High transparency, improved in vivo safety. J. Tissue Eng. 2019, 10, 204173141882338. [Google Scholar] [CrossRef]
- Zhang, B.; Xue, Q.; Hu, H.; Yu, M.; Gao, L.; Luo, Y.; Li, Y.; Li, J.; Ma, L.; Yao, Y.; et al. Integrated 3D bioprinting-based geometry-control strategy for fabricating corneal substitutes. J. Zhejiang Univ. B 2019, 20, 945–959. [Google Scholar] [CrossRef]
- Kim, K.W.; Lee, S.J.; Park, S.H.; Kim, J.C. Ex Vivo Functionality of 3D Bioprinted Corneal Endothelium Engineered with Ribonuclease 5-Overexpressing Human Corneal Endothelial Cells. Adv. Healthc. Mater. 2018, 7, 1800398. [Google Scholar] [CrossRef]
| Corneal Layer | Bioprinting Method | Materials for Bioink | Cell Type | Cell Elongation | Mechanical Properties | Transparency Evaluation | Reference |
|---|---|---|---|---|---|---|---|
| Native cornea | [60] | ||||||
| Stroma | CSKs/LSSCs | Dendritic up to 100 µm | 100–250 kPa | 80% at 380 nm and | |||
| Epithelium | CEpCs/LECs | Round/polygonal tight monolayer | Fragile | >90% at 500–1300 nm | |||
| Endothelium | CECs | Hexagonal tight monolayer | Fragile | ||||
| Stroma | Laser | Matrigel-COL I bioink // LN-COL IV support sheet | Human LECs+ ADSCs | Filopodial elongation up to 50 µm | n.a. | Qualitative: slightly opaque | [84] |
| Extrusion | 1.3% ALG-2.7% COL I bioink // FRESH support | Human CSKs | Round cells | n.a. | Qualitative: see through gel | [85] | |
| Drop-on-demand | 0.2% COL I-0.5% AG bioink // no support | Human CSKs | Filopodial elongation up to 100 µm | 15–20 kPa | Qualitative: see through gel | [86] | |
| Extrusion | 15% GelMa bioink // reinforced with PEG-PCL fibers | Rat LSSCs | Filopodial elongation up to 50 µm | 60–120 kPa | Qualitative: see through gel | [87] | |
| Extrusion | 15% GelMa bioink // no support | Human CSKs | Round cells | 10–20 kPa | Quantitative: 80% at 700 nm 5% at UVB | [88] | |
| Extrusion | Cornea-derived dECM bioink // no support | Human TDMSCs with keratocyte induction | Filopodial elongation up to 50 µm | 70–100 kPa | Quantitative: 80% at 700 nm 70% at UVB | [89] | |
| Epithelium | Extrusion | 15% GelMa bioink // 15% GelMa dome-shaped mold | Human CEpCs line | Round cells | 50 kPa | Qualitative: see through gel | [90] |
| Endothelium | Extrusion | Gelatin-RGD bioink // amniotic membrane dECM support | Human CECs | Round cells | n.a. | n.a. | [91] |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fuest, M.; Yam, G.H.-F.; Mehta, J.S.; Duarte Campos, D.F. Prospects and Challenges of Translational Corneal Bioprinting. Bioengineering 2020, 7, 71. https://doi.org/10.3390/bioengineering7030071
Fuest M, Yam GH-F, Mehta JS, Duarte Campos DF. Prospects and Challenges of Translational Corneal Bioprinting. Bioengineering. 2020; 7(3):71. https://doi.org/10.3390/bioengineering7030071
Chicago/Turabian StyleFuest, Matthias, Gary Hin-Fai Yam, Jodhbir S. Mehta, and Daniela F. Duarte Campos. 2020. "Prospects and Challenges of Translational Corneal Bioprinting" Bioengineering 7, no. 3: 71. https://doi.org/10.3390/bioengineering7030071
APA StyleFuest, M., Yam, G. H.-F., Mehta, J. S., & Duarte Campos, D. F. (2020). Prospects and Challenges of Translational Corneal Bioprinting. Bioengineering, 7(3), 71. https://doi.org/10.3390/bioengineering7030071








