Culture Time Needed to Scale up Infrapatellar Fat Pad Derived Stem Cells for Cartilage Regeneration: A Systematic Review
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Design and Literature Search
- Infrapatellar fat pad
- Stem cell OR mesenchymal stem cell OR adipose-derived mesenchymal stem cell
2.2. Study Selection and Eligibility Criteria
2.3. Data Extraction
2.4. Quality Control and Assessment
2.5. Minimum Time Required for Regenerative Therapy Using IFP-Derived hADSCs
3. Results
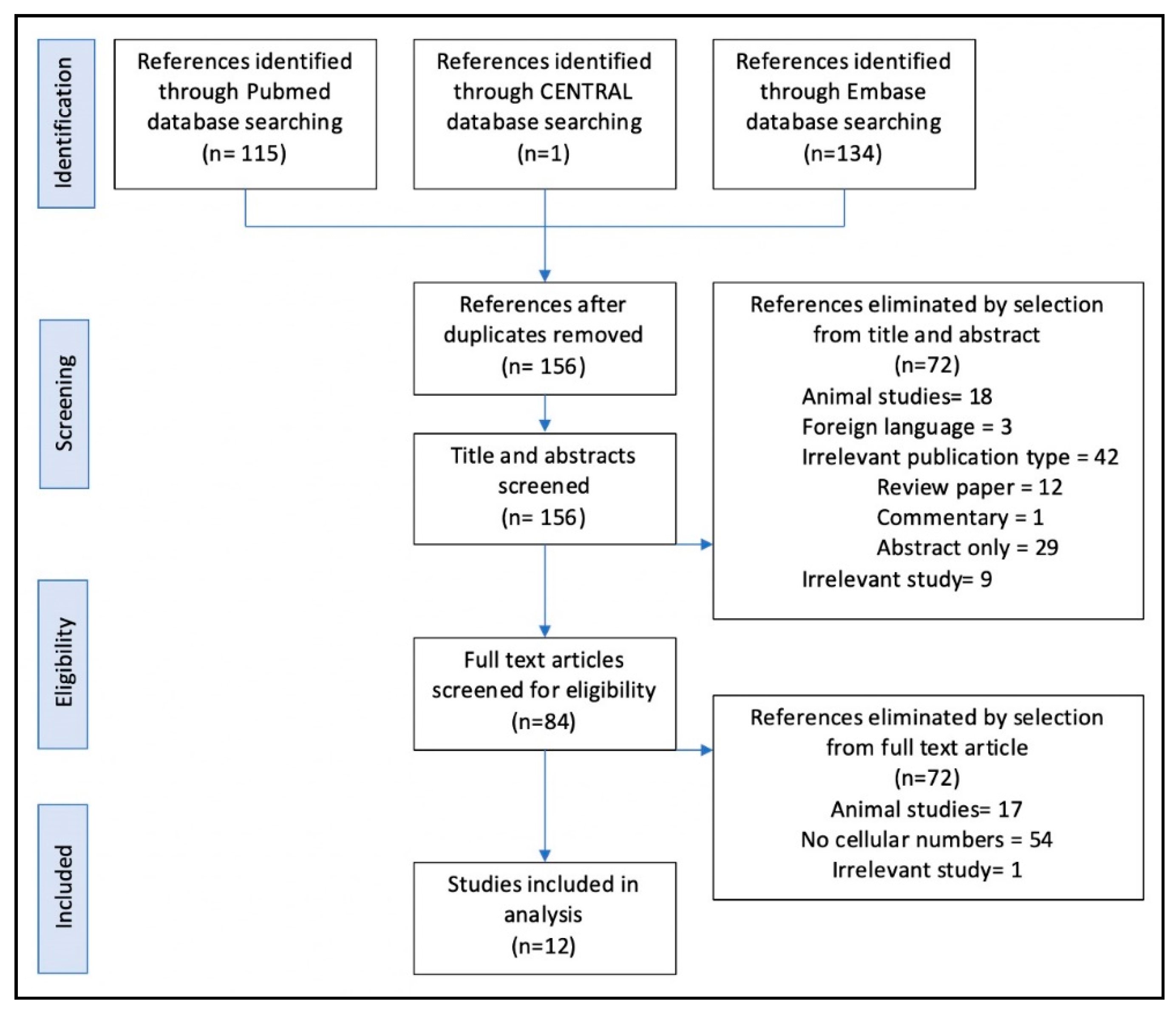
3.1. Search Results
3.2. Included Studies
3.3. Study Outcomes
3.3.1. Arthroscopic Based Harvest of IFP
3.3.2. Open Arthrotomy Based Harvest of IFP
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| SVF | Stromal vascular fraction |
| IFP | Infrapatellar fat pad |
| MSCs | Mesenchymal stem cells |
| hADSCs | Human adipose-derived mesenchymal stem cell |
| CENTRAL | Cochrane Central Register of Controlled Trials |
| PRISMA | Preferred Reporting Items for Systematic Reviews and Meta-Analyses |
References
- Bunnell, B.A.; Flaat, M.; Gagliardi, C.; Patel, B.; Ripoll, C. Adipose-Derived stem cells: Isolation, expansion and differentiation. Methods 2008, 45, 115–120. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zuk, P.A.; Zhu, M.; Mizuno, H.; Huang, J.; Futrell, J.W.; Katz, A.J.; Benhaim, P.; Lorenz, H.P.; Hedrick, M.H. Multilineage Cells from Human Adipose Tissue: Implications for Cell-Based Therapies. Tissue Eng. 2001, 7, 211–228. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Becker, A.; Mc Culloch, C.E.; Till, J.E. Cytological demonstration of the clonal nature of spleen colonies derived from transplanted mouse marrow cells. Nature 1963, 197, 452–454. [Google Scholar] [CrossRef] [PubMed]
- Friedenstein, A.J.; Deriglasova, U.F.; Kulagina, N.N.; Panasuk, A.F.; Rudakowa, S.F.; Luriá, E.A.; Ruadkow, I.A. Precursors for fibroblasts in different populations of hematopoietic cells as detected by the in vitro colony assay method. Exp. Hematol. 1974, 2, 83–92. [Google Scholar] [PubMed]
- Pittenger, M.; Mackay, A.; Beck, S.; Jaiswal, R.; Douglas, R.; Mosca, J.; Moorman, M.; Simonetti, D.; Craig, S.; Marshak, D. Multilineage Potential of Adult Human Mesenchymal Stem Cells. Science 1999, 284, 143–147. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rada, T.; Reis, R.L.; Gomes, M.E. Adipose Tissue-Derived Stem Cells and Their Application in Bone and Cartilage Tissue Engineering. Tissue Eng. Part B Rev. 2009, 15, 113–125. [Google Scholar] [CrossRef] [Green Version]
- Veronesi, F.; Maglio, M.; Tschon, M.; Aldini, N.N.; Fini, M. Adipose-Derived mesenchymal stem cells for cartilage tissue engineering: State-of-The-Art in in vivo studies. J. Biomed. Mater. Res. Part A 2013, 102, 2448–2466. [Google Scholar] [CrossRef]
- Ioan-Facsinay, A.; Kloppenburg, M. An emerging player in knee osteoarthritis: The infrapatellar fat pad. Arthritis Res. 2013, 15, 225. [Google Scholar] [CrossRef] [Green Version]
- Stanco, D.; De Girolamo, L.; Sansone, V.; Moretti, M. Donor-Matched mesenchymal stem cells from knee infrapatellar and subcutaneous adipose tissue of osteoarthritic donors display differential chondrogenic and osteogenic commitment. Eur. Cells Mater. 2014, 27, 298–311. [Google Scholar] [CrossRef]
- Sheng, G. The developmental basis of mesenchymal stem/stromal cells (MSCs). BMC Dev. Biol. 2015, 15, 44. [Google Scholar] [CrossRef] [Green Version]
- Spasovski, D.; Spasovski, V.; Bascarevic, Z.; Stojiljkovic, M.; Vreca, M.; Andelkovic, M.; Pavlović, S. Intra-articular injection of autologous adipose-derived mesenchymal stem cells in the treatment of knee osteoarthritis. J. Gene Med. 2018, 20, e3002. [Google Scholar] [CrossRef]
- Di Bella, C.; Duchi, S.; O’Connell, C.D.; Blanchard, R.; Augustine, C.; Yue, Z.; Thompson, F.; Richards, C.; Beirne, S.; Onofrillo, C.; et al. In situ handheld three-dimensional bioprinting for cartilage regeneration. J. Tissue Eng. Regen. Med. 2017, 12, 611–621. [Google Scholar] [CrossRef] [PubMed]
- Vahedi, P.; Jarolmasjed, S.; Shafaei, H.; Roshangar, L.; Rad, J.S.; Ahmadian, E. In vivo articular cartilage regeneration through infrapatellar adipose tissue derived stem cell in nanofiber polycaprolactone scaffold. Tissue Cell 2019, 57, 49–56. [Google Scholar] [CrossRef] [PubMed]
- Halme, D.G.; Kessler, D.A. FDA Regulation of Stem-Cell–Based Therapies. N. Engl. J. Med. 2006, 355, 1730–1735. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hunziker, E. Articular cartilage repair: Basic science and clinical progress. A review of the current status and prospects. Osteoarthr. Cartil. 2002, 10, 432–463. [Google Scholar] [CrossRef] [Green Version]
- Hjelle, K.; Solheim, E.; Strand, T.; Muri, R.; Brittberg, M. Articular cartilage defects in 1000 knee arthroscopies. Arthroscopy 2002, 18, 730–734. [Google Scholar] [CrossRef]
- Garcia, J.; Wright, K.; Roberts, S.; Kuiper, J.H.; Mangham, C.; Richardson, J.; Mennan, C. Characterisation of synovial fluid and infrapatellar fat pad derived mesenchymal stromal cells: The influence of tissue source and inflammatory stimulus. Sci. Rep. 2016, 6, 24295. [Google Scholar] [CrossRef] [Green Version]
- Kokai, L.E.; Traktuev, D.O.; Zhang, L.; Merfeld-Clauss, S.; Dibernardo, G.; Lu, H.; Marra, K.G.; Donnenberg, A.; Meyer, E.M.; Fodor, P.B.; et al. Adipose Stem Cell Function Maintained with Age: An Intra-Subject Study of Long-Term Cryopreserved Cells. Aesthet. Surg. J. 2016, 37. [Google Scholar] [CrossRef] [Green Version]
- Tsekouras, A.; Mantas, D.; Tsilimigras, D.I.; Moris, D.; Kontos, M.; Zografos, C.G. Comparison of the Viability and Yield of Adipose-Derived Stem Cells (ASCs) from Different Donor Areas. In Vivo 2017, 31, 1229–1234. [Google Scholar] [CrossRef] [Green Version]
- Dominici, M.; Le Blanc, K.; Mueller, I.; Slaper-Cortenbach, I.; Marini, F.; Krause, D.; Deans, R.; Keating, A.; Prockop, D.; Horwitz, E. Minimal criteria for defining multipotent mesenchymal stromal cells. The International Society for Cellular Therapy position statement. Cytotherapy 2006, 8, 315–317. [Google Scholar] [CrossRef]
- Krampera, M.; Galipeau, J.; Shi, Y.; Tarte, K.; Sensebe, L. MSC Committee of the International Society for Cellular Therapy (ISCT). Immunological characterization of multipotent mesenchymal stromal cells—The International Society for Cellular Therapy (ISCT) working proposal. Cytotherapy 2013, 15, 8. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liberati, A.; Altman, U.G.; Tetzlaff, J.; Mulrow, C.; Gøtzsche, P.C.; Ioannidis, J.P.A.; Clarke, M.; Devereaux, P.J.; Kleijnen, J.; Moher, D. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate healthcare interventions: Explanation and elaboration. BMJ 2009, 339, b2700. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mantripragada, V.P.; Piuzzi, N.S.; Bova, W.A.; Boehm, C.; Obuchowski, N.A.; Lefebvre, V.; Midura, R.J.; Muschler, G. Donor-Matched comparison of chondrogenic progenitors resident in human infrapatellar fat pad, synovium, and periosteum—Implications for cartilage repair. Connect. Tissue Res. 2019, 60, 597–610. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lopez-Ruiz, E.; Jimenez, G.; Kwiatkowski, W.; Montanez, E.; Arrebola, F.; Carrillo, E.; Choe, S.; Marchal, J.A.; Perán, M. Impact of TGF-beta family-related growth factors on chondrogenic differentiation of adipose-derived stem cells isolated from lipoaspirates and infrapatellar fat pads of osteoarthritic patients. Eur. Cell Mater. 2018, 35, 209–224. [Google Scholar] [CrossRef]
- Bravo, B.; Guisasola, M.C.; Vaquero, J.; Tirado, I.; Gortazar, A.R.; Forriol, F. Gene expression, protein profiling, and chemotactic activity of infrapatellar fat pad mesenchymal stem cells in pathologies of the knee joint. J. Cell. Physiol. 2019, 234, 18917–18927. [Google Scholar] [CrossRef]
- Wang, B.; Liu, W.; Xing, D.; Li, R.; Lv, C.; Li, Y.; Yan, X.; Ke, Y.; Xu, Y.; Du, Y.; et al. Injectable nanohydroxyapatite-chitosan-gelatin micro-scaffolds induce regeneration of knee subchondral bone lesions. Sci. Rep. 2017, 7, 16709. [Google Scholar] [CrossRef] [Green Version]
- Dragoo, J.; Chang, W. Arthroscopic Harvest of Adipose-Derived Mesenchymal Stem Cells from the Infrapatellar Fat Pad. Am. J. Sports Med. 2017, 45, 3119–3127. [Google Scholar] [CrossRef]
- Dragoo, J.L.; Samimi, B.; Zhu, M.; Hame, S.L.; Thomas, B.J.; Lieberman, J.R.; Hedrick, M.H.; Benhaim, P. Tissue-engineered cartilage and bone using stem cells from human infrapatellar fat pads. J. Bone Jt. Surg. Br. Vol. 2003, 85, 740–747. [Google Scholar] [CrossRef] [Green Version]
- Muñoz-Criado, I.; Meseguer-Ripolles, J.; Mellado-López, M.; Alastrue-Agudo, A.; Griffeth, R.J.; Forteza-Vila, J.; Cugat, R.; García, M.; Moreno-Manzano, V. Human Suprapatellar Fat Pad-Derived Mesenchymal Stem Cells Induce Chondrogenesis and Cartilage Repair in a Model of Severe Osteoarthritis. Stem Cells Int. 2017, 2017, 1–12. [Google Scholar] [CrossRef] [Green Version]
- Neri, S.; Guidotti, S.; Lilli, N.L.; Cattini, L.; Mariani, E. Infrapatellar fat pad-derived mesenchymal stromal cells from osteoarthritis patients: In vitro genetic stability and replicative senescence. J. Orthop. Res. 2016, 35, 1029–1037. [Google Scholar] [CrossRef] [Green Version]
- Tangchitphisut, P.; Srikaew, N.; Numhom, S.; Tangprasittipap, A.; Woratanarat, P.; Wongsak, S.; Kijkunasathian, C.; Hongeng, S.; Murray, I.R.; Tawonsawatruk, T. Infrapatellar Fat Pad: An Alternative Source of Adipose-Derived Mesenchymal Stem Cells. Arthritis 2016, 2016, 1–10. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jurgens, W.J.; Van Dijk, A.; Doulabi, B.Z.; Niessen, F.B.; Ritt, M.J.; Van Milligen, F.J.; Helder, M.N. Freshly isolated stromal cells from the infrapatellar fat pad are suitable for a one-step surgical procedure to regenerate cartilage tissue. Cytotherapy 2009, 11, 1052–1064. [Google Scholar] [CrossRef] [PubMed]
- Wickham, M.Q.; Erickson, G.R.; Gimble, J.M.; Vail, T.P.; Guilak, F. Multipotent Stromal Cells Derived from the Infrapatellar Fat Pad of the Knee. Clin. Orthop. Relat. Res. 2003, 412, 196–212. [Google Scholar] [CrossRef] [PubMed]
- Koh, Y.-G.; Choi, Y.-J. Infrapatellar fat pad-derived mesenchymal stem cell therapy for knee osteoarthritis. Knee 2012, 19, 902–907. [Google Scholar] [CrossRef] [PubMed]
| Inclusion Criteria | Exclusion Criteria |
|---|---|
| Human participants ≥ 18 years old | Conference abstracts and non-original papers (commentaries, reviews, letters or editorials) |
| Studies written in English | Animal studies |
| Clinical studies, multi-centre studies, observational studies, clinical trials, randomised control trials, meta-analyses and systematic reviews | Studies conducted in children, adolescents or pregnant women |
| Measurement of MSCs derived from IFP only | Non-IFP-derived MSCs or studies lacking specific data relating to IFP or MSC numbers |
| Explicit measurement of the quantitative value of MSCs found per participant | Off-topic studies |
| Study | Country | Patient Demographics | Harvest Procedure (Surgical Indication) | IFP Tissue Yield | Cellular Data/Yield |
|---|---|---|---|---|---|
| Mantripragada et al. 2019 [23] | USA | n = 28 Mean age: 63.1 (37–82) F = 16, M = 12 | TKA (Idiopathic OA) | 2.5 mL of IFP | SVF = 4320 cells per mL of IFP |
| Lopez-Ruiz et al. 2018 [24] | Spain | n = 8 Mean age: 66.9 (57–74) F = 4, M = 4 | TKA (Idiopathic OA) | Not described | SVF = 1.1 × 105 cells per gram of IFP (0.9–1.3 × 105) |
| Bravo et al. 2019 [25] | Spain | n = 21 ≥ Arthroscopic ACL repair Median age: 32 (21–50) F = 4, M = 17 n = 21 ≥ TKA Median age: 74 (57–84) F = 15, M = 6 | Arthroscopic repair (ACL rupture) Or TKA (OA) | ≥3 mL of adipose extracted from IFP ≥3 mL of adipose extracted from IFP | 6.0 × 105–8.0 × 105 cells |
| Wang et al. 2017 [26] | China | n = 4 | TKA (No stated indication) | 10 g of IFP | Primary culture (SVF) ≥ Approx. 5 × 105/g of IFP |
| Dragoo et al. 2017 [27] | USA | n = 7 Median age: 35.14 (17–52) F = 6, M = 1 | Arthroscopic repair (ACL rupture with no evidence of OA) | Not described | SVF = 4.86 × 105 cells 0.19–0.33 × 105 cells per gram of IFP |
| Munoz-Criado et al. 2017 [29] | Spain | n = 24 Ages: 50–80 | TKA (Idiopathic OA) | Not described | SVF = 7.8 × 105 ± 2.8 × 105 cells |
| Neri et al. 2017 [30] | Italy | n = 11 Mean age: 69.4 ± 6.5 | TKA (Idiopathic OA) | 5–15 g IFP obtained | SVF = Average of 7.0 × 105 cells per gram of IFP |
| Tangchitphisut et al. 2016 [31] | Thailand | n = 5 Mean age: 65.8 (53–77) F = 5, M = 0 | TKA (Idiopathic OA) | Average IFP weight 12.12 ± 2.57 g (8.48–14.75) | SVF: 3.94 × 106 ± 3.73 × 105 cells |
| Koh et al. 2012 [34] | South Korea | n = 25 Mean age: 54.2 ± 9.3 (34–69) F = 17, M = 8 | Arthroscopic harvest (secondary OA) | Average IFP weight 9.4 g (6.9–11.2) | SVF (described as MSC count in the paper) = 1.89 × 106 (1.2–2.3 × 106) |
| Jurgens et al. 2009 [32] | Netherlands | n = 53 Median age: 72 (43–89) | TKA (Idiopathic OA) | Average IFP weight 15.1 ± 5.8 g (8.7–26.3) | SVF: 4.0 × 106 ± 4.45 × 105 cells |
| Dragoo et al. 2003 [28] | USA | n = 5 Mean age: 74 (53 to 86) | TKA (no indication stated) | Average IFP volume 20.6 mL (15–25) | SVF mean yield of 5.5 × 106 extracted cells per IFP (2.0 × 106 to 1.2 × 107) |
| Wickham et al. 2003 [33] | USA | n = 16 Mean age: 68 ± 11.1 (49–82) | TKA (no indication stated) | Average IFP weight 21.5 ± 8.8 g | Not described |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Francis, S.L.; Yao, A.; Choong, P.F.M. Culture Time Needed to Scale up Infrapatellar Fat Pad Derived Stem Cells for Cartilage Regeneration: A Systematic Review. Bioengineering 2020, 7, 69. https://doi.org/10.3390/bioengineering7030069
Francis SL, Yao A, Choong PFM. Culture Time Needed to Scale up Infrapatellar Fat Pad Derived Stem Cells for Cartilage Regeneration: A Systematic Review. Bioengineering. 2020; 7(3):69. https://doi.org/10.3390/bioengineering7030069
Chicago/Turabian StyleFrancis, Sam L., Angela Yao, and Peter F. M. Choong. 2020. "Culture Time Needed to Scale up Infrapatellar Fat Pad Derived Stem Cells for Cartilage Regeneration: A Systematic Review" Bioengineering 7, no. 3: 69. https://doi.org/10.3390/bioengineering7030069
APA StyleFrancis, S. L., Yao, A., & Choong, P. F. M. (2020). Culture Time Needed to Scale up Infrapatellar Fat Pad Derived Stem Cells for Cartilage Regeneration: A Systematic Review. Bioengineering, 7(3), 69. https://doi.org/10.3390/bioengineering7030069




