Material Characterization and Substrate Suitability Assessment of Chicken Manure for Dry Batch Anaerobic Digestion Processes
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Analytical Methods
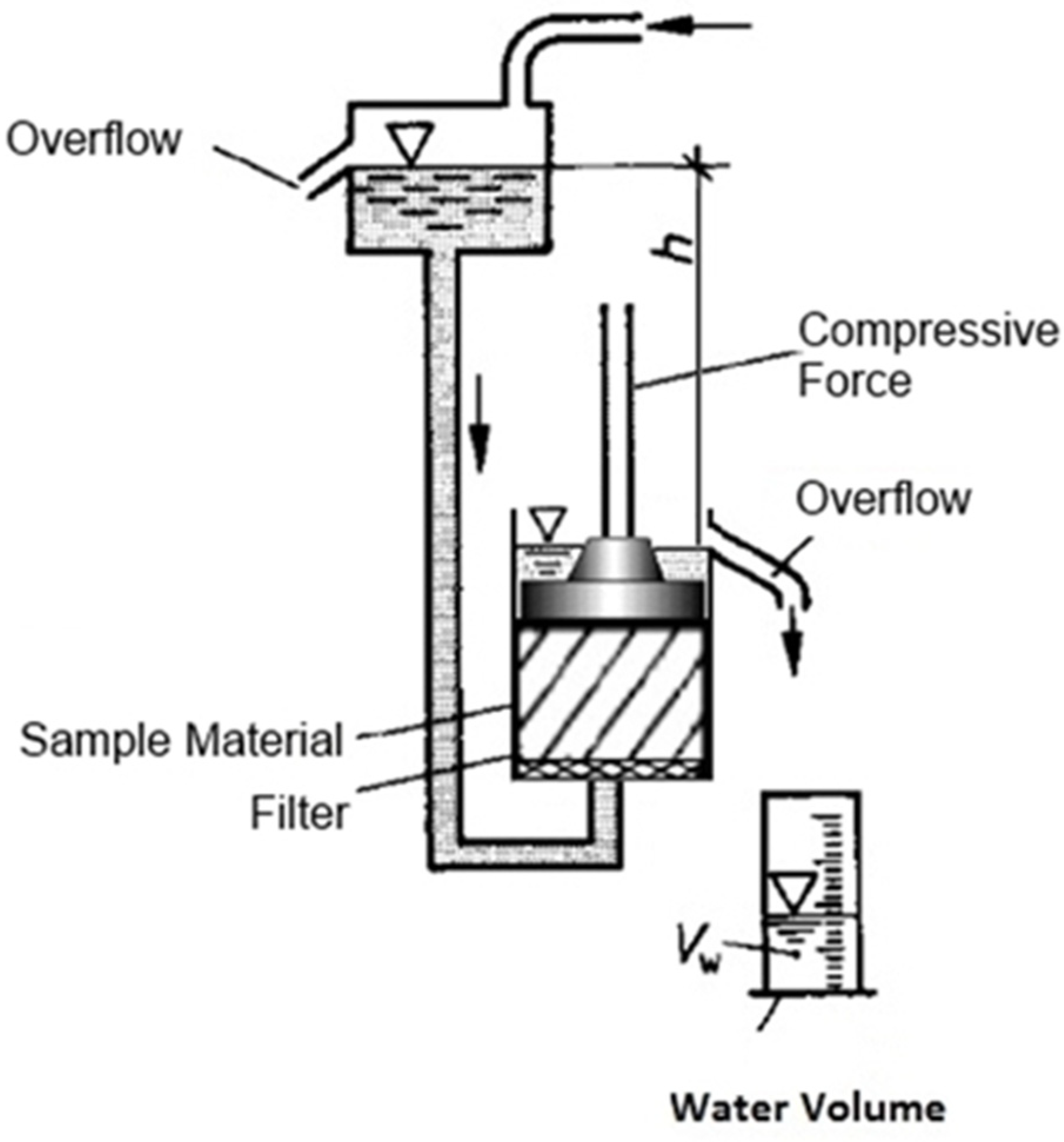
2.3. Measurement of the Compressibility and Permeability under Compaction
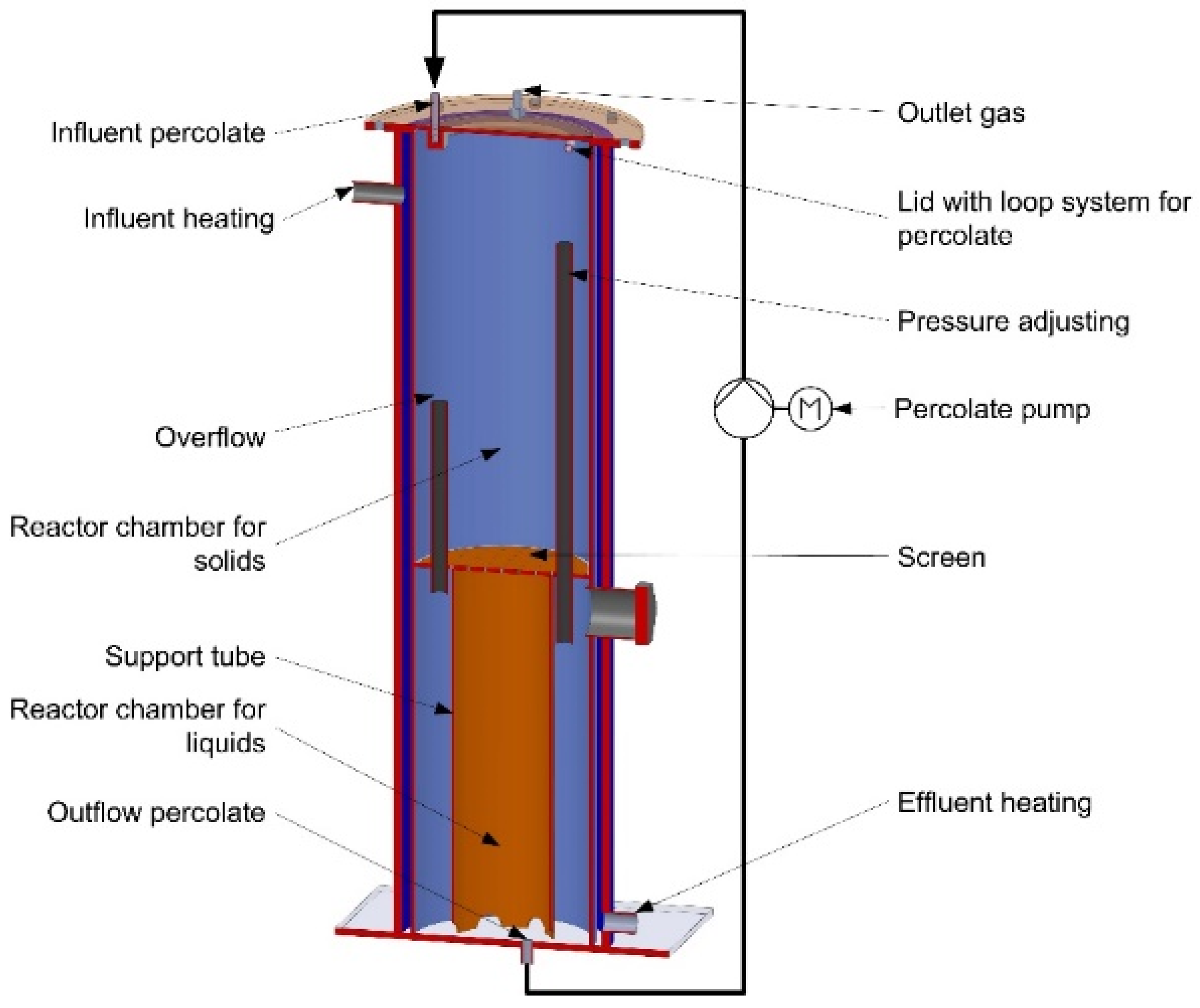
2.4. Dry Anaerobic Batch Digestion Trials
2.5. Statistical Analyses
3. Results and Discussion
3.1. Material Characteristics of Chicken Manure
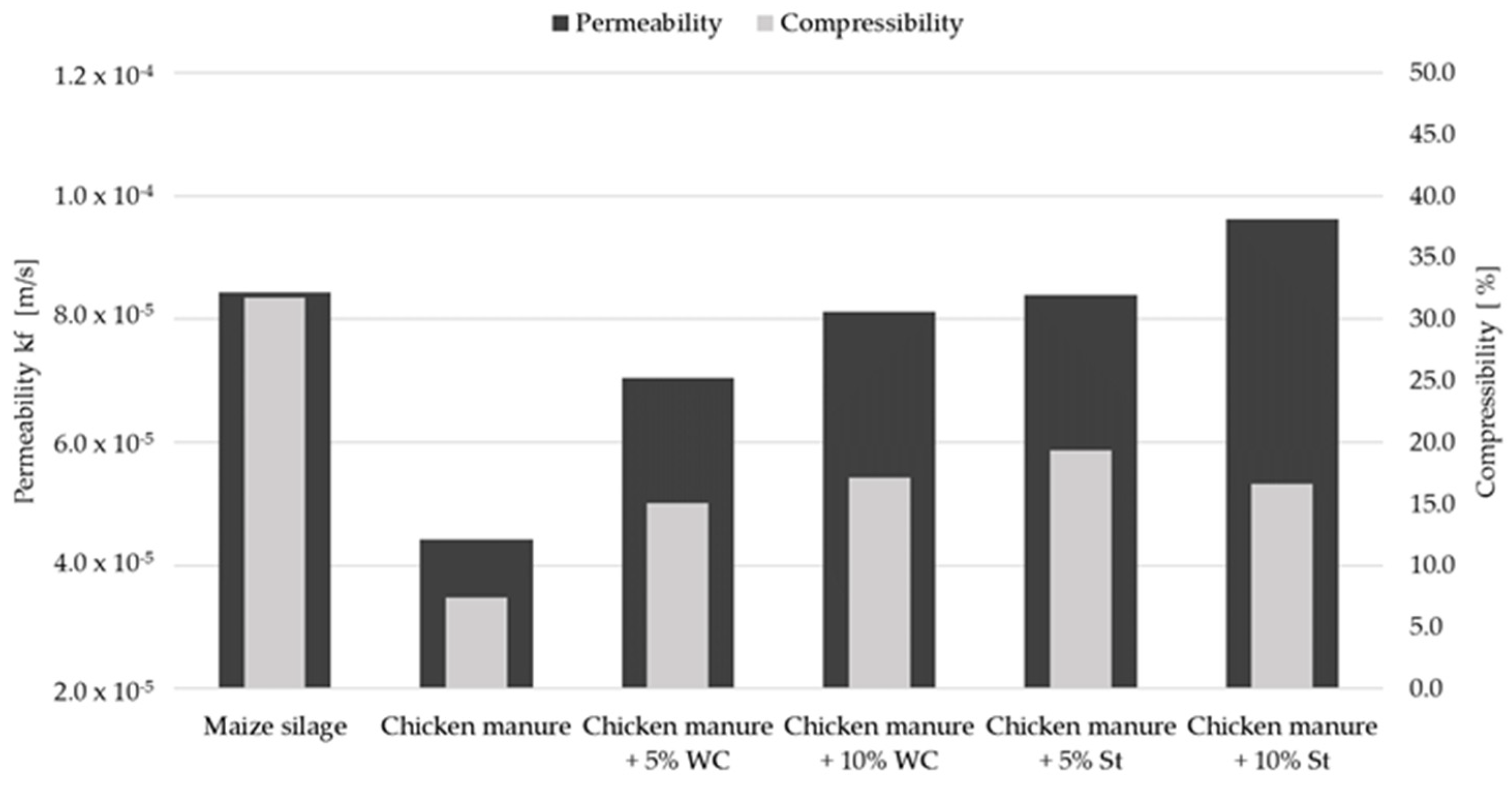
3.2. Material Permeability of Chicken Manure
3.3. Results of Dry Batch Anaerobic Digestion Trials
3.3.1. Process Conditions
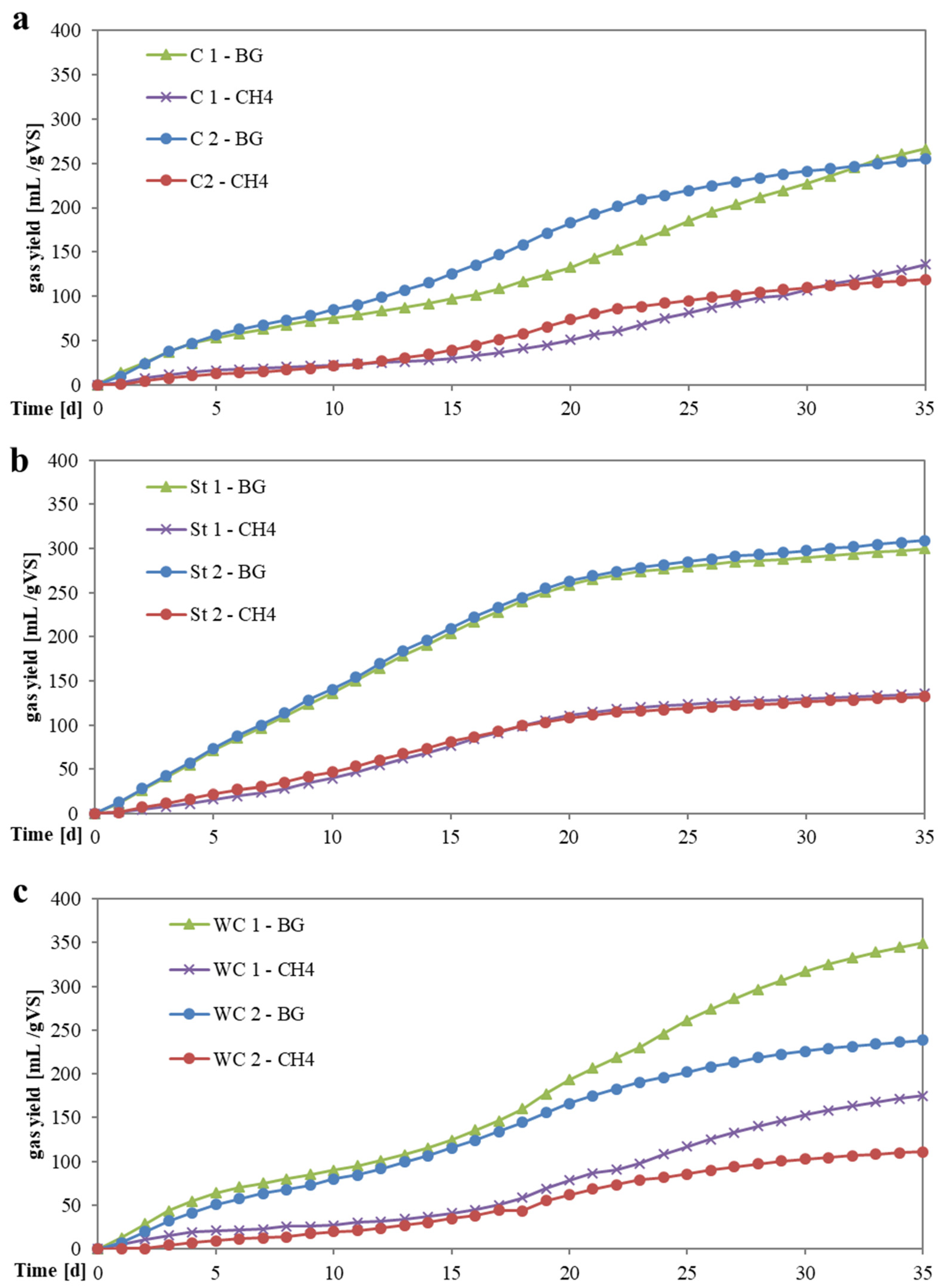
3.3.2. Specific Methane Yield
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- FAO. Nitrogen Inputs to Agricultural Soils from Livestock Manure. New Statistics; Integrated crop management; Food and Agriculture Organization, FAO: Rome, Italy, 2018; Volume 24, p. 17. ISBN 978-92-5-130024-4. [Google Scholar]
- BMEL-Statistik. Federal Ministry of Food and Agriculture—BMEL. November 2019. Available online: https://www.bmel-statistik.de/ernaehrung-fischerei/versorgungsbilanzen/eier/ (accessed on 14 July 2020).
- Nebel, U.; Kühnel, M. Survey of the Different Chicken Housing Systems and Accumulating Form of Manure/Slurry for the Derivative of a Standardised Form of Veterinary Drug Decomposition in Expositions Scenarios; Federal Environment Agency Germany–Texte: Dessau-Roßlau, Germany, 2010; Volume 17, p. 74, ISSN 1862-4804.
- Haenel, H.-D.; Rösemann, C.; Dämmgen, U.; Döring, U.; Wulf, S.; Eurich-Menden, B.; Freibauer, A.; Döhler, H.; Schreiner, C.; Osterburg, B. Calculatons of Gaseous and Particulate Emissions from German Agriculture 1990–2016: Report on Methods and Data (RMD) 2018; Johann Heinrich von Thünen-Institut: Braunschweig, Germany, 2018; p. 424. [Google Scholar] [CrossRef]
- Rajagopal, R.; Massé, D. Start-up of dry anaerobic digestion system for processing solid poultry litter using adapted liquid inoculum. Process Saf. Environ. Prot. 2016, 102, 495–502. [Google Scholar] [CrossRef]
- Federal Ministry of Food and Agriculture—FNR. Guide to Biogas from Production to Use. July 2012. Available online: https://mediathek.fnr.de/broschuren/fremdsprachige-publikationen/english-books/guide-to-biogas-from-production-to-use.html (accessed on 14 July 2020).
- Bayrakdar, A.; Sürmeli, R.-Ö.; Çalli, B. Dry anaerobic digestion of chicken manure coupled with membrane separation of ammonia. Bioresour. Technol. 2017, 244, 816–823. [Google Scholar] [CrossRef] [PubMed]
- Nie, H.; Jacobi, H.F.; Strach, K.; Xua, C.; Zhoua, H.; Liebetrau, J. Monofermentation of chicken manure: Ammonia inhibition and recirculation of the digestate. Bioresour. Technol. 2015, 178, 238–246. [Google Scholar] [CrossRef]
- Luning, L.; Van Zundert, E.H.M.; Brinkmann, A.J.F. Comparison of dry and wet digestion for solid waste. Water Sci. Technol. 2003, 48, 15–20. [Google Scholar] [CrossRef] [PubMed]
- Rocamora, I.; Wagland, S.T.; Villa, R.; Simpson, E.W.; Fernández, O.; Bajón-Fernández, Y. Dry anaerobic digestion of organic waste—A review of operational parameters and their impact on process performance. Bioresour. Technol. 2020, 299, 122681. [Google Scholar] [CrossRef]
- Di Maria, F.; Barratta, M.; Biaconi, F.; Placidi, P.; Passeri, D. Solid anaerobic digestion batch with liquid digestate recirculation and wet anaerobic digestion of organic waste: Comparison of system performances and identification of microbial guilds. Waste Manag. 2017, 59, 172–180. [Google Scholar] [CrossRef]
- Ge, X.; Xu, F.; Li, Y. Solid-state anaerobic digestion of lignocellulosic biomass: Recent progress and perspectives. Bioresour. Technol. 2016, 205, 238–249. [Google Scholar] [CrossRef]
- Olivier, F.; Gourc, J.P. Hydro-mechanical behavior of Municipal Solid Waste subject to leachate recirculation in a large-scale compression reactor cell. Waste Manag. 2007, 27, 44–58. [Google Scholar] [CrossRef]
- Cysneiros, D.; Banks, C.J.; Heaven, S.; Karatzas, K.-A.G. The role of phase separation and feed cycle length in leach beds coupled to methanogenic reactors for digestion of a solid substrate (Part 2): Hydrolysis, acidification and methanogenesis in a two-phase system. Bioresour. Technol. 2011, 102, 7393–7400. [Google Scholar] [CrossRef]
- DIN EN 12880. Characterization of Sludges—Determination of Dry Residue and Water Content; DIN Deutsches Institut für Normung e. V.: Berlin, Germany, 2001.
- DIN EN 15935:2012-11. Sludge, Treated Biowaste, Soil and Waste—Determination of Loss on Ignition; DIN Deutsches Institut für Normung e. V.: Berlin, Germany, 2012.
- Liebetrau, J.; Pfeiffer, D.; Thrän, D. (Eds.) Collection of Measurement Methods for Biogas—Methods to Determine Parameters for Analysis Purposes and Parameters That Describe Processes in the Biogas Sector; Series of the Funding Programme “Biomass Energy Use”; Deutsches Biomasseforschungszentrum gemeinnützige GmbH: Leipzig, Germany, 2016; Volume 7, ISSN 2364-897X; Available online: https://www.energetische-biomassenutzung.de/fileadmin/user_upload/Downloads/Ver%C3%B6ffentlichungen/07_MMS_Biogas_en_web.pdf (accessed on 30 October 2019).
- Wedwitschka, H.; Jenson, E.; Liebetrau, J. Feedstock Characterization and Suitability Assessment for Dry Anaerobic Batch Digestion. Chem. Eng. Technol. 2016, 39, 665–672. [Google Scholar] [CrossRef]
- VDI 4630: 2006. Fermentation of Organic Materials—Characterisation of the Substrate, Sampling, Collection of Material Data, Fermentation Tests; Beuth Verlag GmbH: Berlin, Germany, 2006.
- Massé, D.; Saady, N.M.C.; Gilbert, Y. Psychrophilic dry anaerobic digestion of cow feces and wheat straw—Feasibility studies. Biomass Bioenergy 2015, 77, 1–8. [Google Scholar] [CrossRef]
- Hendriks, A.T.W.M.; Zeeman, G. Pretreatments to enhance the digestibility of lignocellulosic biomass. Bioresour. Technol. 2009, 100, 10–18. [Google Scholar] [CrossRef] [PubMed]
- André, L.; Durante, M.; Pauss, A.; Lespinard, O.; Ribeiro, T.; Lamy, E. Quantifying physical structure changes and non-uniform water flow in cattle manure during dry anaerobic digestion process at lab scale—Implication for biogas production. Bioresour. Technol. 2015, 192, 660–669. [Google Scholar] [CrossRef] [PubMed]
- Hayes, A.C.; Enongene, E.S.; Mervin, S.; Jenson, E. Techno-economic evaluation of a tandem dry batch, garage-style digestion-compost process for remote work camp environments. Waste Manag. 2016, 58, 70–80. [Google Scholar] [CrossRef]
- Shewani, A.; Horgue, P.; Pommier, S.; Debenest, G.; Lefebvre, X.; Gandon, E.; Paul, E. Assessment of percolation through a solid leach bed in dry batch anaerobic digestion processes. Bioresour. Technol. 2015, 178, 209–216. [Google Scholar] [CrossRef]
- Hafner, S.D.; Bisogni, J.J. Modeling of ammonia speciation in anaerobic digesters. Water Res. 2009, 43, 4105–4114. [Google Scholar] [CrossRef]
- Ying, J.; Ewan, M.; Yue, Z.; Sonia, H.; Charles, B.; Philp, L. Ammonia inhibition and toxicity in anaerobic digestion: A critical review. J. Water Process. Eng. 2019, 32. [Google Scholar] [CrossRef]
- Bujoczek, G.; Oleszkiewicz, J.; Sparling, R.; Cenkowski, S. High Solid Anaerobic Digestion of Chicken Manure. J. Agric. Eng. Res. 2000, 76, 51–60. [Google Scholar] [CrossRef]
- Yenigün, O.; Demirel, B. Ammonia inhibition in anaerobic digestion—A review. Process. Biochem. 2013, 48, 901–911. [Google Scholar] [CrossRef]
- Niu, Q.; Qiao, W.; Qiang, H.; Hojo, T.; Li, Y.Y. Mesophilic methane fermentation of chicken manure at a wide range of ammonia concentration: Stability, inhibition and recovery. Bioresour. Technol. 2013, 137, 358–367. [Google Scholar] [CrossRef]
- Marchioro, V.; Steinmetz, R.L.R.; do Amaral, A.C.; Gaspareto, T.C.; Treichel, H.; Kunz, A. Poultry Litter Solid State Anaerobic Digestion: Effect of Digestate Recirculation Intervals and Substrate/Inoculum Ratios on Process Efficiency. Frontiers 2018, 2, 1–10. [Google Scholar] [CrossRef]
- Bi, S.; Westerholm, M.; Qiao, W.; Xiong, L.; Mahdy, A.; Yin, D.; Song, Y.; Dong, R. Metabolic performance of anaerobic digestion of chicken manure under wet, high solid, and dry conditions. Bioresour. Technol. 2020, 296, 122342. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Gabauer, W.; Li, Z.; Ortner, M.; Fuchs, W. Improving exploitation of chicken manure via two-stage anaerobic digestion with an intermediate membrane contactor to extract ammonia. Bioresour. Technol. 2018, 268, 811–814. [Google Scholar] [CrossRef] [PubMed]

| Designation | Structure Material | Structure Material Addition (w%) | |
|---|---|---|---|
| Trial 1 | C1 | - | - |
| Trial 2 | C2 | - | - |
| Trial 3 | S1 | Straw | 5 |
| Trial 4 | S2 | Straw | 10 |
| Trial 5 | W1 | Woodchips | 10 |
| Trial 6 | W2 | Woodchips | 10 |
| TS | VS | NH4-N | TKN | Raw Protein | Raw Fat | Raw Fibre | |
|---|---|---|---|---|---|---|---|
| n = 50 | n = 50 | n = 44 | n = 42 | n = 42 | n = 38 | n = 44 | |
| %FM | %TS | %TS | %TS | %TS | %TS | %TS | |
| Mean | 48.3 | 69.5 | 0.8 | 4.7 | 21.6 | 3.6 | 14.0 |
| Max | 77.0 | 86.4 | 2.3 | 8.7 | 46.2 | 12.9 | 32.5 |
| Min | 28.6 | 56.7 | 0.1 | 1.5 | 1.5 | 0.1 | 1.7 |
| Sample | Structure Material | P1 | P2 | C2 | P3 | C3 |
|---|---|---|---|---|---|---|
| Material | Addition [w%] | [m/s] | [m/s] | [%] | [m/s] | [%] |
| MS | - | 1.59 × 10−4 | 1.08 × 10−4 | 22.94 | 8.39 × 10−5 | 31.76 |
| 1 | - | 1.91 × 10−4 | n.p. | n.m. | n.p. | n.m. |
| 2 | - | 4.36 × 10−5 | 9.58 × 10−6 | n.m. | n.p. | n.m. |
| 2 | 5 (PSC) | 2.14 × 10−4 | 1.36 × 10−4 | 4.47 | 1.38 × 10−4 | 19.42 |
| 3 | - | 1.91 × 10−4 | 1.38 × 10−4 | 8.43 | 9.89 × 10−5 | 18.54 |
| 4 | - | 1.02 × 10−4 | 6.34 × 10−5 | 3.28 | 4.32 × 10−5 | 7.38 |
| 4 | 5 (WC) | 1.43 × 10−4 | 9.95 × 10−5 | 7.53 | 6.87 × 10−5 | 15.07 |
| 4 | 10 (WC) | 1.49 × 10−4 | 1.01 × 10−4 | 13.70 | 8.02 × 10−5 | 17.12 |
| 4 | 5 (St) | 1.71 × 10−4 | 1.10 × 10−4 | 15.00 | 8.20 × 10−5 | 19.38 |
| 4 | 10 (St) | 1.80 × 10−4 | 1.15 × 10−4 | 14.29 | 9.39 × 10−5 | 16.67 |
| SMY1 | SMY2 | SMYmean | ||
|---|---|---|---|---|
| [mL/g VS] | [mL/g VS] | [mL/g VS] | [mL/g FM] | |
| Control | 136 | 119 | 127 ± 12 | 70 ± 6 |
| Straw | 138 | 132 | 135 ± 4 | 75 ± 2 |
| Woodchips | 175 | 111 | 143 ± 45 | 79 ± 25 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wedwitschka, H.; Gallegos Ibanez, D.; Schäfer, F.; Jenson, E.; Nelles, M. Material Characterization and Substrate Suitability Assessment of Chicken Manure for Dry Batch Anaerobic Digestion Processes. Bioengineering 2020, 7, 106. https://doi.org/10.3390/bioengineering7030106
Wedwitschka H, Gallegos Ibanez D, Schäfer F, Jenson E, Nelles M. Material Characterization and Substrate Suitability Assessment of Chicken Manure for Dry Batch Anaerobic Digestion Processes. Bioengineering. 2020; 7(3):106. https://doi.org/10.3390/bioengineering7030106
Chicago/Turabian StyleWedwitschka, Harald, Daniela Gallegos Ibanez, Franziska Schäfer, Earl Jenson, and Michael Nelles. 2020. "Material Characterization and Substrate Suitability Assessment of Chicken Manure for Dry Batch Anaerobic Digestion Processes" Bioengineering 7, no. 3: 106. https://doi.org/10.3390/bioengineering7030106
APA StyleWedwitschka, H., Gallegos Ibanez, D., Schäfer, F., Jenson, E., & Nelles, M. (2020). Material Characterization and Substrate Suitability Assessment of Chicken Manure for Dry Batch Anaerobic Digestion Processes. Bioengineering, 7(3), 106. https://doi.org/10.3390/bioengineering7030106




