Electrospun Scaffolds and Induced Pluripotent Stem Cell-Derived Cardiomyocytes for Cardiac Tissue Engineering Applications
Abstract
1. Introduction
2. Tissue Engineered Scaffolds
2.1. Polymers Used for Scaffold Fabrication
2.2. Biocompatibility of Scaffolds
2.3. Physical and Mechanical Properties of TE Scaffolds
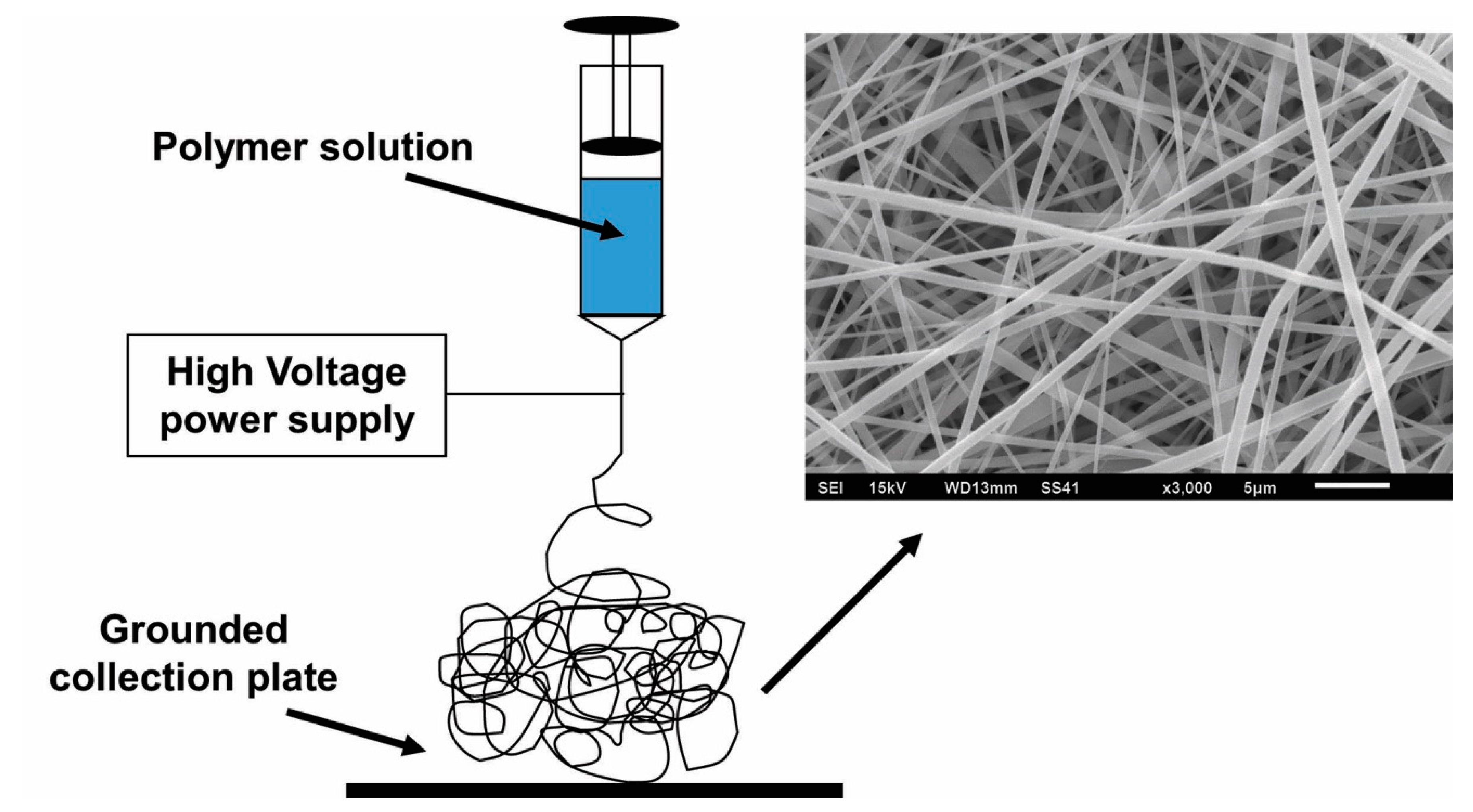
3. Electrospinning
Polymers and Fabrication of Electrospun TE Scaffolds
4. Induced Pluripotent Stem Cell-Derived Cardiomyocytes
4.1. Cardiac Differentiation of iPSCs
4.2. iPSC-CMs Used for Tissue Engineering
4.3. iPSC-CM Maturity for TE Applications
5. Electrospun Scaffolds Seeded with iPSC-CMs
6. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Benjamin, E.J.; Virani, S.S.; Callaway, C.W.; Chamberlain, A.M.; Chang, A.R.; Cheng, S.; Chiuve, S.E.; Cushman, M.; Delling, F.N.; Deo, R.; et al. Heart Disease and Stroke Statistics-2018 Update: A Report From the American Heart Association. Circulation 2018, 137, e67–e492. [Google Scholar] [CrossRef] [PubMed]
- Heron, M. Deaths: Leading Causes for 2015. Natl. Vital Stat. Rep. 2017, 66, 1–76. [Google Scholar] [PubMed]
- Lloyd-Jones, D.M.; Adams, R.; Carnethon, M.; De Simone, G.; Ferguson, T.B.; Flegal, K.M.; Ford, E.; Furie, K.; Go, A. Heart Disease and Stroke Statistics-2009 Update. Circulation 2009, 119, 480–486. [Google Scholar] [CrossRef]
- Health Resources and Services Administration: Scientific Registry of Transplant Recipients. OPTN/SRTR 2018 Annual Data Report: Heart. U.S. Department of Health and Human Services. Available online: https://onlinelibrary.wiley.com/doi/full/10.1111/ajt.15676 (accessed on 14 April 2020).
- Iyer, R.K.; Chiu, L.L.Y.; Reis, L.A.; Radisic, M. Engineered cardiac tissues. Curr. Opin. Biotechnol. 2011, 22, 706–714. [Google Scholar] [CrossRef] [PubMed]
- Huang, N.; Serpooshan, V.; Morris, V.B.; Sayed, N.; Pardon, G.; Abilez, O.; Nakayama, K.H.; Pruitt, B.L.; Wu, S.M.; Yoon, Y.-S.; et al. Big bottlenecks in cardiovascular tissue engineering. Commun. Boil. 2018, 1. [Google Scholar] [CrossRef]
- Pinto, A.R.; Ilinykh, A.; Ivey, M.J.; Kuwabara, J.T.; D’Antoni, M.L.; Debuque, R.; Chandran, A.; Wang, L.; Arora, K.; Rosenthal, N.A.; et al. Revisiting Cardiac Cellular Composition. Circ. Res. 2015, 118, 400–409. [Google Scholar] [CrossRef]
- Gray, G.A.; Toor, I.; Castellan, R.; Crisan, M.; Meloni, M. Resident cells of the myocardium: More than spectators in cardiac injury, repair and regeneration. Curr. Opin. Physiol. 2018, 1, 46–51. [Google Scholar] [CrossRef]
- Zhang, J.; Wilson, G.F.; Soerens, A.G.; Koonce, C.H.; Yu, J.; Palecek, S.P.; Thomson, J.A.; Kamp, T.J. Functional cardiomyocytes derived from human induced pluripotent stem cells. Circ. Res. 2009, 104, 30–41. [Google Scholar] [CrossRef]
- Bruyneel, A.A.; McKeithan, W.L.; Feyen, D.A.; Mercola, M. Will iPSC-cardiomyocytes revolutionize the discovery of drugs for heart disease? Curr. Opin. Pharmacol. 2018, 42, 55–61. [Google Scholar] [CrossRef]
- Tirziu, D.; Giordano, F.J.; Simons, M. Cell communications in the heart. Circulation 2010, 122, 928–937. [Google Scholar] [CrossRef]
- Orlova, Y.; Magome, N.; Liu, L.; Chen, Y.; Agladze, K. Electrospun nanofibers as a tool for architecture control in engineered cardiac tissue. Biomaterials 2011, 32, 5615–5624. [Google Scholar] [CrossRef] [PubMed]
- Opie, L.H. Heart Physiology: From Cell to Circulation; Lippincott Williams & Wilkins: Philadelphia, PA, USA, 2003. [Google Scholar]
- Matsuda, N.; Shimizu, T.; Yamato, M.; Okano, T. Tissue Engineering Based on Cell Sheet Technology. Adv. Mater. 2007, 19, 3089–3099. [Google Scholar] [CrossRef]
- Bursac, N.; Loo, Y.; Leong, K.W.; Tung, L. Novel anisotropic engineered cardiac tissues: Studies of electrical propagation. Biochem. Biophys. Res. Commun. 2007, 361, 847–853. [Google Scholar] [CrossRef] [PubMed]
- Jafari, M.; Paknejad, Z.; Rad, M.R.; Motamedian, S.R.; Eghbal, M.J.; Nadjmi, N.; Khojasteh, A. Polymeric scaffolds in tissue engineering: A literature review. J. Biomed. Mater. Res. Part B: Appl. Biomater. 2015, 105, 431–459. [Google Scholar] [CrossRef]
- Lannutti, J.J.; Reneker, D.; Ma, T.; Tomasko, D.; Farson, D. Electrospinning for tissue engineering scaffolds. Mater. Sci. Eng. C 2007, 27, 504–509. [Google Scholar] [CrossRef]
- Khorshidi, S.; Solouk, A.; Mirzadeh, H.; Mazinani, S.; Lagarón, J.M.; Sharifi, S.; Ramakrishna, S. A review of key challenges of electrospun scaffolds for tissue-engineering applications. J. Tissue Eng. Regen. Med. 2015, 10, 715–738. [Google Scholar] [CrossRef]
- Da Silva, D.; Kaduri, M.; Poley, M.; Adir, O.; Krinsky, N.; Shainsky-Roitman, J.; Schroeder, A. Biocompatibility, biodegradation and excretion of polylactic acid (PLA) in medical implants and theranostic systems. Chem. Eng. J. 2018, 340, 9–14. [Google Scholar] [CrossRef]
- Gupta, B.; Revagade, N.; Hilborn, J. Poly(lactic acid) fiber: An overview. Prog. Polym. Sci. 2007, 32, 455–482. [Google Scholar] [CrossRef]
- Gentile, P.; Chiono, V.; Carmagnola, I.; Hatton, P. An Overview of Poly(lactic-co-glycolic) Acid (PLGA)-Based Biomaterials for Bone Tissue Engineering. Int. J. Mol. Sci. 2014, 15, 3640–3659. [Google Scholar] [CrossRef]
- Grayson, A.C.; Voskerician, G.; Lynn, A.; Anderson, J.M.; Cima, M.J.; Langer, R. Differential degradation rates in vivo and in vitro of biocompatible poly(lactic acid) and poly(glycolic acid) homo- and co-polymers for a polymeric drug-delivery microchip. J. Biomater. Sci. Polym. Ed. 2004, 15, 1281–1304. [Google Scholar] [CrossRef]
- Salgado, C.L.; Sanchez, E.M.S.; Zavaglia, C.A.C.; Granja, P.L. Biocompatibility and biodegradation of polycaprolactone-sebacic acid blended gels. J. Biomed. Mater. Res. Part A 2011, 100, 243–251. [Google Scholar] [CrossRef] [PubMed]
- Malikmammadov, E.; Tanir, T.E.; Kiziltay, A.; Hasirci, N.; Hasirci, N. PCL and PCL-based materials in biomedical applications. J. Biomater. Sci. Polym. Ed. 2017, 29, 863–893. [Google Scholar] [CrossRef] [PubMed]
- Naahidi, S.; Jafari, M.; Logan, M.; Wang, Y.; Yuan, Y.; Bae, H.; Dixon, B.; Chen, P. Biocompatibility of hydrogel-based scaffolds for tissue engineering applications. Biotechnol. Adv. 2017, 35, 530–544. [Google Scholar] [CrossRef] [PubMed]
- Anderson, J.M. Biocompatibility and Bioresponse to Biomaterials. In Principles of Regenerative Medicine; Academic Press: Cambridge, UK, 2019; pp. 675–694. [Google Scholar]
- Reid, J.A.; Callanan, A. Hybrid cardiovascular sourced extracellular matrix scaffolds as possible platforms for vascular tissue engineering. J. Biomed. Mater. Res. Part B: Appl. Biomater. 2019, 108, 910–924. [Google Scholar] [CrossRef]
- Woodruff, M.A.; Hutmacher, D.W. The return of a forgotten polymer—Polycaprolactone in the 21st century. Prog. Polym. Sci. 2010, 35, 1217–1256. [Google Scholar] [CrossRef]
- Anderson, J.M.; Jiang, S. Implications of the Acute and Chronic Inflammatory Response and the Foreign Body Reaction to the Immune Response of Implanted Biomaterials. In The Immune Response to Implanted Materials and Devices; Springer Science and Business Media LLC: Berlin, Germany, 2016; pp. 15–36. [Google Scholar]
- Brown, B.N.; Badylak, S.F. The Role of the Host Immune Response in Tissue Engineering and Regenerative Medicine. Princ. Tissue Eng. 2014, 497–509. [Google Scholar] [CrossRef]
- Katti, D.; Vasita, R.; Shanmugam, K. Improved Biomaterials for Tissue Engineering Applications: Surface Modification of Polymers. Curr. Top. Med. Chem. 2008, 8, 341–353. [Google Scholar] [CrossRef]
- Boehler, R.M.; Graham, J.G.; Shea, L.D. Tissue engineering tools for modulation of the immune response. Biotechniques. 2011, 51, 239–254. [Google Scholar] [CrossRef]
- Riolobos, L.; Hirata, R.K.; Turtle, C.J.; Wang, P.-R.; Gornalusse, G.G.; Zavajlevski, M.; Riddell, S.R.; Russell, D.W. HLA Engineering of Human Pluripotent Stem Cells. Mol. Ther. 2013, 21, 1232–1241. [Google Scholar] [CrossRef]
- Gourraud, P.-A.; Gilson, L.; Girard, M.; Peschanski, M. The Role of Human Leukocyte Antigen Matching in the Development of Multiethnic “Haplobank” of Induced Pluripotent Stem Cell Lines. STEM CELLS 2012, 30, 180–186. [Google Scholar] [CrossRef]
- Gorabi, A.M.; Tafti, S.H.A.; Soleimani, M.; Panahi, Y.; Sahebkar, A. Cells, Scaffolds and Their Interactions in Myocardial Tissue Regeneration. J. Cell. Biochem. 2017, 118, 2454–2462. [Google Scholar] [CrossRef] [PubMed]
- Lyu; Schley, J.; Loy, B.; Lind, D.; Hobot, C.; Sparer, R.; Untereker, D. Kinetics and Time−Temperature Equivalence of Polymer Degradation. Biomacromolecules 2007, 8, 2301–2310. [Google Scholar] [CrossRef]
- Zhang, H.; Zhou, L.; Zhang, W. Control of Scaffold Degradation in Tissue Engineering: A Review. Tissue Eng. Part B Rev. 2014, 20, 492–502. [Google Scholar] [CrossRef]
- Cairns, M.-L.; Dickson, G.R.; Orr, J.F.; Farrar, D.; Hardacre, C.; Sá, J.; Lemoine, P.; Mughal, M.; Buchanan, F.J. The potential of electron beam radiation for simultaneous surface modification and bioresorption control of PLLA. J. Biomed. Mater. Res. Part A 2012, 100, 2223–2229. [Google Scholar] [CrossRef] [PubMed]
- Li, P.; Feng, X.; Jia, X.; Fan, Y. Influences of tensile load on in vitro degradation of an electrospun poly(l-lactide-co-glycolide) scaffold. Acta Biomater. 2010, 6, 2991–2996. [Google Scholar] [CrossRef] [PubMed]
- Jun, I.; Han, H.-S.; Edwards, J.R.; Jeon, H. Electrospun Fibrous Scaffolds for Tissue Engineering: Viewpoints on Architecture and Fabrication. Int. J. Mol. Sci. 2018, 19, 745. [Google Scholar] [CrossRef] [PubMed]
- Balguid, A.; Mol, A.; Van Marion, M.H.; Bank, R.A.; Bouten, C.V.; Baaijens, F.P. Tailoring Fiber Diameter in Electrospun Poly(ɛ-Caprolactone) Scaffolds for Optimal Cellular Infiltration in Cardiovascular Tissue Engineering. Tissue Eng. Part A 2009, 15, 437–444. [Google Scholar] [CrossRef] [PubMed]
- Martins, A.; Araújo, J.V.; Reis, R.L.; Neves, N. Electrospun nanostructured scaffolds for tissue engineering applications. Future Med. 2007, 2, 929–942. [Google Scholar] [CrossRef]
- Rogina, A. Electrospinning process: Versatile preparation method for biodegradable and natural polymers and biocomposite systems applied in tissue engineering and drug delivery. Appl. Surf. Sci. 2014, 296, 221–230. [Google Scholar] [CrossRef]
- Dattola, E.; Parrotta, E.I.; Scalise, S.; Perozziello, G.; Limongi, T.; Candeloro, P.; Coluccio, M.L.; Maletta, C.; Bruno, L.; De Angelis, M.T.; et al. Development of 3D PVA scaffolds for cardiac tissue engineering and cell screening applications. RSC Adv. 2019, 9, 4246–4257. [Google Scholar] [CrossRef]
- Wang, C.; Cheng, Y.-W.; Hsu, C.-H.; Chien, H.-S.; Tsou, S.-Y. How to manipulate the electrospinning jet with controlled properties to obtain uniform fibers with the smallest diameter?—A brief discussion of solution electrospinning process. J. Polym. Res. 2010, 18, 111–123. [Google Scholar] [CrossRef]
- Anderson, R.H.; Ho, S.Y.; Redmann, K.; Sánchez-Quintana, D.; Lunkenheimer, P.P. The anatomical arrangement of the myocardial cells making up the ventricular mass. Eur. J. Cardio-Thoracic Surg. 2005, 28, 517–525. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Wu, Y.; Hu, T.; Guo, B.; Ma, P.X. Electrospun conductive nanofibrous scaffolds for engineering cardiac tissue and 3D bioactuators. Acta Biomater. 2017, 59, 68–81. [Google Scholar] [CrossRef] [PubMed]
- Robinson, T.F.; Cohen-Gould, L.; Factor, S.M.; Eghbali, M.; Blumenfeld, O.O. Structure and function of connective tissue in cardiac muscle: Collagen types I and III in endomysial struts and pericellular fibers. Scanning Microsc. 1988, 2, 1005–1015. [Google Scholar] [PubMed]
- Fleischer, S.; Miller, J.; Hurowitz, H.; Shapira, A.; Dvir, T. Effect of fiber diameter on the assembly of functional 3D cardiac patches. Nanotechnology 2015, 26, 291002. [Google Scholar] [CrossRef]
- Bettinger, C.J.; Langer, R.; Borenstein, J.T. Engineering Substrate Topography at the Micro- and Nanoscale to Control Cell Function. Angew. Chem. Int. Ed. 2009, 48, 5406–5415. [Google Scholar] [CrossRef]
- Li, M.; Guo, Y.; Wei, Y.; MacDiarmid, A.G.; Lelkes, P.I. Electrospinning polyaniline-contained gelatin nanofibers for tissue engineering applications. Biomaterials 2006, 27, 2705–2715. [Google Scholar] [CrossRef]
- Mirjalili, M.; Zohoori, S. Review for application of electrospinning and electrospun nanofibers technology in the textile industry. J. Nanostructure Chem. 2006, 6, 207–213. [Google Scholar] [CrossRef]
- Zafar, M.S.; Najeeb, S.; Khurshid, Z.; Vazirzadeh, M.; Zohaib, S.; Najeeb, B.; Sefat, F. Potential of Electrospun Nanofibers for Biomedical and Dental Applications. Materials 2016, 9, 73. [Google Scholar] [CrossRef]
- Correia, D.; Ribeiro, C.; Ferreira, J.C.; Botelho, G.; Ribelles, J.L.G.; Lanceros-Méndez, S.; Sencadas, V. Influence of electrospinning parameters on poly(hydroxybutyrate) electrospun membranes fiber size and distribution. Polym. Eng. Sci. 2013, 54, 1608–1617. [Google Scholar] [CrossRef]
- Zhao, G.; Zhang, X.; Lu, T.J.; Xu, F. Recent Advances in Electrospun Nanofibrous Scaffolds for Cardiac Tissue Engineering. Adv. Funct. Mater. 2015, 25, 5726–5738. [Google Scholar] [CrossRef]
- Pillay, V.; Dott, C.; Choonara, Y.E.; Tyagi, C.; Tomar, L.; Kumar, P.; Du Toit, L.C.; Ndesendo, V.M.K. A Review of the Effect of Processing Variables on the Fabrication of Electrospun Nanofibers for Drug Delivery Applications. J. Nanomater. 2013, 2013, 1–22. [Google Scholar] [CrossRef]
- Hsiao, C.-W.; Bai, M.-Y.; Chang, Y.; Chung, M.-F.; Lee, T.-Y.; Wu, C.-T.; Maiti, B.; Liao, Z.-X.; Li, R.-K.; Sung, H.-W. Electrical coupling of isolated cardiomyocyte clusters grown on aligned conductive nanofibrous meshes for their synchronized beating. Biomaterials 2013, 34, 1063–1072. [Google Scholar] [CrossRef] [PubMed]
- Zong, X.; Bien, H.; Chung, C.-Y.; Yin, L.; Fang, D.; Hsiao, B.S.; Chu, B.; Entcheva, E. Electrospun fine-textured scaffolds for heart tissue constructs. Biomaterials 2005, 26, 5330–5338. [Google Scholar] [CrossRef] [PubMed]
- Shokraei, N.; Asadpour, S.; Shokraei, S.; Sabet, M.N.; Faridi-Majidi, R.; Ghanbari, H. Development of electrically conductive hybrid nanofibers based on CNT-polyurethane nanocomposite for cardiac tissue engineering. Microsc. Res. Tech. 2019, 82, 1316–1325. [Google Scholar] [CrossRef]
- Takahashi, K.; Yamanaka, S. Induction of Pluripotent Stem Cells from Mouse Embryonic and Adult Fibroblast Cultures by Defined Factors. Cell 2006, 126, 663–676. [Google Scholar] [CrossRef]
- Mauritz, C.; Schwanke, K.; Reppel, M.; Neef, S.; Katsirntaki, K.; Maier, L.S.; Nguemo, F.; Menke, S.; Haustein, M.; Hescheler, J.; et al. Generation of Functional Murine Cardiac Myocytes From Induced Pluripotent Stem Cells. Circulation 2008, 118, 507–517. [Google Scholar] [CrossRef]
- Takahashi, K.; Tanabe, K.; Ohnuki, M.; Narita, M.; Ichisaka, T.; Tomoda, K.; Yamanaka, S. Induction of Pluripotent Stem Cells from Adult Human Fibroblasts by Defined Factors. Cell 2007, 131, 861–872. [Google Scholar] [CrossRef]
- Mummery, C.; Zhang, J.; Ng, E.S.; Elliott, D.A.; Elefanty, A.G.; Kamp, T.J. Differentiation of human embryonic stem cells and induced pluripotent stem cells to cardiomyocytes: A methods overview. Circ. Res. 2012, 111, 344–358. [Google Scholar] [CrossRef]
- Le, T.Y.L.; Chong, J.J.H. Cardiac progenitor cells for heart repair. Cell Death Discov. 2016, 2, 16052. [Google Scholar] [CrossRef]
- Pettinato, G.; Wen, X.; Zhang, N. Engineering Strategies for the Formation of Embryoid Bodies from Human Pluripotent Stem Cells. Stem Cells Dev. 2015, 24, 1595–1609. [Google Scholar] [CrossRef] [PubMed]
- Kehat, I.; Kenyagin-Karsenti, D.; Snir, M.; Segev, H.; Amit, M.; Gepstein, A.; Livne, E.; Binah, O.; Itskovitz-Eldor, J.; Gepstein, L. Human embryonic stem cells can differentiate into myocytes with structural and functional properties of cardiomyocytes. J. Clin. Investig. 2001, 108, 407–414. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Raval, K.K.; Lian, X.; Herman, A.M.; Wilson, G.F.; Barron, M.R.; Yu, J.; Palecek, S.P.; Thompson, J.A.; Kamp, T.J. Matrix-promoted efficient cardiac differentiation of human iPS and ES cells. Circulation 2010, 122, A20724. [Google Scholar]
- Evseenko, D.; Zhu, Y.; Schenke-Layland, K.; Kuo, J.; Latour, B.; Ge, S.; Scholes, J.; Dravid, G.; Li, X.; MacLellan, W.R.; et al. Mapping the first stages of mesoderm commitment during differentiation of human embryonic stem cells. Proc. Natl. Acad. Sci. USA 2010, 107, 13742–13747. [Google Scholar] [CrossRef] [PubMed]
- Jeziorowska, D.; Fontaine, V.; Jouve, C.; Villard, E.; Dussaud, S.; Akbar, D.; Letang, V.; Cervello, P.; Itier, J.-M.; Pruniaux, M.-P.; et al. Differential Sarcomere and Electrophysiological Maturation of Human iPSC-Derived Cardiac Myocytes in Monolayer vs. Aggregation-Based Differentiation Protocols. Int. J. Mol. Sci. 2017, 18, 1173. [Google Scholar] [CrossRef] [PubMed]
- Lewandowski, J.; Kolanowski, T.; Kurpisz, M. Techniques for the induction of human pluripotent stem cell differentiation towards cardiomyocytes. J. Tissue Eng. Regen. Med. 2016, 11, 1658–1674. [Google Scholar] [CrossRef] [PubMed]
- Correia, C.; Koshkin, A.; Duarte, P.; Hu, D.; Carido, M.; Sebastião, M.J.; Gomes-Alves, P.; Elliott, D.A.; Domian, I.J.; Teixeira, A.P.; et al. 3D aggregate culture improves metabolic maturation of human pluripotent stem cell derived cardiomyocytes. Biotechnol. Bioeng. 2017, 115, 630–644. [Google Scholar] [CrossRef]
- Lian, X.; Zhang, J.; Azarin, S.M.; Zhu, K.; Hazeltine, L.B.; Bao, X.; Hsiao, C.; Kamp, T.J.; Palecek, S.P. Directed cardiomyocyte differentiation from human pluripotent stem cells by modulating Wnt/β-catenin signaling under fully defined conditions. Nat. Protoc. 2012, 8, 162–175. [Google Scholar] [CrossRef]
- Lundy, S.D.; Zhu, W.-Z.; Regnier, M.; Laflamme, M. Structural and Functional Maturation of Cardiomyocytes Derived from Human Pluripotent Stem Cells. Stem Cells Dev. 2013, 22, 1991–2002. [Google Scholar] [CrossRef]
- Liaw, N.Y.; Zimmermann, W.-H. Mechanical stimulation in the engineering of heart muscle. Adv. Drug Deliv. Rev. 2016, 96, 156–160. [Google Scholar] [CrossRef]
- Hirt, M.N.; Boeddinghaus, J.; Mitchell, A.; Schaaf, S.; Börnchen, C.; Müller, C.; Schulz, H.; Hubner, N.; Stenzig, J.; Stöhr, A.; et al. Functional improvement and maturation of rat and human engineered heart tissue by chronic electrical stimulation. J. Mol. Cell. Cardiol. 2014, 74, 151–161. [Google Scholar] [CrossRef] [PubMed]
- Khan, M.; Xu, Y.; Hua, S.; Johnson, J.; Belevych, A.; Janssen, P.M.L.; Gyorke, S.; Guan, J.; Angelos, M.G. Evaluation of Changes in Morphology and Function of Human Induced Pluripotent Stem Cell Derived Cardiomyocytes (HiPSC-CMs) Cultured on an Aligned-Nanofiber Cardiac Patch. PLoS ONE 2015, 10, e0126338. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Minami, I.; Yu, L.; Tsuji, K.; Nakajima, M.; Qiao, J.; Suzuki, M.; Shimono, K.; Nakatsuji, N.; Kotera, H.; et al. Extracellular Recordings of Patterned Human Pluripotent Stem Cell-Derived Cardiomyocytes on Aligned Fibers. Stem Cells Int. 2016, 2016, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Wanjare, M.; Hou, L.; Nakayama, K.H.; Kim, J.J.; Mezak, N.P.; Abilez, O.; Tzatzalos, E.; Wu, J.C.; Huang, N. Anisotropic microfibrous scaffolds enhance the organization and function of cardiomyocytes derived from induced pluripotent stem cells. Biomater. Sci. 2017, 5, 1567–1578. [Google Scholar] [CrossRef]
- Han, J.; Wu, Q.; Xia, Y.; Wagner, M.B.; Xu, C. Cell alignment induced by anisotropic electrospun fibrous scaffolds alone has limited effect on cardiomyocyte maturation. Stem Cell Res. 2016, 16, 740–750. [Google Scholar] [CrossRef]
- Joanne, P.; Kitsara, M.; Boitard, S.E.; Naemetalla, H.; Vanneaux, V.; Pernot, M.; Larghero, J.; Forest, P.; Chen, Y.; Menasche, P.; et al. Nanfibrous clinical-grade collagen scaffolds seeded with human cardiomyocytes induces cardiac remodeling in dilated cardiomyopathy. Biomaterials 2016, 80, 167–168. [Google Scholar] [CrossRef]
- Chun, Y.W.; Balikov, D.A.; Feaster, T.K.; Williams, C.; Sheng, C.C.; Lee, J.-B.; Boire, T.C.; Neely, M.D.; Bellan, L.M.; Ess, K.C.; et al. Combinatorial polymer matrices enhance in vitro maturation of human induced pluripotent stem cell-derived cardiomyocytes. Biomaterials 2015, 67, 52–64. [Google Scholar] [CrossRef]
- Westfall, M.V.; Rust, E.M.; Metzger, J.M. Slow skeletal troponin I gene transfer, expression, and myofilament incorporation enhances adult cardiac myocyte contractile function. Proc. Natl. Acad. Sci. USA 1997, 94, 5444–5449. [Google Scholar] [CrossRef]
- Bedada, F.B.; Chan, S.S.; Metzger, S.K.; Zhang, L.; Zhang, J.; Garry, D.J.; Kamp, T.J.; Kyba, M.; Metzger, J.M. Acquisition of a quantitative, stoichiometrically conserved ratiometric marker of maturation status in stem cell-derived cardiac myocytes. Stem Cell Rep. 2014, 3, 594–605. [Google Scholar] [CrossRef]
- Siedner, S.; Kruger, M.; Schroeter, M.; Metzler, D.; Roell, W.; Fleischmann, B.K.; Hescheler, J.; Pfitzer, G.; Stehle, R. Developmental changes in contractility and sarcomeric proteins from the early embryonic to the adult state in the mouse heart. J. Physiol. 2003, 2, 493–505. [Google Scholar]
| Publication | Material | Fabrication | Cells | Requirement/Characteristics | Outcome |
|---|---|---|---|---|---|
| Wang et al. (2017) [47] | PLA/PANI blend | Electrospinning | H9c2 rat cardiomyoblasts | Incorporate PANI into electrospun PLA scaffolds to promote electrical propagation for functional coupling of CMs | Promoted differentiation into CMs; enhancement of cell-cell signaling and maturation of CMs; promotion of spontaneous beating within CMs |
| Hsiao et al. (2012) [57] | Composite PLGA and PANI doped with HCl | Electrospinning | Neonatal CMs from Lewis rats | Create electrospun mesh that serves as an electrically active scaffold to coordinate the synchronous beating of CMs, thus mimicking electroconductive properties of cardiac ECM | All CMs within each cluster demonstrated synchronous beating, implying fully developed electrical coupling between cells; beating rates within isolated CM cell culture could be synchronized via electrical stimulation designed to mimic human heart |
| Zong et al. (2005) [58] | PLGA | Electrospinning | Primary CMs from Sprague Dawley rats | Develop sub-micron features within electrospun meshes to mimic cardiac ECM | SEM revealed the development of sub-micron features successful; primary CMs cultured on electrospun scaffolds developed into tissue-like constructs; scaffold provided appropriate electrochemical modulation |
| Orlova et al. (2011) [12] | PMGI, some suspended on PDMS | Electrospinning | Cardiac cells from Wistar rats | Address tissue thickness limitations for engineered cardiac constructs via varying architectural configuration of electrospun scaffolds | Different architectural configurations in electrospun meshes achieved by varying positioning density and degree of alignment; cardiac cells proliferated into contractile tissue filaments, open-worked tissue meshes, and continuous anisotropic cell sheets |
| Shokraei et al. (2019) [59] | Poly-urethane with multi-walled carbon nanotubes | Electrospinning + electrospraying | H9c2 cells and human umbilical vein endothelial cells (HUVECs) | Use a simultaneous electrospinning + electrospraying method to create electroconductive nanofibrous patches that biomimic cell-cell communication capacity of the human heart in vivo | The increased conductivity of scaffold; high viability and proliferation of cells with increased cell/scaffold interactions |
| Publication | Cells Used | Maturation Method | Results/Conclusion |
|---|---|---|---|
| Lundy et al. (2013) [73] | Human iPSC-CMs and ESC-CMs | Long-term culture to facilitate morphological, contractile, and electrophysiological maturation | Late-stage (i.e., cultured for longer) iPSC-CMs and ESC-CMs demonstrated higher morphological, contractile, electrophysical, and genetic maturity |
| Hirt et al. (2014) [75] | Neonatal rodent CMs or human iPSC-CMs | Application of continuous electrical stimulation | Higher CM density, increased connexin-43 abundance, and shift of Ca2+ response curve towards physiological values |
| Liaw et al. (2015) [74] | Neonatal rodent CMs | Mechanical recreation of pumping action in the human heart | Flexible loading most effective for the facilitation of contractions |
| Publication | Electrospun Scaffold Material | Aim | Outcome |
|---|---|---|---|
| Joanne et al. (2016) [80] | Collagen | Use ES collagen scaffolds to deliver iPSC-CMs to the heart to induce cardiac remodeling in dilated cardiomyopathy | Collagen scaffolds exhibited high biocompatibility; iPSC-CMs delivered by ES scaffolds demonstrated improved cardiac function, scaffold vascularization, and scaffold adherence |
| Li et al. (2016) [77] | PMGI | Observe the activity of patterned human iPSC-CMs on aligned ES PMGI fibers through extracellular recording | Recordings showed iPSC-CMs organized into mature tissues oriented anisotropically along aligned ES fibers; recordings showed premature CM beating, higher signal amplitude, and higher T-wave detection probability compared to iPSC-CMs on non-aligned fibers; recordings showed that iPSC-CMs on aligned scaffolds exhibited anisotropic field potential propagation |
| Wanjare et al. (2017) [78] | PCL | Mimic highly ordered physiology and function of native CMs using anisotropic, microfibrous, ES PCL scaffolds seeded with human iPSC-CMs; compare the cellular response to anisotropic scaffolds vs. randomly oriented scaffolds | ES scaffolds with anisotropically aligned microfibers induced iPSC-CM alignment 2 days post-seeding, and promoted greater iPSC-CM maturation and higher maximum contraction velocity of iPSC-CMs, compared to ES scaffolds with randomly oriented fibers |
| Han et al. (2016) [79] | PCL | Seed human iPSC-CMs onto ES PCL scaffolds, using the anisotropic alignment of the PCL fibers to facilitate human iPSC-CMs’ mimicry of the longitudinal alignment into parallel bundles exhibited by CMs in in vivo adult myocardium | Cell alignment alone is insufficient to facilitate increased maturation in iPSC-CMs, based on the assessment of various gene expressions |
| Khan et al. (2015) [76] | PLGA | Compare morphological and functional changes in human iPSC-CMs cultured on highly-aligned, nanofibrous ES PLGA scaffold vs. standard flat culture plate | iPSC-CMs aligned symmetrically to ES PLGA fibers and demonstrated more rapid calcium cycling than CMs cultured on a flat plate; CMs expressed α-actinin, TnT, and Cx43 in vitro |
| Chun et al. (2015) [81] | Combinatorial polymer of PCL, PEG, and cPCL | Use ES combinatorial polymer matrices to facilitate in vitro maturation of iPSC-CMs | iPSC-CMs cultured onto 4%PEG-96%PCL exhibited the greatest contractility and mitochondrial function, TnI isoform switch from fetal ssTNI to postnatal cTNI, and increased expression of genes encoding intermediate filaments that transduce integrin-mediated mechanical signals to microfilaments |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Suh, T.C.; Amanah, A.Y.; Gluck, J.M. Electrospun Scaffolds and Induced Pluripotent Stem Cell-Derived Cardiomyocytes for Cardiac Tissue Engineering Applications. Bioengineering 2020, 7, 105. https://doi.org/10.3390/bioengineering7030105
Suh TC, Amanah AY, Gluck JM. Electrospun Scaffolds and Induced Pluripotent Stem Cell-Derived Cardiomyocytes for Cardiac Tissue Engineering Applications. Bioengineering. 2020; 7(3):105. https://doi.org/10.3390/bioengineering7030105
Chicago/Turabian StyleSuh, Taylor Cook, Alaowei Y. Amanah, and Jessica M. Gluck. 2020. "Electrospun Scaffolds and Induced Pluripotent Stem Cell-Derived Cardiomyocytes for Cardiac Tissue Engineering Applications" Bioengineering 7, no. 3: 105. https://doi.org/10.3390/bioengineering7030105
APA StyleSuh, T. C., Amanah, A. Y., & Gluck, J. M. (2020). Electrospun Scaffolds and Induced Pluripotent Stem Cell-Derived Cardiomyocytes for Cardiac Tissue Engineering Applications. Bioengineering, 7(3), 105. https://doi.org/10.3390/bioengineering7030105





