Hepatic Differentiation of Stem Cells in 2D and 3D Biomaterial Systems
Abstract
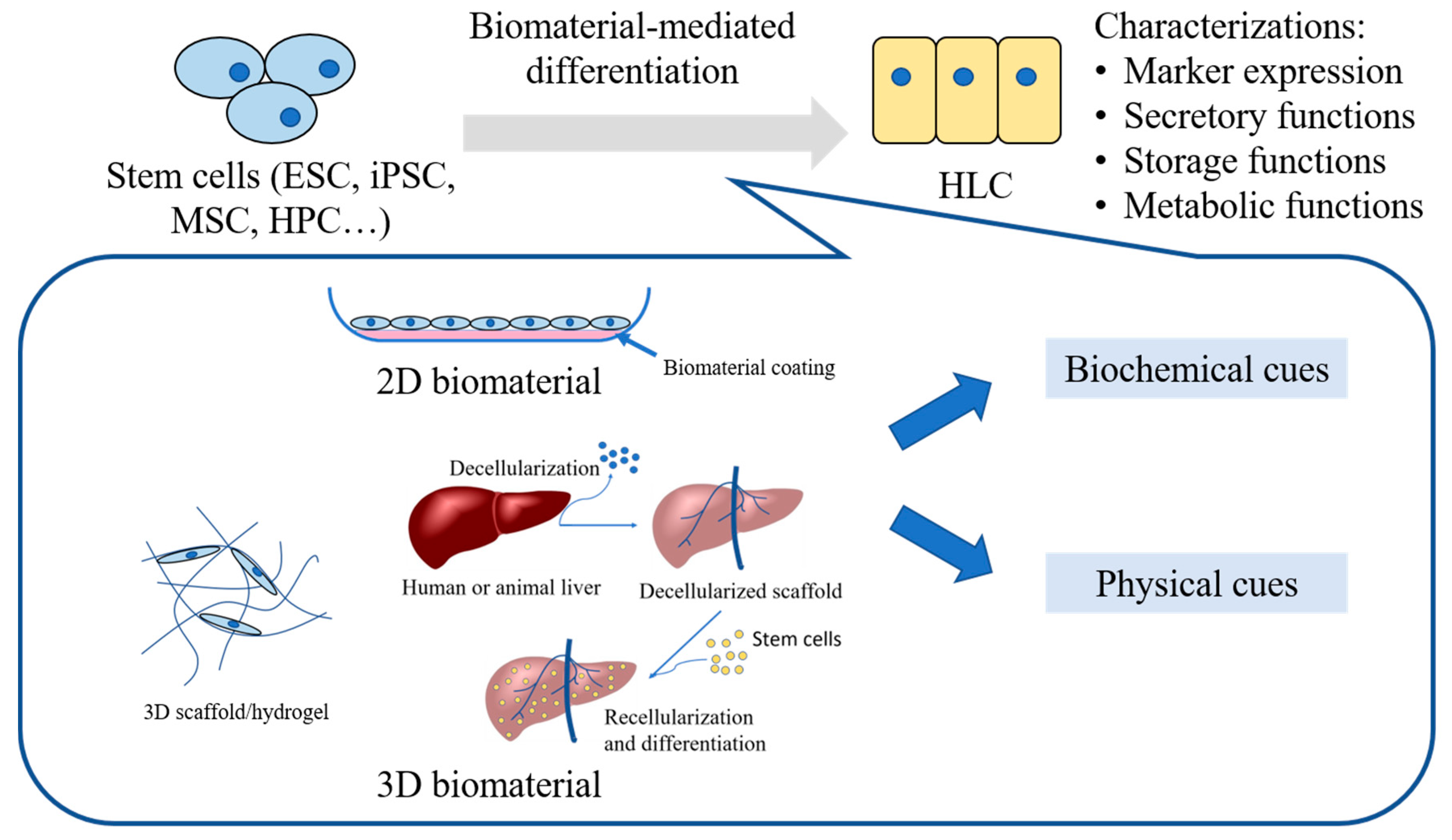
1. Introduction
2. Sources of Stem Cells
3. Biomaterial Systems Employed in Hepatic Differentiation of Stem Cells
3.1. Biomaterials Presenting Biochemical Cues
3.1.1. Natural and Composite Biomaterials
3.1.2. Synthetic Biomaterials
3.2. Biomaterial Systems Presenting Physical Cues
4. Comparison between 2D and 3D Biomaterial Systems
5. Conclusions and Future Perspectives
Author Contributions
Funding
Conflicts of Interest
References
- Alqahtani, S.A. Update in liver transplantation. Curr. Opin. Gastroenterol. 2012, 28, 230–238. [Google Scholar] [CrossRef] [PubMed]
- Asrani, S.K.; Devarbhavi, H.; Eaton, J.; Kamath, P.S. Burden of liver diseases in the world. J. Hepatol. 2019, 70, 151–171. [Google Scholar] [CrossRef] [PubMed]
- Bodzin, A.S.; Baker, T.B. Liver Transplantation Today: Where We Are Now and Where We Are Going. Liver Transplant. 2018, 24, 1470–1475. [Google Scholar] [CrossRef]
- Muraca, M.; Gerunda, G.E.; Neri, D.; Vilei, M.T.; Granato, A.; Feltracco, P.; Giron, G.; Burlina, A.B. Hepatocyte transplantation as a treatment for glycogen storage disease type IA. J. Hepatol. 2002, 36, 41. [Google Scholar] [CrossRef]
- Langer, R.; Vacanti, J. Advances in tissue engineering. J. Pediatr. Surg. 2015, 51, 8–12. [Google Scholar] [CrossRef]
- Demetriou, A.A.; Brown, R.S., Jr.; Busuttil, R.W.; Fair, J.; McGuire, B.M.; Rosenthal, P.; Am Esch, J.S. Prospective, Randomized, Multicenter, Controlled Trial of a Bioartificial Liver in Treating Acute Liver Failure. Ann. Surg. 2004, 239, 660–667. [Google Scholar] [CrossRef]
- Ang, L.T.; Tan, A.K.Y.; Autio, M.I.; Goh, S.H.; Choo, S.H.; Lee, K.L.; Tan, J.; Pan, B.; Lee, J.J.H.; Lum, J.J.; et al. A Roadmap for Human Liver Differentiation from Pluripotent Stem Cells. Cell Rep. 2018, 22, 2190–2205. [Google Scholar] [CrossRef]
- Cotovio, J.P.; Fernandes, T.G. Production of Human Pluripotent Stem Cell-Derived Hepatic Cell Lineages and Liver Organoids: Current Status and Potential Applications. Bioengineering 2020, 7, 36. [Google Scholar] [CrossRef]
- McKee, C.; Chaudhry, G.R. Advances and challenges in stem cell culture. Colloids Surfaces B Biointerfaces 2017, 159, 62–77. [Google Scholar] [CrossRef]
- Baxter, M.; Withey, S.; Harrison, S.; Segeritz, C.-P.; Zhang, F.; Atkinson-Dell, R.; Rowe, C.; Gerrard, D.; Sison-Young, R.; Jenkins, R.; et al. Phenotypic and functional analyses show stem cell-derived hepatocyte-like cells better mimic fetal rather than adult hepatocytes. J. Hepatol. 2014, 62, 581–589. [Google Scholar] [CrossRef]
- Yu, Y.; Liu, H.; Ikeda, Y.; Amiot, B.P.; Rinaldo, P.; Duncan, S.A.; Nyberg, S.L. Hepatocyte-like cells differentiated from human induced pluripotent stem cells: Relevance to cellular therapies. Stem Cell Res. 2012, 9, 196–207. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.-H.; Park, H.-J.; Jang, I.; Kim, H.-E.; Lee, D.-H.; Park, J.-K.; Lee, S.-K.; Yoon, H.H. In Vitro Differentiation of Human Liver-derived Stem Cells with Mesenchymal Characteristics into Immature Hepatocyte-like Cells. Transplant. Proc. 2014, 46, 1633–1637. [Google Scholar] [CrossRef] [PubMed]
- Kadota, S.; Pabon, L.; Reinecke, H.; Murry, C.E. In Vivo Maturation of Human Induced Pluripotent Stem Cell-Derived Cardiomyocytes in Neonatal and Adult Rat Hearts. Stem Cell Rep. 2017, 8, 278–289. [Google Scholar] [CrossRef] [PubMed]
- Clause, K.C.; Barker, T.H. Extracellular matrix signaling in morphogenesis and repair. Curr. Opin. Biotechnol. 2013, 24, 830–833. [Google Scholar] [CrossRef]
- Yu, Y.-D.; Kim, K.-H.; Lee, S.-G.; Choi, S.-Y.; Kim, Y.-C.; Byun, K.-S.; Cha, I.-H.; Park, K.-Y.; Cho, C.-H.; Choi, D.-H. Hepatic Differentiation from Human Embryonic Stem Cells Using Stromal Cells. J. Surg. Res. 2011, 170, e253–e261. [Google Scholar] [CrossRef]
- Pettinato, G.; Lehoux, S.; Ramanathan, R.; Salem, M.M.; He, L.-X.; Muse, O.; Flaumenhaft, R.; Thompson, M.T.; Rouse, E.A.; Cummings, R.D.; et al. Generation of fully functional hepatocyte-like organoids from human induced pluripotent stem cells mixed with Endothelial Cells. Sci. Rep. 2019, 9, 8920. [Google Scholar] [CrossRef]
- Takebe, T.; Sekine, K.; Enomura, M.; Koike, H.; Kimura, M.; Ogaeri, T.; Zhang, R.; Ueno, Y.; Zheng, Y.-W.; Koike, N.; et al. Vascularized and functional human liver from an iPSC-derived organ bud transplant. Nature 2013, 499, 481–484. [Google Scholar] [CrossRef]
- Aufschnaiter, R.; Zamir, E.A.; Little, C.D.; Özbek, S.; Münder, S.; David, C.N.; Li, L.; Sarras, M.P.; Zhang, X. In vivo imaging of basement membrane movement: ECM patterning shapes Hydra polyps. J. Cell Sci. 2011, 124, 4027–4038. [Google Scholar] [CrossRef] [PubMed]
- Hassan, A.; Sapir, L.; Nitsan, I.; Ben-El, R.T.G.; Halachmi, N.; Salzberg, A.; Tzlil, S. A Change in ECM Composition Affects Sensory Organ Mechanics and Function. Cell Rep. 2019, 27, 2272–2280.e4. [Google Scholar] [CrossRef]
- Sato, E.; Zhang, L.-J.; Dorschner, R.A.; Adase, C.A.; Choudhury, B.P.; Gallo, R.L. Activation of Parathyroid Hormone 2 Receptor Induces Decorin Expression and Promotes Wound Repair. J. Investig. Dermatol. 2017, 137, 1774–1783. [Google Scholar] [CrossRef]
- Mouw, J.K.; Ou, G.; Weaver, V.M. Extracellular Matrix Assembly: A Multiscale Deconstruction. Nat. Rev. Mol. Cell Biol. 2014, 15, 771–785. [Google Scholar] [CrossRef] [PubMed]
- Engler, A.J.; Sen, S.; Sweeney, H.L.; E Discher, D. Matrix Elasticity Directs Stem Cell Lineage Specification. Cell 2006, 126, 677–689. [Google Scholar] [CrossRef] [PubMed]
- Lee, B.L.-P.; Tang, Z.; Wang, A.; Huang, F.; Yan, Z.; Wang, N.; Chu, J.S.; Dixit, N.; Yang, L.; Li, S. Synovial stem cells and their responses to the porosity of microfibrous scaffold. Acta Biomater. 2013, 9, 7264–7275. [Google Scholar] [CrossRef] [PubMed]
- Yim, E.K.; Pang, S.W.; Leong, K.W. Synthetic nanostructures inducing differentiation of human mesenchymal stem cells into neuronal lineage. Exp. Cell Res. 2007, 313, 1820–1829. [Google Scholar] [CrossRef]
- Du, J.; Chen, X.; Liang, X.; Zhang, G.; Xu, J.; He, L.; Zhan, Q.; Feng, X.-Q.; Chien, S.; Yang, C. Integrin activation and internalization on soft ECM as a mechanism of induction of stem cell differentiation by ECM elasticity. Proc. Natl. Acad. Sci. USA 2011, 108, 9466–9471. [Google Scholar] [CrossRef]
- Dombrowski, C.; Song, S.J.; Chuan, P.; Lim, X.; Susanto, E.; Sawyer, A.A.; Woodruff, M.A.; Hutmacher, D.W.; Nurcombe, V.; Cool, S.M. Heparan Sulfate Mediates the Proliferation and Differentiation of Rat Mesenchymal Stem Cells. Stem Cells Dev. 2009, 18, 661–670. [Google Scholar] [CrossRef]
- Chen, A.E.; Egli, D.; Niakan, K.; Deng, J.; Akutsu, H.; Yamaki, M.; Cowan, C.; Fitz-Gerald, C.; Zhang, K.; Melton, D.A.; et al. Optimal Timing of Inner Cell Mass Isolation Increases the Efficiency of Human Embryonic Stem Cell Derivation and Allows Generation of Sibling Cell Lines. Cell Stem Cell 2009, 4, 103–106. [Google Scholar] [CrossRef]
- Takahashi, K.; Tanabe, K.; Ohnuki, M.; Narita, M.; Ichisaka, T.; Tomoda, K.; Yamanaka, S. Induction of Pluripotent Stem Cells from Adult Human Fibroblasts by Defined Factors. Cell 2007, 131, 861–872. [Google Scholar] [CrossRef]
- Carpentier, A.; Nimgaonkar, I.; Chu, V.; Xia, Y.; Hu, Z.; Liang, T.J. Hepatic differentiation of human pluripotent stem cells in miniaturized format suitable for high-throughput screen. Stem Cell Res. 2016, 16, 640–650. [Google Scholar] [CrossRef]
- Si-Tayeb, K.; Noto, F.K.; Nagaoka, M.; Li, J.; Battle, M.A.; Duris, C.; North, P.E.; Dalton, S.; Duncan, S.A. Highly efficient generation of human hepatocyte-like cells from induced pluripotent stem cells. Hepatology 2010, 51, 297–305. [Google Scholar] [CrossRef]
- Iii, R.L.G.; Hannan, N.R.F.; Bort, R.; Hanley, N.; Drake, R.A.L.; Cameron, G.W.W.; Wynn, T.A.; Vallier, L. Maturation of Induced Pluripotent Stem Cell Derived Hepatocytes by 3D-Culture. PLoS ONE 2014, 9, e86372. [Google Scholar] [CrossRef]
- Imamura, T.; Cui, L.; Teng, R.; Johkura, K.; Okouchi, Y.; Asanuma, K.; Ogiwara, N.; Sasaki, K. Embryonic Stem Cell-Derived Embryoid Bodies in Three-Dimensional Culture System Form Hepatocyte-Like Cells in Vitro and in Vivo. Tissue Eng. 2004, 10, 1716–1724. [Google Scholar] [CrossRef] [PubMed]
- Nagaoka, M.; Kobayashi, M.; Kawai, C.; Mallanna, S.K.; Duncan, S.A. Design of a Vitronectin-Based Recombinant Protein as a Defined Substrate for Differentiation of Human Pluripotent Stem Cells into Hepatocyte-Like Cells. PLoS ONE 2015, 10, e0136350. [Google Scholar] [CrossRef] [PubMed]
- Cameron, K.; Tan, R.; Schmidt-Heck, W.; Campos, G.; Lyall, M.J.; Wang, Y.; Lucendo-Villarin, B.; Szkolnicka, D.; Bates, N.; Kimber, S.J.; et al. Recombinant Laminins Drive the Differentiation and Self-Organization of Hesc-Derived Hepatocytes. Stem Cell Rep. 2015, 5, 1250–1262. [Google Scholar] [CrossRef] [PubMed]
- Tsuchiya, A.; Takeuchi, S.; Watanabe, T.; Yoshida, T.; Nojiri, S.; Ogawa, M.; Terai, S. Mesenchymal stem cell therapies for liver cirrhosis: MSCs as "conducting cells" for improvement of liver fibrosis and regeneration. Inflamm. Regen. 2019, 39, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Hass, R.; Kasper, C.; Böhm, S.; Jacobs, R. Different populations and sources of human mesenchymal stem cells (MSC): A comparison of adult and neonatal tissue-derived MSC. Cell Commun. Signal. 2011, 9, 12. [Google Scholar] [CrossRef]
- Lee, K.-D.; Kuo, T.K.-C.; Whang-Peng, J.; Chung, Y.-F.; Lin, C.-T.; Chou, S.-H.; Chen, J.-R.; Chen, Y.-P.; Lee, O.K.-S. In vitro hepatic differentiation of human mesenchymal stem cells. Hepatol. 2004, 40, 1275–1284. [Google Scholar] [CrossRef]
- Darwiche, H.; Petersen, B.E. Biology of the Adult Hepatic Progenitor Cell. Prog. Mol. Biol. Transl. Sci. 2010, 97, 229–249. [Google Scholar] [CrossRef]
- Liu, W.; Wang, Y.; Sun, Y.; Wu, Y.; Ma, Q.; Shi, Y.; He, R.; Zhang, T.; Ma, Y.; Zuo, W.; et al. Clonal expansion of hepatic progenitor cells and differentiation into hepatocyte-like cells. Dev. Growth Differ. 2019, 61, 203–211. [Google Scholar] [CrossRef]
- Jang, M.; Kleber, A.; Ruckelshausen, T.; Betzholz, R.; Manz, A. Differentiation of the human liver progenitor cell line (HepaRG) on a microfluidic-based biochip. J. Tissue Eng. Regen. Med. 2019, 13, 482–494. [Google Scholar] [CrossRef]
- Conigliaro, A.; Colletti, M.; Cicchini, C.; Guerra, M.T.; Manfredini, R.; Zini, R.; Bordoni, V.; Siepi, F.; Leopizzi, M.; Tripodi, M.; et al. Isolation and characterization of a murine resident liver stem cell. Cell Death Differ. 2007, 15, 123–133. [Google Scholar] [CrossRef] [PubMed]
- Ai, H.; Anderson, J.; Anseth, K.; Antoniac, I.; Barbosa, M.; Basu, B.; Best, S.; Bettini, R.; Bezuidenhout, D.; Bizios, R.; et al. Attendees at Chengdu Definitions in Biomaterials Conference 2019. In Proceedings of the Definitions of Biomaterials for the Twenty-First Century; Elsevier: Amsterdam, The Netherlands, 2019. [Google Scholar]
- Reis, R.L. 2nd Consensus conference on definitions on biomaterials science. J. Tissue Eng. Regen. Med. 2020, 14, 561–562. [Google Scholar] [CrossRef] [PubMed]
- Kohane, D.S.; Langer, R. Polymeric Biomaterials in Tissue Engineering. Pediatr. Res. 2008, 63, 487–491. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.; Abdeen, A.A.; Zhang, D.; Kilian, K.A. Directing stem cell fate on hydrogel substrates by controlling cell geometry, matrix mechanics and adhesion ligand composition. Biomaterials 2013, 34, 8140–8148. [Google Scholar] [CrossRef]
- Cunha, C.; Panseri, S.; Villa, O.; Silva, D.; Gelain, F. 3D culture of adult mouse neural stem cells within functionalized self-assembling peptide scaffolds. Int. J. Nanomed. 2011, 6, 943–955. [Google Scholar] [CrossRef]
- Jeong, H.-J.; Nam, H.; Jang, J.; Lee, S.-J. 3D Bioprinting Strategies for the Regeneration of Functional Tubular Tissues and Organs. Bioengineering 2020, 7, 32. [Google Scholar] [CrossRef]
- Mirdamadi, M.E.S.; Kalhori, D.; Zakeri, N.; Azarpira, N.; Solati-Hashjin, M. Liver Tissue Engineering as an Emerging Alternative for Liver Disease Treatment. Tissue Eng. Part B: Rev. 2020, 26, 145–163. [Google Scholar] [CrossRef]
- Morais, A.D.S.; Vieira, S.; Zhao, X.; Mao, Z.; Gao, C.; Oliveira, J.M.; Reis, R.L. Advanced Biomaterials and Processing Methods for Liver Regeneration: State-of-the-Art and Future Trends. Adv. Heal. Mater. 2020, 9, e1901435. [Google Scholar] [CrossRef]
- Song, R.; Murphy, M.; Li, C.; Ting, K.; Soo, C.; Zheng, Z. Current development of biodegradable polymeric materials for biomedical applications. Drug Des. Dev. Ther. 2018, 12, 3117–3145. [Google Scholar] [CrossRef]
- Lucendo-Villarin, B.; Rashidi, H.; Cameron, K.; Hay, D.C. Pluripotent stem cell derived hepatocytes: Using materials to define cellular differentiation and tissue engineering. J. Mater. Chem. B 2016, 4, 3433–3442. [Google Scholar] [CrossRef]
- Hay, D.C.; Fletcher, J.; Payne, C.; Terrace, J.D.; Gallagher, R.C.J.; Snoeys, J.; Black, J.R.; Wojtacha, D.; Samuel, K.; Hannoun, Z.; et al. Highly efficient differentiation of hESCs to functional hepatic endoderm requires ActivinA and Wnt3a signaling. Proc. Natl. Acad. Sci. USA 2008, 105, 12301–12306. [Google Scholar] [CrossRef] [PubMed]
- Sullivan, G.; Hay, D.C.; Park, I.-H.; Fletcher, J.; Hannoun, Z.; Payne, C.M.; Dalgetty, N.; Black, J.R.; Ross, J.A.; Samuel, K.; et al. Generation of functional human hepatic endoderm from human induced pluripotent stem cells. Hepatol. 2010, 51, 329–335. [Google Scholar] [CrossRef] [PubMed]
- Touboul, T.; Vallier, L.; Weber, A. Robust Differentiation of Fetal Hepatocytes from Human Embryonic Stem Cells and Ips. Med. Sci. (Paris) 2010, 26, 1061–1066. [Google Scholar] [CrossRef]
- Schwartz, R.E.; Reyes, M.; Koodie, L.; Jiang, Y.; Blackstad, M.; Lund, T.; Lenvik, T.; Johnson, S.; Hu, W.S.; Verfaillie, C.M. Multipotent Adult Progenitor Cells from Bone Marrow Differentiate into Functional Hepatocyte-Like Cells. J. Clin. Investig. 2002, 109, 1291–1302. [Google Scholar] [CrossRef]
- Serban, M.A.; Prestwich, G.D. Modular extracellular matrices: Solutions for the puzzle. Methods 2008, 45, 93–98. [Google Scholar] [CrossRef]
- Zhu, J.; E Marchant, R. Design properties of hydrogel tissue-engineering scaffolds. Expert Rev. Med Devices 2011, 8, 607–626. [Google Scholar] [CrossRef]
- Goddard, E.; Hill, R.C.; Barrett, A.; Betts, C.; Guo, Q.; Maller, O.; Borges, V.F.; Hansen, K.C.; Schedin, P. Quantitative extracellular matrix proteomics to study mammary and liver tissue microenvironments. Int. J. Biochem. Cell Biol. 2016, 81, 223–232. [Google Scholar] [CrossRef] [PubMed]
- Brill, S. The role of fetal and adult hepatocyte extracellular matrix in the regulation of tissue-specific gene expression in fetal and adult hepatocytes. Eur. J. Cell Biol. 2002, 81, 43–50. [Google Scholar] [CrossRef]
- Kim, T.H.; Mars, W.M.; Stolz, D.B.; Petersen, B.E.; Michalopoulos, G.K. Extracellular Matrix Remodeling at the Early Stages of Liver Regeneration in the Rat. Hepatology 1997, 26, 896–904. [Google Scholar] [CrossRef]
- Nakai, S.; Shibata, I.; Shitamichi, T.; Yamaguchi, H.; Takagi, N.; Inoue, T.; Nakagawa, T.; Kiyokawa, J.; Wakabayashi, S.; Miyoshi, T.; et al. Collagen vitrigel promotes hepatocytic differentiation of induced pluripotent stem cells into functional hepatocyte-like cells. Biol. Open 2019, 8, bio042192. [Google Scholar] [CrossRef]
- Azandeh, S.; Gharravi, A.M.; Orazizadeh, M.; Khodadi, A.; Tabar, M.H. Improvement of mesenchymal stem cell differentiation into the endoderm lineage by four step sequential method in biocompatible biomaterial. BioImpacts 2016, 6, 9–13. [Google Scholar] [CrossRef]
- Maguire, T.; Novik, E.; Schloss, R.; Yarmush, M. Alginate-PLL microencapsulation: Effect on the differentiation of embryonic stem cells into hepatocytes. Biotechnol. Bioeng. 2006, 93, 581–591. [Google Scholar] [CrossRef]
- Sun, J.; Tan, H. Alginate-Based Biomaterials for Regenerative Medicine Applications. Mater. 2013, 6, 1285–1309. [Google Scholar] [CrossRef] [PubMed]
- Yang, W.; Xia, R.; Zhang, Y.; Zhang, H.; Bai, L. Decellularized Liver Scaffold for Liver Regeneration. In Advanced Structural Safety Studies; Springer Science and Business Media LLC: Berlin, Germany, 2017; pp. 11–23. [Google Scholar]
- Rana, D.; Zreiqat, H.; Benkirane-Jessel, N.; Ramakrishna, S.; Ramalingam, M. Development of decellularized scaffolds for stem cell-driven tissue engineering. J. Tissue Eng. Regen. Med. 2015, 11, 942–965. [Google Scholar] [CrossRef] [PubMed]
- Wu, Q.; Tang, J.; Li, Y.; Li, L.; Wang, Y.; Bao, J.; Bu, H. Hepatic differentiation of mouse bone marrow-derived mesenchymal stem cells using a novel 3D culture system. Mol. Med. Rep. 2017, 16, 9473–9479. [Google Scholar] [CrossRef] [PubMed]
- Jiang, W.-C.; Cheng, Y.-H.; Yen, M.-H.; Chang, Y.; Yang, V.W.; Lee, O.K. Cryo-chemical decellularization of the whole liver for mesenchymal stem cells-based functional hepatic tissue engineering. Biomater. 2014, 35, 3607–3617. [Google Scholar] [CrossRef] [PubMed]
- Navarro-Tableros, V.; Sanchez, M.B.H.; Figliolini, F.; Romagnoli, R.; Tetta, C.; Camussi, G. Recellularization of Rat Liver Scaffolds by Human Liver Stem Cells. Tissue Eng. Part A 2015, 21, 1929–1939. [Google Scholar] [CrossRef]
- Wang, B.; Jakus, A.E.; Baptista, P.M.; Soker, S.; Soto-Gutierrez, A.; Abecassis, M.; Shah, R.N.; Wertheim, J.A. Functional Maturation of Induced Pluripotent Stem Cell Hepatocytes in Extracellular Matrix—A Comparative Analysis of Bioartificial Liver Microenvironments. STEM CELLS Transl. Med. 2016, 5, 1257–1267. [Google Scholar] [CrossRef]
- Zhang, X.; Dong, J. Direct comparison of different coating matrix on the hepatic differentiation from adipose-derived stem cells. Biochem. Biophys. Res. Commun. 2015, 456, 938–944. [Google Scholar] [CrossRef]
- Wang, B.; Li, W.; Dean, D.; Mishra, M.K.; Wekesa, K.S. Enhanced hepatogenic differentiation of bone marrow derived mesenchymal stem cells on liver ECM hydrogel. J. Biomed. Mater. Res. Part A 2017, 106, 829–838. [Google Scholar] [CrossRef]
- Adamski, M.; Fontana, G.; Gershlak, J.R.; Gaudette, G.R.; Le, H.D.; Murphy, W.L. Two Methods for Decellularization of Plant Tissues for Tissue Engineering Applications. J. Vis. Exp. 2018, e57586. [Google Scholar] [CrossRef] [PubMed]
- Aleahmad, F.; Talaei-Khozani, T.; Rajabi-Zeleti, S.; Sani, M.; Jalili-Firoozinezhad, S.; Bonakdar, S.; Heshmat-Azad, S.; Azarnia, M.; Jaberipour, M. Fabrication and Characterization of Heparin/Collagen Sponge for in Vitro Differentiation of Wharton’s Jelly-Derived Mesenchymal Stem Cells into Hepatocytes. Zahedan J. Res. Med Sci. 2017, 17, 58724. [Google Scholar] [CrossRef]
- Chitrangi, S.; Nair, P.; Khanna, A. Three-dimensional polymer scaffolds for enhanced differentiation of human mesenchymal stem cells to hepatocyte-like cells: A comparative study. J. Tissue Eng. Regen. Med. 2016, 11, 2359–2372. [Google Scholar] [CrossRef] [PubMed]
- Malinen, M.M.; Kanninen, L.; Corlu, A.; Isoniemi, H.M.; Lou, Y.-R.; Yliperttula, M.; Urtti, A. Differentiation of liver progenitor cell line to functional organotypic cultures in 3D nanofibrillar cellulose and hyaluronan-gelatin hydrogels. Biomater. 2014, 35, 5110–5121. [Google Scholar] [CrossRef] [PubMed]
- Abdulghani, S.; Mitchell, G.R. Biomaterials for In Situ Tissue Regeneration: A Review. Biomol. 2019, 9, 750. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Tao, R.; Wu, W.; Cao, H.; Xin, J.; Guo, J.; Jiang, L.; Gaoa, C.; Demetriou, A.A.; Farkas, D.L.; et al. 3D PLGA Scaffolds Improve Differentiation and Function of Bone Marrow Mesenchymal Stem Cell–Derived Hepatocytes. Stem Cells Dev. 2010, 19, 1427–1436. [Google Scholar] [CrossRef]
- Wang, Y.; Lee, J.-H.; Shirahama, H.; Seo, J.; Glenn, J.S.; Cho, N.-J. Extracellular Matrix Functionalization and Huh-7.5 Cell Coculture Promote the Hepatic Differentiation of Human Adipose-Derived Mesenchymal Stem Cells in a 3D ICC Hydrogel Scaffold. ACS Biomater. Sci. Eng. 2016, 2, 2255–2265. [Google Scholar] [CrossRef]
- Prowse, A.B.J.; Chong, F.; Gray, P.P.; Munro, T.P. Stem cell integrins: Implications for ex-vivo culture and cellular therapies. Stem Cell Res. 2011, 6, 1–12. [Google Scholar] [CrossRef]
- Chan, H.F.; Zhang, Y.; Ho, Y.-P.; Chiu, Y.-L.; Jung, Y.; Leong, K.W. Rapid formation of multicellular spheroids in double-emulsion droplets with controllable microenvironment. Sci. Rep. 2013, 3, 3462. [Google Scholar] [CrossRef]
- Janoštiak, R.; Pataki, A.C.; Brábek, J.; Rosel, D. Mechanosensors in integrin signaling: The emerging role of p130Cas. Eur. J. Cell Biol. 2014, 93, 445–454. [Google Scholar] [CrossRef]
- Hwang, Y.; Goh, M.; Kim, M.; Tae, G. Injectable and detachable heparin-based hydrogel micropatches for hepatic differentiation of hADSCs and their liver targeted delivery. Biomater. 2018, 165, 94–104. [Google Scholar] [CrossRef]
- Mittal, N.; Tasnim, F.; Yue, C.; Qu, Y.; Phan, D.; Choudhury, Y.; Tan, M.-H.; Yu, H. Substrate Stiffness Modulates the Maturation of Human Pluripotent Stem-Cell-Derived Hepatocytes. ACS Biomater. Sci. Eng. 2016, 2, 1649–1657. [Google Scholar] [CrossRef]
- Cozzolino, A.M.; Noce, V.; Battistelli, C.; Marchetti, A.; Grassi, G.; Cicchini, C.; Tripodi, M.; Amicone, L. Modulating the Substrate Stiffness to Manipulate Differentiation of Resident Liver Stem Cells and to Improve the Differentiation State of Hepatocytes. Stem Cells Int. 2016, 2016, 1–12. [Google Scholar] [CrossRef] [PubMed]
- You, J.; Park, S.-A.; Shin, D.-S.; Patel, D.; Raghunathan, V.K.; Kim, M.; Murphy, C.J.; Tae, G.; Revzin, A. Characterizing the Effects of Heparin Gel Stiffness on Function of Primary Hepatocytes. Tissue Eng. Part A 2013, 19, 2655–2663. [Google Scholar] [CrossRef]
- Lee, H.-J.; Son, M.J.; Ahn, J.; Oh, S.J.; Lee, M.; Kim, A.; Jeung, Y.-J.; Kim, H.-G.; Won, M.; Lim, J.H.; et al. Elasticity-based development of functionally enhanced multicellular 3D liver encapsulated in hybrid hydrogel. Acta Biomater. 2017, 64, 67–79. [Google Scholar] [CrossRef] [PubMed]
- Rajendran, D.; Hussain, A.; Yip, D.; Parekh, A.; Shrirao, A.; Cho, C.H. Long-term liver-specific functions of hepatocytes in electrospun chitosan nanofiber scaffolds coated with fibronectin. J. Biomed. Mater. Res. Part A 2017, 105, 2119–2128. [Google Scholar] [CrossRef]
- Bishi, D.K.; Mathapati, S.; Venugopal, J.R.; Guhathakurta, S.; Cherian, K.M.; Ramakrishna, S.; Verma, R.S. Trans-Differentiation of Human Mesenchymal Stem Cells Generates Functional Hepatospheres on Poly(L-Lactic Acid)-Co-Poly(Epsilon-Caprolactone)/Collagen Nanofibrous Scaffolds. J Mater Chem B 2013, 1, 3972–3984. [Google Scholar] [CrossRef]
- Asonuma, K.; Gilbert, J.C.; Stein, J.E.; Takeda, T.; Vacanti, J.P. Quantitation of transplanted hepatic mass necessary to cure the gunn rat model of hyperbilirubinemia. J. Pediatr. Surg. 1992, 27, 298–301. [Google Scholar] [CrossRef]
- Ranucci, C.S.; Kumar, A.; Batra, S.P.; Moghe, P.V. Control of hepatocyte function on collagen foams: Sizing matrix pores toward selective induction of 2-D and 3-D cellular morphogenesis. Biomater. 2000, 21, 783–793. [Google Scholar] [CrossRef]
- Wang, X.; Ding, B.; Li, B. Biomimetic electrospun nanofibrous structures for tissue engineering. Mater. Today 2013, 16, 229–241. [Google Scholar] [CrossRef]
- Andalib, M.N.; Lee, J.S.; Ha, L.; Dzenis, Y.A.; Lim, J.Y. Focal adhesion kinase regulation in stem cell alignment and spreading on nanofibers. Biochem. Biophys. Res. Commun. 2016, 473, 920–925. [Google Scholar] [CrossRef] [PubMed]
- Cooper, A.; Leung, M.; Zhang, M. Polymeric Fibrous Matrices for Substrate-Mediated Human Embryonic Stem Cell Lineage Differentiation. Macromol. Biosci. 2012, 12, 882–892. [Google Scholar] [CrossRef] [PubMed]
- Morgan, K.; Bryans, A.; Brzeszczynski, F.; Samuel, M.K.; Treskes, P.; Brzeszczynska, J.; Morley, S.; Hayes, P.; Gadegaard, N.; Nelson, L.; et al. Oxygen plasma substrate and specific nanopattern promote early differentiation of HepaRG progenitors. Tissue Eng. Part A 2020. [Google Scholar] [CrossRef] [PubMed]
- Duval, K.E.A.; Grover, H.; Han, L.-H.; Mou, Y.; Pegoraro, A.F.; Fredberg, J.; Chen, Z. Modeling Physiological Events in 2D vs. 3D Cell Culture. Physiol. 2017, 32, 266–277. [Google Scholar] [CrossRef]
- Chan, H.F.; Zhang, Y.; Leong, K.W. Efficient One-Step Production of Microencapsulated Hepatocyte Spheroids with Enhanced Functions. Small 2016, 12, 2720–2730. [Google Scholar] [CrossRef]
- Bratt-Leal, A.M.; Carpenedo, R.L.; McDevitt, T.C. Engineering the embryoid body microenvironment to direct embryonic stem cell differentiation. Biotechnol. Prog. 2009, 25, 43–51. [Google Scholar] [CrossRef]
- Richardson, T.; Kumta, P.N.; Banerjee, I. Alginate Encapsulation of Human Embryonic Stem Cells to Enhance Directed Differentiation to Pancreatic Islet-Like Cells. Tissue Eng. Part A 2014, 20, 3198–3211. [Google Scholar] [CrossRef]
- Hong, H.; Stegemann, J.P. 2D and 3D collagen and fibrin biopolymers promote specific ECM and integrin gene expression by vascular smooth muscle cells. J. Biomater. Sci. Polym. Ed. 2008, 19, 1279–1293. [Google Scholar] [CrossRef]
- McClelland, R.; Wauthier, E.; Uronis, J.; Reid, L. Gradients in the Liver’s Extracellular Matrix Chemistry from Periportal to Pericentral Zones: Influence on Human Hepatic Progenitors. Tissue. Eng. Part A 2008, 14, 59–70. [Google Scholar] [CrossRef]
- Doddapaneni, R.; Chawla, Y.K.; Das, A.; Kalra, J.K.; Ghosh, S.; Chakraborti, A. Overexpression of microRNA-122 enhances in vitro hepatic differentiation of fetal liver-derived stem/progenitor cells. J. Cell. Biochem. 2013, 114, 1575–1583. [Google Scholar] [CrossRef]
- Jung, K.H.; McCarthy, R.L.; Zhou, C.; Uprety, N.; Barton, M.C.; Beretta, L. MicroRNA Regulates Hepatocytic Differentiation of Progenitor Cells by Targeting YAP1. STEM CELLS 2016, 34, 1284–1296. [Google Scholar] [CrossRef] [PubMed]
- Liu, T.; Zhang, S.; Xiang, D.; Wang, Y. Induction of Hepatocyte-Like Cells from Mouse Embryonic Stem Cells by Lentivirus-Mediated Constitutive Expression of Foxa2/Hnf4a. J. Cell Biochem. 2013, 114, 2531–2541. [Google Scholar] [CrossRef] [PubMed]
| Biomaterial Systems | Stem Cell Sources | Differentiation Efficiency (% of Albumin-Positive Cells) | Ref. |
|---|---|---|---|
| Biochemical cues | |||
| 2D | |||
| Collagen | iPSC | 54.3% (day 25) | [61] |
| Decellularized liver ECM | MSC | 26.7% (day 21) | [71] |
| Laminin | ESC | 91.3% (day 18) | [34] |
| Matrigel | ESC, iPSC, MSC | 80.9% (day 20) [30]; 90% (day 17) [52]; 91% (day 14) [53] | [30,51,52,53,54,55] |
| Vitronectin | ESC, iPSC | Not provided | [33] |
| 3D | |||
| Alginate | ESC, MSC | 87% (day 20) [63] | [62,63] |
| Collagen | ESC | Not provided | [31,32] |
| Cellulose, hyaluronan-gelatin | HepaRG | Not provided | [76] |
| Decellularized liver ECM | ESC, iPSC, MSC | Not provided | [67,68,70] |
| Dextran-gelatin, chitosan-hyaluronic acid, gelatin-vinyl acetate | MSC | Dextran-gelatin: 57.2% (day 28); chitosan-hyaluronic acid: 62.8% (day 28); gelatin-vinyl acetate: 68.1% (day 28) | [75] |
| Heparin-collagen | MSC | Not provided | [74] |
| PEG-collagen/fibronectin | MSC | Not provided | [79] |
| PLGA-collagen | MSC | Not provided | [78] |
| Physical cues (stiffness/topography/porosity and pore size) | |||
| 2D | |||
| Heparin (stiffness) | MSC | ~60% (day 21) | [83] |
| Polyacrylamide (stiffness) | ESC, iPSC, Resident liver stem cells | Not provided | [84,85] |
| 3D | |||
| Chitosan (topography) | ESC | Not provided | [94] |
| PEG/hyaluronic acid (stiffness) | HepaRG | Not provided | [87] |
| Poly(L-lactic acid)-co-poly (ε-caprolactone) (PLACL)/collagen (porosity) | MSC | Not provided | [89] |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhao, X.; Zhu, Y.; Laslett, A.L.; Chan, H.F. Hepatic Differentiation of Stem Cells in 2D and 3D Biomaterial Systems. Bioengineering 2020, 7, 47. https://doi.org/10.3390/bioengineering7020047
Zhao X, Zhu Y, Laslett AL, Chan HF. Hepatic Differentiation of Stem Cells in 2D and 3D Biomaterial Systems. Bioengineering. 2020; 7(2):47. https://doi.org/10.3390/bioengineering7020047
Chicago/Turabian StyleZhao, Xiaoyu, Yanlun Zhu, Andrew L. Laslett, and Hon Fai Chan. 2020. "Hepatic Differentiation of Stem Cells in 2D and 3D Biomaterial Systems" Bioengineering 7, no. 2: 47. https://doi.org/10.3390/bioengineering7020047
APA StyleZhao, X., Zhu, Y., Laslett, A. L., & Chan, H. F. (2020). Hepatic Differentiation of Stem Cells in 2D and 3D Biomaterial Systems. Bioengineering, 7(2), 47. https://doi.org/10.3390/bioengineering7020047





