Bioprinting for Liver Transplantation
Abstract
1. Introduction
2. 3D Bioprinting Techniques
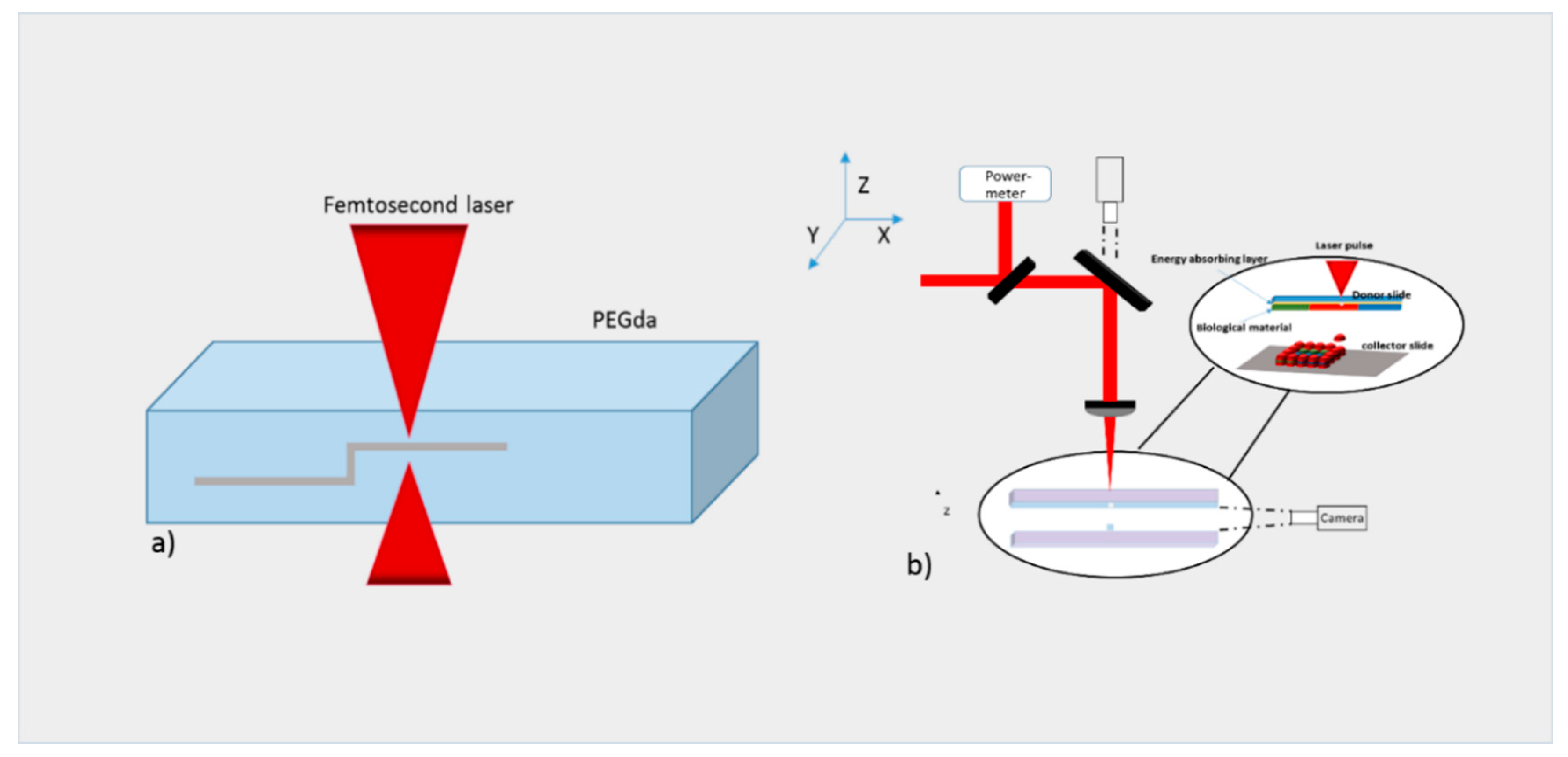
2.1. Laser Bioprinting
2.2. Inkjet Bioprinting
2.3. Extrusion Bioprinting
3. Tissue and Liver Bioprinting
3.1. Micropatterned 2D and 3D Liver Models
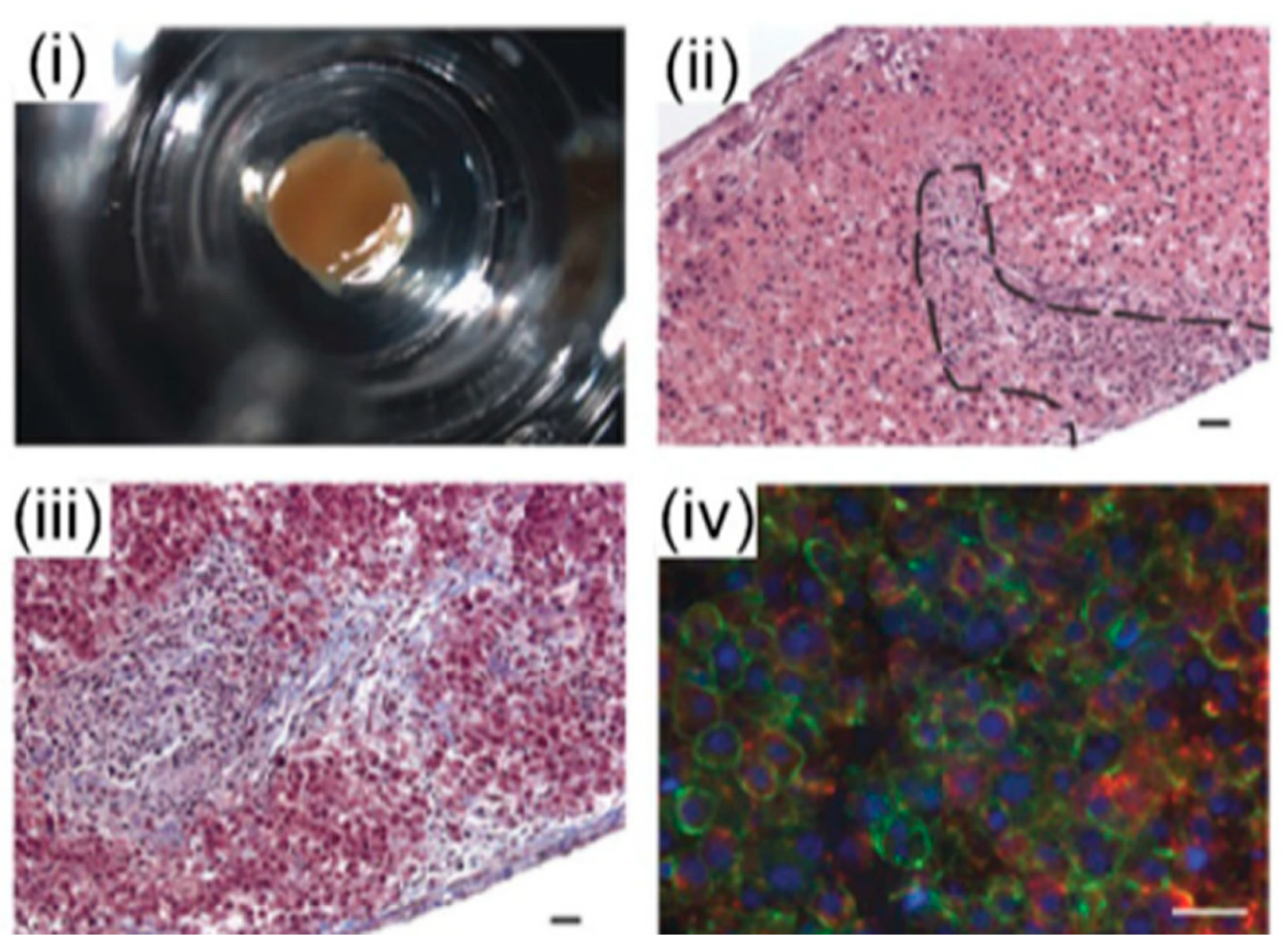
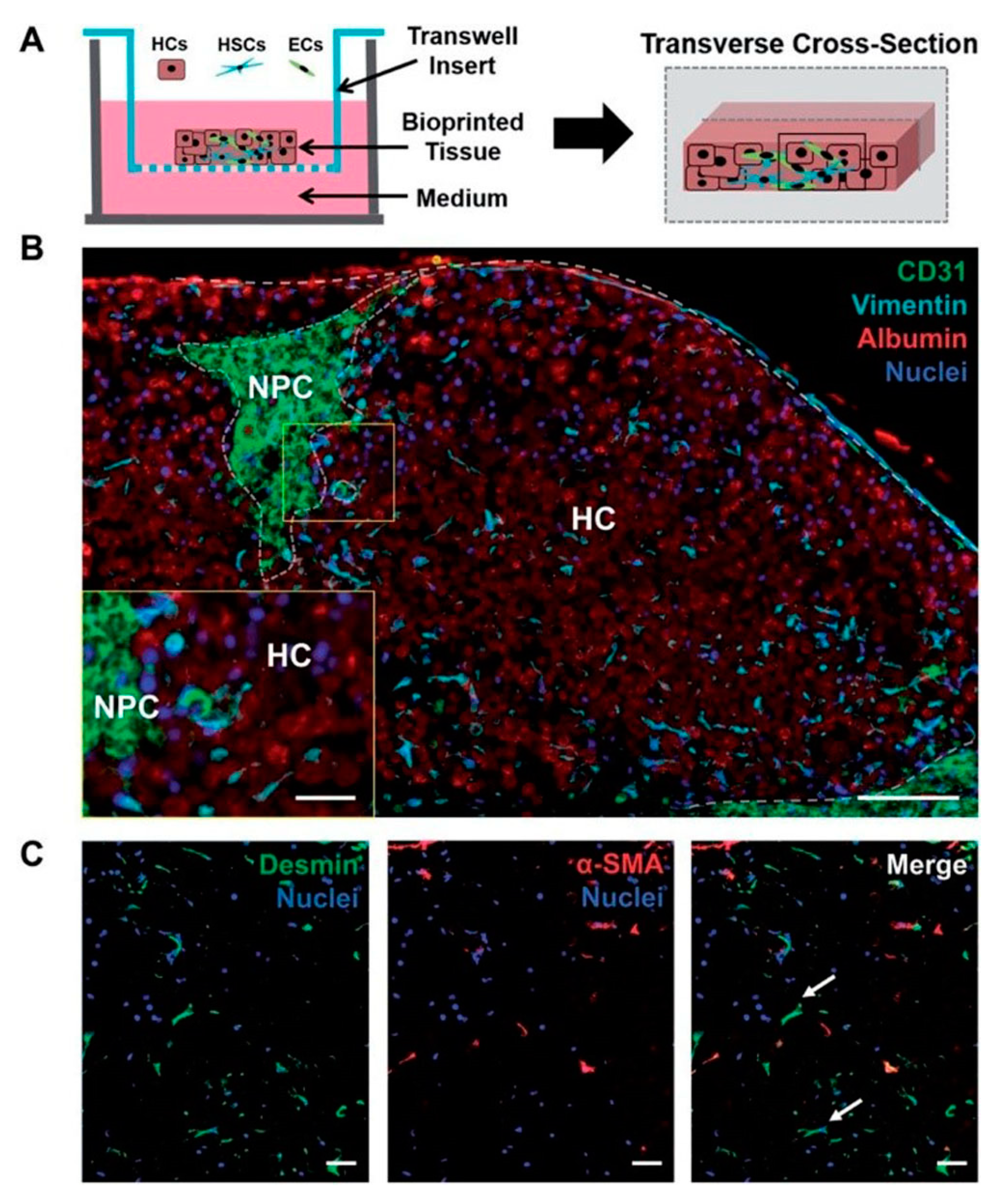
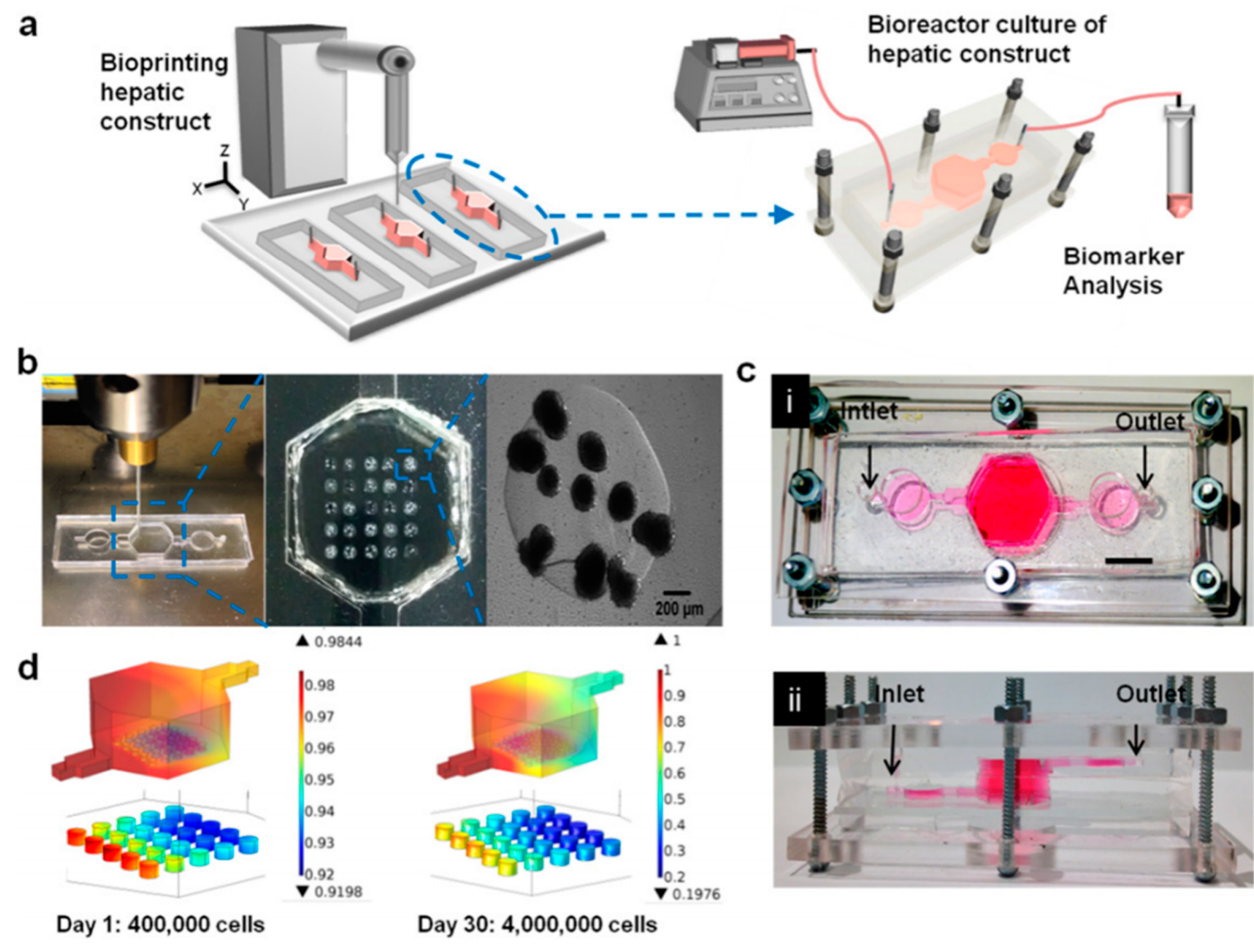
3.2. 3D Bioprinting for Liver Models
3.3. Liver-on-Chip Platforms
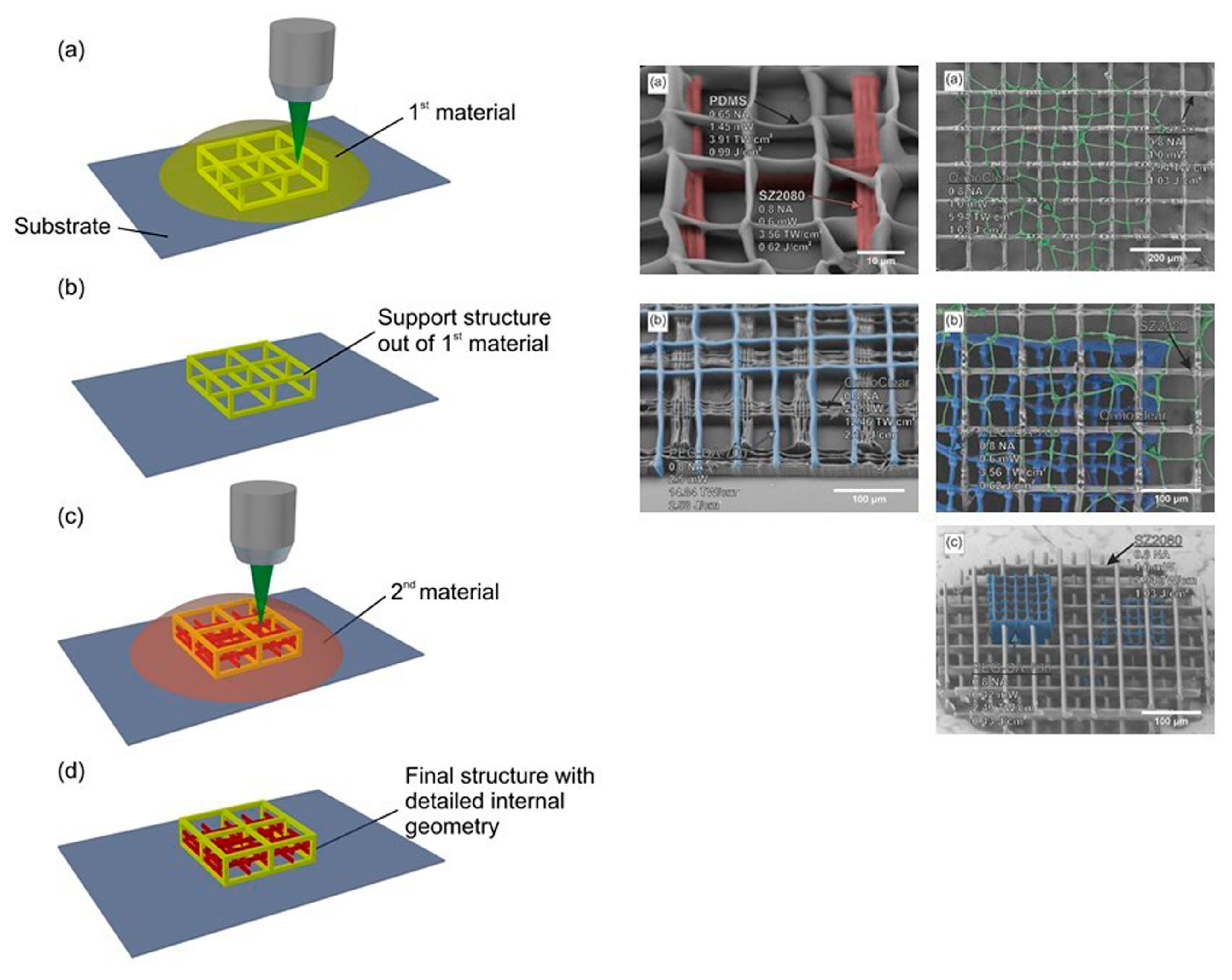
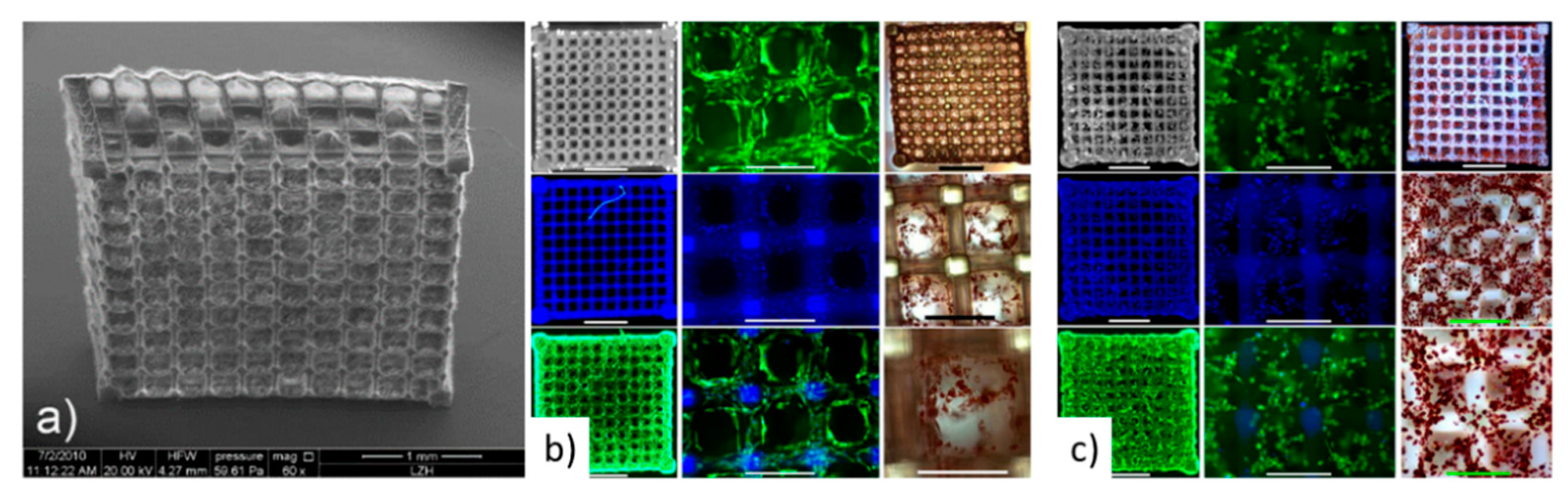
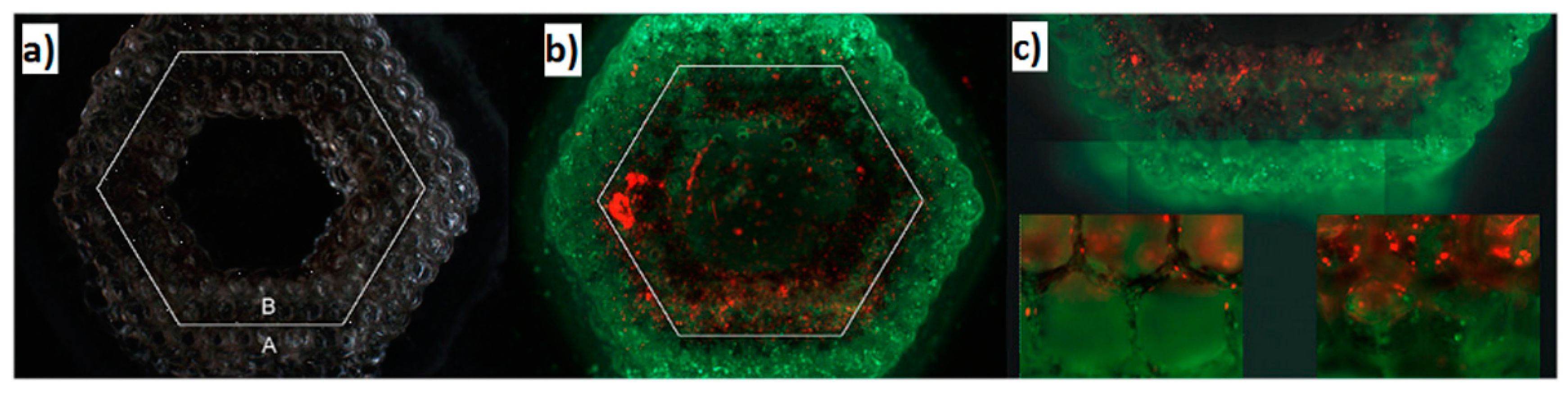
4. Scaffolds Fabrication Methods
4.1. Laser-Based Methods
4.2. Inkjet Printing
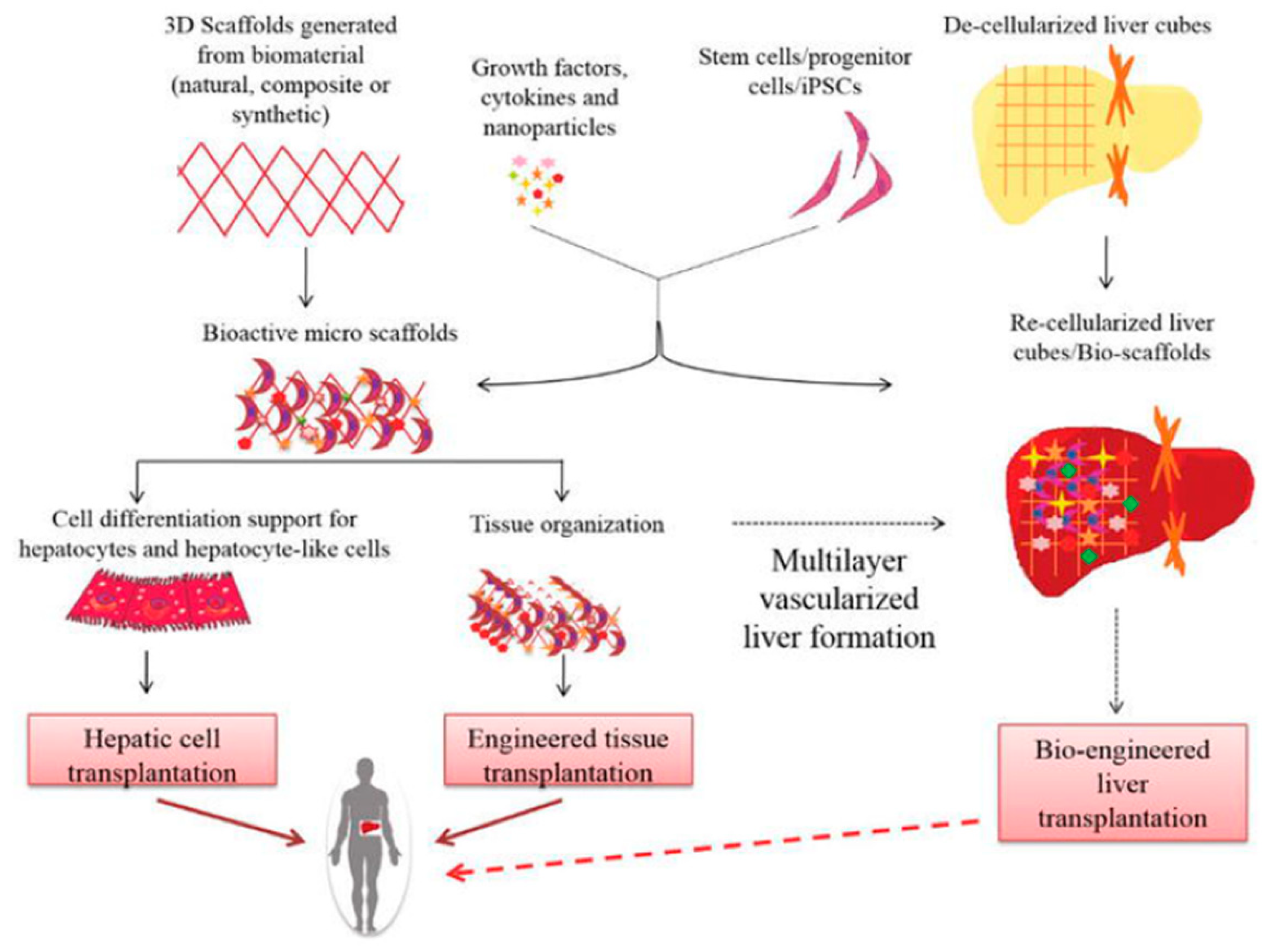
5. Scaffolds for Liver Tissue Engineering
6. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Kruth, J. Material Incress Manufacturing by Rapid Prototyping Techniques. CIRP Ann. 1991, 40, 603–614. [Google Scholar] [CrossRef]
- Heller, T.B.; Hill, R.M.; Saggal, A.F. Method of and Apparatus for Forming a Solid Three-Dimensional Article from a Liquid Medium. U.S. Patent 5,071,337, 10 December 1991. [Google Scholar]
- Hull, C.W. Apparatus for Production of Three-Dimensional Objects by Stereolithography. U.S. Patent 4,575,330, 11 March 1986. [Google Scholar]
- Nakamura, M.; Iwanaga, S.; Henmi, C.; Arai, K.; Nishiyama, Y. Biomatrices and biomaterials for future developments of bioprinting and biofabrication. Biofabrication 2010, 2, 014110. [Google Scholar] [CrossRef] [PubMed]
- Murphy, S.V.; Atala, A. 3D bioprinting of tissues and organs. Nat. Biotechnol. 2014, 32, 773–785. [Google Scholar] [CrossRef] [PubMed]
- Munaz, A.; Vadivelu, R.K.; John, J.S.; Barton, M.; Kamble, H.; Nguyen, N.T. Three-dimensional printing of biological matters. J. Sci. Adv. Mater. Devices 2016, 1, 1–17. [Google Scholar] [CrossRef]
- Derakhshanfar, S.; Mbeleck, R.K.; Xu, K.; Zhang, X.; Zhong, W.; Xing, M. 3D bioprinting for biomedical devices and tissue engineering: A review of recent trends and advances. Bioact. Mater. 2018, 3, 144–156. [Google Scholar] [CrossRef] [PubMed]
- Bertassoni, L.E.; Cecconi, M.; Manoharan, V.; Nikkhah, M.; Hjortnaes, J.; Cristino, A.L.; Barabaschi, G.; Demarchi, D.; Dokmeci, M.R.; Yang, Y.; et al. Hydrogel bioprinted microchannel networks for vascularization of tissue engineering constructs. Lab Chip 2014, 14, 2202–2211. [Google Scholar] [CrossRef] [PubMed]
- Moya, M.L.; Hsu, Y.H.; Lee, A.P.; Hughes, C.C.; George, S.C. In Vitro Perfused Human Capillary Networks. Tissue Eng. Part C Methods 2013, 19, 730–737. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Chen, Y.; Li, P.C.H. A simple and fast microfluidic approach of same-single-cell analysis (SASCA) for the study of multidrug resistance modulation in cancer cells. Lab Chip 2011, 11, 1378–1384. [Google Scholar] [CrossRef]
- Li, X.J.; Nie, Z.H.; Cheng, C.M.; Goodale, A.B.; Whitesides, G.M. Paper-based electrochemical ELISA. Proc. Micro Total Anal. Syst. 2010, 14, 1487–1489. [Google Scholar]
- Salieb-Beugelaar, G.B.; Simone, G.; Arora, A.; Philippi, A.; Manz, A. Latest Developments in Microfluidic Cell Biology and Analysis Systems. Anal. Chem. 2010, 82, 4848–4864. [Google Scholar] [CrossRef]
- Jang, K.; Sato, K.; Igawa, K.; Chung, U.I.; Kitamori, T. Development of an osteoblast-based 3D continuous-perfusion microfluidic system for drug screening. Anal. Bioanal. Chem. 2008, 390, 825–832. [Google Scholar] [CrossRef] [PubMed]
- Chang, C.C.; Boland, E.D.; Williams, S.K.; Hoying, J.B. Direct-write bioprinting three-dimensional biohybrid systems for future regenerative therapies. J. Biomed. Mater. Res. Part B Appl. Biomater. 2011, 98, 160–170. [Google Scholar] [CrossRef] [PubMed]
- Kurobe, H.; Maxfield, M.W.; Breuer, C.K.; Shinoka, T. Concise Review: Tissue-Engineered Vascular Grafts for Cardiac Surgery: Past, Present, and Future. Stem Cells Transl. Med. 2012, 1, 566–571. [Google Scholar] [CrossRef] [PubMed]
- Freeman, R.B.; Steffick, D.E., Jr.; Guidinger, M.K.; Farmer, D.G.; Berg, C.L.; Merion, R.M. Liver and Intestine Transplantation in the United States 1998–2006. Am. J. Transplant. 2008, 8, 958–976. [Google Scholar] [CrossRef] [PubMed]
- Powers, M.J.; Janigian, D.M.; Wack, K.E.; Baker, C.S.; Stolz, D.B.; Griffith, L.G. Functional Behavior of Primary Rat Liver Cells in a Three-Dimensional Perfused Microarray Bioreactor. Tissue Eng. 2002, 8, 499–513. [Google Scholar] [CrossRef] [PubMed]
- Allen, J.W.; Bhatia, S.N. Improving the next generation of bioartificial liver devices. Semin. Cell Dev. Biol. 2002, 13, 447–454. [Google Scholar] [CrossRef] [PubMed]
- Bao, J.; Fisher, J.; Nyberg, S.L. Liver Regeneration and Tissue Engineering. In Tissue Engineering in Regenerative Medicine; Humana Press: New York, NY, USA, 2011; pp. 315–332. [Google Scholar]
- Melchels, F.P.W.; Domingos, M.A.N.; Klein, T.J.; Malda, J.; Bartolo, P.J.; Hutmacher, D.W. Additive Manufacturing of Tissues and Organs. Prog. Polym. Sci. 2012, 37, 1079–1104. [Google Scholar] [CrossRef]
- Jose, R.R.; Rodriguez, M.J.; Dixon, T.A.; Omenetto, F.G.; Kaplan, D.L. Evolution of Bioinks and Additive Manufacturing Technologies for 3D Bioprinting. ACS Biomater. Sci. Eng. 2016, 2, 1662–1678. [Google Scholar] [CrossRef]
- Gross, B.C.; Erkal, J.L.; Lockwood, S.Y.; Chen, C.; Spence, D.M. Evaluation of 3D Printing and Its Potential Impact on Biotechnology and the Chemical Sciences. Anal. Chem. 2014, 86, 3240–3253. [Google Scholar] [CrossRef] [PubMed]
- Choi, J.Y.; Das, S.; Theodore, N.D. Advances in 2D/3D Printing of Functional Nanomaterials and Their Applications. ECS J. Solid State Sci. Technol. 2015, 4, 3001–3009. [Google Scholar] [CrossRef]
- Wang, X.; Yan, Y.; Zhang, R. Rapid prototyping as a tool for manufacturing bioartificial livers. Trends Biotechnol. 2007, 25, 505–513. [Google Scholar] [CrossRef] [PubMed]
- Derby, B. Printing and prototyping of tissues and scaffolds. Science 2012, 338, 921–926. [Google Scholar] [CrossRef] [PubMed]
- Choi, Y.J.; Yi, H.G.; Kim, S.W.; Cho, D.W. 3D Cell Printed Tissue Analogues: A New Platform for Theranostics. Theranostics 2017, 7, 3118–3137. [Google Scholar] [CrossRef] [PubMed]
- Iwami, K.; Noda, T.; Ishida, K.; Morishima, K.; Nakamura, M.; Umeda, N. Bio rapid prototyping by extruding/aspirating/refilling thermoreversible hydrogel. Biofabrication 2010, 2, 014108. [Google Scholar] [CrossRef] [PubMed]
- Xu, T.; Zhao, W.; Zhu, J.M.; Albanna, M.Z.; Yoo, J.J.; Atala, A. Complex heterogeneous tissue constructs containing multiple cell types prepared by inkjet printing technology. Biomaterials 2013, 34, 130–139. [Google Scholar] [CrossRef]
- Guillemot, F.; Souquet, A.; Catros, S.; Guillotin, B.; Lopez, J.; Faucon, M.; Pippenger, B.; Bareille, R.; Remy, M.; Bellance, S.; et al. High-throughput laser printing of cells and biomaterials for tissue engineering. Acta Biomater. 2010, 6, 2494–2500. [Google Scholar] [CrossRef]
- Guillotin, B.; Souquet, A.; Catros, S.; Duocastella, M.; Pippenger, B.; Bellance, S.; Bareille, R.; Remy, M.; Bordenave, L.; Amedee, J.; et al. Laser assisted bioprinting of engineered tissue with high cell density and microscale organization. Biomaterials 2010, 31, 7250–7256. [Google Scholar] [CrossRef]
- Bohandy, J.; Kim, B.F.; Adrian, F.J. Metal deposition from a supported metal film using an excimer laser. J. Appl. Phys. 1986, 60, 1538–1539. [Google Scholar] [CrossRef]
- Guillemot, F.; Souquet, A.; Catros, S.; Guillotin, B. Laser-assisted cell printing: Principle, physical parameters versus cell fate and perspectives in tissue engineering. Nanomedicine 2010, 5, 507–515. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Zhang, Y. Tissue Engineering Applications of Three-Dimensional Bioprinting. Cell Biophys. 2015, 72, 777–782. [Google Scholar] [CrossRef] [PubMed]
- Karaiskou, A.; Zergioti, I.; Fotakis, C.; Kapsetaki, M.; Kafetzopoulos, D. Microfabrication of biomaterials by the sub-ps laser-induced forward transfer process. Appl. Surf. Sci. 2003, 208, 245–249. [Google Scholar] [CrossRef]
- Othon, C.M.; Wu, X.; Anders, J.J.; Ringeisen, B.R. Single-cell printing to form three-dimensional lines of olfactory ensheathing cells. Biomed. Mater. 2008, 3, 034101. [Google Scholar] [CrossRef] [PubMed]
- Gruene, M.; Deiwick, A.; Koch, L.; Schlie, S.; Unger, C.; Hofmann, N.; Bernemann, I.; Glasmacher, B.; Chichkov, B. Laser Printing of Stem Cells for Biofabrication of Scaffold-Free Autologous Grafts. Tissue Eng. Part C Methods 2011, 17, 79–87. [Google Scholar] [CrossRef] [PubMed]
- Koch, L.; Deiwick, A.; Schlie, S.; Michael, S.; Gruene, M.; Coger, V.; Zychlinski, D.; Schambach, A.; Reimers, K.; Vogt, P.M.; et al. Skin tissue generation by laser cell printing. Biotechnol. Bioeng. 2012, 109, 1855–1863. [Google Scholar] [CrossRef] [PubMed]
- Koch, L.; Kuhn, S.; Sorg, H.; Gruene, M.; Schlie, S.; Gaebel, R.; Polchow, B.; Reimers, K.; Stoelting, S.; Ma, N.; et al. Laser Printing of Skin Cells and Human Stem Cells. Tissue Eng. Part C Methods 2010, 16, 847–854. [Google Scholar] [CrossRef] [PubMed]
- Catros, S.; Guillotin, B.; Bacakova, M.; Fricain, J.C.; Guillemot, F. Effect of laser energy, substrate film thickness and bioink viscosity on viability of endothelial cells printed by Laser-Assisted Bioprinting. Appl. Surf. Sci. 2011, 257, 5142–5147. [Google Scholar] [CrossRef]
- Keriquel, V.; Guillemot, F.; Arnault, I.; Guillotin, B.; Miraux, S.; Amedee, J.; Fricain, J.C.; Catros, S. In vivobioprinting for computer- and robotic-assisted medical intervention: Preliminary study in mice. Biofabrication 2010, 2, 014101. [Google Scholar] [CrossRef] [PubMed]
- Gudapati, H.; Dey, M.; Ozbolat, I. A comprehensive review on droplet-based bioprinting: Past, present and future. Biomaterials 2016, 102, 20–42. [Google Scholar] [CrossRef]
- Hansen, C.J.; Saksena, R.; Kolesky, D.B.; Vericella, J.J.; Kranz, S.J.; Muldowney, G.P.; Christensen, K.T.; Lewis, J.A. High-Throughput Printing via Microvascular Multinozzle Arrays. Adv. Mater. 2013, 25, 96–102. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.D.; Choi, J.S.; Kim, B.S.; Choi, Y.C.; Cho, Y.W. Piezoelectric inkjet printing of polymers: Stem cell patterning on polymer substrates. Polymer 2010, 51, 2147–2154. [Google Scholar] [CrossRef]
- Xu, T.; Jin, J.; Gregory, C.; Hickman, J.J.; Boland, T. Inkjet printing of viable mammalian cells. Biomaterials 2005, 26, 93–99. [Google Scholar] [CrossRef] [PubMed]
- Skardal, A.; Zhang, J.; Prestwich, G.D. Bioprinting vessel-like constructs using hyaluronan hydrogels crosslinked with tetrahedral polyethylene glycol tetracrylates. Biomaterials 2010, 31, 6173–6181. [Google Scholar] [CrossRef] [PubMed]
- Phillippi, J.A.; Miller, E.; Weiss, L.; Huard, J.; Waggoner, A.; Campbell, P. Microenvironments Engineered by Inkjet Bioprinting Spatially Direct Adult Stem Cells Toward Muscle- and Bone-Like Subpopulations. Stem Cells 2008, 26, 127–134. [Google Scholar] [CrossRef] [PubMed]
- Skardal, A.; Mack, D.; Kapetanovic, E.; Atala, A.; Jackson, J.D.; Yoo, J.; Soker, S. Bioprinted Amniotic Fluid-Derived Stem Cells Accelerate Healing of Large Skin Wounds. Stem Cells Transl. Med. 2012, 1, 792–802. [Google Scholar] [CrossRef] [PubMed]
- Cui, X.; Breitenkamp, K.; Finn, M.; Lotz, M.; D’Lima, D.D. Direct Human Cartilage Repair Using Three-Dimensional Bioprinting Technology. Tissue Eng. Part A 2012, 18, 1304–1312. [Google Scholar] [CrossRef] [PubMed]
- Marga, F.; Jakab, K.; Khatiwala, C.; Shephard, B.; Dorfman, S.; Forgacs, G. Organ printing: A novel tissue engineering paradigm. In Proceedings of the 5th European Conference of the International Federation for Medical and Biological Engineering, Budapest, Hungary, 14–18 September 2011; pp. 27–30. [Google Scholar]
- Marga, F.; Jakab, K.; Khatiwala, C.; Shepherd, B.; Dorfman, S.; Hubbard, B.; Colbert, S.; Gabor, F. Toward engineering functional organ modules by additive manufacturing. Biofabrication 2012, 4, 022001. [Google Scholar] [CrossRef] [PubMed]
- Mironov, V.; Kasyanov, V.; Markwald, R.R. Organ printing: From bioprinter to organ biofabrication line. Curr. Opin. Biotechnol. 2011, 22, 667–673. [Google Scholar] [CrossRef] [PubMed]
- Kolesky, D.B.; Homan, K.A.; Skylar-Scott, M.A.; Lewis, J.A. Three-dimensional bioprinting of thick vascularized tissues. Proc. Natl. Acad. Sci. USA 2016, 113, 3179–3184. [Google Scholar] [CrossRef] [PubMed]
- Chang, R.; Nam, J.; Sun, W. Effects of dispensing pressure and nozzle diameter on cell survival from solid freeform fabrication-based direct cell writing. Tissue Eng. Part A 2008, 14, 41–48. [Google Scholar] [CrossRef] [PubMed]
- Duan, B.; Hockaday, L.A.; Kang, K.H.; Butcher, J.T. 3D bioprinting of heterogeneous aortic valve conduits with alginate/gelatin hydrogels. J. Biomed. Mater. Res. A 2013, 101, 1255–1264. [Google Scholar] [CrossRef]
- Chang, R.; Nam, J.; Sun, W. Direct Cell Writing of 3D Microorgan for In Vitro Pharmacokinetic Model. Tissue Eng. Part C Methods 2008, 14, 157–166. [Google Scholar] [CrossRef] [PubMed]
- Widmaier, E.P.; Raff, H.; Strang, K.T. Vander’s Human Physiology; McGraw-Hill Education: Boston, NY, USA, 2010. [Google Scholar]
- Betts, J.G.; Desaix, P.; Johnson, J.E.; Korol, O.; Kruse, D.; Poe, B.; Womble, M.D. Anatomy & Physiology; Rice University: Houston, TX, USA, 2013. [Google Scholar]
- Eguchi, S.; Chen, S.C.; Rozga, J.; Demetriou, A.A. Tissue Engineering: Liver. In Yearbook of Cell and Tissue Transplantation 1996–1997; Kluwer Academic Publishers: Dordrecht, The Netherlands, 1996; pp. 247–252. [Google Scholar] [CrossRef]
- Tayyeb, A.; Azam, F.; Nisar, R.; Nawaz, R.; Qaisar, U.; Ali, G. Regenerative Medicine in Liver Cirrhosis: Promises and Pitfalls. In Liver Cirrhosis—Update and Current Challenges; BoD Publisher: Norderstedt, Germany, 2017. [Google Scholar] [CrossRef]
- Khetani, S.R.; Bhatia, S.N. Microscale culture of human liver cells for drug development. Nat. Biotechnol. 2008, 26, 120–126. [Google Scholar] [CrossRef] [PubMed]
- Hewitt, N.J.; Gómez Lechón, M.J.; Houston, J.B.; Hallifax, D.; Brown, H.S.; Maurel, P.; Kenna, J.G.; Gustavsson, L.; Lohmann, C.; Skonberg, C.; et al. Primary hepatocytes: Current understanding of the regulation of metabolic enzymes and transporter proteins, and pharmaceutical practice for the use of hepatocytes in metabolism, enzyme induction, transporter, clearance, and hepatotoxicity studies. Drug Metab. Rev. 2007, 39, 159–234. [Google Scholar] [CrossRef] [PubMed]
- Griffith, L.G.; Wu, B.; Cima, M.J.; Powers, M.J.; Chaignaud, B.; Vacanti, J.P. In vitro organogenesis of liver tissue. Ann. N. Y. Acad. Sci. 1997, 831, 382–397. [Google Scholar] [CrossRef] [PubMed]
- Takayama, K.; Kawabata, K.; Nagamoto, Y.; Kishimoto, K.; Tashiro, K.; Sakurai, F.; Tachibana, M.; Kanda, K.; Hayakawa, T.; Furue, M.K.; et al. 3D spheroid culture of hESC/hiPSC-derived hepatocyte-like cells for drug toxicity testing. Biomaterials 2013, 34, 1781–1789. [Google Scholar] [CrossRef] [PubMed]
- Gaskell, H.; Sharma, P.; Colley, H.E.; Murdoch, C.; Williams, D.P.; Webb, S.D. Characterization of a functional C3A liver spheroid model. Toxicol. Res. (Camb.) 2016, 5, 1053–1065. [Google Scholar] [CrossRef]
- Ho, C.T.; Lin, R.Z.; Chen, R.J.; Chin, C.K.; Gong, S.E.; Chang, H.Y.; Peng, H.L.; Hsu, L.; Yew, T.R.; Chang, S.F.; et al. Liver-cell patterning Lab Chip: Mimicking the morphology of liver lobule tissue. Lab Chip 2013, 13, 3578–3587. [Google Scholar] [CrossRef]
- Berger, D.R.; Ware, B.R.; Davidson, M.D.; Allsup, S.R.; Khetani, S.R. Enhancing the functional maturity of iPSC-derived human hepatocytes via controlled presentation of cell-cell interactions in vitro. Hepatology 2014, 1, 1370–1381. [Google Scholar] [CrossRef]
- Taub, R. Liver regeneration: From myth to mechanism. Nat. Rev. Mol. Cell Biol. 2004, 5, 836–847. [Google Scholar] [CrossRef]
- Wang, X.; Yan, Y.; Pan, Y.; Xiong, Z.; Liu, H.; Cheng, J.; Liu, F.; Lin, F.; Wu, R.; Zhang, R.; et al. Generation of three-dimensional hepatocyte/gelatin structures with rapid prototyping system. Tissue Eng. 2006, 12, 83–90. [Google Scholar] [CrossRef]
- Robbins, J.B.; Gorgen, V.; Min, P.; Shepherd, B.R.; Presnell, S.C. A novel in vitro three dimensional bioprinted liver tissue system for drug development. FASEB J. 2013, 27, 812–872. [Google Scholar]
- Zein, N.N.; Hanouneh, I.A.; Bishop, P.D.; Samaan, M.; Eghtesad, B.; Quintini, C.; Miller, C.; Yerian, L.; Klatte, R. Three-dimensional print of a liver for preoperative planning in living donor liver transplantation. Liver Transpl. 2013, 19, 1304–1310. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, D.G.; Funk, J.; Robbins, J.B.; Crogan-Grundy, C.; Presnell, S.C.; Singer, T.; Roth, A.B. Bioprinted 3D primary liver tissues allow assessment of organ-level response to clinical drug induced toxicity in vitro. PLoS ONE 2016, 11, 0158674. [Google Scholar] [CrossRef] [PubMed]
- Leva, V.; Chatzipetrou, M.; Alexopoulos, L.; Tzeranis, D.S.; Zergioti, I. Direct Laser Printing of Liver Cells on Porous Collagen Scaffolds. JLMN J. Laser Micro Nanoeng. 2018, 13, 234–237. [Google Scholar] [CrossRef]
- Arai, K.; Yoshida, T.; Okabe, M.; Goto, M.; Mir, T.A.; Soko, C.; Tsukamoto, Y.; Akaike, T.; Nikaido, T.; Zhou, K.; et al. Fabrication of 3D-culture platform with sandwich architecture for preserving liver-specific functions of hepatocytes using 3D bioprinter. J. Biomed. Mater. Res. Part A 2017, 105, 1583–1592. [Google Scholar] [CrossRef] [PubMed]
- Matsusaki, M.; Sakaue, K.; Kadowaki, K.; Akashi, M. Three dimensional human tissue chips fabricated by rapid and automatic inkjet cell printing. Adv. Healthc. Mater. 2013, 2, 534–539. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.; Kang, K.; Jeong, J.; Paik, S.S.; Kim, J.S.; Park, S.A.; Kim, W.D.; Park, J.; Choi, D. Three-dimensional (3D) printing of mouse primary hepatocytes to generate 3D hepatic structure. Ann. Surg. Treat. Res. 2017, 92, 67–72. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.W.; Choi, Y.J.; Yong, W.J.; Pati, F.; Shim, J.H.; Kang, K.S.; Kang, I.H.; Park, J.; Cho, D.W. Development of a 3D cell printed construct considering angiogenesis for liver tissue engineering. Biofabrication 2016, 8, 015007. [Google Scholar] [CrossRef]
- Skardal, A.; Devarasetty, M.; Kang, H.W.; Mead, I.; Bishop, C.; Shupe, T.; Lee, S.J.; Jackson, J.; Yoo, J.; Soker, S. A hydrogel bioink toolkit for mimicking native tissue biochemical and mechanical properties in bioprinted tissue constructs. Acta Biomater. 2015, 25, 24–34. [Google Scholar] [CrossRef]
- Mazzocchi, A.; Devarasetty, M.; Huntwork, R.C.; Soker, S.; Skardal, A. Optimization of collagen type I-hyaluronan hybrid bioink for 3D bioprinted liver microenvironments. Biofabrication 2018, 11, 015003. [Google Scholar] [CrossRef]
- Lee, H.; Han, W.; Kim, H.; Ha, D.H.; Jang, J.; Kim, B.S.; Cho, D.W. Development of Liver Decellularized Extracellular Matrix Bioink for Three-Dimensional Cell Printing-Based Liver Tissue Engineering. Biomacromolecules 2017, 18, 1229–1237. [Google Scholar] [CrossRef] [PubMed]
- Hiller, T.; Berg, J.; Elomaa, L.; Röhrs, V.; Ullah, I.; Schaar, K.; Dietrich, A.C.; Al-Zeer, M.A.; Kurtz, A.; Hocke, A.C.; et al. Generation of a 3D Liver Model Comprising Human Extracellular Matrix in an Alginate/Gelatin-Based Bioink by Extrusion Bioprinting for Infection and Transduction Studies. Int. J. Mol. Sci. 2018, 19, 3129. [Google Scholar] [CrossRef] [PubMed]
- Faulkner-Jones, A.; Greenhough, S.; King, J.A.; Gardner, J.; Courtney, A.; Shu, W. Development of a valve-based cell printer for the formation of human embryonic stem cell spheroid aggregates. Biofabrication 2013, 5, 015013. [Google Scholar] [CrossRef] [PubMed]
- Faulkner-Jones, A.; Fyfe, C.; Cornelissen, D.J.; Gardner, J.; King, J.; Courtney, A.; Shu, W. Bioprinting of human pluripotent stem cells and their directed differentiation into hepatocyte-like cells for the generation of mini-livers in 3D. Biofabrication 2015, 7, 044102. [Google Scholar] [CrossRef] [PubMed]
- Lei, M.; Wang, X. Biodegradable Polymers and Stem Cells for Bioprinting. Molecules 2016, 21, 539. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.; Kang, K.; Yoon, S.; Kim, J.S.; Park, S.A.; Kim, W.D.; Lee, S.B.; Ryu, K.Y.; Jeong, J.; Choi, D. Prolongation of liver-specific function for primary hepatocytes maintenance in 3D printed architectures. Organogenesis 2018, 14, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Orlando, G.; Baptista, P.; Birchall, M.; Coppi, P.D.; Farney, A.; Guimaraes-Souza, N.K.; Opara, E.; Rogers, J.; Seliktar, D.; Shapira-Schweitzer, K.; et al. Regenerative medicine as applied to solid organ transplantation: Current status and future challenges. Transpl. Int. 2011, 24, 223–232. [Google Scholar] [CrossRef] [PubMed]
- Chaudhari, P.; Tian, L.; Deshmukh, A.; Jang, Y.-Y. Expression kinetics of hepatic progenitor markers in cellular models of human liver development recapitulating hepatocyte and biliary cell fate commitment. Exp. Biol. Med. 2016, 241, 1653–1662. [Google Scholar] [CrossRef]
- Maza, G.; Rombouts, K.; Hall, A.R.; Urbani, L.; Luong, T.V.; Al-Akkad, W.; Longato, L.; Brown, D.; Maghsoudlou, P.; Dhillon, A.P.; et al. Decellularized human liver as a natural 3D-scaffold for liver bioengineering and transplantation. Sci. Rep. 2015, 5, 13079. [Google Scholar] [CrossRef]
- Hussein, K.H.; Park, K.M.; Ghim, J.H.; Yang, S.R.; Woo, H.M. Three dimensional culture of HepG2 liver cells on a rat decellularized liver matrix for pharmacological studies. J. Biomed. Mater. Res. Part B Appl. Biomater. 2016, 104, 263–273. [Google Scholar] [CrossRef]
- Kizawa, H.; Nagao, E.; Shimamura, M.; Zhang, G.; Torii, H. Scaffold-free 3D bio-printed human liver tissue stably maintains metabolic functions useful for drug discovery. Biochem. Biophys. Rep. 2017, 10, 186–191. [Google Scholar] [CrossRef] [PubMed]
- Schepers, A.; Li, C.; Chhabra, A.; Seney, B.T.; Bhatia, S. Engineering a perfusable 3D human liver platform from iPS cells. Lab Chip 2016, 16, 2644–2653. [Google Scholar] [CrossRef] [PubMed]
- Shamir, E.R.; Ewald, A.J. Three-dimensional organotypic culture: Experimental models of mammalian biology and disease. Nat. Rev. Mol. Cell Biol. 2014, 15, 647. [Google Scholar] [CrossRef] [PubMed]
- Qian, X.; Nguyen, H.N.; Song, M.M.; Hadiono, C.; Ogden, S.C.; Hammack, C.; Yao, B.; Hamersky, G.R.; Jacob, F.; Zhong, C.; et al. Brain-Region-Specific Organoids Using Mini-bioreactors for Modeling ZIKV Exposure. Cell 2016, 165, 1238–1254. [Google Scholar] [CrossRef] [PubMed]
- Frey, O.; Misun, P.M.; Fluri, D.A.; Hengstler, J.G.; Hierlemann, A. Reconfigurable microfluidic hanging drop network for multi-tissue interaction and analysis. Nat. Commun. 2014, 5, 4250. [Google Scholar] [CrossRef] [PubMed]
- Tung, Y.C.; Hsiao, A.Y.; Allen, S.; Torisawa, Y.S.; Ho, M.; Takayama, S. High-throughput 3D spheroid culture and drug testing using a 384 hanging drop array. Analyst 2011, 136, 473–478. [Google Scholar] [CrossRef] [PubMed]
- Norona, L.M.; Nguyen, D.G.; Gerber, D.A.; Presnell, S.C.; Lecluyse, E.L. Editor’s Highlight: Modeling Compound-Induced Fibrogenesis In Vitro Using Three-Dimensional Bioprinted Human Liver Tissues. Toxicol. Sci. 2016, 154, 354–367. [Google Scholar] [CrossRef] [PubMed]
- Kwon, R.Y.; Meays, D.R.; Tang, W.J.; Frangos, J.A. Microfluidic Enhancement of Intramedullary Pressure Increases Interstitial Fluid Flow and Inhibits Bone Loss in Hindlimb Suspended Mice. J. Bone Miner. Res. 2010, 25, 1798–1807. [Google Scholar] [CrossRef] [PubMed]
- Chung, B.G.; Flanagan, L.A.; Rhee, S.W.; Schwartz, P.H.; Lee, A.P.; Monuki, E.S.; Jeon, N.L. Human neural stem cell growth and differentiation in a gradient-generating microfluidic device. Lab Chip 2005, 5, 401–406. [Google Scholar] [CrossRef]
- Andersson, H.; Van Den Berg, A. Microfabrication and microfluidics for tissue engineering: State of the art and future opportunities. Lab Chip 2004, 4, 98–103. [Google Scholar] [CrossRef]
- Takayama, S.; Ostuni, E.; LeDuc, P.; Naruse, K.; Ingber, D.E.; Whitesides, G.M. Subcellular positioning of small molecules. Nature 2001, 411, 1016. [Google Scholar] [CrossRef] [PubMed]
- Saxena, V.; Nyberg, L.; Pauly, M.; Dasgupta, A.; Nyberg, A.; Piasecki, B.; Winston, B.; Redd, J.; Ready, J.; Terrault, N.A. Safety and Efficacy of Simeprevir/Sofosbuvir in Hepatitis C-Infected Patients with Compensated and Decompensated Cirrhosis. Hepatology 2015, 62, 715–725. [Google Scholar] [CrossRef] [PubMed]
- Geerts, A. History, heterogeneity, developmental biology, and functions of quiescent hepatic stellate cells. Semin. Liver Dis. 2001, 21, 311–336. [Google Scholar] [CrossRef] [PubMed]
- Prot, J.M.; Aninat, C.; Griscom, L.; Razan, F.; Guillouzo, C.G.; Corlu, A.; Brochot, C.; Legallais, C.; Leclerc, E. Improvement of HepG2/C3a cell functions in a microfluidic biochip. Biotechnol. Bioeng. 2011, 108, 1704–1715. [Google Scholar] [CrossRef] [PubMed]
- Kim, C.; Kasuya, J.; Jeon, J.; Chung, S.; Kamm, R.D. A quantitative microfluidic angiogenesis screen for studying anti-angiogenic therapeutic drugs. Lab Chip 2015, 15, 301–310. [Google Scholar] [CrossRef] [PubMed]
- Korin, N.; Kanapathipillai, M.; Matthews, B.D.; Crescente, M.; Brill, A.; Mammoto, T.; Ghosh, K.; Jurek, S.; Bencherif, S.A.; Bhatta, D.; et al. Shear-activated nanotherapeutics for drug targeting to obstructed blood vessels. Science 2012, 337, 738–742. [Google Scholar] [CrossRef] [PubMed]
- Kang, Y.B.A.; Sodunke, T.R.; Lamontagne, J.; Cirillo, J.; Rajiv, C.; Bouchard, M.J.; Noh, M. Liver sinusoid on a chip: Long-term layered co-culture of primary rat hepatocytes and endothelial cells in microfluidic platforms. Biotechnol. Bioeng. 2015, 112, 2571–2582. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Q.; Patel, D.; Kwa, T.; Haque, A.; Matharu, Z.; Stybayeva, G.; Gao, Y.; Diehl, A.M.; Revzin, A. Liver injury-on-a-chip: Microfluidic co-cultures with integrated biosensors for monitoring liver cell signaling during injury. Lab Chip 2015, 15, 4467–4478. [Google Scholar] [CrossRef]
- Kietzmann, T. Metabolic zonation of the liver: The oxygen gradient revisited. Redox Biol. 2017, 11, 622–630. [Google Scholar] [CrossRef] [PubMed]
- Allen, J.W.; Bhatia, S.N. Formation of steady-state oxygen gradients in vitro:Application to liver zonation. Biotechnol. Bioeng. 2003, 82, 253–262. [Google Scholar] [CrossRef]
- McCarty, W.J.; Usta, O.B.; Yarmush, M.L. A microfabricated platform for generating physiologically-relevant hepatocyte zonation. Sci. Rep. 2016, 6, 26868. [Google Scholar] [CrossRef] [PubMed]
- Bhise, N.S.; Manoharan, V.; Massa, S.; Tamayol, A.; Ghaderi, M.; Miscuglio, M.; Lang, Q.; Zhang, Y.S.; Shin, S.R.; Calzone, G.; et al. A liver-on-a-chip platform with bioprinted hepatic spheroids. Biofabrication 2016, 8, 014101. [Google Scholar] [CrossRef] [PubMed]
- Chang, R.; Emami, K.; Wu, H.; Sun, W. Biofabrication of a three-dimensional liver micro-organ as anin vitrodrug metabolism model. Biofabrication 2010, 2, 045004. [Google Scholar] [CrossRef] [PubMed]
- Novik, E.; Maguire, T.J.; Chao, P.; Cheng, K.C.; Yarmush, M.L. A microfluidic hepatic coculture platform for cell-based drug metabolism studies. Biochem. Pharmacol. 2010, 79, 1036–1044. [Google Scholar] [CrossRef] [PubMed]
- Cho, C.H.; Park, J.; Tilles, A.W.; Berthiaume, F.; Toner, M.; Yarmush, M.L. Layered patterning of hepatocytes in co-culture systems using microfabricated stencils. Biotechniques 2010, 48, 47–52. [Google Scholar] [CrossRef] [PubMed]
- Vernetti, L.A.; Senutovitch, N.; Boltz, R.; DeBiasio, R.; Shun, T.Y.; Gough, A.; Taylor, D.L. A human liver microphysiology platform for investigating physiology, drug safety, and disease models. Exp. Biol. Med. (Maywood) 2016, 241, 101–114. [Google Scholar] [CrossRef] [PubMed]
- Wong, S.F.; No, D.Y.; Choi, Y.Y.; Kim, D.S.; Chung, B.G.; Lee, S.H. Concave microwell based size-controllable hepatosphere as a three-dimensional liver tissue model. Biomaterials 2011, 32, 8087–8096. [Google Scholar] [CrossRef] [PubMed]
- Holt, E.; Lunde, P.K.; Sejersted, O.M.; Christensen, G. Electrical stimulation of adult rat cardiomyocytes in culture improves contractile properties and is associated with altered calcium handling. Basic Res. Cardiol. 1997, 92, 289–298. [Google Scholar] [CrossRef] [PubMed]
- Karageorgiou, V.; Kaplan, D. Porosity of 3D biomaterial scaffolds and osteogenesis. Biomaterials 2005, 26, 5474–5491. [Google Scholar] [CrossRef] [PubMed]
- Lee, K.W.; Wang, S.; Dadsetan, M.; Yaszemski, M.J.; Lu, L. Enhanced cell ingrowth and proliferation through three-dimensional nanocomposite scaffolds with controlled pore structures. Biomacromolecules 2010, 11, 682–689. [Google Scholar] [CrossRef]
- Nguyen, A.K.; Narayan, R.J. Two-photon polymerization for biological applications. Mater. Today 2017, 20, 314–322. [Google Scholar] [CrossRef]
- Ovsianikov, A.; Gruene, M.; Pflaum, M.; Koch, L.; Maiorana, F.; Wilhelmi, M.; Haverich, A.; Chichkov, B. Laser printing of cells into 3D scaffolds. Biofabrication 2010, 2, 014104. [Google Scholar] [CrossRef] [PubMed]
- Rekštyte, S.; Kaziulionyte, E.; Balciunas, E.; Kaškelyte, D.; Malinauskas, M. Direct laser fabrication of composite material 3D microstructured scaffolds. JLMN J. Laser Micro Nanoeng. 2014, 9. [Google Scholar] [CrossRef]
- Ovsianikov, A.; Deiwick, A.; Van Vlierberghe, S.; Pflaum, M.; Wilhelmi, M.; Dubruel, P.; Chichkov, B. Laser Fabrication of 3D Gelatin Scaffolds for the Generation of Bioartificial Tissues. Materials 2011, 4, 288–299. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Y.C.; Zhao, Y.Y.; Zheng, M.L.; Chen, S.L.; Liu, J.; Jin, F.; Dong, X.Z.; Zhao, Z.S.; Duan, X.M. Cucurbit uril-Carbazole Two-Photon Photoinitiators for the Fabrication of Biocompatible Three-Dimensional Hydrogel Scaffolds by Laser Direct Writing in Aqueous Solutions. ACS Appl. Mater. Interfaces 2019, 11, 1782–1789. [Google Scholar] [CrossRef] [PubMed]
- Koroleva, A.; Gittard, S.; Schlie, S.; Deiwick, A.; Jockenhoevel, S.; Chichkov, B. Fabrication of fibrin scaffolds with controlled microscale architecture by a two-photon polymerization–micromolding technique. Biofabrication 2012, 4, 015001. [Google Scholar] [CrossRef] [PubMed]
- Du, Y.; Liu, H.; Yang, Q.; Wang, S.; Wang, J.; Ma, J.; Noh, I.; Mikos, A.G.; Zhang, S. Selective laser sintering scaffold with hierarchical architecture and gradient composition for osteochondral repair in rabbits. Biomaterials 2017, 137, 37–48. [Google Scholar] [CrossRef] [PubMed]
- Lohfeld, S.; Tyndyk, M.A.; Cahill, S.; Flaherty, N.; Barron, V.; McHugh, P.E. A method to fabricate small features on scaffolds for tissue engineering via selective laser sintering. J. Biomed. Sci. Eng. 2010, 3, 138–147. [Google Scholar] [CrossRef]
- Negro, A.; Cherbuin, T.; Lutolf, M.P. 3D Inkjet Printing of Complex, Cell-Laden Hydrogel Structures. Sci. Rep. 2018, 8, 17099. [Google Scholar] [CrossRef] [PubMed]
- Mi, S.; Yang, S.; Liu, T.; Du, Z.; Xu, Y.; Li, B.; Sun, W. A Novel Controllable Cell Array Printing Technique on Microfluidic Chips. IEEE Trans. Biomed. Eng. 2019, 66, 2512–2520. [Google Scholar] [CrossRef] [PubMed]
- Derby, B. Bioprinting: Inkjet printing proteins and hybrid cell-containing materials and structures. J. Mater. Chem. 2008, 18, 5717–5721. [Google Scholar] [CrossRef]
- Saunders, R.E.; Derby, B. Inkjet printing biomaterials for tissue engineering: Bioprinting. Int. Mater. Rev. 2014, 59, 430–448. [Google Scholar] [CrossRef]
- Roth, E.A.; Xu, T.; Das, M.; Gregory, C.; Hickman, J.J.; Boland, T. Inkjet printing for high-throughput cell patterning. Biomaterials 2004, 25, 3707–3715. [Google Scholar] [CrossRef] [PubMed]
- Xu, T.; Baicu, C.; Aho, M.; Zile, M.; Boland, T. Fabrication and characterization of bio-engineered cardiac pseudo tissues. Biofabricatioin 2009, 1, 035001. [Google Scholar] [CrossRef] [PubMed]
- Cui, X.; Boland, T. Human microvasculature fabrication using thermal inkjet printing technology. Biomaterials 2009, 30, 6221–6227. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Wen, X.; Vyavahare, N.R.; Boland, T. Synthesis and characterization of biodegradable elastomeric polyurethane scaffolds fabricated by the inkjet tech-nique. Biomaterials 2008, 29, 3781–3791. [Google Scholar] [CrossRef] [PubMed]
- Delaney, J.T.; Liberski, A.R.; Perelaer, J.; Schubert, U.S. Reactive inkjet printing of calcium alginate hydro- gel porogens—A new strategy to open-pore structured matrices with controlled geometry. Soft Matter 2010, 6, 866–869. [Google Scholar] [CrossRef]
- Di Biase, M.; Saunders, R.E.; Tirelli, N.; Derby, B. Inkjet printing and cell seeding thermoreversible photocur- able gel structures. Soft Matter 2011, 7, 2639–2646. [Google Scholar] [CrossRef]
- Xu, T.; Gregory, C.A.; Molnar, P.; Cui, X.; Jalota, S.; Bhaduri, S.B.; Boland, T. Viability and electrophysiology of neural cell structures generated by the inkjet printing method. Biomaterials 2006, 27, 3580–3588. [Google Scholar] [CrossRef] [PubMed]
- Lewis, P.L.; Green, R.M.; Shah, R.N. 3D-printed gelatin scaffolds of differing pore geometry modulate hepatocyte function and gene expression. Acta Biomater. 2018, 69, 63–70. [Google Scholar] [CrossRef] [PubMed]
- Kang, K.; Kim, Y.; Jeon, H.; Lee, S.B.; Kim, J.S.; Park, S.A.; Kim, W.D.; Yang, H.M.; Kim, S.J.; Jeong, J.; et al. Three-Dimensional Bioprinting of Hepatic Structures with Directly Converted Hepatocyte-Like Cells. Tissue Eng. Part A 2018, 24, 576–583. [Google Scholar] [CrossRef] [PubMed]
- Jeon, H.; Kang, K.; Park, S.A.; Kim, W.D.; Paik, S.S.; Lee, S.H.; Jeong, J.; Choi, D. Generation of Multilayered 3D Structures of HepG2 Cells Using a Bio-printing Technique. Gut Liver 2017, 11, 121–128. [Google Scholar] [CrossRef] [PubMed]
- Gong, H.; Agustin, J.; Wootton, D.; Zhou, J.G. Biomimetic design and fabrication of porous chitosan–gelatin liver scaffolds with hierarchical channel network. J. Mater. Sci. Mater. Med. 2014, 25, 113–120. [Google Scholar] [CrossRef] [PubMed]
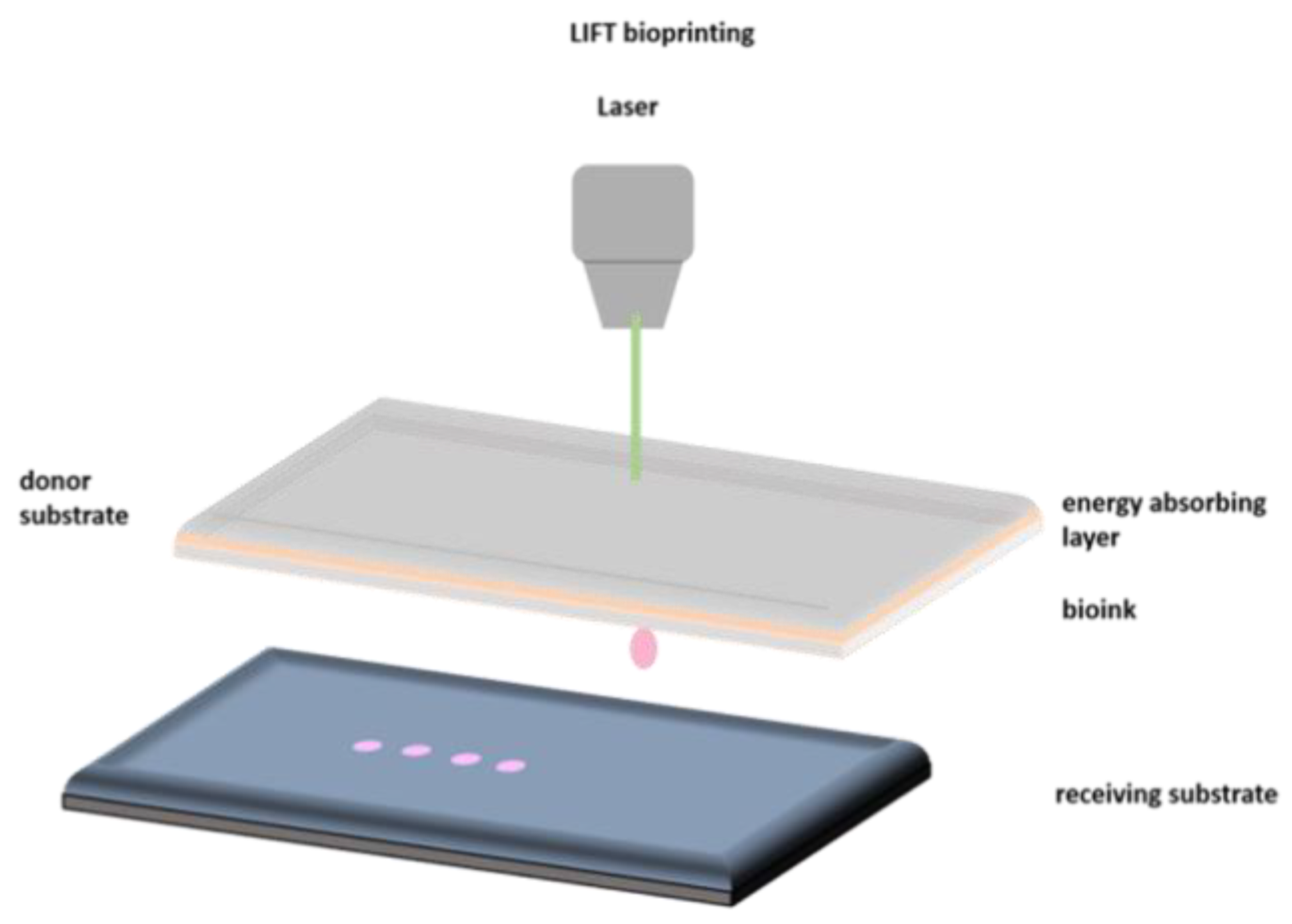
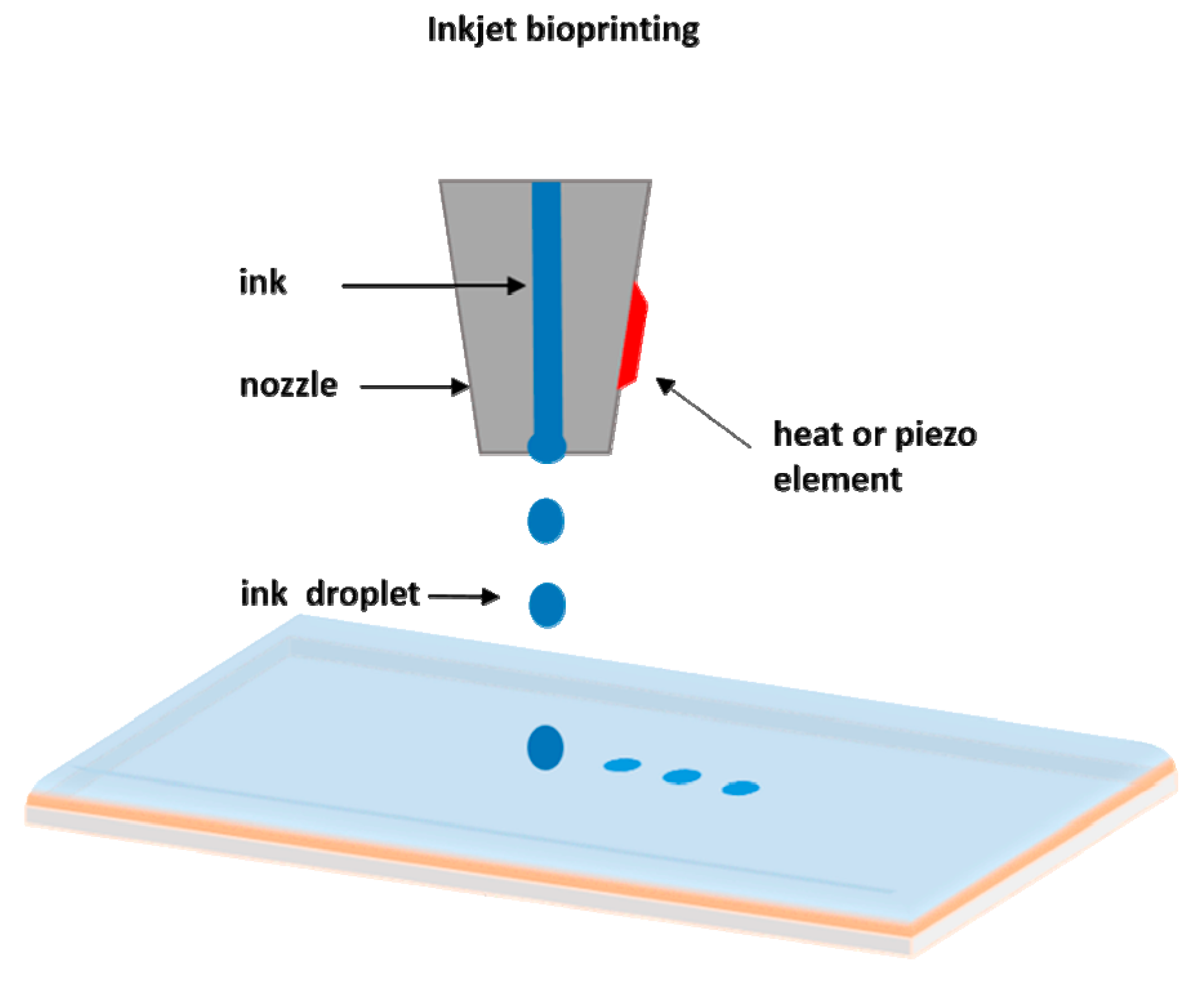
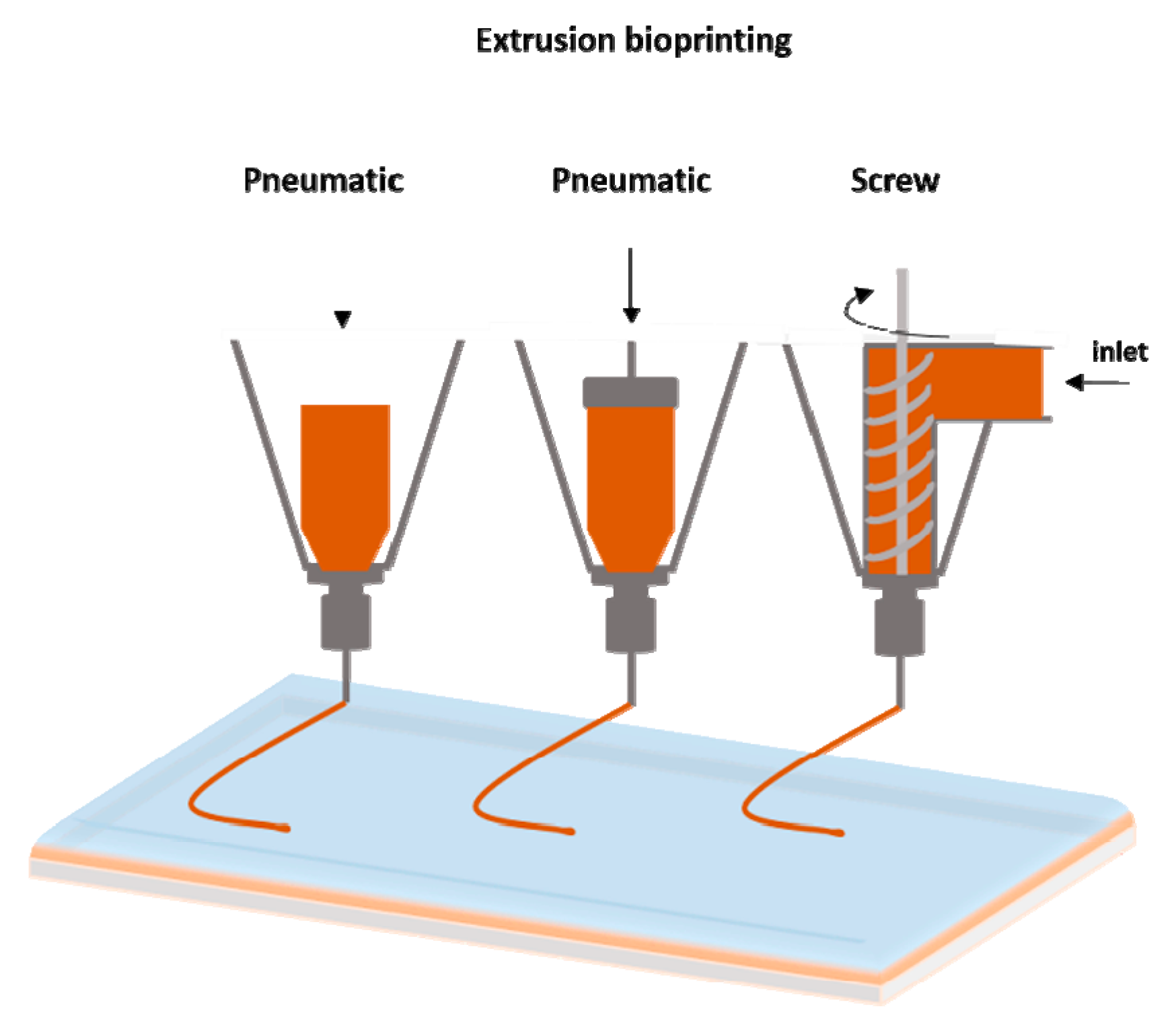



| Laser Assisted Bioprinting | Inkjet | Extrusion | |
|---|---|---|---|
| Advantages | High resolution, deposition of biomaterials in solid or liquid phase, and nozzle free and non-contact printing. | Ability to print low viscosity biomaterials, fast fabrication speed, low cost, high resolution, multi-material printing, Simple operation. | Simple, capable of printing various biomaterials, ability to print high cell densities, multi-material printing, and ability to control ejection speed. |
| Drawbacks | High cost, thermal damage due to nanosecond/femtosecond laser irritation, metallic residuals possible damage of tissue from use of laser lights, slow printing speed, and difficulty in handling heterogenous cells. | Inherent inability to provide a continuous flow, poor functionality for vertical structures, low cell densities, clogging of nozzle, imposing thermal or acoustic stress to cells, and limited variety of bioink. | Only applicable for viscous liquids, gelation and solidification, and limited material selection (shear thinning ability required). |
| Speed | Medium | Fast | Slow |
| Cell viability | <85% | ~80% | >90% |
| Resolution | 10 μm | 50 μm | 100 μm |
| Cell density | Medium | Low | High |
| Viscosity | 1–300 mPa s | <10 mPa s | 30–6 × 107 mPa s |
| Scalability | Low | Low | Low–Medium |
| Structural integrity | Low | Low | High |
| Cost | High | Low | Low–Medium |
| Printing Method | Cell Type/Bioink | Achievements | Reference |
|---|---|---|---|
| Extrusion bioprinting | Hepatocytes Gelatin | The laminated hepatocytes remained viable and performed biological functions for more than 2 months | [68] |
| Extrusion-based bioprinting | Primary human hepatocytes, hepatic stellates, HUVEC cells, and non-parenchymal cells/NovoGelR 2.0 hydrogel (concentration not mentioned) | Viable up to 28 days (% not mentioned) Inkjet bioprinting Galactosylated alginate (12 mg/mL) Primary mouse hepatocytes (isolated from the liver tissue of male 6–8-weeks-old ICR 12 mice) Data not available >85% after 2 Days test of hepatotoxicity of trovafloxacin and Levofloxacin | [71] |
| Inkjet bioprinting | Primary mouse hepatocytes (isolated from the liver tissue of male 6-to-8-week-old ICR 12 mice)/Galactosylated alginate (12 mg/mL) | >85% after 2 days | [73] |
| Inkjet bioprinting | HUVEC | Multilayered organ tissue model test of hepatotoxicity of troglitazone (Rezulin) | [74] |
| Extrusion-based bioprinting | Primary mouse hepatocytes (isolated from the livers of 6–8 weeks old mice)/Alginate (3% w/v) | Viable up to 14 days (% not mentioned) | [75] |
| Extrusion bioprinting | HepG2, BMMSCs/decellularized extracellular matrix (dECM) | Liver tissue model | [79] |
| Microvalve bioprinting | hiPSCs (human-induced pluripotent stem cell lines, RCi-22 and RCi-50); hESCs human embryonic stem cell lines, RC-6 and RC-10)/Alginate (1.5% w/v) | >55% after 1 day | [81] |
| Extrusion-based bioprinting | Primary hepatocytes | Viable up to 60 days (% not mentioned) | [82] |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kryou, C.; Leva, V.; Chatzipetrou, M.; Zergioti, I. Bioprinting for Liver Transplantation. Bioengineering 2019, 6, 95. https://doi.org/10.3390/bioengineering6040095
Kryou C, Leva V, Chatzipetrou M, Zergioti I. Bioprinting for Liver Transplantation. Bioengineering. 2019; 6(4):95. https://doi.org/10.3390/bioengineering6040095
Chicago/Turabian StyleKryou, Christina, Valentina Leva, Marianneza Chatzipetrou, and Ioanna Zergioti. 2019. "Bioprinting for Liver Transplantation" Bioengineering 6, no. 4: 95. https://doi.org/10.3390/bioengineering6040095
APA StyleKryou, C., Leva, V., Chatzipetrou, M., & Zergioti, I. (2019). Bioprinting for Liver Transplantation. Bioengineering, 6(4), 95. https://doi.org/10.3390/bioengineering6040095