Mechanical Response of Porcine Liver Tissue under High Strain Rate Compression
Abstract
:1. Introduction
2. Methodology
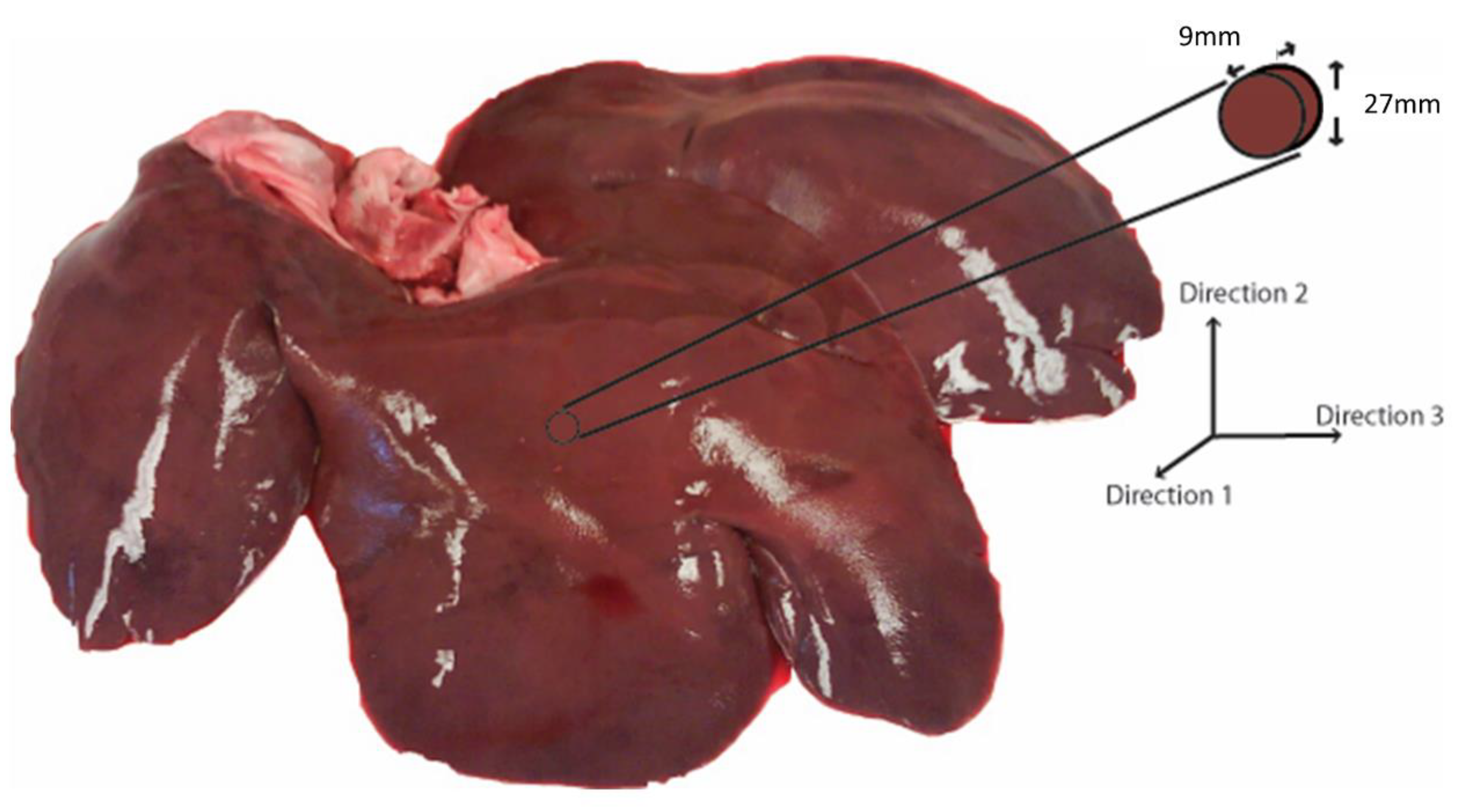
2.1. Sample Preparation
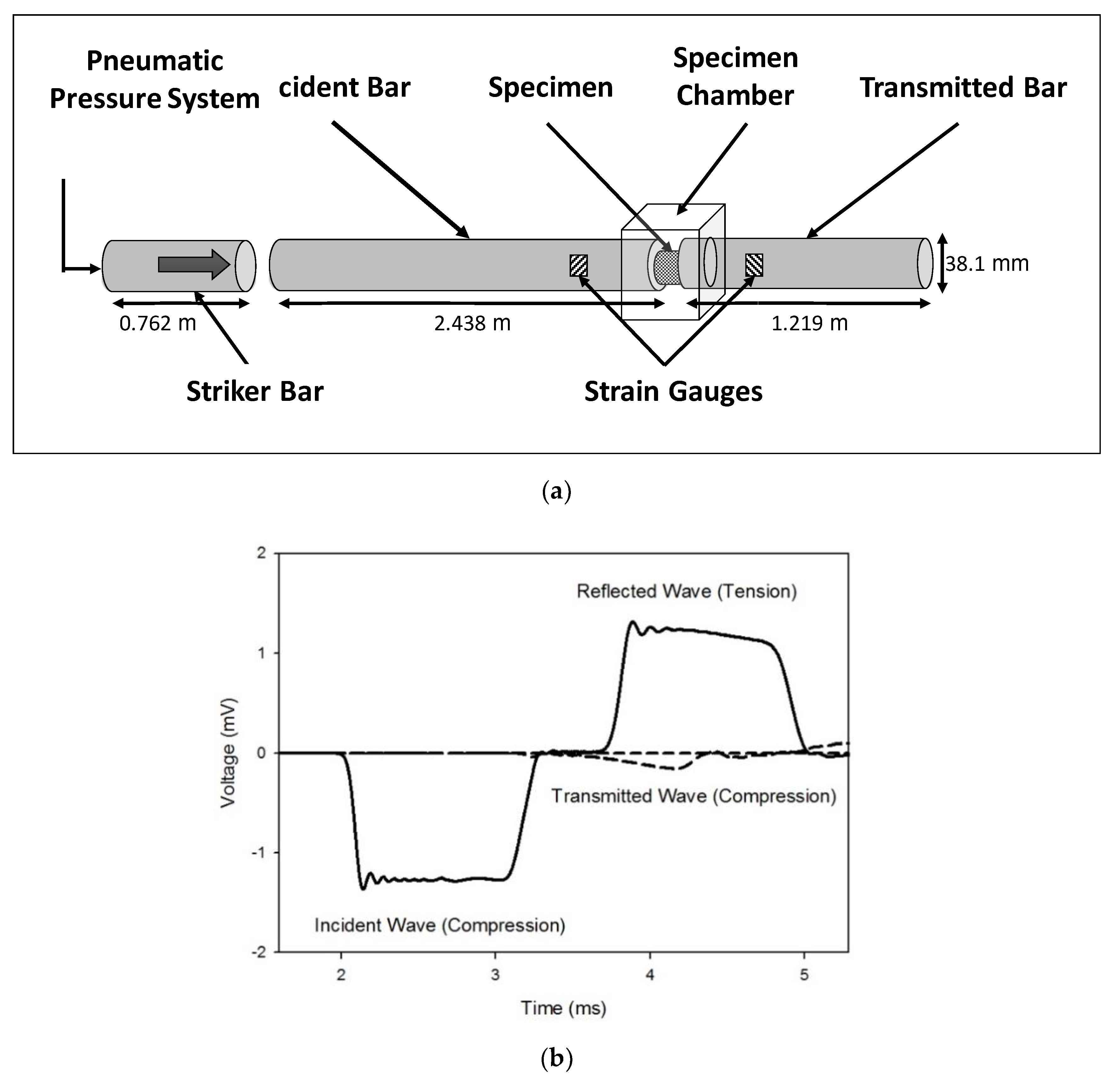
2.2. High Strain Rate Testing Using a Polymeric Split-Hopkinson Pressure Bar (PSHPB)
2.3. Microstructural Analysis
2.4. Finite Element Modeling
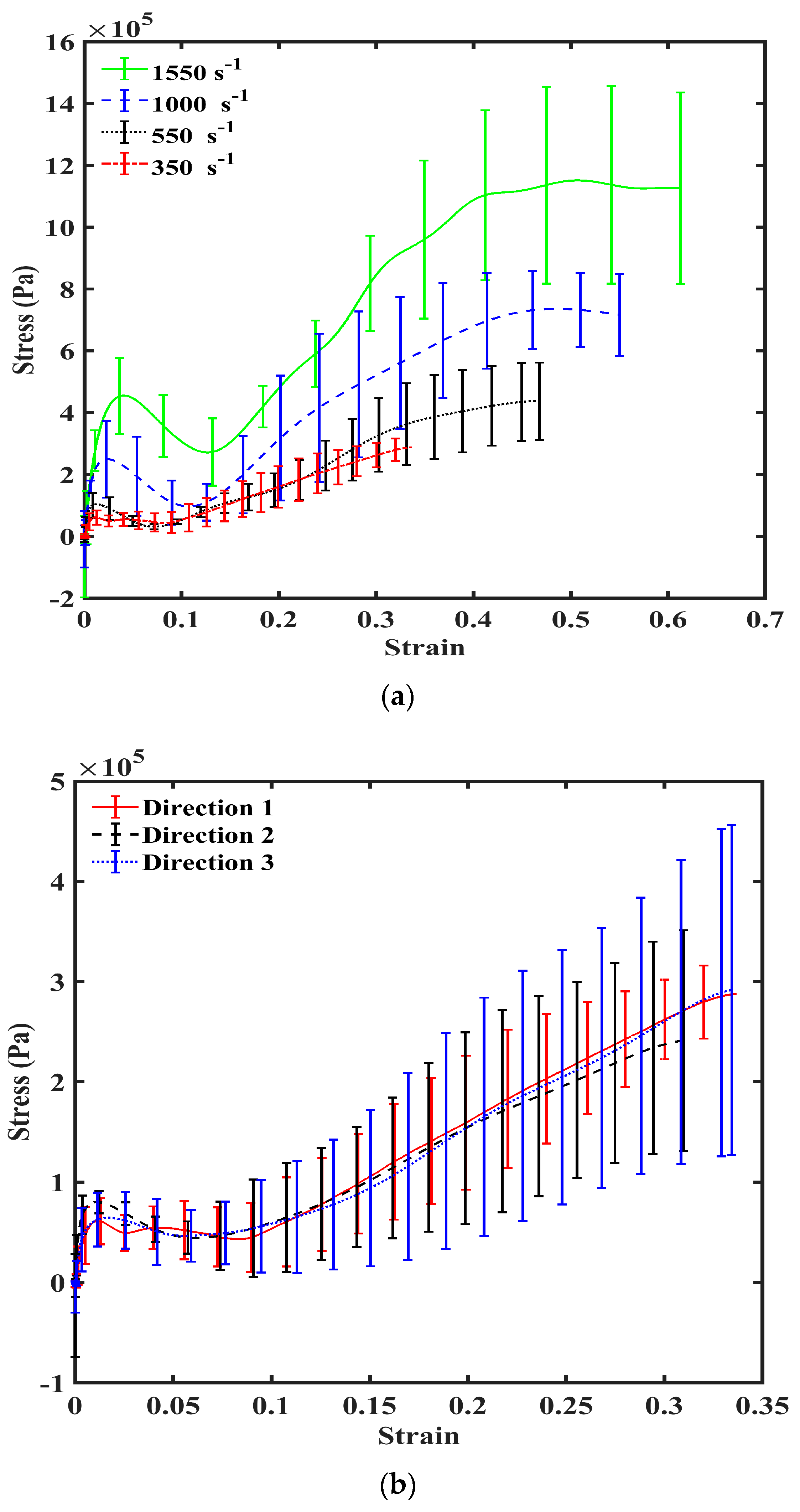
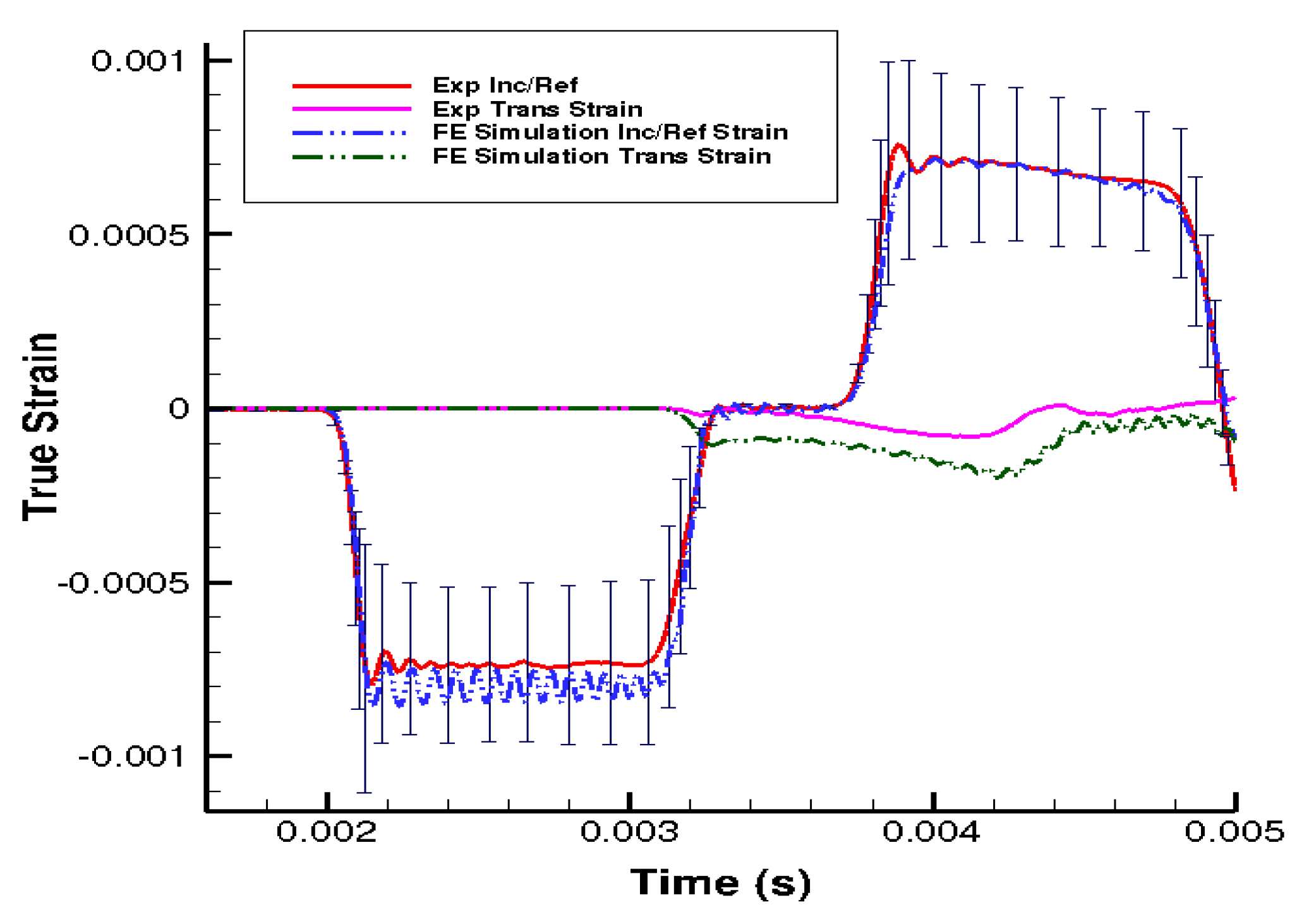
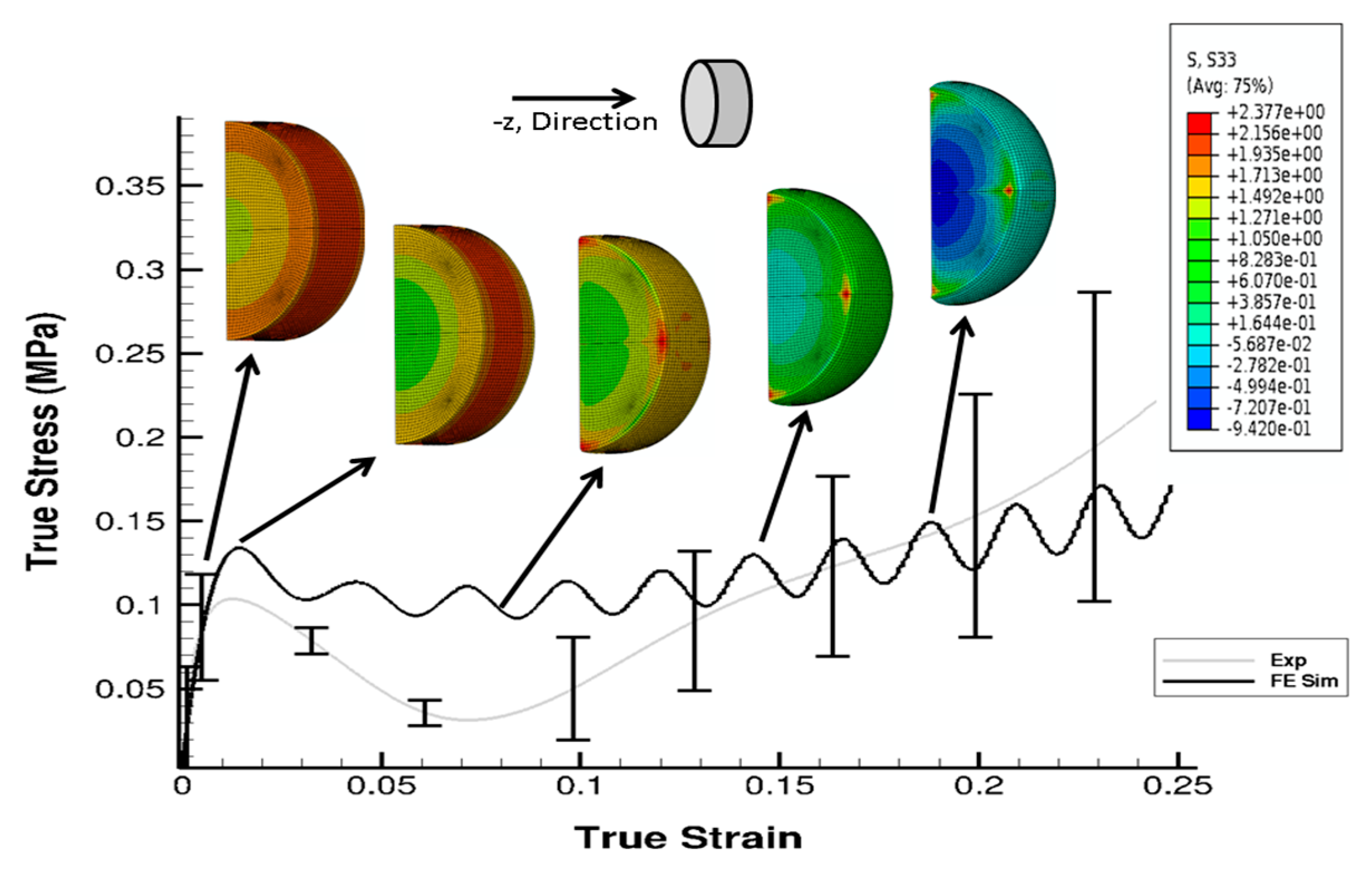
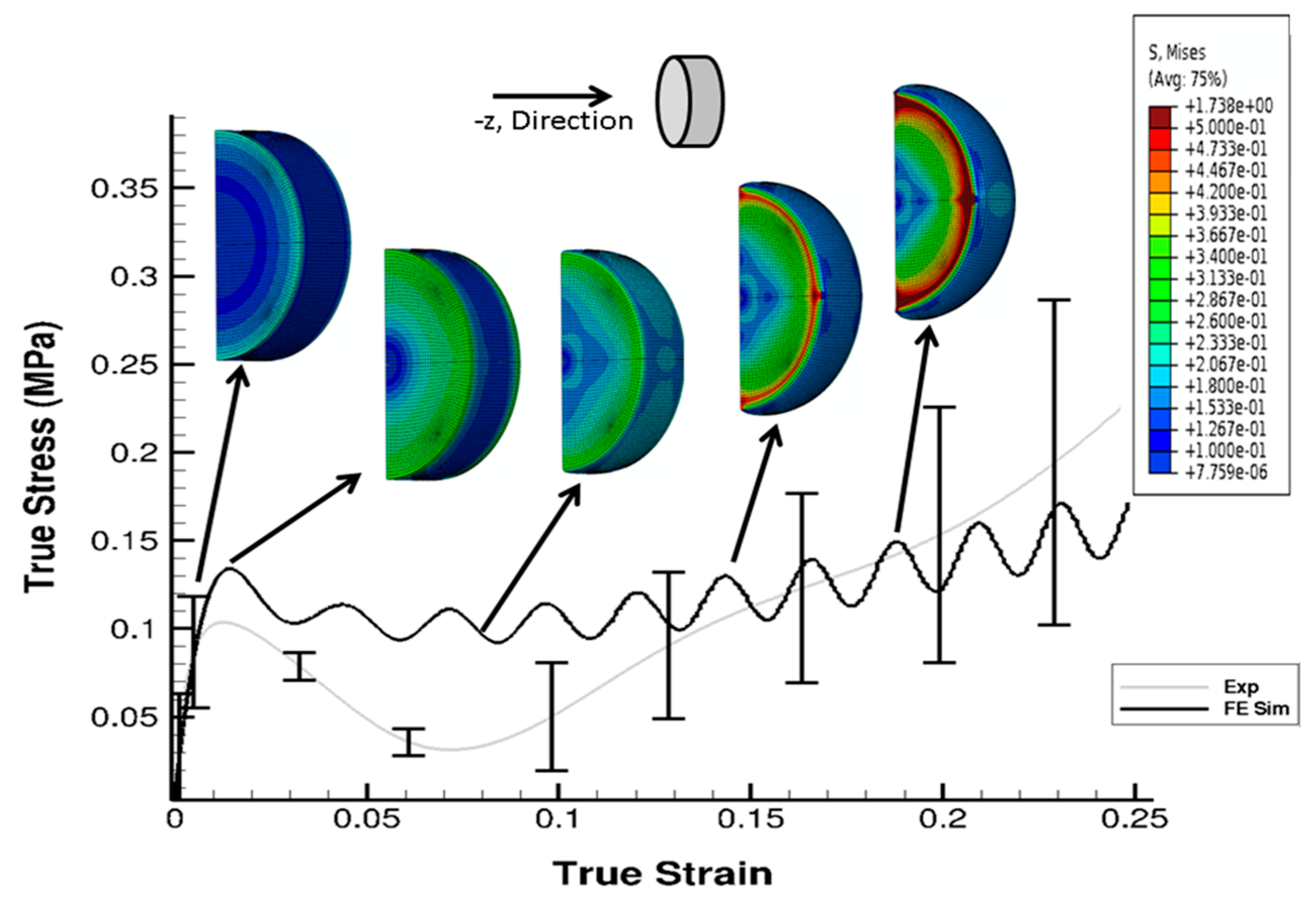
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Disclaimer
Appendix A. Working Principle of Split Hopkinson Pressure Bar (PSHPB)
Appendix B. Mechanism of Rayleigh Viscous Damping
References
- Feliciano, D.V. Surgery for liver trauma. Surg. Clin. North Am. 1989, 69, 273–284. [Google Scholar] [CrossRef]
- Rouhana, S.W.; Foster, M.E. Lateral impact—An analysis of the statistics in the NCSS. In Proceedings of the 29th Stapp Car Crash Conference, Society of Automotive Engineers, Warrendale, PA, USA, 9–11 October 1985. [Google Scholar]
- Elhagediab, A.M.; Rouhana, R.B. Patterns of abdominal injury in frontal automotive crashes. In Proceedings of the 16th International ESV Conference Proceedings, NHTSA, Windsor, ON, Canada, 31 May–4 June 1998. [Google Scholar]
- O’Neill, B. Preventing passenger vehicle occupant injuries by vehicle design—A historical perspective from IIHS. Traffic Inj. Prev. 2009, 10, 113–126. [Google Scholar] [CrossRef]
- Mertz, H.J. NHTSA Docket 74-14. Notice 32. Enclosure 2 of Attachment 1 of Part III of General Motors Submission USG2284; USG: Chicago, IL, USA, 1984. [Google Scholar]
- Mertz, H.J.; Prasad, P.; Irwin, A.I. Injury risk curves for children and adults in frontal and rear collisions. In Forty-First Stapp Car Crash Conference; SAE 973318; SAE: Warrendale, PA, USA, 1997. [Google Scholar]
- Deng, Y.-C.; Kong, W.; Ho, H. Development of a finite element human thorax model for impact injury studies. In SAE Technical Paper Series; 1999-01-0715; SAE: Warrendale, PA, USA, 1999. [Google Scholar]
- Haug, E. Biomechanical models in vehicle accident simulation. In Proceedings of the 1996 NATO Advanced Study Institute on Crashworthiness of Transportation Systems: Structural Impact and Occupant Protection, Tróia, Portugal, 7–19 July 1996. [Google Scholar]
- Iwamoto, M.; Kisanuki, Y.; Watanabe, I.; Furusu, K.; Miki, K. Development of a finite element model of the total human model for safety (THUMS) and application to injury protection. In Proceedings of the 2002 International Conference on the Biomechanics of Impact, Munich, Germany, 18–20 September 2002. [Google Scholar]
- Kimpara, H.; Iwamoto, M.; Miki, K.; Lee, J.B.; Begeman, P.; Yang, K.H.; King, A.I. Biomechanical properties of the male and female chest subjected to frontal and lateral impacts. In Proceedings of the 2003 International IRCOBI Conference on the Biomechanics of Impact, Lisbon, Portugal, 25–26 September 2003. [Google Scholar]
- Van Sligtenhorst, C.; Cronin, D.S.; Brodland, G.W. High strain rate compressive properties of bovine muscle tissue determined using a split Hopkinson bar apparatus. J. Biomech. 2006, 39, 1852–1858. [Google Scholar] [CrossRef]
- Song, B.; Chen, W.; Ge, Y.; Weerasooriya, T. Dynamic and quasi-static compressive response of porcine muscle. J. Biomech. 2007, 40, 2999–3005. [Google Scholar] [CrossRef] [PubMed]
- Sparks, J.; Dupaix, R. Constitutive modeling of rate-dependent stress–strain behavior of human liver in blunt impact loading. Ann. Biomed. Eng. 2008, 36, 1883–1892. [Google Scholar] [CrossRef]
- Ottensmeyer, M.P.; Kerdok, A.E.; Howe, R.D.; Dawson, S.L. The Effects of testing environment on the viscoelastic properties of soft tissues. In Medical Simulation; Cotin, S., Metaxas, D., Eds.; Springer: Berlin/Heidelberg, Germany, 2004; pp. 9–18. [Google Scholar]
- Kerdok, A.E.; Ottensmeyer, M.P.; Howe, R.D. Effects of perfusion on the viscoelastic characteristics of liver. J. Biomech. 2006, 39, 2221–2231. [Google Scholar] [CrossRef]
- Bouvard, J.L.; Francis, D.K.; Tschopp, M.A.; Marin, E.B.; Bammann, D.J.; Horstemeyer, M.F. An internal state variable material model for predicting the time, thermomechanical, and stress state dependence of amorphous glassy polymers under large deformation. Int. J. Plast. 2013, 42, 168–193. [Google Scholar] [CrossRef]
- Whittington, W.R.; Oppedal, A.L.; Turnage, S.; Hammi, Y.; Rhee, H.; Allison, P.G.; Crane, C.K.; Horstemeyer, M.F. Capturing the effect of temperature, strain rate, and stress state on the plasticity and fracture of rolled homogeneous armor (RHA) steel. Mater. Sci. Eng. A 2014, 594, 82–88. [Google Scholar] [CrossRef]
- Zhao, H.; Gary, G.; Klepaczko, J.R. On the use of a viscoelastic split Hopkinson pressure bar. Int. J. Impact Eng. 1997, 19, 319–330. [Google Scholar] [CrossRef]
- Pervin, F.; Chen, W.W.; Weerasooriya, T. Dynamic compressive response of bovine liver tissues. J. Mech. Behav. Biomed. Mater. 2011, 4, 76–84. [Google Scholar] [CrossRef]
- Liu, Q.; Subhash, G. Characterization of viscoelastic properties of polymer bar using iterative deconvolution in the time domain. Mech. Mater. 2006, 38, 1105–1117. [Google Scholar] [CrossRef]
- Kwon, J.; Subhash, G. Compressive strain rate sensitivity of ballistic gelatin. J. Biomech. 2010, 43, 420–425. [Google Scholar] [CrossRef]
- Song, B.; Chen, W. Dynamic stress equilibration in split Hopkinson pressure bar tests on soft materials. Exp. Mech. 2004, 44, 300–312. [Google Scholar] [CrossRef]
- Prabhu, R.; Horstemeyer, M.F.; Tucker, M.T.; Marin, E.B.; Bouvard, J.L.; Sherburn, J.A.; Liao, J.; Williams, L.N. Coupled experiment/finite element analysis on the mechanical response of porcine brain under high strain rates. J. Mech. Behav. Biomed. Mater. 2011, 4, 1067–1080. [Google Scholar] [CrossRef]
- Clemmer, J.; Prabhu, R.; Chen, J.; Colebeck, E.; Priddy, L.B.; Mccollum, M.; Brazile, B.; Whittington, W.; Wardlaw, J.L.; Rhee, H.; et al. Experimental observation of high strain rate responses of porcine brain, liver, and tendon. J. Mech. Med. Biol. 2016, 16, 1650032. [Google Scholar] [CrossRef]
- Prabhu, R.; Whittington, W.R.; Patnaik, S.S.; Mao, Y.; Begonia, M.T.; Williams, L.N.; Liao, J.; Horstemeyer, M.F. A coupled experiment-finite element modeling methodology for assessing high strain rate mechanical response of soft biomaterials. J. Vis. Exp. 2015, 99, e51545. [Google Scholar] [CrossRef]
- Subhash, C.; Ravichandran, G. Split-Hopkinson pressure bar testing of ceramics. In ASM Handbook Vol 8, Mechanical Testing and Evaluation; ASM Int.: Materials Park, OH, USA, 2000; pp. 427–428. [Google Scholar]
- Uenishi, A.; Yoshida, H.; Kuriyama, Y.; Takahashi, M. Material characterization at high strain rates for optimizing car body structures for crash events. Nippon Steel Tech. Rep. 2003, 88, 21–24. [Google Scholar]
- Roan, E.; Vemaganti, K. The nonlinear material properties of liver tissue determined from no-slip uniaxial compression experiments. J. Biomech. Eng. 2007, 129, 450–456. [Google Scholar] [CrossRef]
- Saraf, H.; Ramesh, K.T.; Lennon, A.M.; Merkle, A.C.; Roberts, J.C. Mechanical properties of soft human tissues under dynamic loading. J. Biomech. 2007, 40, 1960–1967. [Google Scholar] [CrossRef]
- Chen, J.; Brazile, B.; Prabhu, R.; Patnaik, S.S.; Bertucci, R.; Rhee, H.; Horstemeyer, M.F.; Hong, Y.; Williams, L.N.; Liao, J. Quantitative analysis of tissue damage evolution in porcine liver with interrupted mechanical testing under tension, compression, and shear. J. Biomech. Eng. 2018, 140, 071010. [Google Scholar] [CrossRef]
- Bouvard, J.L.; Brown, H.R.; Marin, E.B.; Wang, P.; Horstemeyer, M.F. Mechanical testing and material modeling of thermoplastics: Polycarbonate, polypropylene and acrylonitrile-butadiene-styrene. In MRS Fall Symposium W 2009; MRS: Cambridge, UK, 2009. [Google Scholar]
- Bouvard, J.L.; Ward, D.K.; Hossain, D.; Marin, E.B.; Bammann, D.J.; Horstemeyer, M.F. A general inelastic internal state variable model for amorphous glassy polymers. Acta Mech. 2010, 213, 71–96. [Google Scholar] [CrossRef]
- Smith, M. ABAQUS/Explicit User’s Manual, Version 6.9; Hibbit, Karlsson, and Sorenson, Inc.: Providence, RI, USA, 2009. [Google Scholar]
- Sakuma, I.; Nishimura, Y.; Chui, C.K.; Kobayashi, E.; Inada, H.; Chen, X.; Hisada, T. In vitro measurement of mechanical properties of liver tissue under compression and elongation using a new test piece holding method with surgical glue. In Surgery Simulation and Soft Tissue Modeling; Ayache, N., Delingette, H., Eds.; Springer: Berlin/Heidelberg, Germany, 2003; pp. 284–292. [Google Scholar]


| Model/Material Constants. | Values |
|---|---|
| μ (MPa) | 29 |
| K (MPa) | 12,492 |
| γvo (s−1) | 99,209.5 |
| m | 1.1 |
| Yo (MPa) | 2 |
| αp | 0 |
| λL | 7 |
| μR | 0.168627 |
| Rs1 | 1.4 |
| ho | 31 |
| ζo1 | 0 |
| ζ*sat | 0.01 |
| ζ*o | 0.3 |
| go | 0.07 |
| Cκ1 (MPa) | 0.4 |
| h1 | 0 |
| eos2 | 0 |
| esats2 | 0.4 |
| Cκ2 (MPa) | 0 |
| Strain Rate (s−1) | Mean Peak Stress/Valley Stress | Mean Ultimate Stress/Valley Stress |
|---|---|---|
| 350 | 5.37 ± 4.59 | 21.92 ± 16.39 |
| 550 | 3.55 ± 1.56 | 13.36 ± 6.09 |
| 1000 | 3.00 ± 0.84 | 12.09 ± 3.56 |
| 1550 | 1.42 ± 0.24 | 12.48 ± 2.12 |
| Direction 1 | Direction 2 | Direction 3 | |
|---|---|---|---|
| Objects | 797 | 795 | 803 |
| Cell nuclear density (/mm2) | 4.08 × 103 | 5.91 × 103 | 4.11 × 103 |
| Area fraction of cell nuclei | 9.79% | 13.4% | 8.06% |
| Mean area of cell nuclei (μm2) | 23.98 ± 15.58 | 22.6 ± 15.71 | 19.59 ± 7.73 |
| Mean nearest neighbor distance (nnd) (μm) | 9.62 ± 3.14 | 8.44 ± 2.40 | 9.29 ± 3.12 |
| Velocity of Striker Bar (mph) | Strain Rate of Liver Tissue (s−1) |
|---|---|
| 6.487 | 350 |
| 9.843 | 550 |
| 13.645 | 1000 |
| 17.001 | 1550 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, J.; Patnaik, S.S.; Prabhu, R.K.; Priddy, L.B.; Bouvard, J.-L.; Marin, E.; Horstemeyer, M.F.; Liao, J.; Williams, L.N. Mechanical Response of Porcine Liver Tissue under High Strain Rate Compression. Bioengineering 2019, 6, 49. https://doi.org/10.3390/bioengineering6020049
Chen J, Patnaik SS, Prabhu RK, Priddy LB, Bouvard J-L, Marin E, Horstemeyer MF, Liao J, Williams LN. Mechanical Response of Porcine Liver Tissue under High Strain Rate Compression. Bioengineering. 2019; 6(2):49. https://doi.org/10.3390/bioengineering6020049
Chicago/Turabian StyleChen, Joseph, Sourav S. Patnaik, R. K. Prabhu, Lauren B. Priddy, Jean-Luc Bouvard, Esteban Marin, Mark F. Horstemeyer, Jun Liao, and Lakiesha N. Williams. 2019. "Mechanical Response of Porcine Liver Tissue under High Strain Rate Compression" Bioengineering 6, no. 2: 49. https://doi.org/10.3390/bioengineering6020049
APA StyleChen, J., Patnaik, S. S., Prabhu, R. K., Priddy, L. B., Bouvard, J.-L., Marin, E., Horstemeyer, M. F., Liao, J., & Williams, L. N. (2019). Mechanical Response of Porcine Liver Tissue under High Strain Rate Compression. Bioengineering, 6(2), 49. https://doi.org/10.3390/bioengineering6020049







