Oxygen Persufflation in Liver Transplantation Results of a Randomized Controlled Trial
Abstract
1. Introduction
2. Patients and Methods
2.1. Study Design
2.2. Study Population
- Adult patients (>18 years of age)
- Recipients undergoing the first liver transplantation
- Willingness and ability to attend regular follow up examinations
- Written informed consent
2.3. Study Procedures
2.4. Objectives and Endpoints
2.5. Surgical Procedure and Immunosuppression
2.6. Definition of Rescue Allocation
2.7. Definition of Early Allograft Dysfunction (EAD)
2.8. Clinical Factors for Outcome Analysis
2.9. Monitoring, Data Safety Monitoring Board
2.10. Sample Size Calculation and Statistical Analysis
3. Results
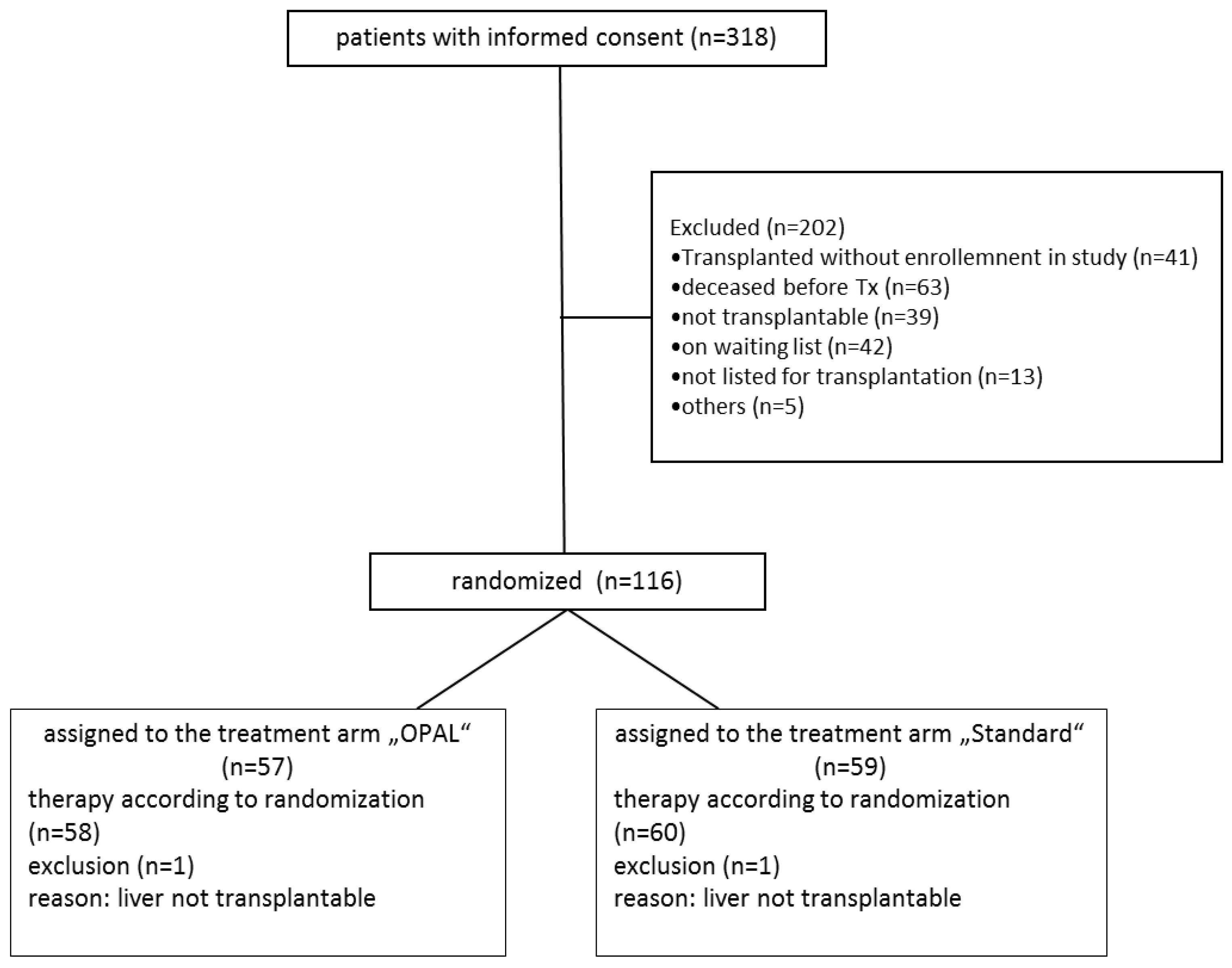
3.1. Recruitment and Follow Up
3.2. Donor, Recipient, and Perioperative Characteristics
3.3. Surgical Study Procedures
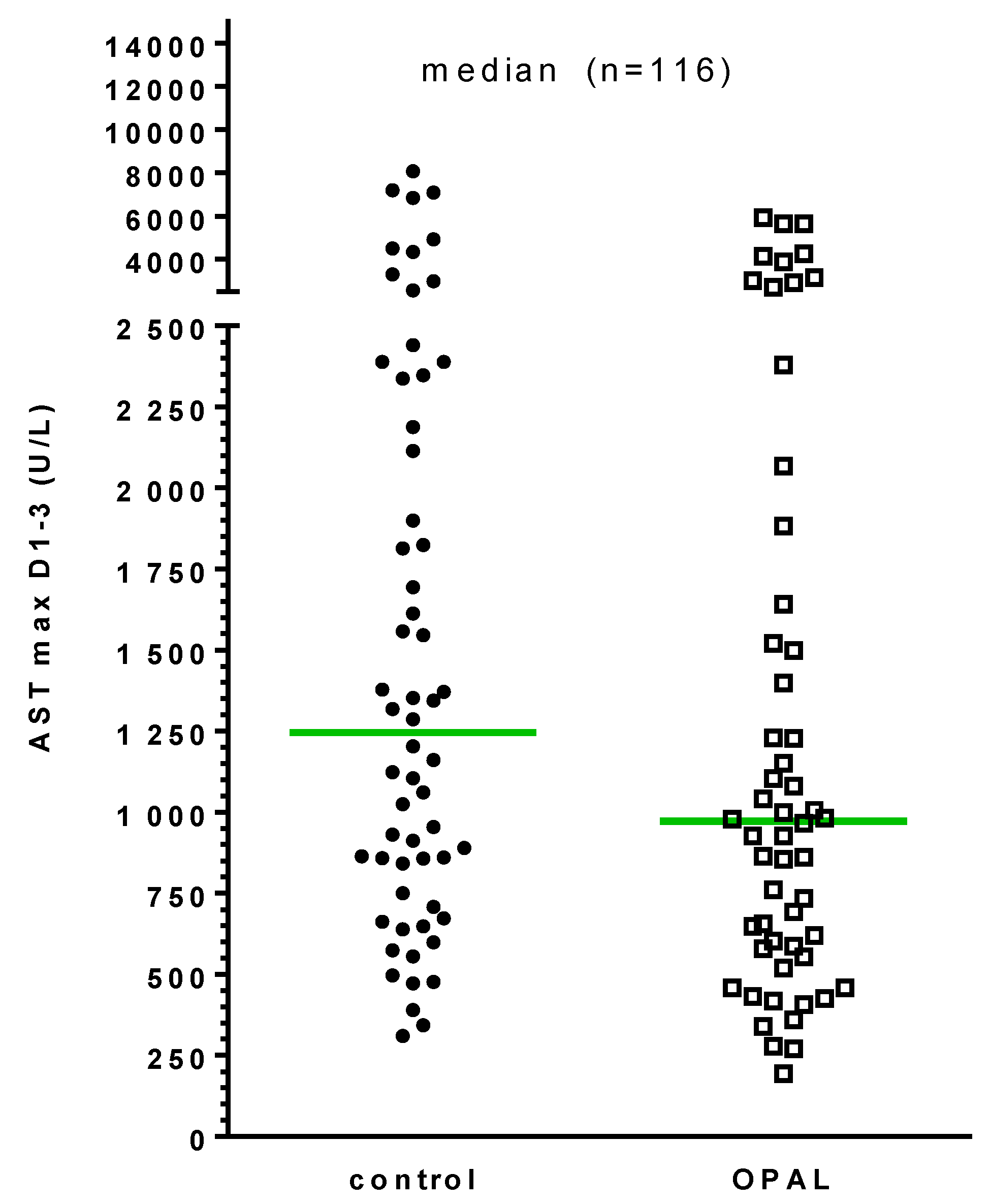
3.4. Primary Endpoint
3.5. Secondary Endpoints
3.6. Patient and Graft Outcome
3.7. Association of Clinical Parameters with Development of EAD
3.8. Association of Clinical Parameters with Patient Survival
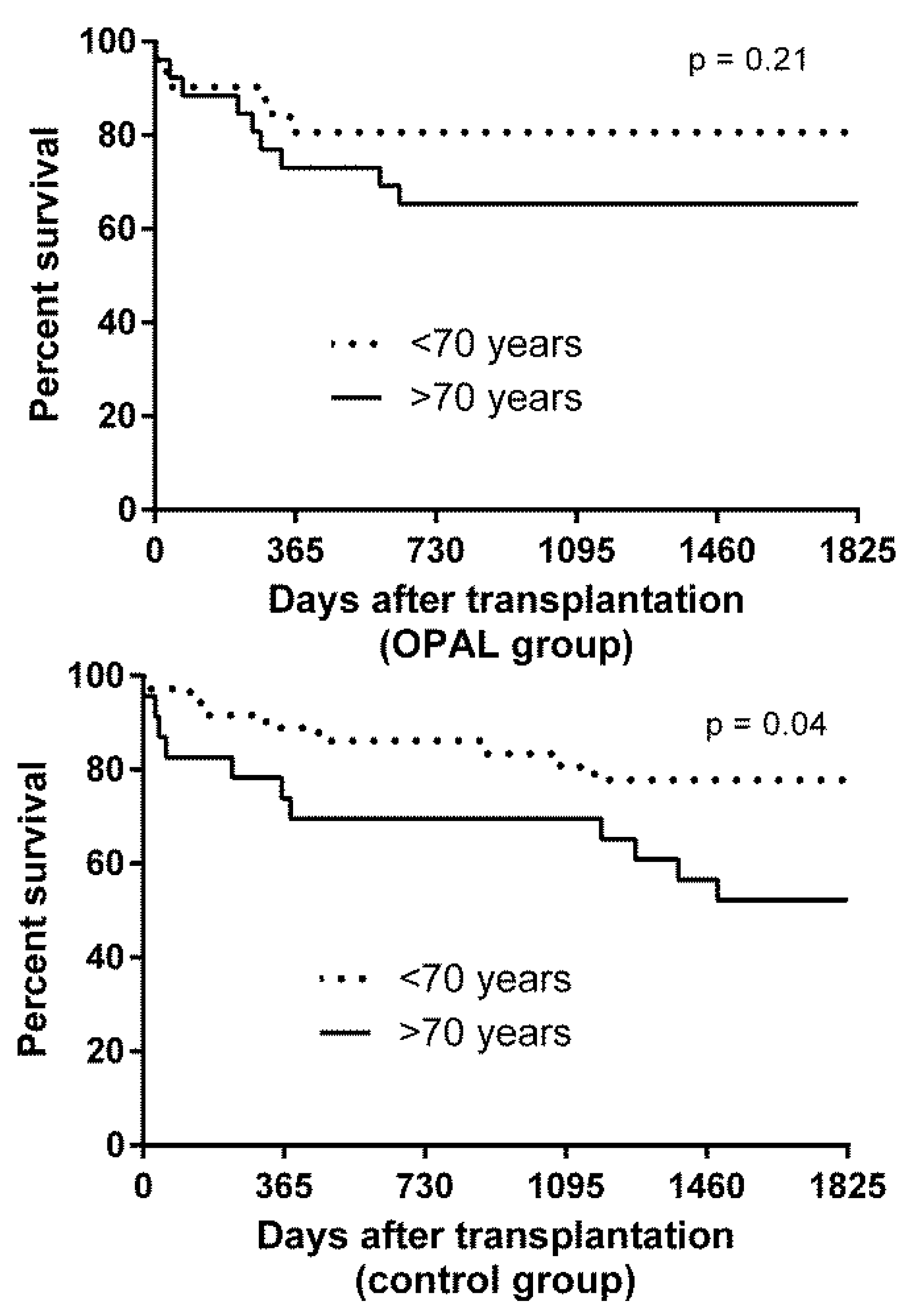
3.9. Subgroup Analysis
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Nickkholgh, A.; Weitz, J.; Encke, J.; Sauer, P.; Mehrabi, A.; Büchler, M.W.; Schmidt, J.; Schemmer, P. Utilization of extended donor criteria in liver transplantation: a comprehensive review of the literature. Nephrol. Dial. Transplant. 2007, 22, viii29–viii36. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Briceño, J.; Marchal, T.; Padillo, J.; Solórzano, G.; Pera, C. Influence of marginal donors on liver preservation injury. Transplantation 2002, 74, 522–526. [Google Scholar] [CrossRef]
- Tector, A.J.; Mangus, R.S.; Chestovich, P.; Vianna, R.; Fridell, J.A.; Milgrom, M.L.; Sanders, C.; Kwo, P.Y. Use of Extended Criteria Livers Decreases Wait Time for Liver Transplantation Without Adversely Impacting Posttransplant Survival. Ann. Surg. 2006, 244, 439–450. [Google Scholar] [CrossRef] [PubMed]
- Markmann, J.F.; Markmann, J.W.; Markmann, D.A.; Bacquerizo, A.; Singer, J.; Holt, C.D.; Gornbein, J.; Yersiz, H.; Morrissey, M.; Lerner, S.M.; et al. Preoperative factors associated with outcome and their impact on resource use in 1148 consecutive primary liver transplants. Transplantation 2001, 72, 1113–1122. [Google Scholar] [CrossRef] [PubMed]
- Moore, D.E.; Feurer, I.D.; Speroff, T.; Gorden, D.L.; Wright, J.K.; Chari, R.S.; Pinson, C.W. Impact of Donor, Technical, and Recipient Risk Factors on Survival and Quality of Life After Liver Transplantation. Arch. Surg. 2005, 140, 273. [Google Scholar] [CrossRef]
- Hoyer, D.P.; Paul, A.; Gallinat, A.; Molmenti, E.P.; Reinhardt, R.; Minor, T.; Saner, F.H.; Canbay, A.; Treckmann, J.W.; Sotiropoulos, G.C.; et al. Donor information based prediction of early allograft dysfunction and outcome in liver transplantation. Liver Int. 2015, 35, 156–163. [Google Scholar] [CrossRef]
- Minor, T.; Saad, S.; Nagelschmidt, M.; Koetting, M.; Fu, Z.; Paul, A.; Isselhard, W. Successful transplantation of porcine livers after warm ischemic insult in situ and cold preservation including postconditioning with gaseous oxygen. Transplantation 1998, 65, 1262–1264. [Google Scholar] [CrossRef]
- Minor, T.; Paul, A. Hypothermic reconditioning in organ transplantation. Curr. Opin. Organ Transplant. 2013, 18, 161–167. [Google Scholar] [CrossRef]
- Fuller, B.J.; Busza, A.L.; Proctor, E. Possible resuscitation of liver function by hypothermic reperfusion in vitro after prolonged (24-hour) cold preservation--a 31p nmr study. Transplantation 1990, 50, 511–512. [Google Scholar] [CrossRef] [PubMed]
- Koetting, M.; Lüer, B.; Efferz, P.; Paul, A.; Minor, T. Optimal time for hypothermic reconditioning of liver grafts by venous systemic oxygen persufflation (vsop) in a large animal model. Transplantation 2011, 91, 42–47. [Google Scholar] [CrossRef] [PubMed]
- Minor, T.; Koetting, M.; Kaiser, G.; Efferz, P.; Lüer, B.; Paul, A. Hypothermic Reconditioning by Gaseous Oxygen Improves Survival After Liver Transplantation in the Pig. Am. J. Transplant. 2011, 11, 2627–2634. [Google Scholar] [CrossRef] [PubMed]
- Minor, T.; Stegemann, J.; Hirner, A.; Koetting, M. Impaired autophagic clearance after cold preservation of fatty livers correlates with tissue necrosis upon reperfusion and is reversed by hypothermic reconditioning. Liver Transplant. 2009, 15, 798–805. [Google Scholar] [CrossRef]
- Kim, J.S.; NItta, T.; Mohuczy, D.; O’Malley, K.A.; Moldawer, L.L.; Dunn, W.A., Jr.; Behrns, K.E. Impaired autophagy: A mechanism of mitochondrial dysfunction in anoxic rat hepatocytes. Hepatology 2008, 47, 1725–1736. [Google Scholar] [CrossRef] [PubMed]
- Gustafsson, Å.B.; Gottlieb, R.A. Recycle or Die: The Role of Autophagy in Cardioprotection. J. Mol. Cell. Cardiol. 2008, 44, 654–661. [Google Scholar] [CrossRef]
- Treckmann, J.; Minor, T.; Saad, S.; Özcelik, A.; Malagó, M.; Broelsch, C.E.; Paul, A. Retrograde oxygen persufflation preservation of human livers: A pilot study. Liver Transplant. 2008, 14, 358–364. [Google Scholar] [CrossRef]
- Khorsandi, S.E.; Jitraruch, S.; Fairbanks, L.; Cotoi, C.; Jassem, W.; Vilca-Melendez, H.; Prachalias, A.; Dhawan, A.; Heaton, N.; Srinivasan, P. The effect of anterograde persufflation on energy charge and hepatocyte function in donation after cardiac death livers unsuitable for transplant. Liver Transplant. 2014, 20, 698–704. [Google Scholar] [CrossRef]
- Minor, T. Vascular oxygen persufflation for preservation and reconditioning of marginal liver grafts. In Organ Preservation and Reengineering, 1st ed.; Uygun, K., Lee, C.E., Eds.; Artech House: Norwood, MA, USA, 2011; pp. 125–134. [Google Scholar]
- Minor, T.; Pütter, C.; Gallinat, A.; Ose, C.; Kaiser, G.; Scherag, A.; Treckmann, J.; Paul, A. Oxygen persufflation as adjunct in liver preservation (OPAL): Study protocol for a randomized controlled trial. Trials 2011, 12, 234. [Google Scholar] [CrossRef] [PubMed]
- Olthoff, K.M.; Kulik, L.; Samstein, B.; Kaminski, M.; Abecassis, M.; Emond, J.; Shaked, A.; Christie, J.D. Validation of a current definition of early allograft dysfunction in liver transplant recipients and analysis of risk factors. Liver Transplant. 2010, 16, 943–949. [Google Scholar] [CrossRef] [PubMed]
- Braat, A.E.; Blok, J.J.; Putter, H.; Adam, R.; Burroughs, A.K.; Rahmel, A.O.; Porte, R.J.; Rogiers, X.; Ringers, J.; European Liver and Intestine Transplant Association (ELITA) and Eurotransplant Liver Intestine Advisory Committee (ELIAC). The Eurotransplant Donor Risk Index in Liver Transplantation: ET-DRI. Am. J. Transplant. 2012, 12, 2789–2796. [Google Scholar] [CrossRef]
- Charlson, M.E.; Pompei, P.; Ales, K.L.; MacKenzie, C. A new method of classifying prognostic comorbidity in longitudinal studies: Development and validation. J. Chronic Dis. 1987, 40, 373–383. [Google Scholar] [CrossRef]
- Isselhard, W.; Witte, J.; Denecke, H.; Berger, M.; Fischer, J.H.; Molzberger, H.; Freiberg, C.; Ammermann, D.; Brunke, M. Function and metabolism of canine kidneys after aerobic ischemia by retrograde persufflation with gaseous oxygen. Exp. Med. 1974, 164, 35–44. [Google Scholar] [CrossRef]
- Ross, H.; Escott, M.L. Gaseous oxygen perfusion of the renal vessels as an adjunct in kidney preservation. Transplantation 1979, 28, 362–364. [Google Scholar] [CrossRef]
- Rolles, K.; Foreman, J.; Pegg, D.E. A pilot clinical study of retrograde oxygen persufflation in renal preservation. Transplantation 1989, 48, 339–342. [Google Scholar]
- Minor, T.; Isselhard, W. Venous oxygen insufflation to prevent reoxygenation injury after ischemia of a solid organ. Transplantation 1994, 58, 121–123. [Google Scholar] [PubMed]
- Minor, T.; Klauke, H.; Vollmar, B.; Isselhard, W.; Menger, M.D. Biophysical aspects of liver aeration by vascular persufflation with gaseous oxygen. Transplantation 1997, 63, 1843–1846. [Google Scholar] [CrossRef] [PubMed]
- Minor, T.; Isselhard, W.; Klauke, H. Reduction in nonparenchymal cell injury and vascular endothelial dysfunction after cold preservation of the liver by gaseous oxygen. Transpl. Int. 1996, 9, S425–S428. [Google Scholar] [CrossRef]
- Minor, T.; Klauke, H.; Nagelschmidt, M.; Isselhard, W. Reduction of proteolysis by venous-systemic oxygen persufflation during rat liver preservation and improved functional outcome after transplantation. Transplantation 1997, 63, 365–368. [Google Scholar] [CrossRef]
- Minor, T.; Akbar, S.; Tolba, R.; Dombrowski, F. Cold preservation of fatty liver grafts: prevention of functional and ultrastructural impairments by venous oxygen persufflation. J. Hepatol. 2000, 32, 105–111. [Google Scholar] [CrossRef]
- Dutkowski, P.; Polak, W.G.; Muiesan, P.; Schlegel, A.; Verhoeven, C.J.; Scalera, I.; DeOliveira, M.L.; Kron, P.; Clavien, P.-A. First comparison of hypothermic oxygenated perfusion versus static cold storage of human donation after cardiac death liver transplants: An international-matched case analysis. Ann. Surg. 2015, 262, 764–771. [Google Scholar] [CrossRef]
- Hoyer, D.P.; Mathe, Z.; Gallinat, A.; Canbay, A.C.; Treckmann, J.W.; Rauen, U.; Paul, A.; Minor, T. Controlled oxygenated rewarming of cold stored livers prior to transplantation: First clinical application of a new concept. Transplantation 2016, 100, 147–152. [Google Scholar] [CrossRef] [PubMed]
- Watson, C.J.E.; Kosmoliaptsis, V.; Randle, L.V.; Russell, N.K.; Griffiths, W.J.H.; Davies, S.; Mergental, H.; Butler, A.J. Preimplant Normothermic Liver Perfusion of a Suboptimal Liver Donated After Circulatory Death. Am. J. Transplant. 2015, 16, 353–357. [Google Scholar] [CrossRef] [PubMed]
| Parameter | OPAL (n = 57) | Control (n = 59) | p-Value |
|---|---|---|---|
| Donor Age (years) | 64 (30–95) | 63 (28–84) | 0.57 |
| Donor Gender (m/f) (%) | 56/44 | 44/56 | 0.27 |
| Donor BMI (kg/m2) | 25(19–42) | 26 (19–51) | 0.38 |
| Donor ICU stay (days) | 3 (1–16) | 4 (1–19) | 0.02 |
| Donor Cause of death (n) Cerebrovascular Hypoxia Trauma Others | 37 10 5 5 | 39 13 4 3 | 0.8 |
| Donor aspartate aminotransferase (AST) (U/L) | 48 (9–501) | 41 (9–607) | 0.8 |
| Donor ALT (U/L) | 32 (6–956) | 33 (6–282) | 0.87 |
| Donor γGT (U/L) | 46 (7–381) | 57 (6–416) | 0.29 |
| Donor Sodium (µmol/L) | 149 (132–163) | 149 (132–169) | 0.71 |
| Donor Creatinin (µmol/L) | 80 (32–689) | 81 (33–265) | 0.87 |
| Donor Bilirubin (µmol/L) | 9 (3.4–30) | 8.2 (2.7–564) | 0.16 |
| Donor INR | 1.13 (0.88–3.50) | 1.12 (0.87–5.60) | 0.81 |
| Donor Risk Index | 1.83 (1.1–2.5) | 1.80 (1.1–2.5) | 0.55 |
| Allograft Histology (n) Macrosteatosis (≥20%) Microsteatosis (%) | 49 13 50 (5–95) | 47 6 40 (0–90) | 0.09 0.36 |
| Perfusion solution HTK/ UW (n) | 53/4 | 52/7 | 0.37 |
| Cold Ischemia Time (min) | 443 (289–1090) | 390 (259–740) | 0.12 |
| Recipient Age (years) | 57 (31/69) | 52 (24–67) | 0.046 |
| Recipient Gender (m/f) (%) | 38/19 | 32/27 | 0.17 |
| Recipient BMI (kg/m2) | 27 (18–44) | 25 (17–41) | 0.14 |
| Underlying disease (%) Viral Hepatitis HCC Cholestative disease Alcohol NASH Others | 8 14 7 14 3 11 | 10 16 4 18 3 8 | 0.83 |
| Charlson Co-morbidity Index | 4 (1–8) | 4 (2–8) | 0.25 |
| Laboratory Model for End-Stage Liver Disease (MELD) | 13 (6–31) | 15 (6–40) | 0.16 |
| Parameter | OPAL (n = 57) | Control (n = 59) | p-Value |
|---|---|---|---|
| Retransplantation (n) | 2 | - | - |
| Early allograft dysfunction (EAD) (n) | 14 | 12 | 0.58 |
| Recipient ICU stay (days) | 3 (1–45) | 3 (1–41) | 0.97 |
| Post Tx dialysis (n) | 5 | 9 | 0.28 |
| Recipient hospital stay (days) | 20 (2–114) | 18 (1–85) | 0.07 |
| 30-day mortality (n) | 3 | 2 | 0.62 |
| In-hospital mortality (n) | 5 | 5 | 0.95 |
| Postop. Comlications (n) Dindo-Clavien IIIa Dindo-Clavien IIIb Dindo-Clavien IVa Dindo-Clavien IVb | 22 7 8 5 2 | 17 5 3 6 3 | 0.26 |
| Rejection within 3 months (n) | 6 | 8 | 0.61 |
| Parameter | OPAL | Control | ||
|---|---|---|---|---|
| Univariate | Multivariate | Univariate | Multivariate | |
| Donor Age (years) | 0.81 | 0.41 | ||
| Donor Age > 70years | 0.7 | 0.65 | ||
| Donor BMI | 0.09 | 3.26 0.07 | 0.67 | 0.14 0.71 |
| Donor cause of death | 0.31 | 0.23 | ||
| Donor ICU stay (days) | 0.49 | 0.79 | ||
| Donor cardiopulmonary resuscitation | 0.47 | 0.12 0.73 | 0.07 | 6.02 0.01 |
| Donor AST (U/L) | 0.87 | 0.1 | ||
| Donor ALT U/L) | 0.38 | 2.54 0.11 | 0.03 | 5.25 0.02 |
| Donor γGT (U/L) | 0.73 | 0.97 0.32 | 0.03 | 1.25 0.26 |
| Donor Bilirubin (µmol/L) | 0.26 | 0.29 | ||
| Donor Risk Index | 0.77 | 0.4 | ||
| Allograft histology: macrosteatosis | 0.18 | 0.67 | ||
| Allograft histology: fibrosis | 0.48 | 0.76 | ||
| Preservation solution | 0.13 | 1.75 0.19 | 0.07 | 1.67 0.2 |
| Cold Ischemia Time (h) | 0.13 | 0.11 | ||
| Warm Ischemia Time (min) | 0.61 | 0.66 | ||
| Duration of Surgical Procedure (min) | 0.89 | 0.86 | ||
| Recipient Age (years) | 0.36 | 0.6 | ||
| Recipient BMI | 0.37 | 1.39 0.24 | 0.08 | 3.24 0.07 |
| Lab-MELD score | 0.52 | 0.43 | ||
| Parameter | OPAL | Control | ||
|---|---|---|---|---|
| Univariate | Multivariate | Univariate | Multivariate | |
| Donor age(years) | 0.63 | 0.138 0.71 | 0.051 | 0.48 0.49 |
| Donor age > 70 years | 0.22 | 0.047 | ||
| Donor BMI | 0.97 | 0.6 | ||
| Donor cause of death | 0.17 | 0.99 | ||
| Donor ICU stay (days) | 0.29 | 0.3 | ||
| Donor cardiopulmonary resuscitation | 0.46 | 0.19 | ||
| Donor AST U/L) | 0.87 | 0.53 | ||
| Donor ALT (U/L) | 0.2 | 0.12 | ||
| Donor γGT (U/L) | 0.67 | 0.69 | ||
| Donor Bilirubin µmol/L) | 0.2 | 0.18 | ||
| Donor Risk Index | 0.35 | 0.65 | ||
| Allograft histology: macrosteatosis | 0.46 | 0.38 0.54 | 0.07 | 6.2 0.01 |
| Allograft histology: fibrosis | 0.09 | 1.17 0.28 | 0.44 | 3.69 0.06 |
| Preservation solution | 0.37 | 0.7 | ||
| Cold Ischemia Time (h) | 0.55 | 0.05 0.83 | 0.09 | 3.55 0.06 |
| Warm Ischemia Time (min) | 0.83 | 0.27 | ||
| Duration of Surgical Procedure (min) | 0.28 | 0.9 | ||
| Recipient Age (years) | 0.71 | 0.17 | ||
| Recipient BMI | 0.48 | 0.34 | ||
| MELD | 0.49 | 0.48 0.49 | 0.054 | 5.06 0.03 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gallinat, A.; Hoyer, D.P.; Sotiropoulos, G.; Treckmann, J.; Benkoe, T.; Belker, J.; Saner, F.; Paul, A.; Minor, T. Oxygen Persufflation in Liver Transplantation Results of a Randomized Controlled Trial. Bioengineering 2019, 6, 35. https://doi.org/10.3390/bioengineering6020035
Gallinat A, Hoyer DP, Sotiropoulos G, Treckmann J, Benkoe T, Belker J, Saner F, Paul A, Minor T. Oxygen Persufflation in Liver Transplantation Results of a Randomized Controlled Trial. Bioengineering. 2019; 6(2):35. https://doi.org/10.3390/bioengineering6020035
Chicago/Turabian StyleGallinat, Anja, Dieter Paul Hoyer, Georgios Sotiropoulos, Jürgen Treckmann, Tamas Benkoe, Jennifer Belker, Fuat Saner, Andreas Paul, and Thomas Minor. 2019. "Oxygen Persufflation in Liver Transplantation Results of a Randomized Controlled Trial" Bioengineering 6, no. 2: 35. https://doi.org/10.3390/bioengineering6020035
APA StyleGallinat, A., Hoyer, D. P., Sotiropoulos, G., Treckmann, J., Benkoe, T., Belker, J., Saner, F., Paul, A., & Minor, T. (2019). Oxygen Persufflation in Liver Transplantation Results of a Randomized Controlled Trial. Bioengineering, 6(2), 35. https://doi.org/10.3390/bioengineering6020035





