Three-Dimensional (3D) Printed Microneedles for Microencapsulated Cell Extrusion
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Methods
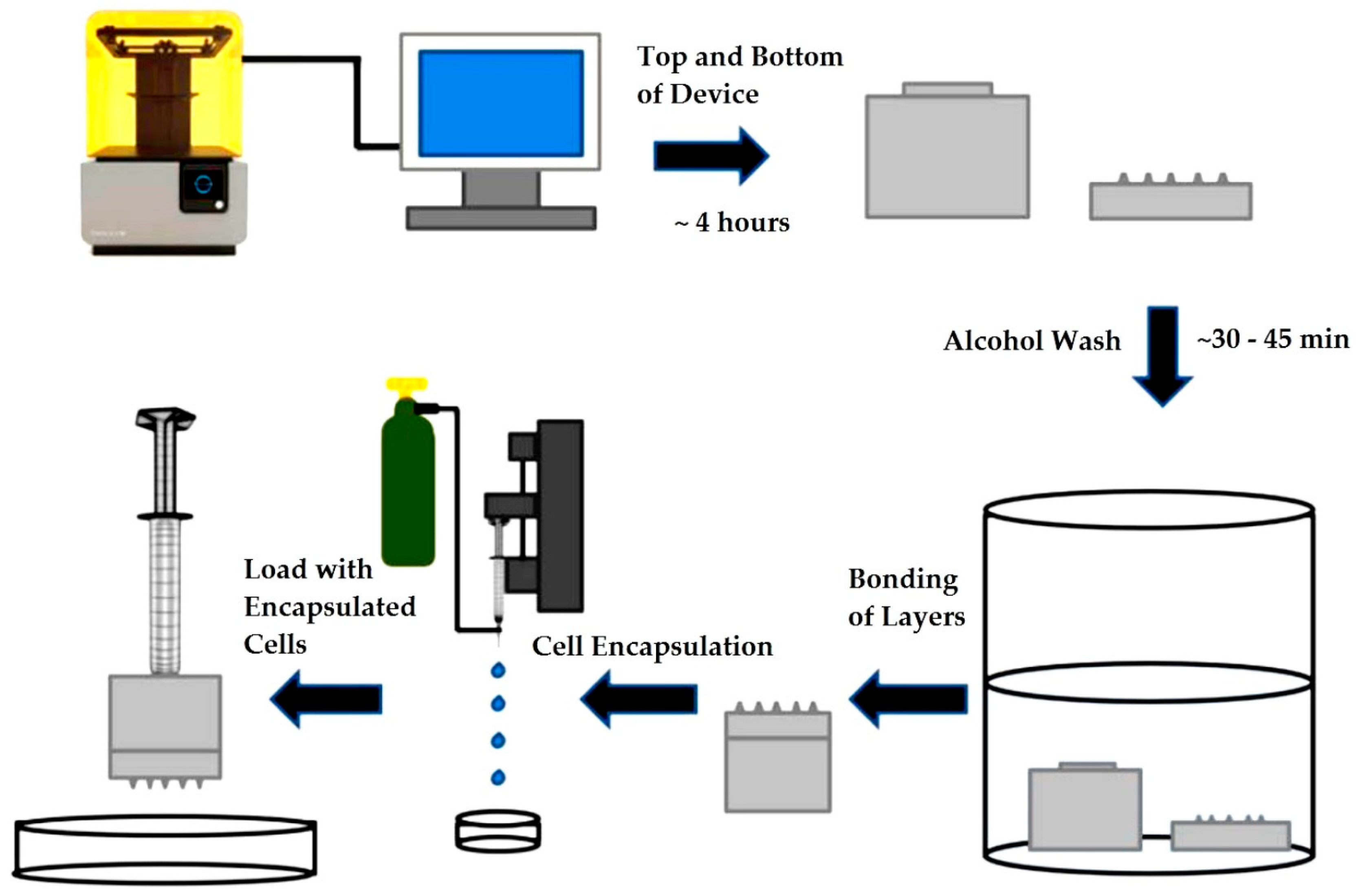
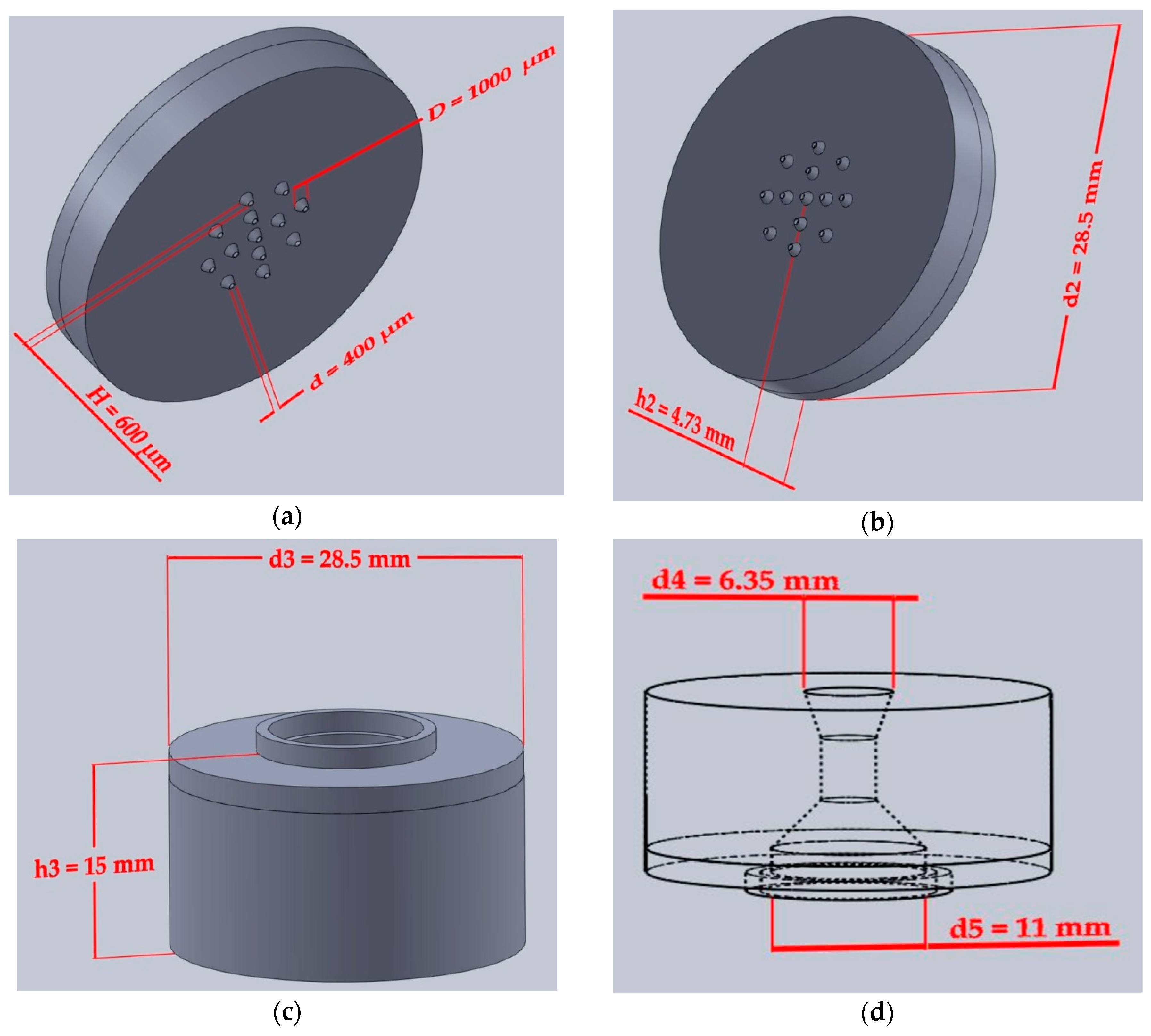
2.2.1. Device Design Printing and Assembly
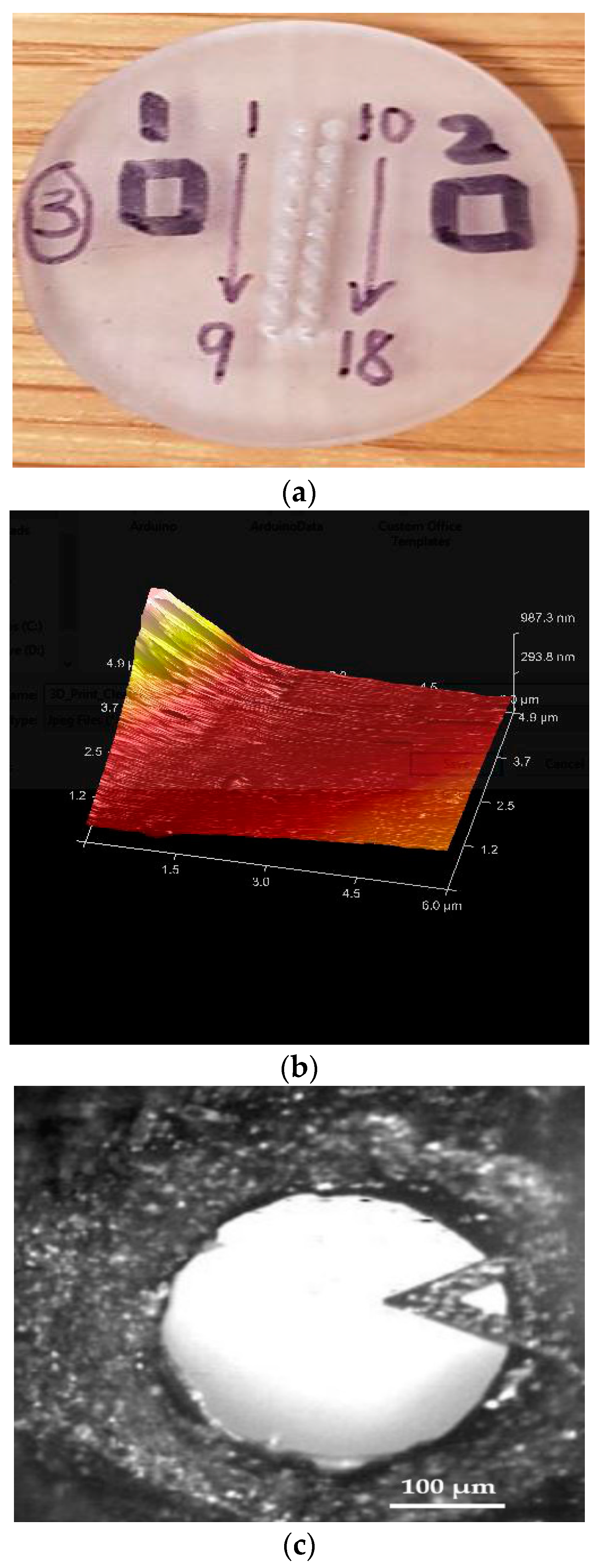
2.2.2. Surface Topography
2.2.3. Mass Flow Capability and Leakage Test
2.2.4. Cell Culture
2.2.5. Cytotoxicity Screening
2.2.6. Microcapsule Fabrication
2.2.7. Microcapsule Compression
2.2.8. Optical Measurements
2.2.9. Microcapsule Extrusion
2.2.10. Viability Testing
2.2.11. Relative Payload Calculation
2.2.12. Statistical Analysis
3. Results
3.1. Device Printing, Sterilization and Assembly
3.2. Surface Topography
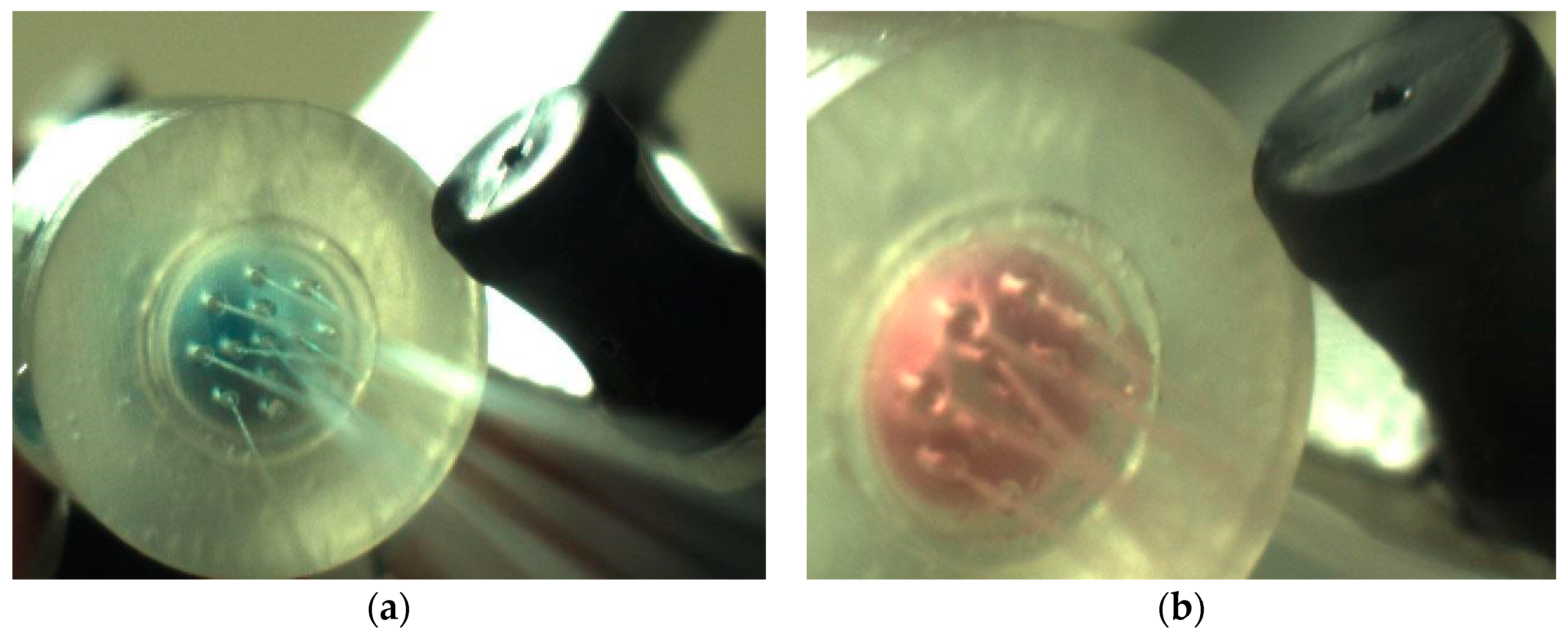
3.3. Mass Flow Capability and Leakage Test
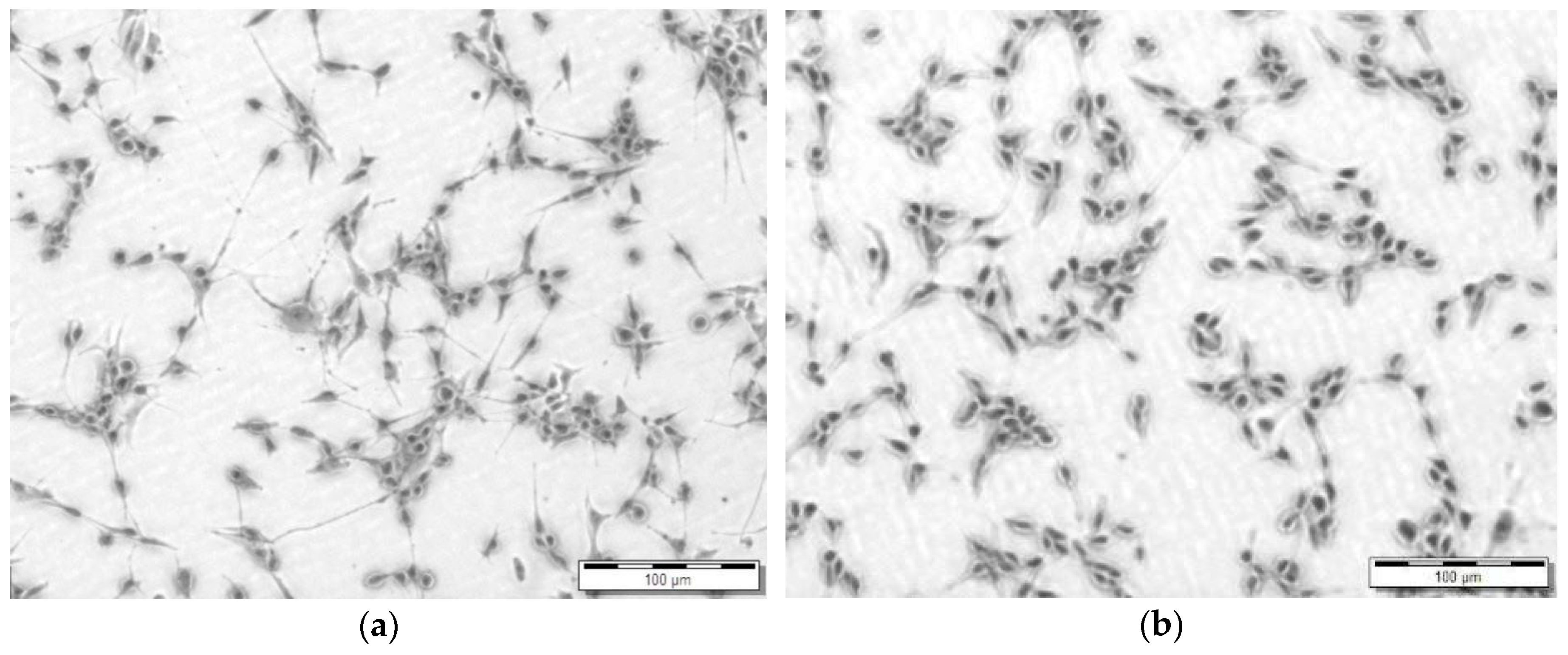
3.4. Cytotoxicity Screening
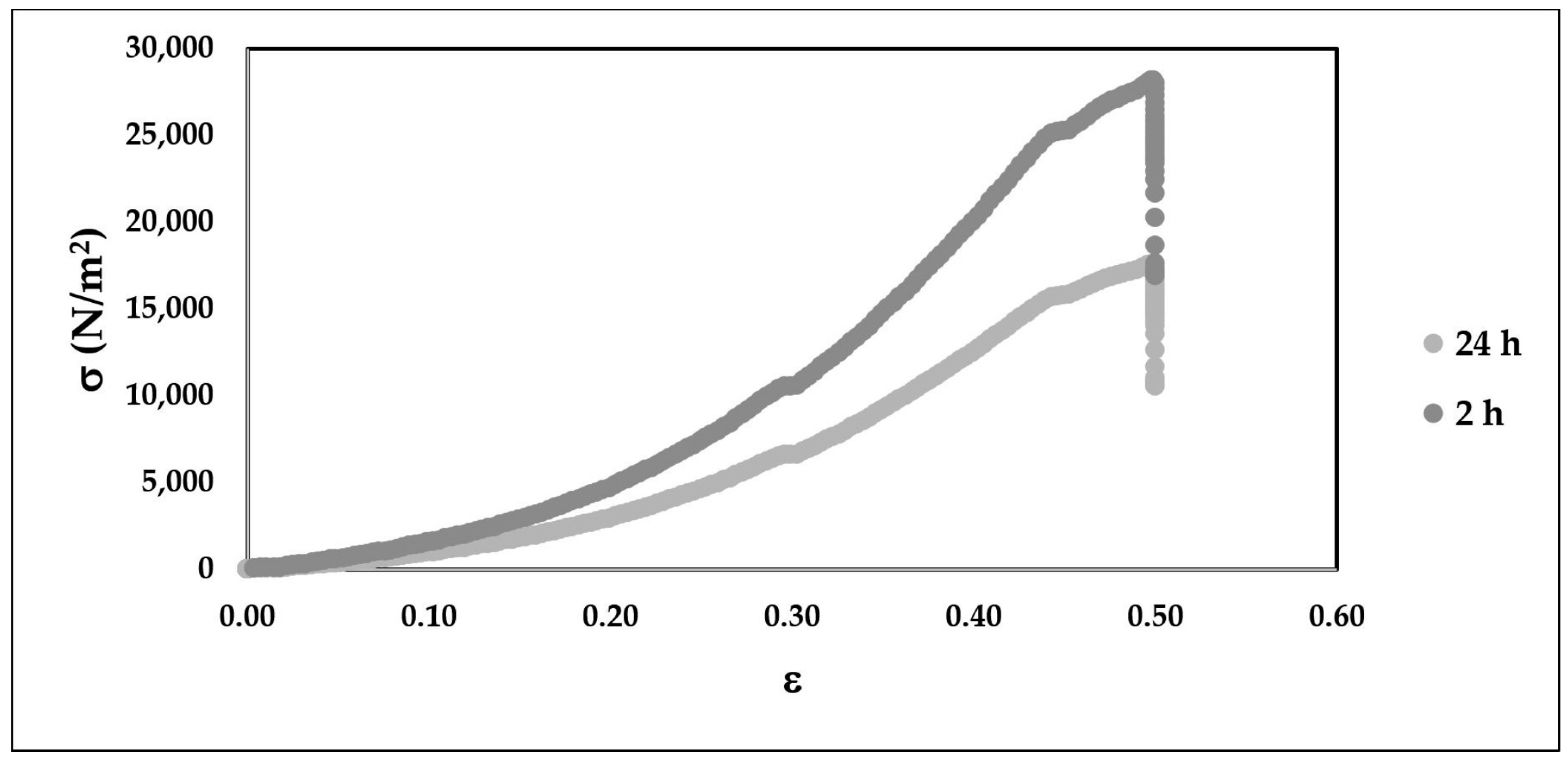
3.5. Microcapsule Compression
3.6. Microcapsule Extrusion
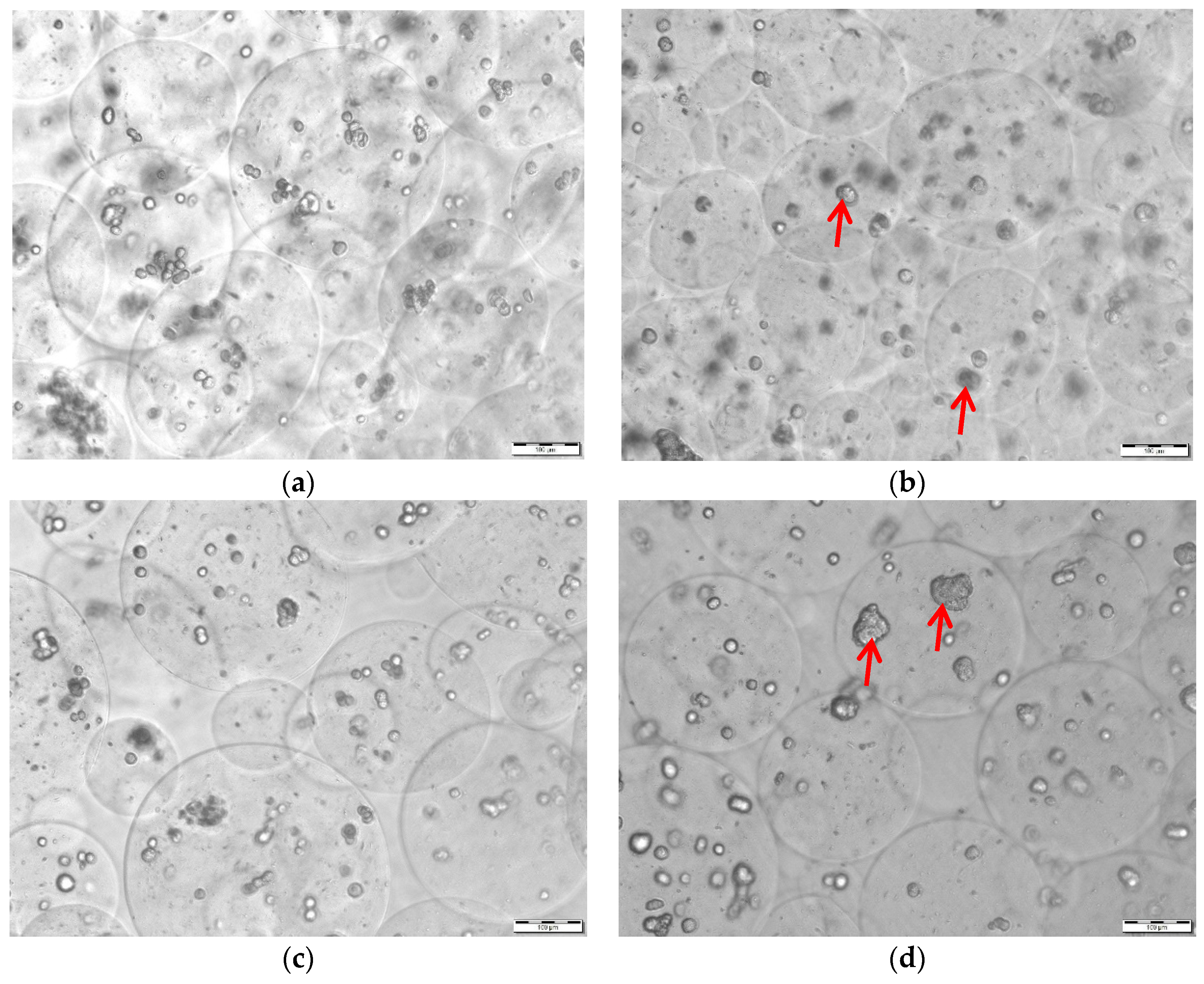
3.7. Viability Post Extrusion and Relative Payload
4. Discussion
5. Conclusions
6. Patent
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| Acronym | Definition |
| ABS | Acrylonitrile Butadiene Styrene |
| AFM | Atomic Force Microscopy |
| AM | Additive manufacturing |
| CAD | Computer-aided design |
| CLIP | Continuous liquid interface production |
| DAB | Droplet-borne air blowing |
| DC-hMN-iSystem | Digitally controlled injection system |
| DHE | Dihydroergotamine mesylate |
| DMA | Dimethacrylate |
| DLP | Digital Light Processing |
| DMEM | Dulbecco’s Modified Eagle Medium |
| EHP | Electrohydrodynamic processing |
| FBS | Fetal Bovine Serum |
| FDM | Fused Deposition Modeling |
| M/G | Mannuronic acid to Guluronic acid ratio |
| GEN | Gentamicin |
| GOx | Glucose Oxidase |
| HA | Hyaluronic Acid |
| HMN | Hollow microneedle |
| IC50 | Half maximal inhibitory concentration |
| MABS | Methyl methacrylate/acrylonitrile/butadiene styrene |
| MN | Microneedle |
| MPP | Multiphoton Polymerization |
| MWCO | Molecular Weight Cut Off |
| NP | Nanoparticles |
| PDMS | Polydimethylsiloxane |
| PEG | Poly (ethylene glycol) |
| PEGDA | Poly (ethylene glycol) diacrylate |
| PFF | Poly (propylene fumarate) |
| PLA | Polylactic Acid |
| PLGA | Poly-lactic-glycolic acid |
| PMMA | Polymethylmethacrylate |
| PVP | Polyvinylpyrrolidone |
| PVs | Polymeric Vesicles |
| RMN | Resorbable microneedle |
| RMS | Root mean square roughness |
| S/V | Surface area to volume ratio |
| SLA | Stereolithography |
| SLS | Selective Laser Sintering |
| TCPS | Tissue culture polystyrene |
| UFP | Ultrafine particle |
References
- Henry, S.; Mcallister, D.V.; Allen, M.G.; Prausnitz, M.R. Microfabricated Microneedles: A Novel Approach to Transdermal Drug Delivery. J. Pharm. Sci. 1998, 87, 922–925. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Ye, Y.; Hochu, G.M.; Sadeghifar, H.; Gu, Z. Enhanced Cancer Immunotherapy by Microneedle Patch-Assisted Delivery of Anti-PD1 Antibody. Nano Lett. 2016, 16, 2334–2340. [Google Scholar] [CrossRef] [PubMed]
- Ye, Y.; Wang, C.; Zhang, X.; Hu, Q.; Zhang, Y.; Liu, Q.; Wen, D.; Milligan, J. A Melanin-mediated Cancer Immunotherapy Patch. Sci. Immunol. 2017, 2. [Google Scholar] [CrossRef] [PubMed]
- Hu, X.; Yu, J.; Qian, C.; Lu, Y.; Kahkoska, A.R.; Xie, Z.; Jing, X.; Buse, J.B.; Gu, Z. H2O2-Responsive Vesicles Integrated with Transcutaneous Patches for Glucose-Mediated Insulin Delivery. ACS Nano 2017, 11, 613–620. [Google Scholar] [CrossRef] [PubMed]
- Lahiji, S.F.; Jang, Y.; Huh, I.; Yang, H.; Jang, M.; Jung, H. Exendin-4-encapsulated dissolving microneedle arrays for efficient treatment of type 2 diabetes. Sci. Rep. 2018, 8, 1170. [Google Scholar] [CrossRef] [PubMed]
- Qiu, Y.; Guo, L.; Zhang, S.; Xu, B.; Gao, Y.; Hu, Y.; Hou, J.; Bai, B.; Shen, H.; Mao, P. DNA-based Vaccination Against Hepatitis B Virus Using Dissolving Microneedle Arrays Adjuvanted by Cationic Liposomes and CpG ODN. Drug Deliv. 2015, 1, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Esser, E.S.; Romanyuk, A.; Vassilieva, E.V.; Jacob, J.; Prausnitz, M.R.; Compans, R.W.; Skountzou, I. Tetanus Vaccination with a Dissolving Microneedle Patch Confers Protective Immune Responses in Pregnancy. J. Control. Release 2016, 236, 47–56. [Google Scholar] [CrossRef] [PubMed]
- Ryu, H.R.; Jeong, H.-R.; Seon-Woo, H.-S.; Kim, J.S.; Lee, S.K.; Kim, H.J.; Baek, J.O.; Park, J.H.; Roh, J.Y. Efficacy of a Bleomycin Microneedle Patch for the Treatment of Warts. Drug Deliv. Transl. Res. 2017, 8, 273–280. [Google Scholar] [CrossRef] [PubMed]
- Maaden, K.V.D.; Heuts, J.; Camps, M.; Pontier, M.; Scheltinga, A.T.V.; Jiskoot, W.; Ossendorp, F.; Bouwstra, J. Hollow Microneedle-mediated Micro-injections of a Liposomal HPV E7 43–63 Synthetic Long Peptide Vaccine for Efficient Induction of Cytotoxic and T-helper Responses. J. Control. Release 2018, 269, 347–354. [Google Scholar] [CrossRef] [PubMed]
- Bhatnagar, S.; Kumari, P.; Pattarabhiran, S.P.; Venuganti, V.V.K. Zein Microneedles for Localized Delivery of Chemotherapeutic Agents to Treat Breast Cancer: Drug Loading, Release Behavior, and Skin Permeation Studies. AAPS PharmSciTech 2018, 19, 1818–1826. [Google Scholar] [CrossRef] [PubMed]
- Ali, A.A.; Mccrudden, C.M.; Mccaffrey, J.; Mcbride, J.W.; Cole, G.; Dunne, N.J.; Robson, T.; Kissenpfennig, A.; Donnelly, R.F.; McCarthy, H.O. DNA Vaccination for Cervical Cancer: A Novel Technology Platform of RALA Mediated Gene Delivery via Polymeric Microneedles. Nanomed. Nanotechnol. Biol. Med. 2017, 13, 921–932. [Google Scholar] [CrossRef] [PubMed]
- González-Vázquez, P.; Larrañeta, E.; Mccrudden, M.T.; Jarrahian, C.; Rein-Weston, A.; Quintanar-Solares, M.; Zehrung, D.; McCarthy, H.; Courtenay, A.J.; Donnelly, R.F. Transdermal Delivery of Gentamicin using Dissolving Microneedle Arrays for Potential Treatment of Neonatal Sepsis. J. Control. Release 2017, 265, 30–40. [Google Scholar] [CrossRef] [PubMed]
- Yan, Q.; Liu, H.; Cheng, Z.; Xue, Y.; Cheng, Z.; Dai, X.; Shan, W.; Chen, F. Immunotherapeutic Effect of BCG-polysaccharide Nucleic Acid Powder on Mycobacterium Tuberculosis-infected Mice using Microneedle Patches. Drug Deliv. 2017, 24, 1648–1653. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Liu, Q.; Yu, J.; Yu, S.; Wang, J.; Qiang, L.; Gu, Z. Locally Induced Adipose Tissue Browning by Microneedle Patch for Obesity Treatment. ACS Nano 2017, 11, 9223–9230. [Google Scholar] [CrossRef] [PubMed]
- Katsumi, H.; Tanaka, Y.; Hitomi, K.; Liu, S.; Quan, Y.-S.; Kamiyama, F.; Sakane, T.; Yamamoto, A. Efficient Transdermal Delivery of Alendronate, a Nitrogen-Containing Bisphosphonate, Using Tip-Loaded Self-Dissolving Microneedle Arrays for the Treatment of Osteoporosis. Pharmaceutics 2017, 9, 29. [Google Scholar] [CrossRef] [PubMed]
- Tas, C.; Joyce, J.C.; Nguyen, H.X.; Eangoor, P.; Knaack, J.S.; Banga, A.K.; Prausnitz, M.R. Dihydroergotamine Mesylate-loaded Dissolving Microneedle Patch Made of Polyvinylpyrrolidone for Management of Acute Migraine Therapy. J. Control. Release 2017, 268, 159–165. [Google Scholar] [CrossRef] [PubMed]
- Gurtner, G.C.; Werner, S.; Barrandon, Y.; Longaker, M.T. Wound Repair and Regeneration. Nature 2008, 453, 314–321. [Google Scholar] [CrossRef] [PubMed]
- Park, S.Y.; Lee, H.U.; Lee, Y.C.; Kim, G.H.; Park, E.C.; Han, S.H.; Lee, J.G.; Choi, S.; Heo, N.S.; Kim, D.L.; et al. Wound healing potential of antibacterial microneedles loaded with green tea extracts. Mater. Sci. Eng. C Mater. Biol. Appl. 2014, 42, 757–762. [Google Scholar] [CrossRef] [PubMed]
- Caffarel-Salvador, E.; Kearney, M.C.; Mairs, R.; Gallo, L.; Stewart, S.A.; Brady, A.J.; Donnelly, R.F. Methylene blue-loaded dissolving microneedles: Potential use in photodynamic antimicrobial chemotherapy of infected wounds. Pharmaceutics 2015, 7, 397–412. [Google Scholar] [CrossRef] [PubMed]
- Saghazadeh, S.; Rinoldi, C.; Schot, M.; Kashaf, S.S.; Sharif, F.; Jalilian, E.; Nuutila, K.; Giatsidis, G.; Mostafalu, P.; Derakhshandeh, H.; et al. Drug delivery systems and materials for wound healing applications. Adv. Drug Deliv. Rev. 2018, 127, 138–166. [Google Scholar] [CrossRef] [PubMed]
- Takada, K.; Ito, Y.; Matsumoto, K.; Sato, Y.; Nishio, M.; Tadano, Y.; Kamei, Y.; Takemura, Y.; Inoue, N.; Akasaka, Y.; et al. Usefulness of basic fibroblast growth factor (bFGF) loaded dissolving microneedles for local therapy of skin wounds. J. Biomater. Nanobiotechnol. 2013, 4, 256–264. [Google Scholar] [CrossRef]
- Liebl, H.; Kloth, L.C. Skin Cell Proliferation Stimulated by Microneedles. J. Am. Coll. Clin. Wound Spec. 2012, 4, 2–6. [Google Scholar] [CrossRef] [PubMed]
- Yeo, D.C.; Balmayor, E.R.; Schantz, J.T.; Xu, C. Microneedle physical contact as a therapeutic for abnormal scars. Eur. J. Med. Res. 2017, 22, 28. [Google Scholar] [CrossRef] [PubMed]
- Omolu, A.; Bailly, M.; Day, R.M. Assessment of solid microneedle rollers to enhance transmembrane delivery of doxycycline and inhibition of MMP activity. Drug Deliv. 2017, 24, 942–951. [Google Scholar] [CrossRef] [PubMed]
- Yang, S.Y.; O’Cearbhaill, E.D.; Sisk, G.C.; Park, K.M.; Cho, W.K.; Villiger, M.; Bouma, B.E.; Pomahac, B.; Karp, J.M. A bio-inspired swellable microneedle adhesive for mechanical interlocking with tissue. Nat. Commun. 2013, 4, 1702. [Google Scholar] [CrossRef] [PubMed]
- Rajabi, M.; Roxhed, N.; Shafagh, R.Z.; Haraldson, T.; Fischer, A.C.; Wijngaart, W.V.D.; Stemme, G.; Niklaus, F. Flexible and Stretchable Microneedle Patches with Integrated Rigid Stainless Steel Microneedles for Transdermal Biointerfacing. PLoS ONE 2016, 11, e0166330. [Google Scholar] [CrossRef] [PubMed]
- Mukerjee, E.V.; Isseroff, R.R.; Nuccitelli, R.; Collins, S.D.; Smith, R.L. Microneedle Array for Measuring Wound Generated Electric Fields. In Proceedings of the 2006 International Conference of the IEEE Engineering in Medicine and Biology Society, New York, NY, USA, 30 August–3 September 2006; Volume 1, pp. 4326–4328. [Google Scholar]
- University of Nebraska-Lincoln. Smart Bandages for Early Detection of Infection. Available online: https://ucare.unl.edu/opportunities/smart-bandages-early-detection-infection (accessed on 8 July 2018).
- Norman, J.J.; Choi, S.-O.; Tong, N.T.; Aiyar, A.R.; Patel, S.R.; Prausnitz, M.R.; Allen, M.G. Hollow Microneedles for Intradermal Injection Fabricated by Sacrificial Micromolding and Selective Electrodeposition. Biomed. Microdevices 2012, 15, 203–210. [Google Scholar] [CrossRef] [PubMed]
- Dardano, P.; Caliò, A.; Palma, V.D.; Bevilacqua, M.F.; Matteo, A.D.; Stefano, L.D. A Photolithographic Approach to Polymeric Microneedles Array Fabrication. Materials 2015, 8, 8661–8673. [Google Scholar] [CrossRef] [PubMed]
- Choi, Y.; Lee, S.G.; Jeong, J.H.; Lee, K.M.; Jeong, K.H.; Yang, H.; Lee, S.; Choi, Y.W. Nanostructured Lipid Carrier-loaded Hyaluronic Acid Microneedles for Controlled Dermal Delivery of a Lipophilic Molecule. Int. J. Nanomed. 2013, 1, 289. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.L.; Zhu, D.D.; Liu, X.B.; Chen, B.Z.; Guo, X.D. Microneedles with Controlled Bubble Sizes and Drug Distributions for Efficient Transdermal Drug Delivery. Sci. Rep. 2016, 6, 28755. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.; Theogarajan, L.S.; Pennathur, S. A Repeatable and Scalable Fabrication Method for Hollow Silicon Microneedles. J. Micromech. Microeng. 2018, 28, 035007. [Google Scholar] [CrossRef]
- Ceyssens, F.; Chaudhri, B.P.; Hoof, C.V.; Puers, R. Fabrication Process for Tall, Sharp, Hollow, High Aspect Ratio Polymer Microneedles on a Platform. J. Micromech. Microeng. 2013, 23, 075023. [Google Scholar] [CrossRef]
- Ruggiero, F.; Vecchione, R.; Bhowmick, S.; Coppola, G.; Coppola, S.; Esposito, E.; Letterad, V.; Ferraroc, P.; Nettia, P.A. Electro-drawn Polymer Microneedle Arrays with Controlled Shape and Dimension. Sens. Actuators B Chem. 2018, 255, 1553–1560. [Google Scholar] [CrossRef]
- Johnson, A.R.; Caudill, C.L.; Tumbleston, J.R.; Bloomquist, C.J.; Moga, K.A.; Ermoshkin, A.; Shirvanyants, D.; Mecham, S.J.; Luft, J.C.; DeSimone, M.J. Single-Step Fabrication of Computationally Designed Microneedles by Continuous Liquid Interface Production. PLoS ONE 2016, 11, e0162518. [Google Scholar] [CrossRef] [PubMed]
- Huh, I.; Kim, S.; Yang, H.; Jang, M.; Kang, G.; Jung, H. Effects of Two Droplet-based Dissolving Microneedle Manufacturing Methods on the Activity of Encapsulated Epidermal Growth Factor and Ascorbic Acid. Eur. J. Pharm. Sci. 2018, 114, 285–292. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.; Yang, H.; Kim, S.; Lee, C.; Jung, H. The Troy Microneedle: A Rapidly Separating, Dissolving Microneedle Formed by Cyclic Contact and Drying on the Pillar (CCDP). PLoS ONE 2015, 10, e0136513. [Google Scholar] [CrossRef] [PubMed]
- Luzuriaga, M.A.; Berry, D.R.; Reagan, J.C.; Smaldone, R.A.; Gassensmith, J.J. Biodegradable 3D Printed Polymer Microneedles for Transdermal Drug Delivery. Lab Chip 2018, 18, 1223–1230. [Google Scholar] [CrossRef] [PubMed]
- Vinayakumar, K.B.; Kulkarni, P.G.; Nayak, M.M.; Dinesh, N.S.; Hegde, G.M.; Ramachandra, S.G.; Rajanna, K. A Hollow Stainless Steel Microneedle Array to Deliver Insulin to a Diabetic Rat. J. Micromech. Microeng. 2016, 26, 065013. [Google Scholar] [CrossRef]
- Mansoor, I.; Liu, Y.; Häfeli, U.O.; Stoeber, B. Arrays of Hollow Out-of-Plane Microneedles Made by Metal Electrodeposition onto Solvent Cast Conductive Polymer Structures. J. Micromech. Microeng. 2013, 23, 085011. [Google Scholar] [CrossRef]
- Ovsianikov, A.; Chichkov, B.; Mente, P.; Monteiro-Riviere, N.A.; Doraiswamy, A.; Narayan, R.J. Two Photon Polymerization of Polymer? Ceramic Hybrid Materials for Transdermal Drug Delivery. Int. J. Appl. Ceram. Technol. 2007, 4, 22–29. [Google Scholar] [CrossRef]
- Boehm, R.D.; Jaipan, P.; Yang, K.-H.; Stewart, T.N.; Narayan, R.J. Microstereolithography-fabricated Microneedles for Fluid Sampling of Histamine-contaminated Tuna. Int. J. Bioprinting 2016, 2. [Google Scholar] [CrossRef]
- Lim, S.H.; Ng, J.Y.; Kang, L. Three-dimensional Printing of a Microneedle Array on Personalized Curved Surfaces for Dual-pronged Treatment of Trigger Finger. Biofabrication 2017, 9, 015010. [Google Scholar] [CrossRef] [PubMed]
- Faraji Rad, Z.; Nordon, R.; Anthony, C.; Bilston, L.; Prewett, P.; Arns, J.-Y.; Arns, C.; Zhang, L.; Davies, G. High Fidelity Replication of Thermoplastic Microneedles with Open Microfluidic Channels. Microsyst. Nanoeng. 2017, 3, 17034. [Google Scholar] [CrossRef]
- ISO/ASTM 52901:2017(en) Additive Manufacturing—General Principles—Requirements for Purchased AM Parts. Available online: https://www.iso.org/obp/ui/#iso:std:iso-astm:52900:ed-1:v1:en in (accessed on 8 July 2018).
- Macdonald, N.P.; Zhu, F.; Hall, C.J.; Reboud, J.; Crosier, P.S.; Patton, E.E.; Wlodkowic, D.; Cooper, J.M. Assessment of Biocompatibility of 3D Printed Photopolymers Using Zebrafish Embryo Toxicity Assays. Lab Chip 2016, 16, 291–297. [Google Scholar] [CrossRef] [PubMed]
- Oskui, S.M.; Diamante, G.; Liao, C.; Shi, W.; Gan, J.; Schlenk, D.; Grover, W.H. Assessing and Reducing the Toxicity of 3D-Printed Parts. Environ. Sci. Technol. Lett. 2015, 3, 1–6. [Google Scholar] [CrossRef]
- Carve, M.; Wlodkowic, D. 3D-Printed Chips: Compatibility of Additive Manufacturing Photopolymeric Substrata with Biological Applications. Micromachines 2018, 9, 91. [Google Scholar] [CrossRef]
- Athanasiou, K. Sterilization, Toxicity, Biocompatibility and Clinical Applications of Polylactic Acid/Polyglycolic Acid Copolymers. Biomaterials 1996, 17, 93–102. [Google Scholar] [CrossRef]
- Kang, L.; Kochhar, C.; Zou, S. Protein Encapsulation in Polymeric Microneedles by Photolithography. Int. J. Nanomed. 2012, 1, 3143. [Google Scholar] [CrossRef] [PubMed]
- Lu, Y.; Mantha, S.N.; Crowder, D.C.; Chinchilla, S.; Shah, K.N.; Yun, Y.H.; Wicker, R.B.; Choi, Ja. Microstereolithography and Characterization of Poly(propylene fumarate)-based Drug-loaded Microneedle Arrays. Biofabrication 2015, 7, 045001. [Google Scholar] [CrossRef] [PubMed]
- Gieseke, M.; Senz, V.; Vehse, M.; Fiedler, S.; Irsig, R.; Hustedt, M.; Sternberg, K.; Nölke, C.; Kaierle, S.; Wesling, V.; et al. Additive Manufacturing of Drug Delivery Systems. Biomed. Eng. Biomed. Tech. 2012, 57. [Google Scholar] [CrossRef]
- Miller, P.R.; Gittard, S.D.; Edwards, T.L.; Lopez, D.M.; Xiao, X.; Wheeler, D.R.; Monteiro-Riviere, N.A.; Brozik, S.M.; Polsky, R.; Narayan, R.J. Integrated Carbon Fiber Electrodes within Hollow Polymer Microneedles for Transdermal Electrochemical Sensing. Biomicrofluidics 2011, 5, 013415. [Google Scholar] [CrossRef] [PubMed]
- Lacan, F.; Coulman, S.A.; Hotston, A.; Petkov, P.; Birchall, J.C. Prototyping Parts with Micro Scale Features Using Additive Manufacturing: Using Microneedles as a Case Study. In Proceedings of the 10th International Conference on Multi-Material Micro Manufacture, San Sebastián, Spain, 8–10 October 2013; Volume 1, pp. 109–346. [Google Scholar] [CrossRef]
- Pere, C.P.P.; Economidou, S.N.; Lall, G.; Ziraud, C.; Boateng, J.S.; Alexander, B.D.; Lamproua, D.A.; Douroumis, D. 3D Printed Microneedles for Insulin Skin Delivery. Int. J. Pharm. 2018, 544, 425–432. [Google Scholar] [CrossRef] [PubMed]
- Liu, T.-L.; Zahedi, S.; Garling, R.J.; Kralickk, F.; Harris, C.A.; Cheng, M.M.-C. Prosthetic Arachnoid Granulations using 3D Printing Technology. In Proceedings of the IEEE 30th International Conference on Micro Electro Mechanical Systems (MEMS), Las Vegas, NV, USA, 22–26 January 2017. [Google Scholar]
- Nagamine, K.; Kubota, J.; Kai, H.; Ono, Y.; Nishizawa, M. An Array of Porous Microneedles for Transdermal Monitoring of Intercellular Swelling. Biomed. Microdevices 2017, 19, 68. [Google Scholar] [CrossRef] [PubMed]
- Lee, D.-S.; Li, C.G.; Ihm, C.; Jung, H. A Three-dimensional and Bevel-angled Ultra high Aspect Ratio Microneedle for Minimally Invasive and Painless Blood Sampling. Sens. Actuators B Chem. 2018, 255, 384–390. [Google Scholar] [CrossRef]
- Wang, P.-C.; Paik, S.-J.; Kim, J.; Kim, S.-H.; Allen, M.G. Hypodermic Needle-like Hollow Polymer Microneedle Array using UV Lithography into Micromolds. J. Microelectromech. Syst. 2011, 23, 991–998. [Google Scholar] [CrossRef]
- Lau, S.; Fei, J.; Liu, H.; Chen, W.; Liu, R. Multilayered Pyramidal Dissolving Microneedle Patches with Flexible Pedestals for Improving Effective Drug Delivery. J. Control. Release 2017, 265, 113–119. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, H.X.; Banga, A.K. Enhanced Skin Delivery of Vismodegib by Microneedle Treatment. Drug Deliv. Transl. Res. 2015, 5, 407–423. [Google Scholar] [CrossRef] [PubMed]
- Gupta, J.; Park, S.S.; Bondy, B.; Felner, E.I.; Prausnitz, M.R. Infusion Pressure and Pain During Microneedle Injection into Skin of Human Subjects. Biomaterials 2011, 32, 6823–6831. [Google Scholar] [CrossRef] [PubMed]
- Gill, H.S.; Denson, D.D.; Burris, B.A.; Prausnitz, M.R. Effect of Microneedle Design on Pain in Human Volunteers. Clin. J. Pain 2008, 24, 585–594. [Google Scholar] [CrossRef] [PubMed]
- Gupta, J.; Gill, H.S.; Andrews, S.N.; Prausnitz, M.R. Kinetics of Skin Resealing after Insertion of Microneedles in Human Subjects. J. Control. Release 2011, 154, 148–155. [Google Scholar] [CrossRef] [PubMed]
- Sasaki, M.; Abe, R.; Fujita, Y.; Ando, S.; Inokuma, D.; Shimizu, H. Mesenchymal Stem Cells Are Recruited into Wounded Skin and Contribute to Wound Repair by Transdifferentiation into Multiple Skin Cell Type. J. Immunol. 2008, 180, 2581–2587. [Google Scholar] [CrossRef] [PubMed]
- Maharlooei, M.K.; Bagheri, M.; Solhjou, Z.; Jahromi, B.M.; Akrami, M.; Rohani, L.; Monabati, A.; Noorafshan, A.; Omrani, G.R. Adipose Tissue Derived Mesenchymal Stem Cell (AD-MSC) Promotes Skin Wound Healing in Diabetic Rats. Diabetes Res. Clin. Pract. 2011, 93, 228–234. [Google Scholar] [CrossRef] [PubMed]
- Maxson, S.; Lopez, E.A.; Yoo, D.; Danilkovitch-Miagkova, A.; Leroux, M.A. Concise Review: Role of Mesenchymal Stem Cells in Wound Repair. STEM Cells Transl. Med. 2012, 1, 142–149. [Google Scholar] [CrossRef] [PubMed]
- Nuschke, A. Activity of Mesenchymal Stem Cells in Therapies for Chronic Skin Wound Healing. Organogenesis 2013, 10, 29–37. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez-Menocal, L.; Shareef, S.; Salgado, M.; Shabbir, A.; Badiavas, E.V. Role of Whole Bone Marrow, Whole Bone Marrow Cultured Cells, and Mesenchymal Stem Cells in Chronic Wound Healing. Stem Cell Res. Ther. 2015, 6, 24. [Google Scholar] [CrossRef] [PubMed]
- Motegi, S.-I.; Ishikawa, O. Mesenchymal Stem Cells: The Roles and Functions in Cutaneous Wound Healing and Tumor Growth. J. Dermatol. Sci. 2017, 86, 83–89. [Google Scholar] [CrossRef] [PubMed]
- Bryant, S.J.; Vernerey, F.J. Programmable Hydrogels for Cell Encapsulation and Neo-Tissue Growth to Enable Personalized Tissue Engineering. Adv. Healthc. Mater. 2017, 7, 1700605. [Google Scholar] [CrossRef] [PubMed]
- ClincalTrials.gov. Available online: https://clinicaltrials.gov/ct2/results?cond=wound+healing&term=stem+cells&cntry=&state=&city=&dist (accessed on 18 June 2018).
- Duscher, D.; Barrera, J.; Wong, V.W.; Maan, Z.N.; Whittam, A.J.; Januszyk, M.; Gurtner, G.C. Stem Cells in Wound Healing: The Future of Regenerative Medicine? A Mini-Review. Gerontology 2015, 62, 216–225. [Google Scholar] [CrossRef] [PubMed]
- Rosemann, A. Why Regenerative Stem Cell Medicine Progresses Slower Than Expected. J. Cell. Biochem. 2014, 115, 2073–2076. [Google Scholar] [CrossRef] [PubMed]
- Kirby, G.T.S.; Mills, S.J.; Cowin, A.J.; Smith, L.E. Stem Cells for Cutaneous Wound Healing. BioMed Res. Int. 2015, 2015, 285869. [Google Scholar] [CrossRef] [PubMed]
- Cerqueira, M.T.; Pirraco, R.P.; Marques, A.P. Stem Cells in Skin Wound Healing: Are We There Yet? Adv. Wound Care 2016, 5, 64–75. [Google Scholar] [CrossRef] [PubMed]
- Dash, B.; Xu, Z.; Lin, L.; Koo, A.; Ndon, S.; Berthiaume, F.; Dardik, A.; Hsia, H. Stem Cells and Engineered Scaffolds for Regenerative Wound Healing. Bioengineering 2018, 5, 23. [Google Scholar] [CrossRef] [PubMed]
- Dickinson, L.E.; Gerecht, S. Engineered Biopolymeric Scaffolds for Chronic Wound Healing. Front. Physiol. 2016, 7, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Bakhshayesh, A.R.D.; Annabi, N.; Khalilov, R.; Akbarzadeh, A.; Samiei, M.; Alizadeh, E.; Alizadeh-Ghodsi, M.; Davaran, S.; Montaseri, A. Recent Advances on Biomedical Applications of Scaffolds in Wound Healing and Dermal Tissue Engineering. Artif. Cells Nanomed. Biotechnol. 2017, 46, 691–705. [Google Scholar] [CrossRef] [PubMed]
- Han, L.-H.; Conrad, B.; Chung, M.T.; Deveza, L.; Jiang, X.; Wang, A.; Butte, M.J.; Longaker, M.T.; Wan, D.; Yang, F. Microribbon-based Hydrogels Accelerate Stem Cell-based Bone Regeneration in a Mouse Critical-size Cranial. J. Biomed. Mater. Res. Part A 2016, 104, 1321–1331. [Google Scholar] [CrossRef] [PubMed]
- Wong, H.; Chang, T. Bioartificial Liver: Implanted Artificial Cells Microencapsulated Living Hepatocytes Increases Survival of Liver Failure Rats. Int. J. Artif. Organs 1986, 9, 335–336. [Google Scholar] [CrossRef] [PubMed]
- Wright, B.; Hopkinson, A.; Leyland, M.; Connon, C.J. The Secretome of Alginate-Encapsulated Limbal Epithelial Stem Cells Modulates Corneal Epithelial Cell Proliferation. PLoS ONE 2013, 8, e70860. [Google Scholar] [CrossRef] [PubMed]
- Schmitt, A.; Rödel, P.; Anamur, C.; Seeliger, C.; Imhoff, A.B.; Herbst, E.; Vogt, S.; van Griensven, M.; Winter, G.; Engert, J. Calcium Alginate Gels as Stem Cell Matrix—Making Paracrine Stem Cell Activity Available for Enhanced Healing after Surgery. PLoS ONE 2015, 10, e0118937. [Google Scholar] [CrossRef] [PubMed]
- Bussche, L.; Harman, R.M.; Syracuse, B.A.; Plante, E.L.; Lu, Y.-C.; Curtis, T.M.; Ma, M.; Van de Walle, G.R. Microencapsulated Equine Mesenchymal Stromal Cells Promote Cutaneous Wound Healing in Vitro. Stem Cell Res. Ther. 2015, 6, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Vegas, A.J.; Veiseh, O.; Gürtler, M.; Millman, J.R.; Pagliuca, F.W.; Bader, A.R.; Doloff, J.C.; Li, J.; Chen, M.; Olejnik, K.; et al. Long-term Glycemic Control Using Polymer-encapsulated Human Stem Cell–derived Beta Cells in Immune-competent Mice. Nat. Med. 2016, 22, 306–311. [Google Scholar] [CrossRef] [PubMed]
- Cooke, M.E.; Pearson, M.J.; Moakes, R.J.A.; Weston, C.J.; Davis, E.T.; Jones, S.W.; Grover, L.M. Geometric Confinement is Required for Recovery and Maintenance of Chondrocyte Phenotype in Alginate. APL Bioeng. 2017, 1, 016104. [Google Scholar] [CrossRef]
- Gualeni, B.; Coulman, S.A.; Shah, D.; Eng, P.F.; Ashraf, H.; Vescovo, P.; Blayney, G.J.; Piveteau, L.-D.; Guy, O.J.; Birchall, J.C.; et al. Minimally invasive and targeted therapeutic cell delivery to the skin using microneedle devices. Br. J. Dermatol. 2018, 178, 731–739. [Google Scholar] [CrossRef] [PubMed]
- Boniface, K.; Taieb, A.; Seneschal, A. Cell Delivery Using Microneedle Devices: A New Approach to Treat Depigmenting Disorders. Br. J. Dermatol. 2018, 178, 583–594. [Google Scholar] [CrossRef] [PubMed]
- Mitteregger, R.; Vogt, G.; Rossmanith, E.; Falkenhagen, D. Rotary Cell Culture System (RCCS): A New Method for Cultivating Hepatocytes on Microcarriers. Int. J. Artif. Organs 1999, 22, 816–822. [Google Scholar] [CrossRef] [PubMed]
- Baudoin, R.; Griscom, L.; Prot, J.M.; Legallais, C.; Leclerc, E. Behavior of HepG2/C3A Cell Cultures in a Microfluidic Bioreactor. Biochem. Eng. J. 2011, 53, 172–181. [Google Scholar] [CrossRef]
- Deng, X.; Cao, Y.; Yan, H.; Yang, J.; Xiong, G.; Yao, H.; Qi, C. Enhanced Liver Functions of HepG2 Cells in the Alginate/Xyloglucan Scaffold. Biotechnol. Lett. 2014, 37, 235–240. [Google Scholar] [CrossRef] [PubMed]
- CLEAR Photoactive Resin SFS Form. Available online: https://formlabs.com/media/upload/Clear-SDS_u324bsC.pdf (accessed on 19 June 2018).
- U.S. Department of Health and Human Services; Food and Drug Administration; Center for Devices and Radiological Health. Use of International Standard ISO 10993-1, “Biological Evaluation of Medical devices—Part 1: Evaluation and Testing within a Risk Management Process”. Available online: https://www.fda.gov/downloads/medicaldevices/deviceregulationandguidance/guidancedocuments/ucm348890.pdf (accessed on 19 June 2018).
- Treiser, M.; Abramson, S.; Langer, R.; Kohn, J. Biomaterials Science, An Introduction to Materials in Medicine, 3rd ed.; Ratner, B.D., Hoffman, A.S., Schoen, F.J., Lemons, J.E., Eds.; Elsevier: Waltham, MA, USA, 2013; Chapter 1.2.6; p. 179. [Google Scholar]
- Mobed-Miremadi, M.; Asi, B.; Parasseril, J.; Wong, E.; Tat, M.; Shan, Y. Comparative Diffusivity Measurements for Alginate-based Atomized and Inkjet-Bioprinted Artificial Cells using Fluorescence Microscopy. Artif. Cells Nanomed. Biotechnol. 2012, 41, 196–201. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Cheah, C.; Fuh, J.; Lu, L. Influence of Process Parameters on Stereolithography Part Shrinkage. Mater. Des. 1996, 17, 205–213. [Google Scholar] [CrossRef]
- Huang, Q.; Zhang, J.; Sabbaghi, A.; Dasgupta, T. Optimal Offline Compensation of Shape Shrinkage for Three-dimensional Printing Processes. IIE Trans. 2014, 47, 431–441. [Google Scholar] [CrossRef]
- Karalekas, D.; Aggelopoulos, A. Study of Shrinkage Strains in a Stereolithography Cured Acrylic Photopolymer Resin. J. Mater. Process. Technol. 2003, 136, 146–150. [Google Scholar] [CrossRef]
- Wallace, J.; Wang, M.O.; Thompson, P.; Busso, M.; Belle, V.; Mammoser, N.; Kim, K.; Fisher, J.P.; Siblani, A.; Xu, Y.; et al. Validating Continuous Digital Light Processing (cDLP) Additive Manufacturing Accuracy and Tissue Engineering Utility of a Dye-initiator Package. Biofabrication 2014, 6, 015003. [Google Scholar] [CrossRef] [PubMed]
- Karrer, P.; Corbel, S.; Andre, J.C.; Lougnot, D.J. Shrinkage Effects in Photopolymerizable Resins Containing Filling Agents: Application to Stereophotolithography. J. Polym. Sci. Part A Polym. Chem. 1992, 30, 2715–2723. [Google Scholar] [CrossRef]
- Vitale, A.; Cabral, J. Frontal Conversion and Uniformity in 3D Printing by Photopolymerisation. Materials 2016, 9, 760. [Google Scholar] [CrossRef] [PubMed]
- Huang, B.; Du, Z.; Yong, T.; Han, W. Preparation of a Novel Hybrid Type Photosensitive Resin for Stereolithography in 3D Printing and Testing on the Accuracy of the Fabricated Parts. J. Wuhan Univ. Technol. Mater. Sci. Ed. 2017, 32, 726–732. [Google Scholar] [CrossRef]
- ASTM. ASTM D256-10e1, Standard Test Methods for Determining the Izod Pendulum Impact Resistance of Plastics. Available online: https://www.astm.org (accessed on 8 July 2018).
- Harrison, R.G.; Todd, P.W.; Rudge, S.R.; Petrides, D. Bioseparations Science and Engineering, 1st ed.; Oxford University Press: Oxford, UK, 2002; ISBN 0195123409. [Google Scholar]
- Prakash, S. Artificial Cells, Cell Engineering and Therapy; Prakash, S., Ed.; Woodhead Publishing: Cambridge, UK, 2007; ISBN 9781845690366. [Google Scholar]
- Mobed-Miremadi, M. High-Throughput Methods for Miniaturization of Implantable Artificial Cells. In Selected Topics in Nanomedicine; Chang, T.M.S., Ed.; World Scientific Publishing: Singapore, 2013; pp. 411–427. ISBN 9789814472852. [Google Scholar]
- Seaton, M.; Hocking, A.; Gibran, N.S. Porcine Models of Cutaneous Wound Healing. ILAR J. 2015, 56, 127–138. [Google Scholar] [CrossRef] [PubMed]
- Ligon, S.C.; Liska, R.; Stampfl, J.; Gurr, M.; Mulhaupt, R. Polymers for 3D Printing and Customized Additive Manufacturing. Chem. Rev. 2017, 117, 10212–10290. [Google Scholar] [CrossRef] [PubMed]
- Tappa, K.; Jammalamadaka, U. Novel Biomaterials Used in Medical 3D Printing Techniques. J. Funct. Biomater. 2018, 9, 17. [Google Scholar] [CrossRef] [PubMed]
- Xcentric Mold & Engineering. Injection Molding Material Considerations for the Medical Industry. Available online: https://www.xcentricmold.com/2018/06/18/material-considerations-for-the-medical-industry-utilizing-injection-molding/ (accessed on 8 July 8 2018).
- Kundu, A.; Ausaf, T.; Rajaraman, S. 3D Printing, Ink Casting and Micromachined Lamination (3D PICLμM): A Makerspace Approach to the Fabrication of Biological Microdevices. Micromachines 2018, 9, 85. [Google Scholar] [CrossRef]
- Jager, M.; Wilke, A. Comprehensive biocompatibility testing of a new PMMA-hA bone cement versus conventional PMMA cement in vitro. Biomater. Sci. Polym. Ed. 2003, 14, 1283–1298. [Google Scholar] [CrossRef]
- Frazer, R.Q.; Byron, R.T.; Osborne, P.B.; West, K.P. PMMA: An essential material in medicine and dentistry. J. Long-Term Eff. Med. Implants 2005, 15, 629–639. [Google Scholar] [CrossRef] [PubMed]
- Husar, B.; Hatzenbichler, M. Photopolymerization-based additive manufacturing for the development of 3D porous scaffolds. In Biomaterials for Bone Regeneration: Novel Techniques and Applications, 1st ed.; Dubruel, P., Van Vlierberght, S., Eds.; Woodhead Publishing: Cambridge, UK, 2014; pp. 149–198. ISBN 9780857098047. [Google Scholar]
- Cheng, W.; Wu, D.; Liu, Y. Michael Addition Polymerization of Trifunctional Amine and Acrylic Monomer: A Versatile Platform for Development of Biomaterials. Biomacromolecules 2016, 17, 3115–3126. [Google Scholar] [CrossRef] [PubMed]
- Herrick, D.; Klein, R. Emerging Health and Safety Issues in Makerspaces. In Proceedings of the 1st International Symposium on Academic Makerspaces, Cambridge, MA, USA, 13–16 November 2016; pp. 100–103. [Google Scholar]
- Azimi, P.; Zhao, D.; Pouzet, C.; Crain, N.E.; Stephens, B. Emissions of Ultrafine Particles and Volatile Organic Compounds from Commercially Available Desktop Three-Dimensional Printers with Multiple Filaments. Environ. Sci. Technol. 2016, 50, 1260–1268. [Google Scholar] [CrossRef] [PubMed]
- Linan, L.Z.; Lima, N.M.N.; Benatti, C.; Xavier, M.; Rodrigues, A.A.; Manenti, F.; Jardini, A.; Filho, R.M.; Gilioli, R. Cytotoxicity Assessment of a Poly(methyl methacrylate) Synthesized for the Direct Fabrication of Bone Tissues. Braz. Arch. Biol. Technol. 2018, 61. [Google Scholar] [CrossRef]
- Simpliciano, C.; Clark, L.; Asi, B.; Chu, N.; Mercado, M.; Diaz, S.; Goedert, M.; Mobed-Miremadi, M. Cross-Linked Alginate Film Pore Size Determination Using Atomic Force Microscopy and Validation Using Diffusivity Determinations. J. Surf. Eng. Mater. Adv. Technol. 2013, 3, 1–12. [Google Scholar] [CrossRef]
- International Standard ISO Specification 10993-5 Biological evaluation of medical Devices. In Part 5: Tests for In Vitro Cytotoxicity, 3rd ed.; International Organization for Standardization: Geneva, Switzerland, 2009.
- AccuDyneTest. Available online: https://www.accudynetest.com/polytable_03.html?sortby=contact_angle (accessed on 8 July 2018).
- Baier, R.E. Surface behavior of biomaterials: The theta surface for biocompatibility. J. Mater. Sci. Mater. Med. 2006, 17, 1057–1062. [Google Scholar] [CrossRef]
- Buergers, R.; Rosentritt, M.; Handel, G. Bacterial adhesion of Streptococcus mutans to provisional fixed prosthodontic material. J. Prosthet. Dent. 2007, 98, 461–469. [Google Scholar] [CrossRef]
- Bollen, C.M.; Lambrechts, P.; Quirynen, M. Comparison of surface roughness of oral hard materials to the threshold surface roughness for bacterial plaque retention: A review of the literature. Dent. Mater. 1997, 13, 258–269. [Google Scholar] [CrossRef]
- He, Q.; Chen, H.; Huang, L.; Dong, J.; Guo, D.; Mao, M.; Lei, W. Porous Surface Modified Bioactive Bone Cement for Enhanced Bone Bonding. PLoS ONE 2012, 7, e42525. [Google Scholar] [CrossRef] [PubMed]
- Dall’Oca, C.; Maluta, T.; Cavani, F.; Morbioli, G.P.; Bernardi, P.; Sbarbati, A.; Magnan, B. The Biocompatibility of Porous vs Non-Porous Bone Cements: A New Methodological Approach. Eur. J. Histochem. 2014, 58, 2255. [Google Scholar] [CrossRef] [PubMed]
- Xie, J.; Wang, C.-H. Electrospray in the dripping mode for cell microencapsulation. J. Colloid Interface Sci. 2007, 312, 247–255. [Google Scholar] [CrossRef] [PubMed]
- Håti, A.G.; Bassett, D.C.; Ribe, J.M.; Sikorski, P.; Weitz, D.A.; Stokke, B.T. Versatile, cell and chip friendly method to gel alginate in microfluidic devices. Lab Chip 2016, 16, 3718–3727. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Li, J.; Wang, X.; Li, X.; Kawazoe, N.; Chen, G. Single mammalian cell encapsulation by in situ polymerization. J. Mater. Chem. B 2016, 47, 7662–7668. [Google Scholar] [CrossRef]
- Jing, D.; Parikh, A.; Tzanakakis, E.S. Cardiac Cell Generation from Encapsulated Embryonic Stem Cells in Static and Scalable Culture Systems. Cell Transplant. 2010, 19, 1397–1412. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; He, X. Encapsulation of Living Cells in Small (~100 μm) Alginate Microcapsules by Electrostatic Spraying: A Parametric Study. ASME. J. Biomech. Eng. 2009, 131. [Google Scholar] [CrossRef] [PubMed]
- Wilson, J.L.; Najia, M.A.; Saeed, R.; McDevitt, T.C. Alginate Encapsulation Parameters Influence the Differentiation of Microencapsulated Embryonic Stem Cell Aggregates. Biotechnol. Bioeng. 2014, 111, 618–631. [Google Scholar] [CrossRef] [PubMed]
- Gryshkov, O.; Pogozhykh, D.; Hofmann, N.; Pogozhykh, O.; Mueller, T.; Glasmacher, B. Encapsulating Non-Human Primate Multipotent Stromal Cells in Alginate via High Voltage for Cell-Based Therapies and Cryopreservation. PLoS ONE 2014, 9, e0107911. [Google Scholar] [CrossRef] [PubMed]
- Ouyang, L.; Yao, R.; Chen, X.i.; Na, J.; Sun, W. 3D printing of HEK 293FT cell-laden hydrogel into macroporous constructs with high cell viability and normal biological functions. Biofabrication 2015, 7, 015010. [Google Scholar] [CrossRef] [PubMed]
- Lu, J.; Zhang, X.; Li, J.; Yu, L.; Chen, E.; Zhu, D.; Zhang, Y.; Li, L. A New Fluidized Bed Bioreactor Based on Diversion-Type Microcapsule Suspension for Bioartificial Liver Systems. PLoS ONE 2016, 11, e0147376. [Google Scholar] [CrossRef] [PubMed]
- Malafaya, P.B.; Silva, G.A.; Reis, R.L. Natural–origin Polymers as Carriers and Scaffolds for Biomolecules and Cell Delivery in Tissue Engineering Applications. Adv. Drug Deliv. Rev. 2007, 59, 207–233. [Google Scholar] [CrossRef] [PubMed]
- Duque, R.; Shan, Y.; Joya, M.; Ravichandran, N.; Asi, B.; Mobed-Miremadi, M.; Mulrooney, S.; McNeil, M.; Prakash, S. Effect of artificial cell miniaturization on urea degradation by immobilized E. coli DH5α (pKAU17). Artif. Cells Nanomed. Biotechnol. 2018, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Zheng, X.; Baker, H.; Hancock, W.S.; Fawaz, F.; McCaman, M.; Pungor, E., Jr. Proteomic Analysis for the Assessment of Different Lots of Fetal Bovine Serum as a Raw Material for Cell Culture. Part IV. Application of Proteomics to the manufacture of biological drugs. Biotechnol. Prog. 2006, 22, 1294–1300. [Google Scholar] [CrossRef] [PubMed]
- Peppas, N.A. Physiologically Responsive Hydrogels. J. Bioact. Compat. Polym. 1991, 6, 241–246. [Google Scholar] [CrossRef]
- Denton, A.R.; Tang, Q. Counterion-Induced Swelling of Ionic Microgels. J. Chem. Phys. 2016, 145, 164901. [Google Scholar] [CrossRef] [PubMed]
- Hwang, Y.S.; Cho, J.; Tay, F.; Heng, J.Y.; Ho, R.; Kazarian, S.G.; Williams, D.R.; Boccaccini, A.R.; Polak, J.M.; Mantalaris, A. The use of murine embryonic stem cells, alginate encapsulation, and rotary microgravity bioreactor in bone tissue engineering. Biomaterials 2009, 30, 499–507. [Google Scholar] [CrossRef] [PubMed]
- Chan, E.S.; Lim, T.; Voo, W.; Ravindra, P.; Tey, B.T.; Zhang, Z. Effect of formulation of alginate beads on their mechanical behavior and stiffness. Particuology 2011, 9, 228–234. [Google Scholar] [CrossRef]
- Briscoe, B.J.; Liu, K.K.; Williams, D.R. Adhesive Contact Deformation of a Single Microelastomeric Sphere. J. Colloid Interface Sci. 1998, 200, 256–264. [Google Scholar] [CrossRef]
- Yeatts, A.B.; Gordon, C.N.; Fisher, J.P. Formation of an Aggregated Alginate Construct in a Tubular Perfusion System. Tissue Eng. Part C Methods 2011, 17, 1171–1178. [Google Scholar] [CrossRef] [PubMed]



© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Farias, C.; Lyman, R.; Hemingway, C.; Chau, H.; Mahacek, A.; Bouzos, E.; Mobed-Miremadi, M. Three-Dimensional (3D) Printed Microneedles for Microencapsulated Cell Extrusion. Bioengineering 2018, 5, 59. https://doi.org/10.3390/bioengineering5030059
Farias C, Lyman R, Hemingway C, Chau H, Mahacek A, Bouzos E, Mobed-Miremadi M. Three-Dimensional (3D) Printed Microneedles for Microencapsulated Cell Extrusion. Bioengineering. 2018; 5(3):59. https://doi.org/10.3390/bioengineering5030059
Chicago/Turabian StyleFarias, Chantell, Roman Lyman, Cecilia Hemingway, Huong Chau, Anne Mahacek, Evangelia Bouzos, and Maryam Mobed-Miremadi. 2018. "Three-Dimensional (3D) Printed Microneedles for Microencapsulated Cell Extrusion" Bioengineering 5, no. 3: 59. https://doi.org/10.3390/bioengineering5030059
APA StyleFarias, C., Lyman, R., Hemingway, C., Chau, H., Mahacek, A., Bouzos, E., & Mobed-Miremadi, M. (2018). Three-Dimensional (3D) Printed Microneedles for Microencapsulated Cell Extrusion. Bioengineering, 5(3), 59. https://doi.org/10.3390/bioengineering5030059



