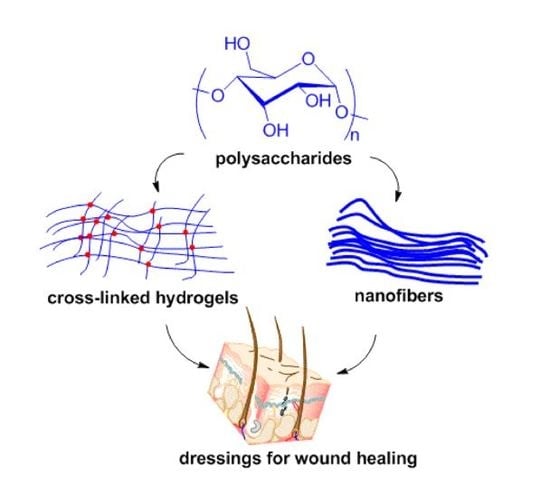
Polysaccharide Fabrication Platforms and Biocompatibility Assessment as Candidate Wound Dressing Materials
Abstract
:1. Introduction
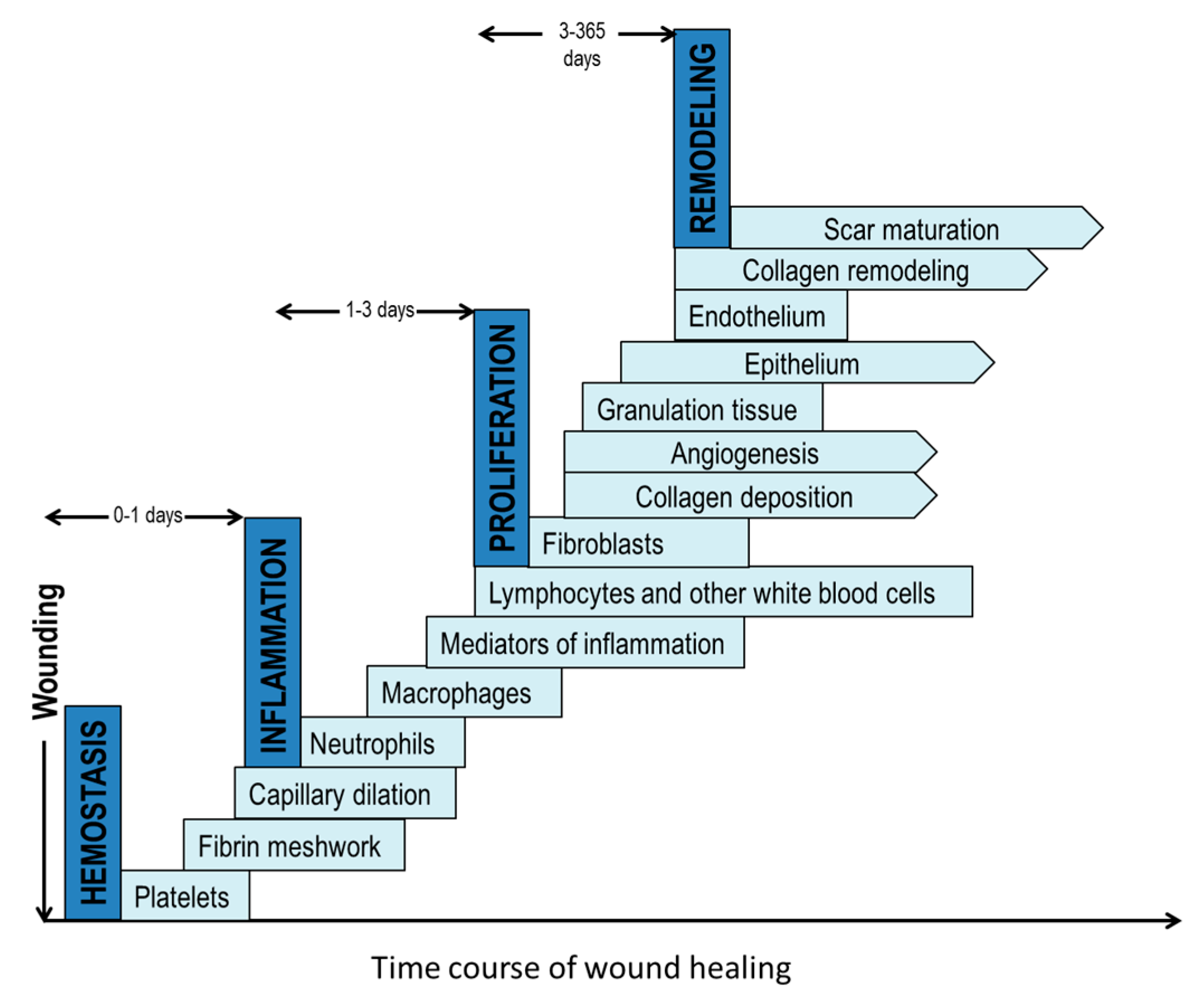
2. The Wound Healing Process
3. Fabrication Platforms of Polysaccharides as Wound Dressing Materials
3.1. Hydrogels
3.1.1. Covalent Crosslinking
3.1.2. Ionic Crosslinking
3.2. Electrospinning
4. Biocompatibility Assessment of Polysaccharides as Wound Dressing Materials
4.1. Cytocompatibility Assessment
4.2. Bioactive Molecule Incorporation and Release
5. Conclusions
Acknowledgments
Conflicts of Interest
References
- Sen, C.K.; Gordillo, G.M.; Roy, S.; Kirsner, R.; Lambert, L.; Hunt, T.K.; Gottrup, F.; Gurtner, G.C.; Longaker, M.T. Human skin wounds: A major and snowballing threat to public health and the economy. Wound Repair Regen. 2009, 17, 763–771. [Google Scholar] [CrossRef] [PubMed]
- Boateng, J.S.; Matthews, K.H.; Stevens, H.N.E.; Eccleston, G.M. Wound healing dressings and drug delivery systems: A review. J. Pharm. Sci. 2008, 97, 2892–2923. [Google Scholar] [CrossRef] [PubMed]
- Thomas, S. Wound Management and Dressings; Pharmaceutical Press: London, UK, 1990. [Google Scholar]
- Sweeney, I.R.; Miraftab, M.; Collyer, G. A critical review of modern and emerging absorbent dressings used to treat exuding wounds. Int. Wound J. 2012, 9, 601–612. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.-P.; Chang, G.-Y.; Chen, J.-K. Electrospun collagen/chitosan nanofibrous membrane as wound dressing. Colloids Surf. A Physicochem. Eng. Asp. 2008, 313, 183–188. [Google Scholar] [CrossRef]
- Noh, H.K.; Lee, S.W.; Kim, J.-M.; Oh, J.-E.; Kim, K.-H.; Chung, C.-P.; Choi, S.-C.; Park, W.H.; Min, B.-M. Electrospinning of chitin nanofibers: Degradation behavior and cellular response to normal human keratinocytes and fibroblasts. Biomaterials 2006, 27, 3934–3944. [Google Scholar] [CrossRef] [PubMed]
- Cai, Z.; Mo, X.; Zhang, K.; Fan, L.; Yin, A.; He, C.; Wang, H. Fabrication of chitosan/silk fibroin composite nanofibers for wound-dressing applications. Int. J. Mol. Sci. 2010, 11, 3529–3539. [Google Scholar] [CrossRef] [PubMed]
- Martin, P. Wound healing—Aiming for perfect skin regeneration. Science 1997, 276, 75–81. [Google Scholar] [CrossRef] [PubMed]
- Petreaca, M.; Martins-Green, M. Cell-ECM interactions in repair and regeneration. In Foundations of Regenerative Medicine; Atala, A., Lanza, R., Thomson, J.A., Nerem, R.M., Eds.; Elsevier: Amsterdam, The Netherlands, 2008. [Google Scholar]
- Cohen, I.K.; Diegelmann, R.F.; Lindblad, W.J.; Hugo, N.E. Wound healing: Biochemical and clinical aspects. Plast. Reconstr. Surg. 1992, 90, 926. [Google Scholar] [CrossRef]
- Black, J.M.; Hawks, J.H.; Keene, A.M. Medical-Surgical Nursing: Clinical Management for Positive Outcomes; WB Saunders Co.: Birmingham, AL, USA, 2001; Volume 1. [Google Scholar]
- Shakespeare, P. Burn wound healing and skin substitutes. Burns 2001, 27, 517–522. [Google Scholar] [CrossRef]
- Davie, E.W.; Fujikawa, K.; Kisiel, W. The coagulation cascade: Initiation, maintenance, and regulation. Biochemistry 1991, 30, 10363–10370. [Google Scholar] [CrossRef] [PubMed]
- Hübner, G.; Hu, Q.; Smola, H.; Werner, S. Strong induction of activin expression after injury suggests an important role of activin in wound repair. Dev. Biol. 1996, 173, 490–498. [Google Scholar] [CrossRef] [PubMed]
- Su, C.-H.; Sun, C.-S.; Juan, S.-W.; Hu, C.-H.; Ke, W.-T.; Sheu, M.-T. Fungal mycelia as the source of chitin and polysaccharides and their applications as skin substitutes. Biomaterials 1997, 18, 1169–1174. [Google Scholar] [CrossRef]
- Senni, K.; Pereira, J.; Gueniche, F.; Delbarre-Ladrat, C.; Sinquin, C.; Ratiskol, J.; Godeau, G.; Fischer, A.-M.; Helley, D.; Colliec-Jouault, S. Marine Polysaccharides: A Source of Bioactive Molecules for Cell Therapy and Tissue Engineering. Mar. Drugs 2011, 9, 1664–1681. [Google Scholar] [CrossRef] [PubMed]
- Kirker, K.R.; Luo, Y.; Nielson, J.H.; Shelby, J.; Prestwich, G.D. Glycosaminoglycan hydrogel films as bio-interactive dressings for wound healing. Biomaterials 2002, 23, 3661–3671. [Google Scholar] [CrossRef]
- Hamidi, M.; Azadi, A.; Rafiei, P. Hydrogel nanoparticles in drug delivery. Adv. Drug Deliv. Rev. 2008, 60, 1638–1649. [Google Scholar] [CrossRef] [PubMed]
- Khan, F.; Tare, R.S.; Oreffo, R.; Bradley, M. Versatile biocompatible polymer hydrogels: Scaffolds for cell growth. Angew. Chem. Int. Ed. 2009, 48, 978–982. [Google Scholar] [CrossRef] [PubMed]
- Ladet, S.; David, L.; Domard, A. Multi-membrane hydrogels. Nature 2008, 452, 76–79. [Google Scholar] [CrossRef] [PubMed]
- Denkbas, E.B.; Ottenbrite, R.M. Perspectives on: Chitosan drug delivery systems based on their geometries. J. Bioact. Compat. Polym. 2006, 21, 351–368. [Google Scholar] [CrossRef]
- Lou, X.; Dalton, P.D.; Chirila, T.V. Hydrophilic sponges based on 2-hydroxyethyl methacrylate Part VII: Modulation of sponge characteristics by changes in reactivity and hydrophilicity of crosslinking agents. J. Mater. Sci. Mater. Med. 2000, 11, 319–325. [Google Scholar] [CrossRef] [PubMed]
- Lou, X.; Chirila, T.V. Swelling behavior and mechanical properties of chemically cross-linked gelatin gels for biomedical use. J. Biomater. Appl. 1999, 14, 184–191. [Google Scholar] [PubMed]
- Cruise, G.M.; Scharp, D.S.; Hubbell, J.A. Characterization of permeability and network structure of interfacially photopolymerized poly (ethylene glycol) diacrylate hydrogels. Biomaterials 1998, 19, 1287–1294. [Google Scholar] [CrossRef]
- Lin, C.-C.; Metters, A.T. Hydrogels in controlled release formulations: Network design and mathematical modeling. Adv. Drug Deliv. Rev. 2006, 58, 1379–1408. [Google Scholar] [CrossRef] [PubMed]
- Berger, J.; Reist, M.; Mayer, J.M.; Felt, O.; Peppas, N.A.; Gurny, R. Structure and interactions in covalently and ionically crosslinked chitosan hydrogels for biomedical applications. Eur. J. Pharm. Biopharm. 2004, 57, 19–34. [Google Scholar] [CrossRef]
- Schepetkin, I.A.; Quinn, M.T. Botanical polysaccharides: Macrophage immunomodulation and therapeutic potential. Int. Immunopharmacol. 2006, 6, 317–333. [Google Scholar] [CrossRef] [PubMed]
- Coviello, T.; Matricardi, P.; Marianecci, C.; Alhaique, F. Polysaccharide hydrogels for modified release formulations. J. Control. Release 2007, 119, 5–24. [Google Scholar] [CrossRef] [PubMed]
- Mogoşanu, G.D.; Grumezescu, A.M. Natural and synthetic polymers for wounds and burns dressing. Int. J. Pharm. 2014, 463, 127–136. [Google Scholar] [CrossRef] [PubMed]
- Bhattarai, N.; Gunn, J.; Zhang, M. Chitosan-based hydrogels for controlled, localized drug delivery. Adv. Drug Deliv. Rev. 2010, 62, 83–99. [Google Scholar] [CrossRef] [PubMed]
- Longinotti, C. The use of hyaluronic acid based dressings to treat burns: A review. Burn. Trauma 2014, 2, 162–168. [Google Scholar] [CrossRef] [PubMed]
- Debels, H.; Hamdi, M.; Abberton, K.; Morrison, W. Dermal matrices and bioengineered skin substitutes. Plast. Reconstr. Surg. Glob. Open 2015, 3, e284. [Google Scholar] [CrossRef] [PubMed]
- Voinchet, V.; Vasseur, P.; Kern, J. Efficacy and safety of hyaluronic acid in the management of acute wounds. Am. J. Clin. Dermatol. 2006, 7, 353–357. [Google Scholar] [CrossRef] [PubMed]
- Tomihata, K.; Ikada, Y. Crosslinking of hyaluronic acid with water-soluble carbodiimide. J. Biomed. Mater. Res. 1997, 37, 243–251. [Google Scholar] [CrossRef]
- Luo, Y.; Kirker, K.R.; Prestwich, G.D. Cross-linked hyaluronic acid hydrogel films: New biomaterials for drug delivery. J. Control. Release 2000, 69, 169–184. [Google Scholar] [CrossRef]
- Baier Leach, J.; Bivens, K.A.; Patrick, C.W., Jr.; Schmidt, C.E. Photocrosslinked hyaluronic acid hydrogels: Natural, biodegradable tissue engineering scaffolds. Biotechnol. Bioeng. 2003, 82, 578–589. [Google Scholar] [CrossRef] [PubMed]
- Burdick, J.A.; Prestwich, G.D. Hyaluronic acid hydrogels for biomedical applications. Adv. Mater. 2011, 23, H41–H56. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Yang, J.; Hu, X.; Liang, J.; Fan, Y.; Zhang, X. Superabsorbent polysaccharide hydrogels based on pullulan derivate as antibacterial release wound dressing. J. Biomed. Mater. Res. Part A 2011, 98A, 31–39. [Google Scholar] [CrossRef] [PubMed]
- Lazaridou, A.; Biliaderis, C.G.; Kontogiorgos, V. Molecular weight effects on solution rheology of pullulan and mechanical properties of its films. Carbohydr. Polym. 2003, 52, 151–166. [Google Scholar] [CrossRef]
- Jiang, Q.; Zhou, W.; Wang, J.; Tang, R.; Zhang, D.; Wang, X. Hypromellose succinate-crosslinked chitosan hydrogel films for potential wound dressing. Int. J. Biol. Macromol. 2016, 91, 85–91. [Google Scholar] [CrossRef] [PubMed]
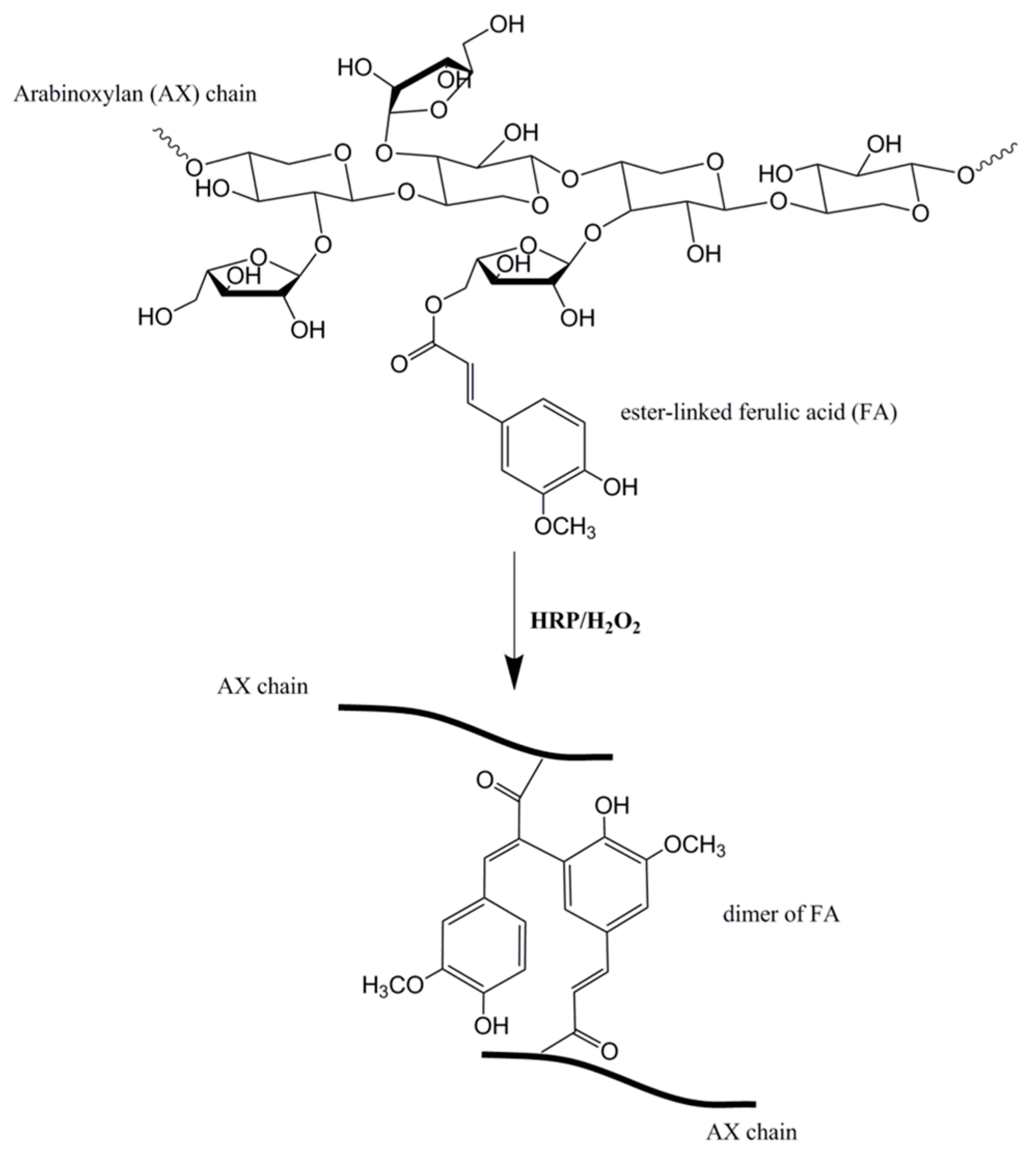
- Carvajal-Millan, E.; Guilbert, S.; Doublier, J.-L.; Micard, V. Arabinoxylan/protein gels: Structural, rheological and controlled release properties. Food Hydrocoll. 2006, 20, 53–61. [Google Scholar] [CrossRef]
- Niño-Medina, G.; Carvajal-Millán, E.; Rascon-Chu, A.; Marquez-Escalante, J.A.; Guerrero, V.; Salas-Muñoz, E. Feruloylated arabinoxylans and arabinoxylan gels: Structure, sources and applications. Phytochem. Rev. 2010, 9, 111–120. [Google Scholar] [CrossRef]
- Iravani, S.; Fitchett, C.S.; Georget, D.M.R. Physical characterization of arabinoxylan powder and its hydrogel containing a methyl xanthine. Carbohydr. Polym. 2011, 85, 201–207. [Google Scholar] [CrossRef]
- Aduba, D.C.; An, S.-S.; Selders, G.S.; Wang, J.; Yeudall, W.A.; Bowlin, G.L.; Kitten, T.; Yang, H. Fabrication, characterization and in vitro evaluation of silver-containing arabinoxylan foams as antimicrobial wound dressing. J. Biomed. Mater. Res. A 2016, 104, 2456–2465. [Google Scholar] [CrossRef] [PubMed]
- Draget, K.I.; Värum, K.M.; Moen, E.; Gynnild, H.; Smidsrød, O. Chitosan cross-linked with Mo(VI) polyoxyanions: A new gelling system. Biomaterials 1992, 13, 635–638. [Google Scholar] [CrossRef]
- Brack, H.P.; Tirmizi, S.A.; Risen, W.M. A spectroscopic and viscometric study of the metal ion-induced gelation of the biopolymer chitosan. Polymer 1997, 38, 2351–2362. [Google Scholar] [CrossRef]
- Chenite, A.; Chaput, C.; Combes, C.; Selmani, A.; Jalal, F. Temperature-Controlled pH-Dependent Formation of Ionic Polysaccharide Gels. U.S. Patent 6,344,488 B1, 5 February 2002. [Google Scholar]
- Jones, V.; Grey, J.E.; Harding, K.G. Wound dressings. BMJ 2006, 332, 777–780. [Google Scholar] [CrossRef] [PubMed]
- Miraftab, M.; Qiao, Q.; Kennedy, J.F.; Anand, S.C.; Groocock, M.R. Fibres for wound dressings based on mixed carbohydrate polymer fibers. Carbohydr. Polym. 2003, 53, 225–231. [Google Scholar] [CrossRef]
- Thomas, A.; Harding, K.G.; Moore, K. Alginates from wound dressings activate human macrophages to secrete tumour necrosis factor-α. Biomaterials 2000, 21, 1797–1802. [Google Scholar] [CrossRef]
- Noda, S.; Funami, T.; Nakauma, M.; Asai, I.; Takahashi, R.; Al-Assaf, S.; Ikeda, S.; Nishinari, K.; Phillips, G.O. Molecular structures of gellan gum imaged with atomic force microscopy in relation to the rheological behavior in aqueous systems. 1. Gellan gum with various acyl contents in the presence and absence of potassium. Food Hydrocoll. 2008, 22, 1148–1159. [Google Scholar] [CrossRef]
- Kubo, W.; Miyazaki, S.; Attwood, D. Oral sustained delivery of paracetamol from in situ-gelling gellan and sodium alginate formulations. Int. J. Pharm. 2003, 258, 55–64. [Google Scholar] [CrossRef]
- Rozier, A.; Mazuel, C.; Grove, J.; Plazonnet, B. Functionality testing of gellan gum, a polymeric excipient material for ophthalmic dosage forms. Int. J. Pharm. 1997, 153, 191–198. [Google Scholar] [CrossRef]
- Jansson, B.; Hägerström, H.; Fransén, N.; Edsman, K.; Björk, E. The influence of gellan gum on the transfer of fluorescein dextran across rat nasal epithelium in vivo. Eur. J. Pharm. Biopharm. 2005, 59, 557–564. [Google Scholar] [CrossRef] [PubMed]
- Cencetti, C.; Bellini, D.; Pavesio, A.; Senigaglia, D.; Passariello, C.; Virga, A.; Matricardi, P. Preparation and characterization of antimicrobial wound dressings based on silver, gellan, PVA and borax. Carbohydr. Polym. 2012, 90, 1362–1370. [Google Scholar] [CrossRef] [PubMed]
- Anton, F. Process and Apparatus for Preparing Artificial Threads. U.S. Patent 1975504, 2 October 1934. [Google Scholar]
- Zahedi, P.; Rezaeian, I.; Ranaei-Siadat, S.; Jafari, S.; Supaphol, P. A review on wound dressings with an emphasis on electrospun nanofibrous polymeric bandages. Polym. Adv. Technol. 2010, 21, 77–95. [Google Scholar] [CrossRef]
- Dabney, S.E. The Use of Electrospinning Technology to Produce Wound Dressings; University of Akron: Akron, OH, USA, 2002. [Google Scholar]
- Schiffman, J.D.; Schauer, C.L. A review: Electrospinning of biopolymer nanofibers and their applications. Polym. Rev. 2008, 48, 317–352. [Google Scholar] [CrossRef]
- McKee, M.G.; Wilkes, G.L.; Colby, R.H.; Long, T.E. Correlations of Solution Rheology with Electrospun Fiber Formation of Linear and Branched Polyesters. Macromolecules 2004, 37, 1760–1767. [Google Scholar] [CrossRef]
- McKee, M.G.; Hunley, M.T.; Layman, J.M.; Long, T.E. Solution rheological behavior and electrospinning of cationic polyelectrolytes. Macromolecules 2006, 39, 575–583. [Google Scholar] [CrossRef]
- Fridrikh, S.V.; Yu, J.H.; Brenner, M.P.; Rutledge, G.C. Controlling the fiber diameter during electrospinning. Phys. Rev. Lett. 2003, 90, 144502. [Google Scholar] [CrossRef] [PubMed]
- Shenoy, S.L.; Bates, W.D.; Frisch, H.L.; Wnek, G.E. Role of chain entanglements on fiber formation during electrospinning of polymer solutions: Good solvent, non-specific polymer–polymer interaction limit. Polymer 2005, 46, 3372–3384. [Google Scholar] [CrossRef]
- Greiner, A.; Wendorff, J.H. Electrospinning: A fascinating method for the preparation of ultrathin fibers. Angew. Chemie Int. Ed. 2007, 46, 5670–5703. [Google Scholar] [CrossRef] [PubMed]
- Reneker, D.H.; Yarin, A.L. Electrospinning jets and polymer nanofibers. Polymer (Guildf) 2008, 49, 2387–2425. [Google Scholar] [CrossRef]
- Lee, K.Y.; Jeong, L.; Kang, Y.O.; Lee, S.J.; Park, W.H. Electrospinning of polysaccharides for regenerative medicine. Adv. Drug Deliv. Rev. 2009, 61, 1020–1032. [Google Scholar] [CrossRef] [PubMed]
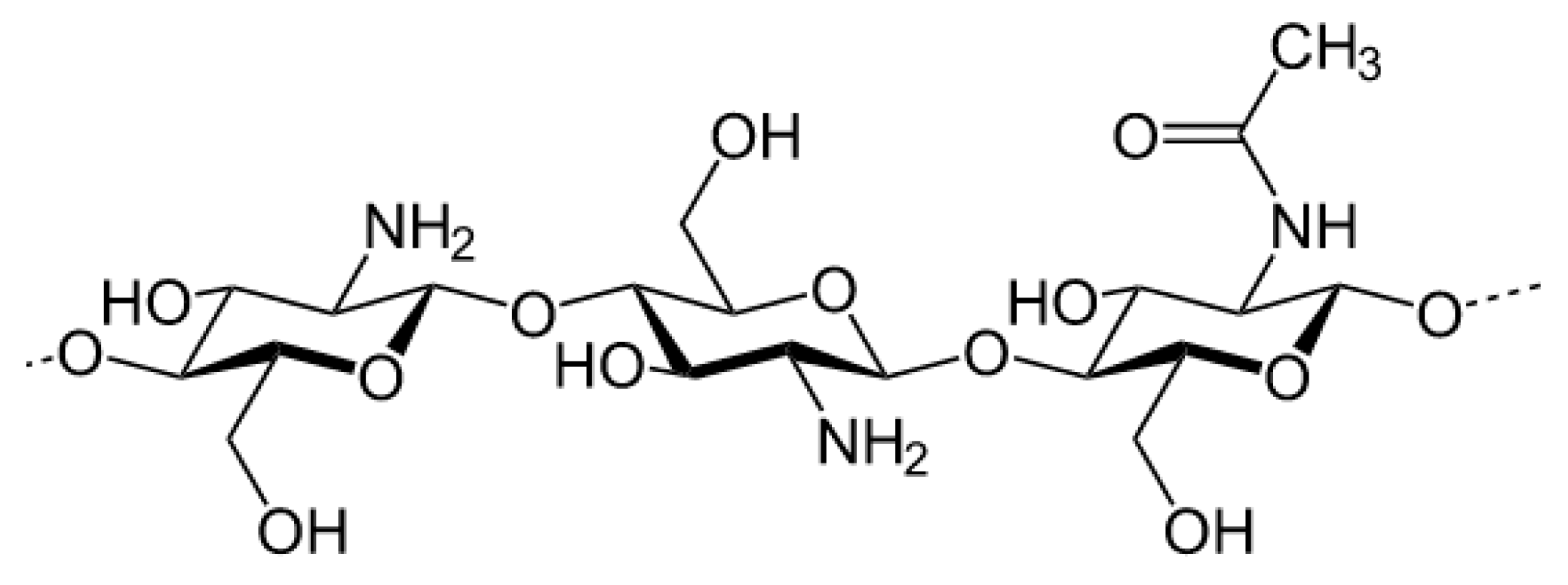
- Rinaudo, M. Chitin and chitosan: Properties and applications. Prog. Polym. Sci. 2006, 31, 603–632. [Google Scholar] [CrossRef]
- Rinaudo, M.; Pavlov, G.; Desbrières, J. Solubilization of chitosan in strong acid medium. Int. J. Polym. Anal. Charact. 1999, 5, 267–276. [Google Scholar] [CrossRef]
- Rinaudo, M.; Pavlov, G.; Desbrières, J. Influence of acetic acid concentration on the solubilization of chitosan. Polymer (Guildf) 1999, 40, 7029–7032. [Google Scholar] [CrossRef]
- Ohkawa, K.; Cha, D.; Kim, H.; Nishida, A.; Yamamoto, H. Electrospinning of chitosan. Macromol. Rapid Commun. 2004, 25, 1600–1605. [Google Scholar] [CrossRef]
- Lee, S.J.; Heo, D.N.; Moon, J.-H.; Ko, W.-K.; Lee, J.B.; Bae, M.S.; Park, S.W.; Kim, J.E.; Lee, D.H.; Kim, E.-C.; et al. Electrospun chitosan nanofibers with controlled levels of silver nanoparticles. Preparation, characterization and antibacterial activity. Carbohydr. Polym. 2014, 111, 530–537. [Google Scholar] [CrossRef] [PubMed]
- Spasova, M.; Manolova, N.; Paneva, D.; Rashkov, I. Preparation of chitosan-containing nanofibres by electrospinning of chitosan/poly (ethylene oxide) blend solutions. e-Polymers 2004, 4, 624–635. [Google Scholar] [CrossRef]
- Geng, X.; Kwon, O.-H.; Jang, J. Electrospinning of chitosan dissolved in concentrated acetic acid solution. Biomaterials 2005, 26, 5427–5432. [Google Scholar] [CrossRef] [PubMed]
- Roberts, G.A.F.; Domszy, J.G. Determination of the Viscometric Constants for Chitosan; Elsevier: Amsterdam, The Netherlands, 1982; Volume 4. [Google Scholar]
- Klossner, R.R.; Queen, H.A.; Coughlin, A.J.; Krause, W.E. Correlation of Chitosan’s Rheological Properties and Its Ability to Electrospin. Biomacromolecules 2008, 9, 2947–2953. [Google Scholar] [CrossRef] [PubMed]
- Shalumon, K.T.; Anulekha, K.H.; Nair, S.V.; Nair, S.V.; Chennazhi, K.P.; Jayakumar, R. Sodium alginate/poly (vinyl alcohol)/nano ZnO composite nanofibers for antibacterial wound dressings. Int. J. Biol. Macromol. 2011, 49, 247–254. [Google Scholar] [CrossRef] [PubMed]
- Bonino, C.A.; Krebs, M.D.; Saquing, C.D.; Jeong, S.I.; Shearer, K.L.; Alsberg, E.; Khan, S.A. Electrospinning alginate-based nanofibers: From blends to crosslinked low molecular weight alginate-only systems. Carbohydr. Polym. 2011, 85, 111–119. [Google Scholar] [CrossRef]
- Lu, J.-W.; Zhu, Y.-L.; Guo, Z.-X.; Hu, P.; Yu, J. Electrospinning of sodium alginate with poly (ethylene oxide). Polymer 2006, 47, 8026–8031. [Google Scholar] [CrossRef]
- Safi, S.; Morshed, M.; Hosseini Ravandi, S.A.; Ghiaci, M. Study of electrospinning of sodium alginate, blended solutions of sodium alginate/poly (vinyl alcohol) and sodium alginate/poly (ethylene oxide). J. Appl. Polym. Sci. 2007, 104, 3245–3255. [Google Scholar] [CrossRef]
- Li, J.; He, A.; Han, C.C.; Fang, D.; Hsiao, B.S.; Chu, B. Electrospinning of hyaluronic acid (HA) and HA/gelatin blends. Macromol. Rapid Commun. 2006, 27, 114–120. [Google Scholar] [CrossRef]
- Raoufi, M.; Aslankoohi, N.; Mollenhauer, C.; Boehm, H.; Spatz, J.P.; Brüggemann, D. Template-assisted extrusion of biopolymer nanofibers under physiological conditions. Integr. Biol. 2016, 8, 1059–1066. [Google Scholar] [CrossRef] [PubMed]
- Mero, A.; Campisi, M. Hyaluronic Acid Bioconjugates for the Delivery of Bioactive Molecules. Polymers (Basel) 2014, 6, 346–369. [Google Scholar] [CrossRef]
- Ramos-Torrecillas, J.; De Luna-Bertos, E.; Diaz-Rodriguez, L.; Garcia-Martinez, O.; Rodriguez-Perez, L.; Ruiz, C. Hyaluronic acid as a treatment option for pressure ulcers. Wounds 2013, 25, 328–332. [Google Scholar] [PubMed]
- Campoccia, D.; Doherty, P.; Radice, M.; Brun, P.; Abatangelo, G.; Williams, D.F. Semisynthetic resorbable materials from hyaluronan esterification. Biomaterials 1998, 19, 2101–2127. [Google Scholar] [CrossRef]
- Iocono, J.A.; Krummel, T.M.; Keefer, K.A.; Allison, G.M.; Ehrlich, H.P. Repeated additions of hyaluronan alters granulation tissue deposition in sponge implants in mice. Wound Repair Regen. 1998, 6, 442–448. [Google Scholar] [CrossRef] [PubMed]
- Ji, Y.; Liang, K.; Shen, X.; Bowlin, G.L. Electrospinning and characterization of chitin nanofibril/polycaprolactone nanocomposite fiber mats. Carbohydr. Polym. 2014, 101, 68–74. [Google Scholar] [CrossRef] [PubMed]
- Xia, G.; Lang, X.; Kong, M.; Cheng, X.; Liu, Y.; Feng, C.; Chen, X. Surface fluid-swellable chitosan fiber as the wound dressing material. Carbohydr. Polym. 2016, 136, 860–866. [Google Scholar] [CrossRef] [PubMed]
- He, P.; Davis, S.S.; Illum, L. In vitro evaluation of the mucoadhesive properties of chitosan microspheres. Int. J. Pharm. 1998, 166, 75–88. [Google Scholar] [CrossRef]
- Ueno, H.; Mori, T.; Fujinaga, T. Topical formulations and wound healing applications of chitosan. Adv. Drug Deliv. Rev. 2001, 52, 105–115. [Google Scholar] [CrossRef]
- Felt, O.; Carrel, A.; Baehni, P.; Buri, P.; Gurny, R. Chitosan as tear substitute: A wetting agent endowed with antimicrobial efficacy. J. Ocul. Pharmacol. Ther. 2000, 16, 261–270. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Guan, Y.; Yang, D.; Li, Z.; Yao, K. Antibacterial action of chitosan and carboxymethylated chitosan. J. Appl. Polym. Sci. 2001, 79, 1324–1335. [Google Scholar]
- Tashiro, T. Antibacterial and Bacterium Adsorbing Macromolecules. Macromol. Mater. Eng. 2001, 286, 63–87. [Google Scholar] [CrossRef]
- Shigemasa, Y.; Minami, S. Applications of chitin and chitosan for biomaterials. Biotechnol. Genet. Eng. Rev. 1996, 13, 383–420. [Google Scholar] [CrossRef] [PubMed]
- Rao, S.B.; Sharma, C.P. Use of chitosan as a biomaterial: Studies on its safety and hemostatic potential. J. Biomed. Mater. Res. 1997, 34, 21–28. [Google Scholar] [CrossRef]
- Ishihara, M.; Nakanishi, K.; Ono, K.; Sato, M.; Kikuchi, M.; Saito, Y.; Yura, H.; Matsui, T.; Hattori, H.; Uenoyama, M. Photocrosslinkable chitosan as a dressing for wound occlusion and accelerator in healing process. Biomaterials 2002, 23, 833–840. [Google Scholar] [CrossRef]
- Park, C.J.; Clark, S.G.; Lichtensteiger, C.A.; Jamison, R.D.; Johnson, A.J.W. Accelerated wound closure of pressure ulcers in aged mice by chitosan scaffolds with and without bFGF. Acta Biomater. 2009, 5, 1926–1936. [Google Scholar] [CrossRef] [PubMed]
- Feng, Z.-Q.; Chu, X.; Huang, N.-P.; Wang, T.; Wang, Y.; Shi, X.; Ding, Y.; Gu, Z.-Z. The effect of nanofibrous galactosylated chitosan scaffolds on the formation of rat primary hepatocyte aggregates and the maintenance of liver function. Biomaterials 2009, 30, 2753–2763. [Google Scholar] [CrossRef] [PubMed]
- Vashisth, P.; Srivastava, A.K.; Nagar, H.; Raghuwanshi, N.; Sharan, S.; Nikhil, K.; Pruthi, P.A.; Singh, R.P.; Roy, P.; Pruthi, V. Drug functionalized microbial polysaccharide based nanofibers as transdermal substitute. Nanomed. Nanotechnol. Biol. Med. 2016, 12, 1375–1385. [Google Scholar] [CrossRef] [PubMed]
- Sun, G.; Zhang, X.; Shen, Y.I.; Sebastian, R.; Dickinson, L.E.; Fox-Talbot, K.; Reinblatt, M.; Steenbergen, C.; Harmon, J.W.; Gerecht, S. Dextran hydrogel scaffolds enhance angiogenic responses and promote complete skin regeneration during burn wound healing. Proc. Natl. Acad. Sci. USA 2011, 108, 20976–20981. [Google Scholar] [CrossRef] [PubMed]
- Jung, W.K.; Koo, H.C.; Kim, K.W.; Shin, S.; Kim, S.H.; Park, Y.H. Antibacterial activity and mechanism of action of the silver ion in Staphylococcus aureus and Escherichia coli. Appl. Environ. Microbiol. 2008, 74, 2171–2178. [Google Scholar] [CrossRef] [PubMed]
- Dongargaonkar, A.A.; Bowlin, G.L.; Yang, H. Electrospun blends of gelatin and gelatin-dendrimer conjugates as a wound-dressing and drug-delivery platform. Biomacromolecules 2013, 14, 4038–4045. [Google Scholar] [CrossRef] [PubMed]
- Sudheesh Kumar, P.T.; Lakshmanan, V.-K.; Anilkumar, T.V.; Ramya, C.; Reshmi, P.; Unnikrishnan, A.G.; Nair, S.V.; Jayakumar, R. Flexible and microporous chitosan hydrogel/nano ZnO composite bandages for wound dressing: In vitro and in vivo evaluation. ACS Appl. Mater. Interfaces 2012, 4, 2618–2629. [Google Scholar] [CrossRef] [PubMed]
- Miraftab, M.; Masood, R.; Edward-Jones, V. Development and Antimicrobial Assessment of a Novel Hybrid Biomaterial Fibre Intended for Wound Dressing Applications. In Proceedings of the International Conference on Material Science and Material Engineering, San Antonio, TX, USA, 6–8 October 2014.
- Ito, T.; Fraser, I.P.; Yeo, Y.; Highley, C.B.; Bellas, E.; Kohane, D.S. Anti-inflammatory function of an in situ cross-linkable conjugate hydrogel of hyaluronic acid and dexamethasone. Biomaterials 2007, 28, 1778–1786. [Google Scholar] [CrossRef] [PubMed]
- Gianolio, D.A.; Philbrook, M.; Avila, L.Z.; MacGregor, H.; Duan, S.X.; Bernasconi, R.; Slavsky, M.; Dethlefsen, S.; Jarrett, P.K.; Miller, R.J. Synthesis and evaluation of hydrolyzable hyaluronan-tethered bupivacaine delivery systems. Bioconj. Chem. 2005, 16, 1512–1518. [Google Scholar] [CrossRef] [PubMed]
- Bhatia, M.; Ahuja, M. Psyllium arabinoxylan: Carboxymethylation, characterization and evaluation for nanoparticulate drug delivery. Int. J. Biol. Macromol. 2015, 72, 495–501. [Google Scholar] [CrossRef] [PubMed]
- De Cicco, F.; Russo, P.; Reverchon, E.; García-González, C.A.; Aquino, R.P.; del Gaudio, P. Prilling and supercritical drying: A successful duo to produce core-shell polysaccharide aerogel beads for wound healing. Carbohydr. Polym. 2016, 147, 482–489. [Google Scholar] [CrossRef] [PubMed]
- Shimizu, N.; Ishida, D.; Yamamoto, A.; Kuroyanagi, M.; Kuroyanagi, Y. Development of a functional wound dressing composed of hyaluronic acid spongy sheet containing bioactive components: Evaluation of wound healing potential in animal tests. J. Biomater. Sci. Polym. Ed. 2014, 25, 1278–1291. [Google Scholar] [CrossRef] [PubMed]
- Matsumoto, Y.; Kuroyanagi, Y. Development of a wound dressing composed of hyaluronic acid sponge containing arginine and epidermal growth factor. J. Biomater. Sci. Polym. Ed. 2010, 21, 715–726. [Google Scholar] [CrossRef] [PubMed]
- Naseri, N.; Algan, C.; Jacobs, V.; John, M.; Oksman, K.; Mathew, A.P. Electrospun chitosan-based nanocomposite mats reinforced with chitin nanocrystals for wound dressing. Carbohydr. Polym. 2014, 109, 7–15. [Google Scholar] [CrossRef] [PubMed]
- Laurienzo, P. Marine polysaccharides in pharmaceutical applications: An overview. Mar. Drugs 2010, 8, 2435–2465. [Google Scholar] [CrossRef] [PubMed]
- Karaki, N.; Aljawish, A.; Humeau, C.; Muniglia, L.; Jasniewski, J. Enzymatic modification of polysaccharides: Mechanisms, properties, and potential applications: A review. Enzym. Microb. Technol. 2016, 90, 1–18. [Google Scholar] [CrossRef] [PubMed]
- Czaja, W.K.; Young, D.J.; Kawecki, M.; Brown, R.M. The future prospects of microbial cellulose in biomedical applications. Biomacromolecules 2007, 8, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Pescosolido, L.; Schuurman, W.; Malda, J.; Matricardi, P.; Alhaique, F.; Coviello, T.; van Weeren, P.R.; Dhert, W.J.A.; Hennink, W.E.; Vermonden, T. Hyaluronic acid and dextran-based semi-IPN hydrogels as biomaterials for bioprinting. Biomacromolecules 2011, 12, 1831–1838. [Google Scholar] [CrossRef] [PubMed]
- Axpe, E.; Oyen, M. Applications of alginate-based bioinks in 3D bioprinting. Int. J. Mol. Sci. 2016, 17, 1976. [Google Scholar] [CrossRef] [PubMed]
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Aduba, D.C., Jr.; Yang, H. Polysaccharide Fabrication Platforms and Biocompatibility Assessment as Candidate Wound Dressing Materials. Bioengineering 2017, 4, 1. https://doi.org/10.3390/bioengineering4010001
Aduba DC Jr., Yang H. Polysaccharide Fabrication Platforms and Biocompatibility Assessment as Candidate Wound Dressing Materials. Bioengineering. 2017; 4(1):1. https://doi.org/10.3390/bioengineering4010001
Chicago/Turabian StyleAduba, Donald C., Jr., and Hu Yang. 2017. "Polysaccharide Fabrication Platforms and Biocompatibility Assessment as Candidate Wound Dressing Materials" Bioengineering 4, no. 1: 1. https://doi.org/10.3390/bioengineering4010001
APA StyleAduba, D. C., Jr., & Yang, H. (2017). Polysaccharide Fabrication Platforms and Biocompatibility Assessment as Candidate Wound Dressing Materials. Bioengineering, 4(1), 1. https://doi.org/10.3390/bioengineering4010001