Abstract
Crude glycerol is generated as a by-product during transesterification process and during hydrolysis of fat in the soap-manufacturing process, and poses a problem for waste management. In the present approach, an efficient process was designed for simultaneous production of 0.2 g/L extracellular ε-polylysine and 64.6% (w/w) intracellular polyhydroxyalkanoate (PHA) in the same fermentation broth (1 L shake flask) utilizing Jatropha biodiesel waste residues as carbon rich source by marine bacterial strain (Bacillus licheniformis PL26), isolated from west coast of India. The synthesized ε-polylysine and polyhydroxyalkanoate PHA by Bacillus licheniformis PL26 was characterized by thermogravimetric analysis (TGA), differential scanning colorimetry (DSC), Fourier transform infrared spectroscopy (FTIR), and 1H Nuclear magnetic resonance spectroscopy (NMR). The PHA produced by Bacillus licheniformis was found to be poly-3-hydroxybutyrate-co-3-hydroxyvalerate (P3HB-co-3HV). The developed process needs to be statistically optimized further for gaining still better yield of both the products in an efficient manner.
1. Introduction
Most of the global economy is driven by petroleum fuels as the main source of energy. However, due to market fluctuation, it is moving towards a sustainable bio based economy as fossil reserves are projected to decline completely by 2050 [1,2,3]. In addition to over exploitation of petroleum deposits, climate change and other negative environmental effects from exhaust gases lead researchers for the search of renewable alternatives such as biodiesel [4]. Biodiesel is an appealing alternative [5,6], which is clean burning, non-toxic and biodegradable [7]. It is a fatty acid methyl ester compound produced by a transesterification process of animal or plant oils with methanol in the presence of a catalyst [8,9,10]. Generally, glycerol is obtained in huge amount as a by-product in production of biodiesel [11,12]. With every 100 lbs of biodiesel produced by transesterification of vegetable oils or animal fats, 10 lbs of crude glycerol is generated [13,14]. However, the tremendous growth of the biodiesel industry has created a glycerol surplus that resulted in a dramatic 10-fold decrease in crude glycerol prices over the last few years. This decrease in prices resulted a problem for the glycerol producing and refining industries and also the economic viability of the biodiesel industry has also been greatly affected [15,16].
For sustainable development and commercialization of biodiesel production, effective utilization of crude glycerol into value added products are desired. However, conversion of crude glycerol will also promote the accretion of integrated biorefineries. 1,3-propanediol, citric acid, PHA’s, ε-polylysine, butanol, hydrogen, ethanol, phytase, lipase, succinic acid, docosahexaenoic acid, eicosapentanoic acid, monoglycerides, lipids and syngas can be produced from crude glycerol through microbial strains [17,18,19,20,21,22,23,24,25,26,27,28,29,30,31]. However, many of the available technologies need further optimization and development in the form of efficient and sustainable form for its incorporation in bio-refineries.
The utilization of low quality glycerol obtained as by-product of biodiesel production is a big challenge as this glycerol cannot be used for direct food and cosmetic uses. An effective usage for conversion of crude glycerol to specific products may cut down the biodiesel production costs. The process for biodiesel preparation and generating value added products simultaneously through the use of waste generated during the biodiesel production is a very effective approach [32]. Clearly, the development of processes to convert crude glycerol into higher-value products is an urgent need.
Various studies on tuning the material properties of the polyhydroxyalkanoate (PHA) polymer are carried out for its higher applicability in diverse areas [33]. Nerve 2010 reported production of ε-polylysine from Streptomyces albulus (CCRC 11814) utilizing crude glycerol as carbon source through aerobic fermentation yielding 0.2 g/L ε-polylysine. However, growth rate of Streptomyces albulus (CCRC 11814) was slow due to other impurities such as methanol and other salts present in crude glycerol.
ε-polylysine and polyhydroxyalkanoates are important biopolymers which can be conjugated with other biopolymers for its various applications e.g., ε-polylysine may be utilized as water absorbable hydrogels, drug carriers and anticancer agents. Simultaneously, PHA may be used in drug delivery systems, atrial septal defect repair and cardiovascular stents. ε-polylysine and polyhydroxyalkanoates are important biopolymers which are synthesized through microbe in an efficient and eco-friendly manner. However, these biopolymers may be conjugated with other biopolymers for its application as water absorbable hydrogels, drug carriers and anticancer agents. The hydrogels prepared from these complex biopolymers may be used for its application in quick peritoneal repair and prevention of post-surgical intraabdominal adhesions.
In the present study, for sustainable development of biodiesel production, efforts have been made for effective utilization of crude glycerol as the carbon source for simultaneous production of ε-polylysine and PHA. The present bioconversion route will effectively convert the waste stream of biodiesel production into value added products. In addition, metabolic engineering may used in future for improving product yield of such strains.
2. Materials and Methods
2.1. Materials
2.1.1. Chemicals
Peptone, yeast extract, iron(III) citrate, NaCl, MgCl2, Na2SO4, CaCl2, KCl, NaHCO3, KBr, SrCl2, H3BO3, Na2O3Si, NaF, (NH4)(NO3) and Na2HPO4 were purchased from M/S Hi-Media Limited Mumbai, and were of the highest purity available. ε-PL was procured from Handary SA, Brussels, Belgium, Polyhydroxybutyrate and 3-hydroxyvalerate from Sigma, Bangalore, India.
2.1.2. Growth and Maintenance of Bacillus licheniformis
Bacillus licheniformis was isolated from sea brine of experimental salt farm, Bhavnagar, India. It was maintained on Zobell marine agar plates containing (g/L) peptone 5.0; yeast extract 1.0; iron(III) citrate 0.1; NaCl 19.45; MgCl2 8.8; Na2SO4 3.24; CaCl2 1.8; KCl 0.55; NaHCO3 0.16; KBr 0.08; SrCl2 0.034; H3BO3 0.022; Na2O3Si 0.004; NaF 0.0024; (NH4)(NO3) 0.0016; Na2HPO4 0.008; agar, 1.5, at pH 7.6 ± 0.2. 5 mL of glycerol was added to the above medium. The slants were incubated at 37 °C for 4 days and then stored at 4 °C.
Experiments were carried out in 250 mL Erlenmeyer flasks with 100 mL of production medium with following components (g/L): yeast extract, 10; glucose, 50; (NH4)2SO4, 15; MgSO4, 0.5; K2HPO4, 0.8; KH2PO4, 1.4; FeSO4, 0.04; ZnSO4, 0.04. The pH of the medium was adjusted to 6.8 with 1 N NaOH before sterilization [34]. 10% (v/v) of a 48-h-old culture (approximately 8.9 × 108 cells/mL) was used as inoculum. Shake flask cultures of the organism were incubated at temperature 37 ± 2 °C with continuous agitation at 150 rpm for 96 h. These fermentation parameters were kept uniform for all the studies conducted. All experiments were carried out in triplicates.
2.2. Fermentation
2.2.1. Culture Media
The strain Bacillus licheniformis PL26 was cultivated in Zobell marine broth, Himedia, Mumbai, India. The media was adjusted to pH 7.6 ± 0.2. The plates were incubated at 37 °C temperature for 48 h.
2.2.2. Inoculum Development
The seed culture inoculated with loopful of Bacillus licheniformis was incubated overnight at 30 °C in an incubator shaker at 120 rpm. The inoculum for the production batch was prepared by using a single colony of B. licheniformis PL26 having 100 mL working volume.
2.2.3. Simultaneous Production of ε-Polylysine and PHA
The marine bacteria was cultured in Zobell marine broth to obtain seed culture having O.D.600 of 2.3. Zobell marine medium comprising (g/L) peptone 5.0; yeast extract 1.0; iron(III) citrate 0.1; NaCl 19.45; MgCl2 8.8; Na2SO4 3.24; CaCl2 1.8; KCl 0.55; NaHCO3 0.16; KBr 0.08; SrCl2 0.034; H3BO3 0.022; Na2O3Si 0.004; NaF 0.0024; (NH4)(NO3) 0.0016; Na2HPO4 0.008 in one liter of the medium maintained at pH 7.6 ± 0.2. 20% seed culture was inoculated in the production medium which contained 20 g crude glycerol, yeast extract 5 g, (NH4)2SO4 10 g, K2HPO4 0.8 g, KH2PO4 1.36 g, MgSO4 0.5 g, ZnSO4 0.04 g, FeSO4 0.03 g in one litre of the medium maintained at pH 8.9 ± 0.2.
2.2.4. Analysis of ε-Polylysine
The culture broth was harvested after fermentation and cells were separated by centrifugation at 15,296× g rcf for 10 min in refrigerated centrifuge. 1 mL of supernatant was added to 1 mL of 1 mM methyl orange, mixed thoroughly under shaking condition along with incubating it at 37 °C for 60 min [35]. Further, the solution was centrifuged at 15,296× g rcf for 10 min in refrigerated centrifuge and absorbance of the supernatant was measured at 465 nm on UV-vis spectrophotometer (Varian, Palo Alto, CA, USA). A standard curve was derived from measurements with known amounts (0.1–2 mg/mL) of standard ε-PL procured from Handary S.A. [36].
2.2.5. Percentage Carbon Utilization of Bacillus licheniformis PL26
Percentage carbon utilized by Bacillus licheniformis PL26 was calculated a
Glycerol estimation was carried out by using “Waters Alliance” high performance chromatographic system equipped with RI detector (Waters 2414 model, Waters India Ltd., Bangalore, India) and separation module (Waters 2695 model, Waters India Ltd., Bangalore, India). Chromatographic separations were performed on an “Aminex HPX-87H” column (300 × 7.8 mm) (Bio-Rad Laboratories, Richmond, CA, USA) with a precolumn (30 × 4.6 mm) of the same stationary phase (DVB-S, hydrogen form, Richmond, CA, USA). Isocratic elution at a flow rate of 0.6 mL/min was carried out using a mixture of 5 mM sulfuric acid. Peak detection was made by keeping the cells of the RI detector at 30 °C. The samples were appropriately degassed, twice diluted with double-distilled water, filtered through a “Whatman” 0.45-μm filter membrane (GE Healthcare Life Sciences, Little Chalfont, Buckinghamshire, UK), and then injected (50-μL loop volume). Data were obtained and processed by using “Waters EMPOWER” software (waters Corporation India, Bangalore, India). Peak identification was carried out by spiking the sample with pure standards and comparing the retention times with those of pure compounds.
2.2.6. Extraction and Purification of PHA
After completion of 96 h fermentation, culture broth was centrifuged at 15,296× g rcf for 10 min in refrigerated centrifuge. The cell pellets were oven dried overnight at 60 °C. Cellular digestion of dried cell pellet was carried out by re-suspending it in 6% (v/v) sodium hypochlorite solution followed by centrifugation at 10,000 rpm for 5 min. Further, the digested cell pellets were washed twice with methanol followed by distilled water to remove the traces of impurities resulting in a purified product, which was further dissolved in chloroform and weighed after air drying [37].
2.3. Purification of ε-Polylysine
2.3.1. Precipitation of Polycationic ε-PL with TPB− Anion from the Supernatant
After removal of cells through centrifugation, the supernatant obtained was treated with sodium tetraphenylborate for precipitating ε-PL as a polyelectrolyte salt with the TPB− anion. The polycationic ε-PL salt with the TPB− anion was further purified by washing the mixed precipitate with acetone to remove triphenylborate and benzene. Thereafter, the precipitate reacted with 1 M HCl for obtaining ε-PL hydrochloride.
2.3.2. Analytical Methods
FT-IR spectra of obtained PHA were recorded on a Perkin-Elmer Spectrum GX (FT-IR System, Waltham, MA, USA) instrument. 1H Nuclear magnetic resonance spectroscopy of PHA was determined on Bruker Avance-II 500 (Ultra shield) spectrometer, Bangalore, India, at 500 MHz, in CDCl3. Proton 1H NMR spectroscopy was also used to determine copolymer composition through running standards of 3HB and 3HV. Differential scanning calorimetry (DSC) of PHA was carried out using a DSC 204 F1 phoenix instrument with Netzsch software (NETZSCH Technologies India Pvt. Ltd., Chennai, India). The PHA samples were scanned from −20 °C to 500 °C with the heating rate of 10 °C/min. Glass transition temperature and onset melting points were determined in the scan between −20 °C and 500 °C in DSC analysis. Thermo-gravimetric analysis (TGA) of PHA was carried out in temperature range of 27–500 °C using TG 209 F1 instrument (NETZSCH Technologies India Pvt. Ltd., Chennai, India).
3. Results and Discussions
3.1. Simultaneous ε-Polylysine and PHA Production by B. licheniformis PL26
After complete submerged fermentation of 96 h at an agitation of 220 rpm, fermentation broth was centrifuged to obtain supernatant containing ε-polylysine and biomass for PHA extraction.
Simultaneous production of ε-polylysine and PHA by B. licheniformis PL26 was obtained utilizing crude glycerol as the carbon source. However, B. licheniformis is able to produce 0.2 g/L ε-polylysine extracellularly in the fermentation broth along with 64.59% PHA with respect to dry cell weight i.e., 1.1 g/L P(3HB-co-3HV) having 96 h production age at 37 °C (Figures S1, S2 and S3).
In the present case, B. licheniformis PL26 is able to produce both ε-polylysine and PHA in the same fermentation broth, which is not reported till date. However, Streptomyces albulus (CCRC 11814) is reported to produce ε-polylysine utilizing crude glycerol [3]. S. albulus being an Actinomycetes, it possesses a relatively slower growth rate as compared to Bacillus sp. As previously reported, S. albulus produces ε-polylysine after 120 h in M3G medium containing glucose as the carbon source, 0.2 g/L of ε-polylysine was produced by Streptomyces albulus (CCRC 11814) after 168 h [3], but in present study, ε-polylysine was produced after 96 h by B. licheniformis at a concentration of 0.2 g/L. In addition, fast growth rate and less production age of B. licheniformis which is producing 64.59% PHA with respect to dry cell weight i.e., 1.1 g/L P(3HB-co-3HV), which may be considered as the additional advantage of the process. Simultaneously, crude glycerol was replaced with analytical grade pure glycerol with similar concentration of the carbon source and it was found that pure glycerol yielded 0.06 g/L ε-polylysine and 0.4 g/L P(3HB-co-3HV).
0.2 g/L of ε-Poly-l-lysine produced from Bacillus licheniformis PL26 is in similar range with respect to its production from a wild strain Streptomyces albulus CCRC 11814 as reported in the literature [3]. However, similar sort of system developed by Moralejo-Ga’rate 2013, wherein using microbial community, simultaneous production of PHA and polyglucose was done [37]. Similarly, using halobacterium Haloferax mediterranei, simultaneously poly(3-hydroxybutyrate-co-3-hydroxyvalerate and extracellular polysaccharide (EPS) was produced [38]. Few microbes or microbial consortium have potential to produce two different polymers simultaneously using single carbon and nitrogen source.
3.2. Percentage Carbon Utilization of Bacillus licheniformis PL26
Carbon utilization percentage of isolated Bacillus licheniformis PL26 is shown in Table 1. Bacillus licheniformis isolated from salt pan has a 30% total carbon utilization percentage as it utilizes 0.21% total carbon from 0.7% total carbon present in the production medium.

Table 1.
Percentage carbon utilization by Bacillus licheniformis.
3.3. Characterization of Purified PHA
The polymer extracted from B. licheniformis PL26 grown in production media was characterized through TGA, DSC, NMR and Fourier transform infrared spectroscopy (FTIR).
Figure 1 indicates TGA analysis to analyze the thermal decomposition of the extracted polymer through the thermogravimetric analyzer. The extracted polymer showed 0.28 g weight loss out of 0.35 g till 500 °C temperature. Mass change of 0.21 g PHA out of 0.35 g PHA was found in the temperature range of 225–325 °C.
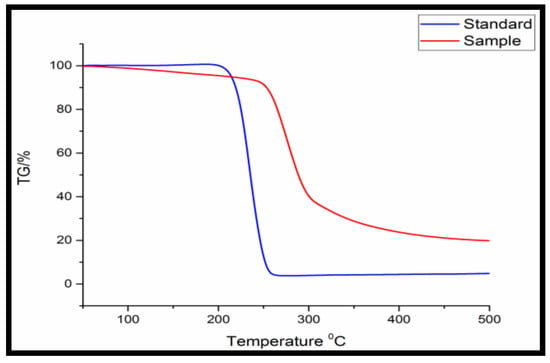
Figure 1.
Thermogravimetric analysis (TGA) of purified PHA obtained from Bacillus licheniformis.
The DSC analysis shown in Figure 2 indicates the melting temperature of the standard sample and the extracted polymer through sodium hypochlorite treatment. The thermal degradation was obtained at 268 °C for the obtained polymer and 250 °C of standard PHA from Sigma Aldrich.
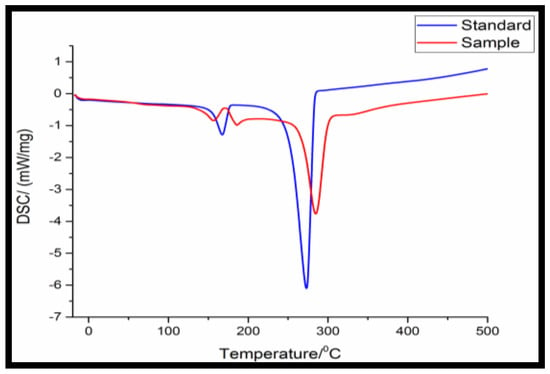
Figure 2.
Differential scanning calorimetry (DSC) of purified PHA from Bacillus licheniformis.
1H NMR spectra of the extracted polymer, standard PHB and standard 3 hydroxy valerate were found to be comparable with respect to each other. Prominent peaks were observed at δ = 1.6 ppm for CH3, δ = 2.4 ppm for CH2 and δ = 5.2 ppm for CH group (Figure 3).
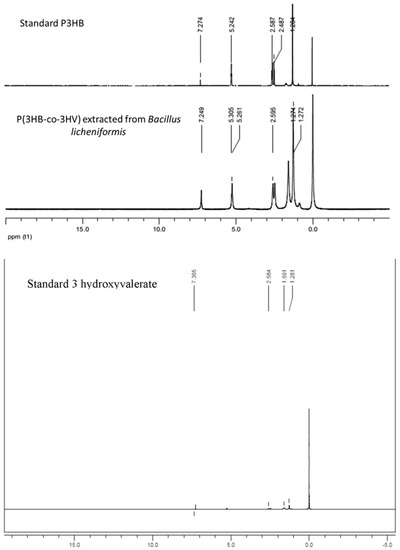
Figure 3.
Nuclear magnetic resonance (NMR) spectra of purified product along with standard PHB and standard 3 hydroxy valerate.
Infra red (IR) spectra (Figure 4) showed intense peaks at 1724 and 1283 cm−1 corresponding to –C=O and –CH group which are in correlation of peaks of standard PHA. Peaks at 1380, 1456 and 2932 correspond to –CH3, –CH2 and –CH group which are in correlation of peaks of standard PHA as shown in Figure 5.
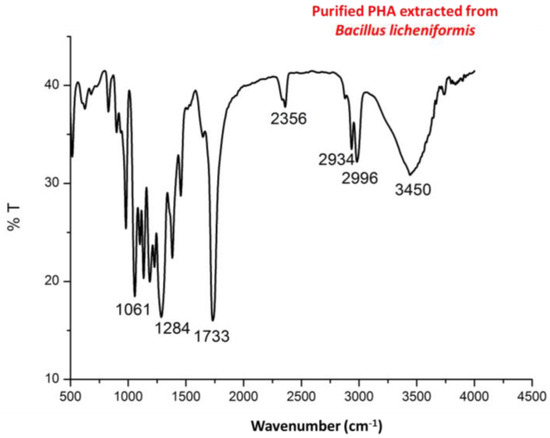
Figure 4.
Fourier transform infrared spectroscopy (FTIR) spectra of purified PHA recovered from Bacillus licheniformis.
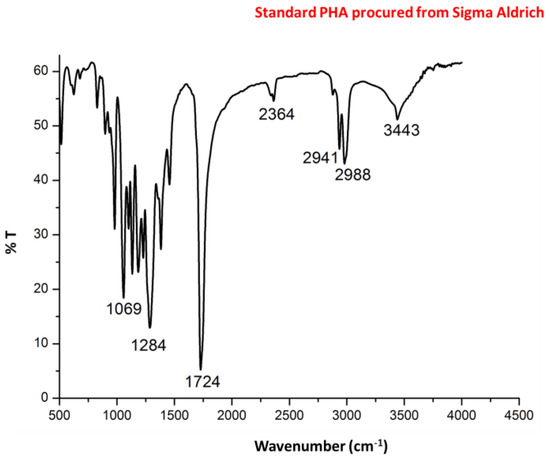
Figure 5.
Fourier transform infrared spectroscopy (FTIR) spectra of standard PHA procured from Sigma Aldrich.
3.4. Characterization of ε-Polylysine
1H NMR of ε-Polylysine in D2O Isolated from B. licheniformis PL26
Protons (Ha, Hc) attached to α-amino groups arrived together as broad singlet at δ 3.76 ppm and protons (Hb, Hd) attached to ε-amino groups arrived together as broad singlet at δ 3.14 ppm. Protons attached to β and β’ carbons come at δ 1.75 ppm as broad singlet. While the other protons attached to carbons come at δ 1.47 ppm (4H, ε and ε’) and δ 1.30 ppm (4H, γ and γ’ respectively (Figure 6). Overall, the peaks showing peptide linkage between α-carboxyl group and the ε-amino group, confirming the structure as ε-polylysine.
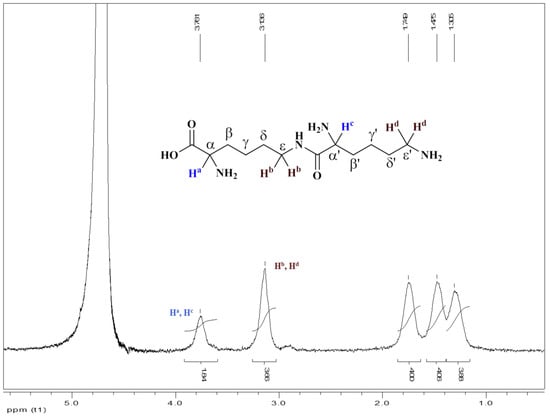
Figure 6.
1H NMR of ε-PL in D2O isolated from Bacillus licheniformis.
As per Jia et al., 2010, chemical shift of ε-H in the ε-polylysine units δεH and at the N-terminal δ’εH are 3.097 and 2.863 of 5 KDa ε-polylysine protein [39]. However, similar results were obtained in the present case, wherein chemical shift of ε-H in the ε-polylysine units, δεH is at δ 3.14 ppm, which are in similar range with respect to the mentioned reports.
The described process showed the potential of utilizing Bacillus licheniformis for the production of PHA (64.59% w/w w.r.t cell dry mass i.e., 1.1 g/L P(3HB-co-3HV)) as well as ε-polylysine (0.2 g/L) using crude glycerol as the carbon source. In addition, there are no such reports as per our knowledge wherein two polymers are produced at a time in the same fermentation broth. Previously, Bera et al., 2014 reported microbial synthesis of such polymer (P(3HB-co-3HV)) by Halomonas hydrothermalis (MTCC accession no. 5445) from seaweed derived levulinic acid at a concentration of 57.5% PHA/dry cell weight and Ghosh et al., 2011 reported microbial synthesis of PHA from biodiesel by-products i.e., from crude glycerol and Jatropha deoiled cake hydrolysate at a concentration of 75% PHA/dry cell weight. However, further optimization will be required for efficient production of PHA and ε-polylysine at larger scale.
The most important parameter responsible for cost of the production in PHA is substrate for carbon and energy source. The economic feasibility of PHA would depend on few important factors like growth rate of microbe for generation of biomass, substrate cost and recovery process including the solvent involved. However, production of other biopolymer along with PHA in the fermentation medium will have additional advantage in further reducing the production cost. PHA production from biodiesel waste stream can reduce production cost and at the same time solves the problem of waste disposal. In order to increase the PHA yield, the concentration of carbon source and other nutrient source desired for the microbial production may be optimized.
4. Conclusions
In the present study, an integrated process for simultaneous production of extracellular ε-polylysine and intracellular P(3HB-co-3HV) developed through marine bacterial strain (Bacillus licheniformis) isolated from west coast of India utilizing Jatropha biodiesel waste residues as carbon rich source. A maximum of 0.2 g/L ε-polylysine content and 64.6% (w/w) P(3HB-co-3HV) production with respect to dry biomass was obtained in the fermentation broth using Bacillus licheniformis. ε-polylysine and PHA are important class of biopolymers which have various applications in food, agriculture, medicine, pharmacy, controlled drug release, tissue engineering, etc. Although, the present approach provides a solution for the effective utilization of biodiesel by-product, still, the developed process needs to be optimized further for gaining still better yield of both the products for its acclamation as cost effective and sustainable process.
Supplementary Materials
The following are available online at http://www.mdpi.com/2306-5354/3/4/34/s1, Figure S1: effect of production age (h.) on ε-polylysine yield at 35 °C, Figure S2: effect of production age (h) on ε-polylysine yield at 37 °C, Figure S3: effect of production age (h) on ε-polylysine yield at 40 °C.
Acknowledgments
Financial assistance from CSIR as a part of EMPOWER scheme is gratefully acknowledged. We also acknowledge analytical science division of CSIR-CSMCRI for their timely support and cooperation. Authors are also grateful to CSC 0105, CSC 0203, OLP 0077 for providing financial support. We extend our gratitude to BDIM for providing PRIS no. CSIR-CSMCRI—043/2016 registration number.
Author Contributions
Sourish Bhattacharya developed the protocol for isolation and screening of potential ε-polylysine halophilic bacteria, followed by optimizing medium composition for simultaneous production of ε-polylysine and polyhydroxyalkanoate in the medium. Further, the extraction, purification and characterization of ε-polylysine was also conducted by him. Priyanka Singh assisted in isolation of bacteria from west coast of India and screening for identifying potential ε-polylysine halophilic bacterial isolate. Anupama Shrivastava and Sonam Dubey optimized the process for extraction and purification of polyhydroxyalkanoate. Sandhya Mishra monitored the overall experiments being performed and helped in the interpretation of results and polishing the manuscript.
Conflicts of Interest
The authors declare no conflict of interest.
References and Notes
- Campbell, C.J.; Laherrère, J.H. The end of cheap oil. Sci. Am. 1998, 3, 78–83. [Google Scholar] [CrossRef]
- Sheehan, J.; Camobreco, V.; Duffield, J.; Graboski, M.; Shapouri, H. Life Cycle Inventory of Biodiesel and Petroleum Diesel for Use in an Urban Bus. Available online: http://www.nrel.gov/docs/legosti/fy98/24089.pdf (accessed on 17 January 2016).
- Nerve, Z. Upgrading of Biodiesel-Derived Glycerol in the Biosynthesis of ε-poly-l-Lysine: An Integrated Biorefinery Approach. Available online: http://wiredspace.wits.ac.za/handle/10539/9253?show=full (accessed on 17 January 2016).
- Vasudevan, P.T.; Briggs, M. Biodiesel production—Current state of the art and challenges. J. Ind. Microbiol. Biotechnol. 2008, 35, 421–430. [Google Scholar] [CrossRef] [PubMed]
- Adhikari, S.; Fernando, S.D.; To, S.D.F.; Bricka, R.M.; Steele, P.H.; Haryanto, A. Conversion of glycerol to hydrogen via a steam reforming process over nickel catalysts. Energy Fuels 2008, 22, 1220–1226. [Google Scholar] [CrossRef]
- Wirawan, S.S.; Tambunan, A.H. The current status and prospects of biodiesel development in Indonesia: A review. In Proceedings of the Third Asia Biomass Workshop, Tsukuba, Japan, 16 November 2006.
- Marchetti, J.M.; Miguel, V.U.; Errazu, A.F. Possible methods for biodiesel production. Renew. Sustain. Energy Rev. 2007, 11, 1300–1311. [Google Scholar] [CrossRef]
- Johnson, D.T.; Taconi, K.A. The glycerin glut: Options for the value-added conversion of crude glycerol resulting from biodiesel production. Environ. Prog. 2007, 26, 338–348. [Google Scholar] [CrossRef]
- Thompson, J.C.; He, B.B. Characterization of crude glycerol from biodiesel production from multiple feedstock. Appl. Eng. Agric. 2006, 22, 261–265. [Google Scholar] [CrossRef]
- Dasari, M.A.; Kiatsimkul, P.P.; Sutterlin, W.R.; Suppes, G.J. Low-pressure hydrogenolysis of glycerol to propylene glycol. Appl. Catal. A Gen. 2005, 281, 225–231. [Google Scholar] [CrossRef]
- Lemke, D. Volumes of Versatility. Auri Ag Innovation News.
- Gallan, M.; Bonet, J.; Sire, R.; Reneaume, J.; Plesu, A.E. From residual to useful oil: Revalorization of glycerine from the biodiesel synthesis. Bioresour. Technol. 2009, 100, 3775–3778. [Google Scholar] [CrossRef] [PubMed]
- McCoy, M. Glycerin surplus. Chem. Eng. News 2006, 84, 7–8. [Google Scholar] [CrossRef]
- Yazdani, S.S.; Gonzalez, R. Anaerobic fermentation of glycerol: A path to economic viability for the biofuels industry. Curr. Opin. Biotechnol. 2007, 18, 213–219. [Google Scholar] [CrossRef] [PubMed]
- Ghosh, P.K.; Mishra, S.C.P.; Gandhi, M.R.; Upadhyay, S.C.; Paul, P.; Anand, P.S.; Popat, K.M.; Shrivastav, A.V.; Mishra, S.K.; Ondhiya, N.; et al. Integrated Process for the Production of Jatropha Methyl Ester and by Products. EU Patent 2,475,754, 18 July 2012. [Google Scholar]
- Yang, F.; Hanna, M.A.; Sun, R. Value-added uses for crude glycerol—A byproduct of biodiesel production. Biotechnol. Biofuels 2012, 5, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Ito, T.; Nakashimada, Y.; Senba, K.; Matsui, T.; Nishio, N. Hydrogen and ethanol production from glycerol-containing wastes discharged after biodiesel manufacturing process. J. Biosci. Bioeng. 2005, 100, 260–265. [Google Scholar] [CrossRef] [PubMed]
- Shrivastav, A.; Mishra, S.K.; Shethia, B.; Pancha, I.; Jain, D.; Mishra, S. Isolation of promising bacterial strains from soil and marine environment for polyhydroxyalkanoates (PHAs) production utilizing Jatropha biodiesel byproduct. Int. J. Biol. Macromol. 2010, 47, 283–287. [Google Scholar] [CrossRef] [PubMed]
- Rymowicz, W.; Rywińska, A.; Marcinkiewicz, M. High-yield production of erythritol from raw glycerol in fed-batch cultures of Yarrowia lipolytica. Biotechnol. Lett. 2009, 31, 377–380. [Google Scholar] [CrossRef] [PubMed]
- Tang, S.; Boehme, L.; Lam, H.; Zhang, Z. Pichia pastoris fermentation for phytase production using crude glycerol from biodiesel production as the sole carbon source. Biochem. Eng. J. 2009, 43, 157–162. [Google Scholar] [CrossRef]
- Volpato, G.; Rodrigues, R.C.; Heck, J.X.; Ayub, M.A.Z. Production of organic solvent tolerant lipase by Staphylococcus caseolyticus EX17 using raw glycerol as substrate. J. Chem. Technol. Biotechnol. 2008, 83, 821–828. [Google Scholar] [CrossRef]
- Scholten, E.; Renz, T.; Thomas, J. Continuous Cultivation Approach for Fermentative Succinic Acid Production from Crude Glycerol by Basfia succiniciproducen DD1. Biotechnol. Lett. 2009, 31, 1947–1951. [Google Scholar] [CrossRef] [PubMed]
- Ethier, S.; Woisard, K.; Vaughan, D.; Wen, Z.Y. Continuous culture of the microalgae Schizochytrium limacinum on biodiesel-derived crude glycerol for producing docosahexaenoic acid. Bioresour. Technol. 2011, 102, 88–93. [Google Scholar] [CrossRef] [PubMed]
- Athalye, S.K.; Garcia, R.A.; Wen, Z.Y. Use of biodiesel-derived crude glycerol for producing eicosapentaenoic acid (EPA) by the fungus Pythium Irregular. J. Agric. Food. Chem. 2009, 57, 2739–2744. [Google Scholar] [CrossRef] [PubMed]
- Choi, W.J.; Hartono, M.R.; Chan, W.H.; Yeo, S.S. Ethanol Production from Biodiesel-Derived Crude Glycerol by Newly Isolated Kluyvera Cryocrescen. Appl. Microbiol. Biotechnol. 2011, 89, 1255–1264. [Google Scholar] [CrossRef] [PubMed]
- Oh, B.R.; Seo, J.W.; Heo, S.Y.; Hong, W.K.; Luo, L.H.; Joe, M.; Park, D.H.; Kim, C.H. Enhancement of ethanol production from glycerol in a Klebsiella pneumoniae mutant strain by the inactivation of lactate dehydrogenase. Bioresour. Technol. 2011, 102, 3918–3922. [Google Scholar] [CrossRef] [PubMed]
- Taconi, K.A.; Venkataramanan, K.P.; Johnson, D.T. Growth and solvent production by Clostridium pasteurianum ATCC® 6013™ utilizing biodiesel-derived crude glycerol as the sole carbon source. Environ. Prog. Sustain. Energy 2009, 28, 100–110. [Google Scholar] [CrossRef]
- Poblete-Castro, I.; Binger, D.; Oehlert, R.; Rohde, M. Comparison of mcl-Poly (3-hydroxyalkanoates) synthesis by different Pseudomonas putida strains from crude glycerol: Citrate accumulates at high titer under PHA-producing conditions. BMC Biotechnol. 2014, 14, 962. [Google Scholar] [CrossRef] [PubMed]
- Moita, R.; Freches, A.; Lemos, P.C. Crude glycerol as feedstock for polyhydroxyalkanoates production by mixed microbial cultures. Water Res. 2014, 58, 9–20. [Google Scholar] [CrossRef] [PubMed]
- Hermann-Krauss, C.; Koller, M.; Muhr, A.; Fasl, H.; Stelzer, F.; Braunegg, G. Archaeal production of polyhydroxyalkanoate (PHA) co-and terpolyesters from biodiesel industry-derived by-products. Archaea 2013, 2013, 129268. [Google Scholar] [CrossRef] [PubMed]
- González-Pajuelo, M.; Andrade, J.C.; Vasconcelos, I. Production of 1,3-propanediol by Clostridium butyricum VPI 3266 using a synthetic medium and raw glycerol. J. Ind. Microbiol. Biotechnol. 2004, 31, 442–446. [Google Scholar] [CrossRef] [PubMed]
- Bera, A.; Dubey, S.; Bhayani, K.; Mondal, D.; Mishra, S.; Ghosh, P.K. Microbial synthesis of polyhydroxyalkanoate using seaweed-derived crude levulinic acid as co-nutrient. Int. J. Biol. Macromol. 2015, 72, 487–494. [Google Scholar] [CrossRef] [PubMed]
- Chheda, A.H.; Vernekar, M.R. Improved production of natural food preservative ε-poly-l-lysine using a novel producer Bacillus cereus. Food Biosci. 2014, 30, 56–63. [Google Scholar] [CrossRef]
- Itzhaki, R.F. Colorimetric method for estimating polylysine and polyarginine. Anal. Biochem. 1972, 50, 569–574. [Google Scholar] [CrossRef]
- Dhangdhariya, J.H.; Dubey, S.; Trivedi, H.B.; Pancha, I.; Bhatt, J.K.; Dave, B.P.; Mishra, S. Polyhydroxyalkanoate from marine Bacillus megaterium using CSMCRI’s Dry Sea Mix as a novel growth medium. Int. J. Biol. Macromol. 2015, 76, 254–261. [Google Scholar] [CrossRef] [PubMed]
- Chheda, A.H.; Vernekar, M.R. Enhancement of ε-poly-l-lysine (ε-PL) production by a novel producer Bacillus cereus using metabolic precursors and glucose feeding. 3 Biotech 2015, 5, 839–846. [Google Scholar] [CrossRef]
- Koller, M.; Chiellini, E.; Braunegg, G. Study on the Production and Re-use of Poly (3-hydroxybutyrate-co-3-hydroxyvalerate) and Extracellular Polysaccharide by the Archaeon Haloferax mediterranei Strain DSM 1411. Chem. Biochem. Eng. Q. 2015, 29, 87–98. [Google Scholar] [CrossRef]
- Moralejo-Gárate, H.; Palmeiro-Sánchez, T.; Kleerebezem, R.; Mosquera-Corral, A.; Campos, J.L.; van Loosdrecht, M. Influence of the cycle length on the production of PHA and polyglucose from glycerol by bacterial enrichments in sequencing batch reactors. Biotechnol. Bioeng. 2013, 110, 3148–3155. [Google Scholar] [CrossRef] [PubMed]
- Jia, S.; Fan, B.; Dai, Y.; Wang, G.; Peng, P.; Jia, Y. Fractionation and Characterization of ε-poly-l-lysine from Streptomyces albulus CGMCC 1986. Food. Sci. Biotechnol. 2010, 19, 361–366. [Google Scholar] [CrossRef]
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).