Applications of Adipose Tissue Micrografts (ATM) and Dermis Micrografts (DMG) in Wound Healing: A Scoping Review of Clinical Studies
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Protocol
2.2. Information Sources, Literature Search, and Eligibility Criteria
2.2.1. Inclusion Criteria
2.2.2. Exclusion Criteria
2.3. Literature Search
2.4. Data Collection and Extraction
3. Results
3.1. Study Selection Process
3.2. Overview of Included Studies and Study Characteristics
3.3. Patient Demographics and Wound Types
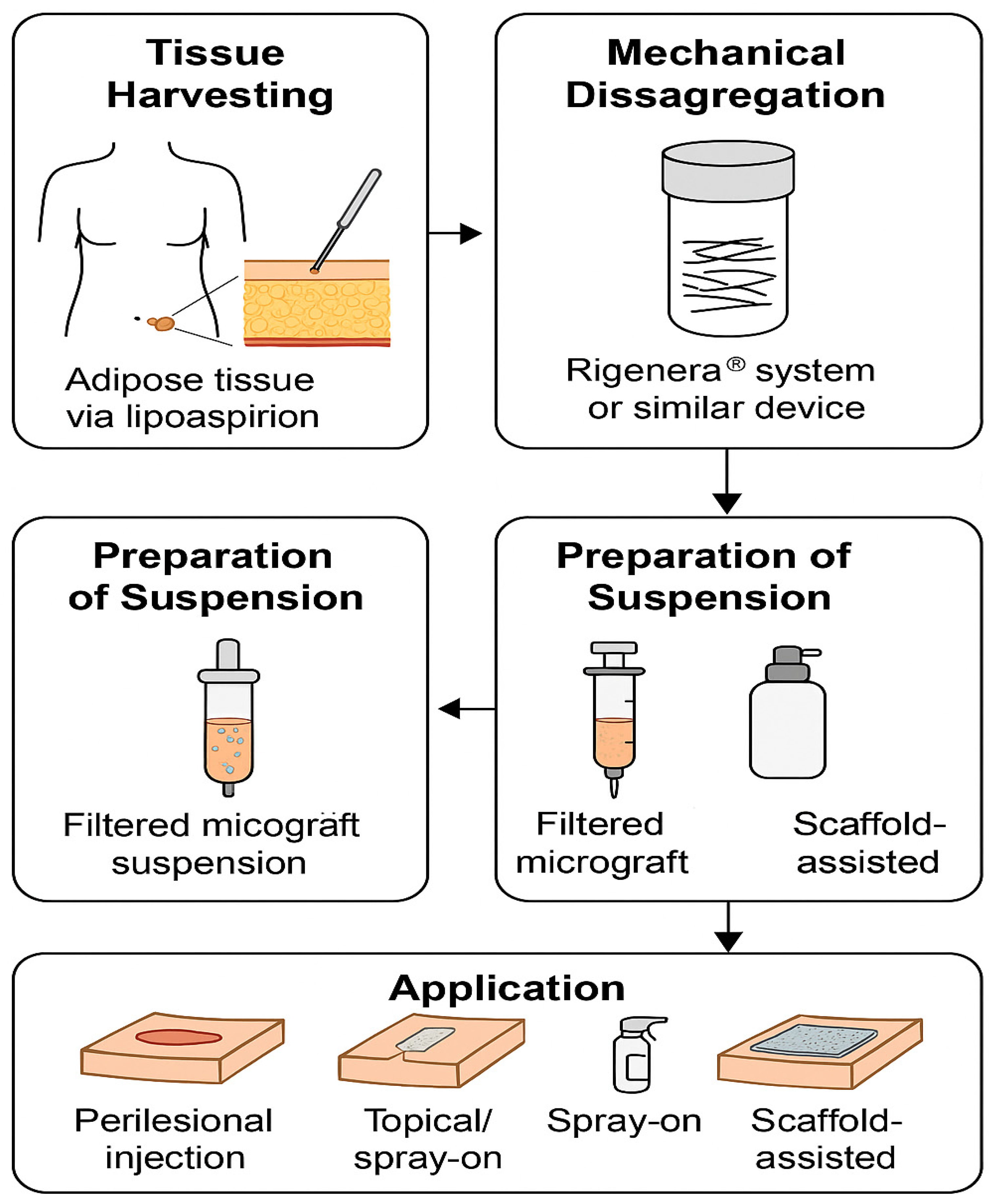
3.4. Preparation and Application
- Composite approach: imbibition into dermal substitute plus infiltration (Tresoldi et al. [22]).
3.5. Outcomes Measured
3.6. Follow-Up and Safety
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| ADSVF | Adipose-Derived Stromal Vascular Fraction |
| ATM | Adipose Tissue Micrograft |
| BCC | Basal Cell Carcinoma |
| BMI | Body Mass Index |
| DM | Diabetes Mellitus |
| DMG | Dermis Micrograft |
| F | Female |
| HTN | Hypertension |
| Integra® | Integra® Dermal Regeneration Template (Integra LifeSciences, Princeton, NJ, USA) |
| M | Male |
| MS | Multiple Sclerosi |
| PRF | Platelet-Rich Fibrin |
| PRISMA-ScR | Preferred Reporting Items for Systematic Reviews and Meta-Analyses Extension for Scoping Reviews |
| QoL | Quality of Life |
| RCT | Randomized Controlled Trial |
| SCC | Squamous Cell Carcinoma |
| SSc | Systemic Sclerosis |
| SVF | Stromal Vascular Fraction |
| TGF-β | Transforming Growth Factor Beta |
| VAS | Visual Analogue Scale |
| VEGF | Vascular Endothelial Growth Factor |
References
- Prasai, A.; Jay, J.W.; Jupiter, D.; Wolf, S.E.; El Ayadi, A. Role of Exosomes in Dermal Wound Healing: A Systematic Review. J. Investig. Dermatol. 2022, 142, 662–678.e8. [Google Scholar] [CrossRef]
- Anestiadou, E.; Kotidis, E.; Deka, I.A.; Tatsis, D.; Bekiari, C.; Loukousia, A.; Ioannidis, O.; Stamiris, S.; Zapsalis, K.; Xylas, C.; et al. Platelet-Rich Therapies in Hernia Repair: A Comprehensive Review of the Impact of Platelet Concentrates on Mesh Integration in Hernia Management. Biomolecules 2024, 14, 921. [Google Scholar] [CrossRef] [PubMed]
- Azevedo, M.; Lisboa, C.; Rodrigues, A. Chronic Wounds and Novel Therapeutic Approaches. Br. J. Community Nurs. 2020, 25, S26–S32. [Google Scholar] [CrossRef]
- European Wound Management Association (EWMA). Wound Healing in a Changing Healthcare Climate. J. Wound Care 2019, 28 (Suppl. S6), S1–S49. [Google Scholar] [CrossRef]
- Augustin, M.; Carville, K.; Clark, M.; J, C.; Flour, M.; J, L.; K, M.; Moffatt, C.; Pattison, M.; Price, P.; et al. International Consensus. Optimising Wellbeing in People Living with a Wound. An Expert Working Group Review; Wounds International: London, UK, 2012. [Google Scholar]
- Dari, S.; O’dea, R.D.; Fadai, N.T. Understanding the Regulation of Chronic Wounds by Tissue Inhibitors of Matrix Metalloproteinases through Mathematical Modelling. J. Theor. Biol. 2025, 604, 112083. [Google Scholar] [CrossRef]
- Gurtner, G.C.; Chapman, M.A. Regenerative Medicine: Charting a New Course in Wound Healing. Adv. Wound Care 2016, 5, 314–328. [Google Scholar] [CrossRef]
- Fu, H.; Wang, C. Micro-Fragmented Adipose Tissue-An Innovative Therapeutic Approach: A Narrative Review. Medicine 2025, 104, e41724. [Google Scholar] [CrossRef]
- Greenwood, V.; Clausen, P.; Matuska, A. Micro-Fragmented Adipose Tissue Cellular Composition Varies by Processing Device and Analytical Method. Sci. Rep. 2022, 12, 1–8. [Google Scholar] [CrossRef]
- Ki, K.I.; Baglioni, E.A.; Perego, F.; Paolin, E.; Abate, A.; Pusceddu, T.; Zavan, B.; Bocchiotti, M.A. Efficacy of Autologous Micrografts Technology: A Promising Approach for Chronic Wound Healing and Tissue Regeneration—A Pilot Study. Front. Med. 2024, 11, 1417920. [Google Scholar] [CrossRef]
- Zapsalis, K.; Ioannidis, O.; Xylas, C.; Siozos, K.; Gemousakakis, G.; Anestiadou, E.; Symeonidis, S.; Bitsianis, S.; Kotidis, E.; Cheva, A.; et al. Platelet Rich Plasma, Adipose Tissue Micrografts, and Regenerative Mimetic Factors for Abdominal Wall Defect Reconstruction: Experimental Study Protocol. World J. Exp. Med. 2025, 15, 99065. [Google Scholar] [CrossRef]
- Trovato, L.; Monti, M.; del Fante, C.; Cervio, M.; Lampinen, M.; Ambrosio, L.; Redi, C.A.; Perotti, C.; Kankuri, E.; Ambrosio, G.; et al. A New Medical Device Rigeneracons Allows to Obtain Viable Micro-Grafts From Mechanical Disaggregation of Human Tissues. J. Cell. Physiol. 2015, 230, 2299–2303. [Google Scholar] [CrossRef] [PubMed]
- Balli, M.; Vitali, F.; Janiszewski, A.; Caluwé, E.; Cortés-Calabuig, A.; Carpentier, S.; Duelen, R.; Ronzoni, F.; Marcelis, L.; Bosisio, F.M.; et al. Autologous Micrograft Accelerates Endogenous Wound Healing Response through ERK-Induced Cell Migration. Cell Death Differ. 2020, 27, 1520–1538. [Google Scholar] [CrossRef] [PubMed]
- Xia, J.; Xu, M.; Ma, X.; Wang, Z.; Shi, X.; Zou, H.; Yang, X.; Wu, B.; Huang, D.; Yu, Z.; et al. Adipose-Derived Regenerative Cells (ADRCs) in Regenerative Medicine: Current Advances and Future Directions. Regenes. Repair Rehabil. 2025, 1, 35–55. [Google Scholar] [CrossRef]
- De Francesco, F.; Graziano, A.; Trovato, L.; Ceccarelli, G.; Romano, M.; Marcarelli, M.; Cusella De Angelis, G.M.; Cillo, U.; Riccio, M.; Ferraro, G.A. A Regenerative Approach with Dermal Micrografts in the Treatment of Chronic Ulcers. Stem Cell Rev. Rep. 2017, 13, 139–148. [Google Scholar] [CrossRef]
- Riccio, M.; Bondioli, E.; Senesi, L.; Zingaretti, N.; Gargiulo, P.; De Francesco, F.; Parodi, P.C.; Zavan, B. Fragmented Dermo-Epidermal Units (FdeU) as an Emerging Strategy to Improve Wound Healing Process: An In Vitro Evaluation and a Pilot Clinical Study. J. Clin. Med. 2023, 12, 6165. [Google Scholar] [CrossRef]
- Uguten, M.; van der Sluis, N.; Vriend, L.; Coert, J.H.; Harmsen, M.C.; van der Lei, B.; van Dongen, J.A. Comparing Mechanical and Enzymatic Isolation Procedures to Isolate Adipose-Derived Stromal Vascular Fraction: A Systematic Review. Wound Repair Regen. 2024, 32, 1008–1021. [Google Scholar] [CrossRef]
- Biswas, A.; Bharara, M.; Hurst, C.; Armstrong, D.G.; Rilo, H. The Micrograft Concept for Wound Healing: Strategies and Applications. J. Diabetes Sci. Technol. 2010, 4, 808–819. [Google Scholar] [CrossRef]
- Tricco, A.C.; Lillie, E.; Zarin, W.; O’Brien, K.K.; Colquhoun, H.; Levac, D.; Moher, D.; Peters, M.D.J.; Horsley, T.; Weeks, L.; et al. PRISMA Extension for Scoping Reviews (PRISMA-ScR): Checklist and Explanation. Ann. Intern. Med. 2018, 169, 467–473. [Google Scholar] [CrossRef]
- Moher, D.; Liberati, A.; Tetzlaff, J.; Altman, D.G. Preferred Reporting Items for Systematic Reviews and Meta-Analyses: The PRISMA Statement. PLoS Med. 2009, 6, e1000097. [Google Scholar] [CrossRef]
- Peters, M.; Godfrey, C.; Mcinerney, P.; Soares, C.; Khalil, H.; Parker, D. Methodology for JBI Scoping Reviews. In The Joanna Briggs Institute Reviewers’ Manual 2015; Aromataris, E., Ed.; The Joanna Briggs Institute: North Adelaide, Australia, 2015; pp. 1–24. [Google Scholar]
- Tresoldi, M.M.; Graziano, A.; Malovini, A.; Faga, A.; Nicoletti, G. The Role of Autologous Dermal Micrografts in Regenerative Surgery: A Clinical Experimental Study. Stem Cells Int. 2019, 2019, 9843407. [Google Scholar] [CrossRef]
- Iglesias, M.; Torre-Villalvazo, I.; Butrón-Gandarillas, P.; Rodríguez-Reyna, T.S.; Torre-Anaya, E.A.; Guevara-Cruz, M.; Flores-Cháirez, M.A.; López-Contreras, D.B.; López-Sánchez, J.Y.; Ruiz-Betanzos, Á.J.; et al. Adipose Derived Stromal Vascular Fraction and Fat Graft for Treating the Hands of Patients with Systemic Sclerosis. A Randomized Clinical Trial. PLoS ONE 2023, 18, e0289594. [Google Scholar] [CrossRef] [PubMed]
- Riccio, M.; Marchesini, A.; Zingaretti, N.; Carella, S.; Senesi, L.; Onesti, M.G.; Parodi, P.C.; Ribuffo, D.; Vaienti, L.; De Francesco, F. A Multicentre Study: The Use of Micrografts in the Reconstruction of Full-Thickness Posttraumatic Skin Defects of the Limbs-A Whole Innovative Concept in Regenerative Surgery. Stem Cells Int. 2019, 2019, 5043518. [Google Scholar] [CrossRef]
- Marcarelli, M.; Trovato, L.; Novarese, E.; Riccio, M.; Graziano, A. Rigenera Protocol in the Treatment of Surgical Wound Dehiscence. Int. Wound J. 2017, 14, 277–281. [Google Scholar] [CrossRef]
- Andreone, A.; den Hollander, D. A Retrospective Study on the Use of Dermis Micrografts in Platelet-Rich Fibrin for the Resurfacing of Massive and Chronic Full-Thickness Burns. Stem Cells Int. 2019, 2019, 8636079. [Google Scholar] [CrossRef]
- Svolacchia, L.; Prisco, C.; Giuzio, F.; Svolacchia, F. Adipose Autologous Micrograft and Its Derived Mesenchymal Stem Cells in a Bio Cross-Linked Hyaluronic Acid Scaffold for Correction Deep Wrinkles, Facial Depressions, Scars, Face Dermis and Its Regenerations: A Pilot Study and Cases Report. Medicina 2022, 58, 1692. [Google Scholar] [CrossRef]
- Miranda, R.; Farina, E.; Farina, M.A. Micrografting Chronic Lower Extremity Ulcers with Mechanically Disaggregated Skin Using a Micrograft Preparation System. J. Wound Care 2018, 27, 60–65. [Google Scholar] [CrossRef]
- Guest, J.F.; Fuller, G.W.; Vowden, P. Cohort Study Evaluating the Burden of Wounds to the UK’s National Health Service in 2017/2018: Update from 2012/2013. BMJ Open 2020, 10, e045253. [Google Scholar] [CrossRef]
- Sen, C.K.; Gordillo, G.M.; Roy, S.; Kirsner, R.; Lambert, L.; Hunt, T.K.; Gottrup, F.; Gurtner, G.C.; Longaker, M.T. Human Skin Wounds: A Major and Snowballing Threat to Public Health and the Economy. Wound Repair Regen. 2009, 17, 763–771. [Google Scholar] [CrossRef]
- Eming, S.A.; Martin, P.; Tomic-Canic, M. Wound Repair and Regeneration: Mechanisms, Signaling, and Translation. Sci. Transl. Med. 2014, 6, 265sr6. [Google Scholar] [CrossRef]
- Yoshimura, K.; Suga, H.; Eto, H. Adipose-Derived Stem/Progenitor Cells: Roles in Adipose Tissue Remodeling and Potential Use for Soft Tissue Augmentation. Regen. Med. 2009, 4, 265–273. [Google Scholar] [CrossRef]
- Tonnard, P.; Verpaele, A.; Peeters, G.; Hamdi, M.; Cornelissen, M.; Declercq, H. Nanofat Grafting: Basic Research and Clinical Applications. Plast. Reconstr. Surg. 2013, 132, 1017–1026. [Google Scholar] [CrossRef]
- Li, K.; Li, X.; Shi, G.; Lei, X.; Huang, Y.; Bai, L.; Qin, C. Effectiveness and Mechanisms of Adipose-Derived Stem Cell Therapy in Animal Models of Parkinson’s Disease: A Systematic Review and Meta-Analysis. Transl. Neurodegener. 2021, 10, 14. [Google Scholar] [CrossRef]
- Adam, A.O.; Rares, H.; Benea, C.; Fotescu, H.M.; Alcal, M.; Cimpean, G.C.; Ciornei, V.; Cernacovschi, A.; Edves, A.R.; Crisan, M. Recent Trends in Adipose Tissue-Derived Injectable Therapies for Osteoarthritis: A Scoping Review of Animal Models. Medicina 2024, 60, 707. [Google Scholar] [CrossRef] [PubMed]
- Astarita, C.; Arora, C.L.; Trovato, L. Tissue Regeneration: An Overview from Stem Cells to Micrografts. J. Int. Med. Res. 2020, 48, 300060520914794. [Google Scholar] [CrossRef] [PubMed]
- De Francesco, F.; Gravina, P.; Busato, A.; Farinelli, L.; Soranzo, C.; Vidal, L.; Zingaretti, N.; Zavan, B.; Sbarbati, A.; Riccio, M.; et al. Stem Cells in Autologous Microfragmented Adipose Tissue: Current Perspectives in Osteoarthritis Disease. Int. J. Mol. Sci. 2021, 22, 10197. [Google Scholar] [CrossRef]
- Mummolo, S.; Mancini, L.; Quinzi, V.; D’Aquino, R.; Marzo, G.; Marchetti, E. Rigenera® Autologous Micrografts in Oral Regeneration: Clinical, Histological, and Radiographical Evaluations. Appl. Sci. 2020, 10, 5084. [Google Scholar] [CrossRef]
- Liu, J.; Li, Y.; Zhang, Y.; Zhao, Z.; Liu, B. Engineered Stromal Vascular Fraction for Tissue Regeneration. Front. Pharmacol. 2025, 16, 1510508. [Google Scholar] [CrossRef]
- Karina, K.; Rosadi, I.; Sobariah, S.; Afini, I.; Widyastuti, T.; Rosliana, I. Comparable Effect of Adipose-Derived Stromal Vascular Fraction and Mesenchymal Stem Cells for Wound Healing: An In Vivo Study. Biomed. Res. Ther. 2019, 6, 3412–3421. [Google Scholar] [CrossRef]
- Liu, Z.-H.; Xie, Q.-Q.; Huang, J.-L. Stromal Vascular Fraction: Mechanisms and Application in Reproductive Disorders. World J. Stem Cells 2025, 17, 101097. [Google Scholar] [CrossRef]
- Wang, K.; Yang, Z.; Zhang, B.; Gong, S.; Wu, Y. Adipose-Derived Stem Cell Exosomes Facilitate Diabetic Wound Healing: Mechanisms and Potential Applications. Int. J. Nanomed. 2024, ume 19, 6015–6033. [Google Scholar] [CrossRef]
- Granel, B.; Daumas, A.; Jouve, E.; Harlé, J.-R.; Nguyen, P.-S.; Chabannon, C.; Colavolpe, N.; Reynier, J.-C.; Truillet, R.; Mallet, S.; et al. Safety, Tolerability and Potential Efficacy of Injection of Autologous Adipose-Derived Stromal Vascular Fraction in the Fingers of Patients with Systemic Sclerosis: An Open-Label Phase I Trial. Ann. Rheum. Dis. 2015, 74, 2175–2182. [Google Scholar] [CrossRef]
- Pinto, P. Efficacy and Safety of an Autologous Micrografting Procedure for Management of Striae Distensae in Women. Dermatol. Ther. 2024, 14, 469–488. [Google Scholar] [CrossRef] [PubMed]
- Gentile, P.; Garcovich, S. Systematic Review: Adipose-Derived Mesenchymal Stem Cells, Platelet-Rich Plasma and Biomaterials as New Regenerative Strategies in Chronic Skin Wounds and Soft Tissue Defects. Int. J. Mol. Sci. 2021, 22, 1538. [Google Scholar] [CrossRef] [PubMed]
- Bora, P.; Majumdar, A.S. Adipose Tissue-Derived Stromal Vascular Fraction in Regenerative Medicine: A Brief Review on Biology and Translation. Stem Cell Res. Ther. 2017, 8, 145. [Google Scholar] [CrossRef]
- Ferrarotti, F.; Romano, F.; Gamba, M.N.; Quirico, A.; Giraudi, M.; Audagna, M.; Aimetti, M. Human Intrabony Defect Regeneration with Micrografts Containing Dental Pulp Stem Cells: A Randomized Controlled Clinical Trial. J. Clin. Periodontol. 2018, 45, 841–850. [Google Scholar] [CrossRef]
- Baena, R.R.Y.; D’Aquino, R.; Graziano, A.; Trovato, L.; Aloise, A.C.; Ceccarelli, G.; Cusella, G.; Pelegrine, A.A.; Lupi, S.M. Autologous Periosteum-Derived Micrografts and PLGA/HA Enhance the Bone Formation in Sinus Lift Augmentation. Front. Cell Dev. Biol. 2017, 5, 87. [Google Scholar] [CrossRef]
- Di Martino, A.; Di Matteo, B.; Papio, T.; Tentoni, F.; Selleri, F.; Cenacchi, A.; Kon, E.; Filardo, G. Platelet-Rich Plasma Versus Hyaluronic Acid Injections for the Treatment of Knee Osteoarthritis: Results at 5 Years of a Double-Blind, Randomized Controlled Trial. Am. J. Sports Med. 2018, 47, 347–354. [Google Scholar] [CrossRef]
- Baria, M.; Pedroza, A.; Kaeding, C.; Durgam, S.; Duerr, R.; Flanigan, D.; Borchers, J.; Magnussen, R. Platelet-Rich Plasma Versus Microfragmented Adipose Tissue for Knee Osteoarthritis: A Randomized Controlled Trial. Orthop. J. Sport. Med. 2022, 10, 23259671221120680. [Google Scholar] [CrossRef]

| Author, Year | Study Design | Patient Demographics | Wound Types | Graft Preparation Method | Application Technique | Outcomes Measured | Follow-Up Duration | Adverse Events |
|---|---|---|---|---|---|---|---|---|
| Marcarelli et al. [25] (2017) | Case series (3 patients) | 3 elderly (61–78 years old); HTN, DM, MS | Surgical wound dehiscence | Rigeneracons mechanical disaggregation of 1 cm2 skin in 1 mL saline over 90–120 s | Perilesional injection & application onto equine collagen sponge scaffold | Time to complete closure (mean 30 days); wound area reduction; re-epithelialization | Weekly up to 1 year | None |
| Andreone et al. [26] (2019) | Retrospective case series (5 patients) | 2 massive burns; 3 chronic burn wounds | Full-thickness burns | Rigeneracons micrografts + autologous PRF | Spray-on via Vivostat® Spraypen (Vivostat A/S, Alleroed, Denmark)onto Integra® dermal template; antimicrobial dressing | Time to re-epithelialization (7–10 days); rate of complete graft incorporation | Mean 7–10 days | None reported |
| Iglesias et al. [23] (2023) | Open-label RCT (20 SSc patients) | >18 y, BMI > 18 kg/m2 | Digital ulcers, Raynaud’s | ADSVF via collagenase digestion + fat micrograft; 60 mL lipoaspirate → 3 mL ADSVF → mixed with 40 mL fat | Injection along radial/ulnar digital pedicles and subcutaneously into palm and dorsum | Time to complete closure (all wounds were closed by week 9), Pain VAS; ulcer count; Raynaud’s frequency/intensity; mobility; thumb opposition; capillary density; hand-function; QoL scores | 0 & 168 days | Donor site pain; ecchymosis (resolved day 5) |
| Baglioni et al. [10] (2024) | Uncontrolled pilot (12 patients) | Patients with postsurgical dehiscence | Chronic wound dehiscence | Rigenera® mechanical micrografts from adipose ± dermis (fluid suspension) | Injection into wound edges and floor under local/regional block | % lesion size reduction; % complete healing (75% of wounds achieved full closure by day 90 (and 91.6% had ≥ 50% reduction by day 90) cellular antioxidant activity; exosome profiling | 90 days | Not reported |
| Author, Year | Study Design | Patient Demographics | Wound Types | Graft Preparation Method | Application Technique | Outcomes Measured | Follow-Up Duration | Adverse Events |
|---|---|---|---|---|---|---|---|---|
| Svolacchia et al. [27] (2016) | Case series (n not stated) | Not reported | Hypertrophic & keloid scars | Rigenera™ mechanical disaggregation of dermal punch biopsies (3 mm) into saline suspension | Intralesional injection | Scar appearance & texture (Vancouver scale); histology (papillary dermis architecture; collagen realignment) | 4 months | None |
| Miranda et al. [28] (2018) | Case series (n = 15 patients) | Age: mean 72.2 ± 8.41 years (range 57–82) | Chronic ulcers (venous, diabetic, pressure, post-traumatic) | Rigeneracons dermal micrografts (collected with a 3 mm diameter punch biopsy) | Topical application on wound bed | Early response, time to complete healing (20–160 days); granulation tissue formation; scar quality neo-angiogenesis | 6 months | No complication noticed. One patient died of cardiovascular causes after 16 weeks (unrelated to procedure) |
| Tresoldi et al. [22] (2019) | RCT (23 units; 20 patients) | median 78 years; 4 F/16 M | Acute postsurgical soft-tissue loss (BCC, SCC, others) | Rigeneracons-derived dermal micrografts in 2.5 mL saline over 90 s | Imbibed into Integra® dermal substitute + perilesional infiltration | Re-epithelialization rate (%) at 4 weeks | 4–6 weeks | 1 wound infection → dropout |
| Riccio et al. [24] (2019) | Multicentre observational | Not reported | Full-thickness posttraumatic limb skin defects | Rigeneracons mechanical micrografts | Spray-on micrograft suspension over wound bed | Time to closure (average 48 days, range 35–84); quality of regeneration (clinical assessment) | Not reported | No complication noticed |
| Study (Citation) | Number of Patients (n) | Patient Age (Mean ± SD or Range) | Micrograft Volume/Formulation | Number of Applications | Adjunct Treatments |
|---|---|---|---|---|---|
| Iglesias et al. [23] (2023) | 20 | 35–72 yrs; mean ≈ 54 yrs | ADSVF + fat micrografts (volume: 10 mL injected into hand) | Single session | Standard wound dressings |
| Baglioni et al. [10] (2024) | 14 | 24–80 yrs (mean ~55 yrs; mode 71–80 yrs) | Fat micrografts: 10 mL lipoaspirate processed 3 min @80 rpm; Dermis micrografts: skin fragment (~lesion/20) in 6 mL saline | Single application | Collagen scaffold + hydrofibre/polyurethane foam dressings |
| Riccio et al. [24] (2019) | 70 | 53 yrs (34–74 yrs) | 1 cm2 dermal biopsy → mechanical disaggregation in two 3 mL saline aliquots; yield ~5 mL suspension | Single application | Equine collagen sponge “biocomplex,” paraffin gauze dressing |
| Andreone et al. [26] (2019) | 5 | 22–46 yrs | 4 × 2 cm2 dermal sample (0.2 mm thick) mechanically disaggregated → 5 mL cell suspension; PRF: 5 mL obtained from 120 mL autologous blood | 1–3 injections per patient (case-dependent) | Integra® dermal template; Acticoat® antimicrobial dressing (Smith & Nephew Medical Ltd., Hull, UK); NPWT in one case |
| Marcarelli et al. [25] (2017) | 3 | Elderly —not specified | Autologous micro-grafts via Rigeneracons (filtered <80 µm) suspended in saline (volume NR) | 1–2 applications per patient | NR |
| Miranda et al. [28] (2018) | 15 | (mean ± standard deviation) 72.2 ± 8.41 | Specimen collected with a 3 mm diameter biopsy punch and dissociated by the Rigenera System | Simple application | NR |
| Tresoldi et al. [22] (2019) | 20 (24 wounds) | NR | Fat micrografts + adipose-derived SVF injections into fingers (volume NR) | Single application | +standard systemic sclerosis medical therapy |
| Svolacchia et al. [27] (2016) | 14 | 41–58 yrs | 1 mL viable adipose micrografts (50 µm-filtered) emulsified in 1 mL cross-linked hyaluronic acid scaffold; lipoaspirate: 3 mL yield ≈1.2 mL final suspension | Single application | Panthenol “Dermal plus 25 High Performance” (Beauty System Pharma S.r.l., Padova, Italy) cross-linked HA; no other adjuncts |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zapsalis, K.; Ioannidis, O.; Anestiadou, E.; Pantelidou, M.; Siozos, K.; Xylas, C.; Gemousakakis, G.; Cheva, A.; Bekiari, C.; Loukousia, A.; et al. Applications of Adipose Tissue Micrografts (ATM) and Dermis Micrografts (DMG) in Wound Healing: A Scoping Review of Clinical Studies. Bioengineering 2025, 12, 948. https://doi.org/10.3390/bioengineering12090948
Zapsalis K, Ioannidis O, Anestiadou E, Pantelidou M, Siozos K, Xylas C, Gemousakakis G, Cheva A, Bekiari C, Loukousia A, et al. Applications of Adipose Tissue Micrografts (ATM) and Dermis Micrografts (DMG) in Wound Healing: A Scoping Review of Clinical Studies. Bioengineering. 2025; 12(9):948. https://doi.org/10.3390/bioengineering12090948
Chicago/Turabian StyleZapsalis, Konstantinos, Orestis Ioannidis, Elissavet Anestiadou, Maria Pantelidou, Konstantinos Siozos, Christos Xylas, Georgios Gemousakakis, Angeliki Cheva, Chryssa Bekiari, Antonia Loukousia, and et al. 2025. "Applications of Adipose Tissue Micrografts (ATM) and Dermis Micrografts (DMG) in Wound Healing: A Scoping Review of Clinical Studies" Bioengineering 12, no. 9: 948. https://doi.org/10.3390/bioengineering12090948
APA StyleZapsalis, K., Ioannidis, O., Anestiadou, E., Pantelidou, M., Siozos, K., Xylas, C., Gemousakakis, G., Cheva, A., Bekiari, C., Loukousia, A., Symeonidis, S., Bitsianis, S., Pramateftakis, M.-G., Kotidis, E., Mantzoros, I., & Angelopoulos, S. (2025). Applications of Adipose Tissue Micrografts (ATM) and Dermis Micrografts (DMG) in Wound Healing: A Scoping Review of Clinical Studies. Bioengineering, 12(9), 948. https://doi.org/10.3390/bioengineering12090948








