Decreasing Bone Resorption by Inducing Anti-Osteoclastogenic IFN-γ and IL-10 Expression in the Spleen Through an Electromagnetic Field on LPS-Induced Osteoporosis Mice
Abstract
1. Introduction
2. Materials and Methods
2.1. LPS-Induced Osteoporosis Mouse Model
2.2. PEMF Exposure System
2.3. Analysis of Serum Bone Biomarker
2.4. Quantitative Analysis of the Gene Expression by qRT-PCR
2.5. Micro-CT
2.6. Hematoxylin and Eosin (H&E) and Masson-Goldner’s Trichrome Staining
2.7. Statistical Analysis
3. Results
3.1. Influence of PEMF on Mouse Body Weight
3.2. Serum Biochemical Analysis
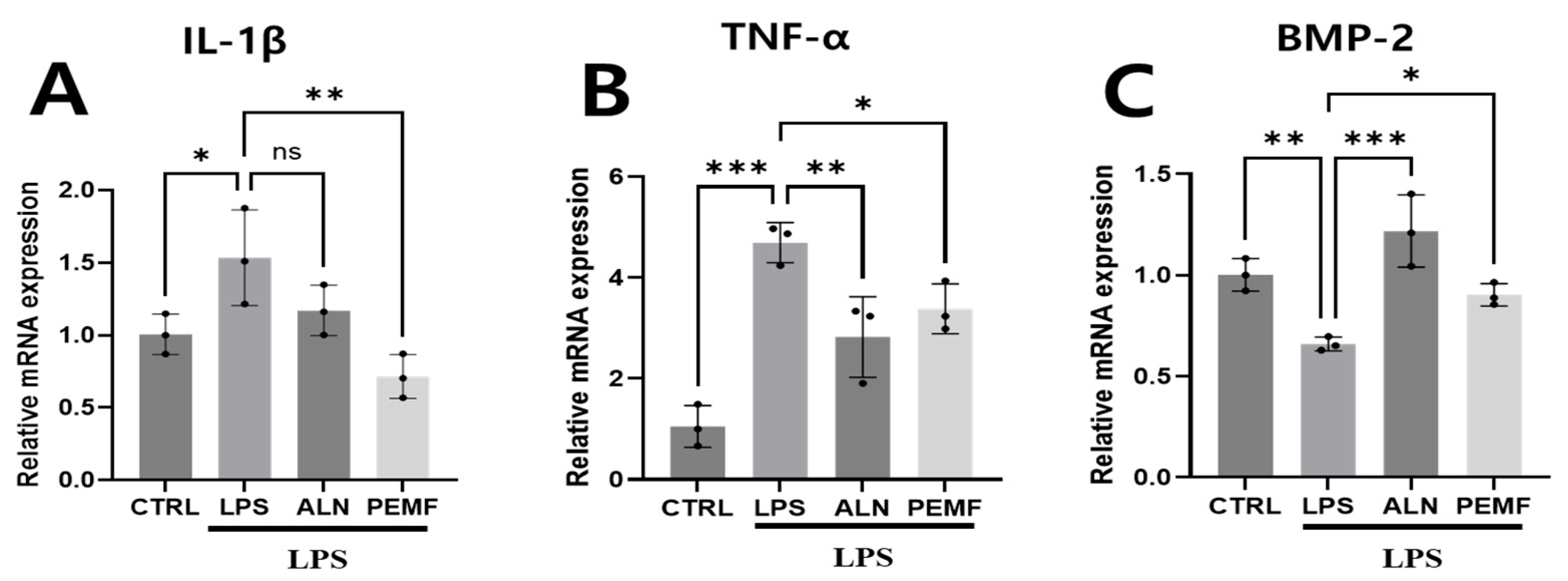
3.3. Effect of PEMF on Osteogenic and Inflammation-Related Gene Expression in Blood Cells
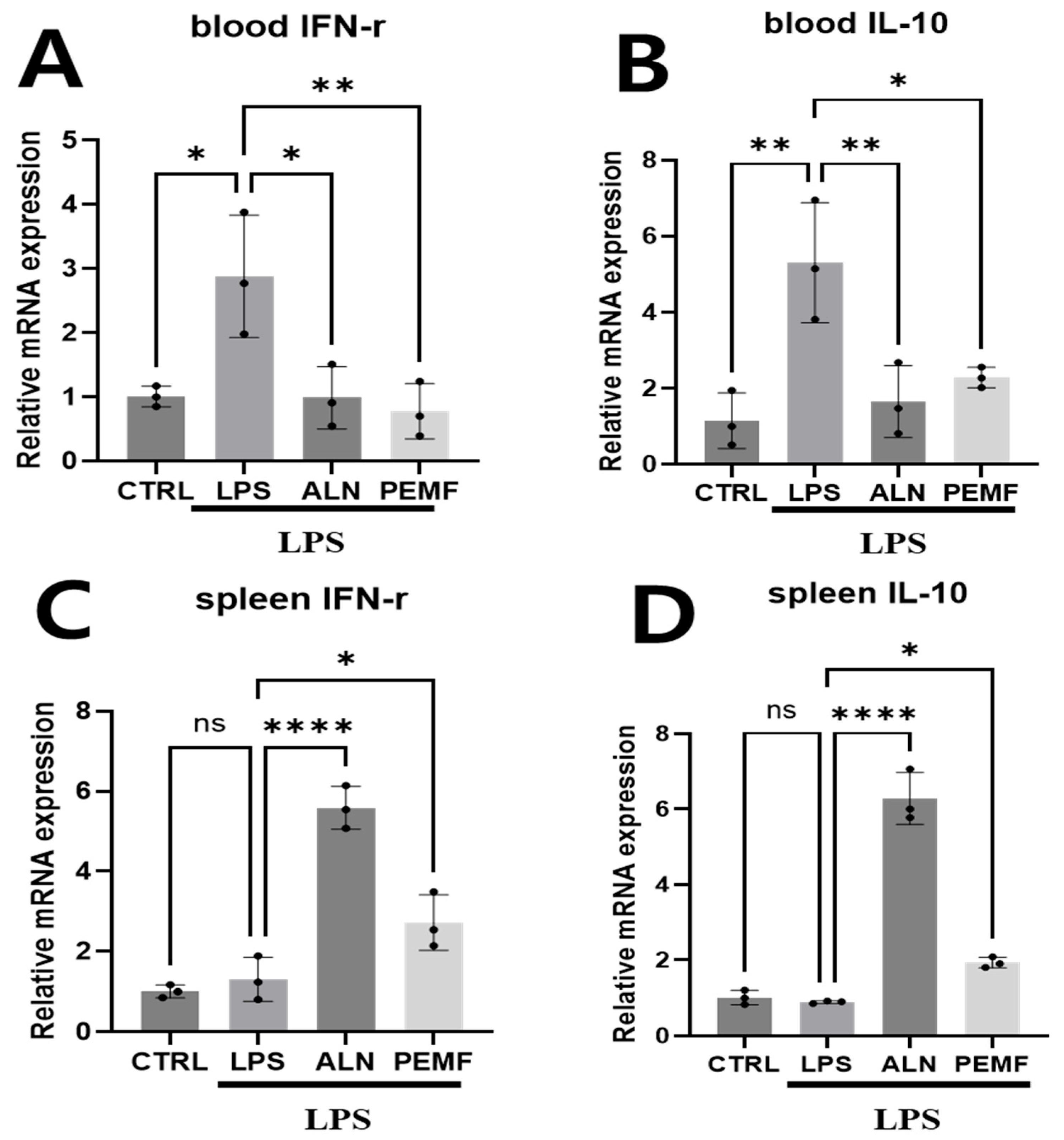
3.4. Effect of PEMF on Anti-Osteoclastogenesis-Related Gene Expression in Blood Cells and Spleen Tissue
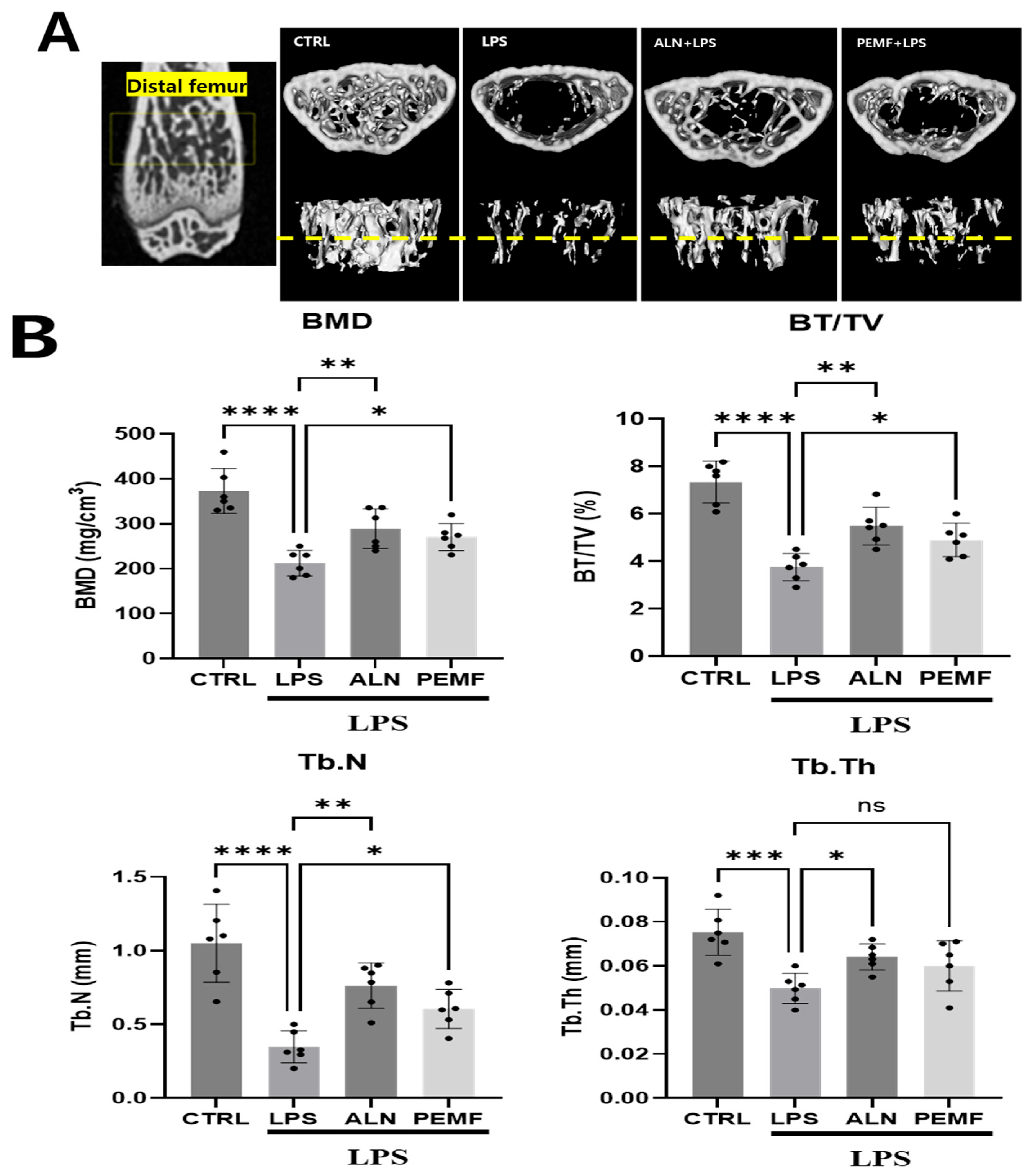
3.5. Effect of PEMF on Micro-CT and Histological Evaluation of Mice Femur
3.6. Effect of PEMF on Micro-CT Bone Evaluation of Mice Calvarial Defects
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- NIH Consensus Development Panel on Osteoporosis Prevention, Diagnosis, and Therapy. Osteoporosis prevention, diagnosis, and therapy. JAMA 2001, 285, 785–795. [Google Scholar] [CrossRef]
- Cooper, C.; Campion, G.; Melton, L.J., 3rd. Hip fractures in the elderly: A world-wide projection. Osteoporos. Int. 1992, 2, 285–289. [Google Scholar] [CrossRef] [PubMed]
- Brown, J.P. Long-Term Treatment of Postmenopausal Osteoporosis. Endocrinol. Metab. 2021, 36, 544–552. [Google Scholar] [CrossRef] [PubMed]
- Herrero, S.; Pico, Y. Treatments for post-menopausal osteoporotic women, what’s new? How can we manage long-term treatment? Eur. J. Pharmacol. 2016, 779, 8–21. [Google Scholar] [CrossRef] [PubMed]
- Jiaoyi, P.; Yongqi, S.; Kechun, G.; Xingyu, L.; Zezhong, L.; Jin Shuai, D.; Youjia, G.; Bing, X.; Xiaofeng, W. Assessing the efficacy and safety of different nonsteroidal anti-inflammatory drugs in the treatment of osteoarthritis: A systematic review and network meta-analysis based on RCT trials. PLoS ONE 2025, 20, e0320379. [Google Scholar] [CrossRef]
- Terpos, E. Bisphosphonate anticancer activity in multiple myeloma. Anticancer Agents Med. Chem. 2012, 12, 123–128. [Google Scholar] [CrossRef]
- Fisher, J.E.; Rogers, M.J.; Halasy, J.M.; Luckman, S.P.; Hughes, D.E.; Masarachia, P.J.; Wesolowski, G.; Russell, R.G.; Rodan, G.A.; Reszka, A.A. Alendronate mechanism of action: Geranylgeraniol, an intermediate in the mevalonate pathway, prevents inhibition of osteoclast formation, bone resorption, and kinase activation in vitro. Proc. Natl. Acad. Sci. USA 1999, 96, 133–138. [Google Scholar] [CrossRef]
- Suzuki, H.; Bando, K.; Tada, H.; Kiyama, T.; Oizumi, T.; Funayama, H.; Sugawara, S.; Takahashi, T.; Endo, Y. Augmentation of Lipopolysaccharide-Induced Production of IL-1α and IL-1β in Mice Given Intravenous Zoledronate (a Nitrogen-Containing Bisphosphonate) and Its Prevention by Clodronate (a Non-nitrogen-containing Bisphosphonate). Biol. Pharm. Bull. 2019, 42, 164–172. [Google Scholar] [CrossRef]
- Tabrah, F.; Hoffmeier, M.; Gilbert, F., Jr.; Batkin, S.; Bassett, C.A. Bone density changes in osteoporosis-prone women exposed to pulsed electromagnetic fields (PEMFs). J. Bone Miner. Res. 1990, 5, 437–442. [Google Scholar] [CrossRef]
- Zhang, Y.; Li, W.; Liu, C.; Yan, J.; Yuan, X.; Wang, W.; Wang, H.; Wu, H.; Yang, Y. Electromagnetic field treatment increases purinergic receptor P2X7 expression and activates its downstream Akt/GSK3β/β-catenin axis in mesenchymal stem cells under osteogenic induction. Stem Cell Res. Ther. 2019, 10, 407. [Google Scholar] [CrossRef]
- Trock, D.H.; Bollet, A.J.; Markoll, R. The effect of pulsed electromagnetic fields in the treatment of osteoarthritis of the knee and cervical spine. Report of randomized, double blind, placebo controlled trials. J. Rheumatol. 1994, 21, 1903–1911. [Google Scholar]
- Wang, P.; Liu, J.; Yang, Y.; Zhai, M.; Shao, X.; Yan, Z.; Zhang, X.; Wu, Y.; Cao, L.; Sui, B.; et al. Differential intensity-dependent effects of pulsed electromagnetic fields on RANKL-induced osteoclast formation, apoptosis, and bone resorbing ability in RAW264.7 cells. Bioelectromagnetics 2017, 38, 602–612. [Google Scholar] [CrossRef]
- Miyamoto, H.; Sawaji, Y.; Iwaki, T.; Masaoka, T.; Fukada, E.; Date, M.; Yamamoto, K. Intermittent pulsed electromagnetic field stimulation activates the mTOR pathway and stimulates the proliferation of osteoblast-like cells. Bioelectromagnetics 2019, 40, 412–421. [Google Scholar] [CrossRef]
- Jing, D.; Li, F.; Jiang, M.; Cai, J.; Wu, Y.; Xie, K.; Wu, X.; Tang, C.; Liu, J.; Guo, W.; et al. Pulsed electromagnetic fields improve bone microstructure and strength in ovariectomized rats through a Wnt/Lrp5/β-catenin signaling-associated mechanism. PLoS ONE 2013, 8, e79377. [Google Scholar] [CrossRef]
- Liu, H.F.; Yang, L.; He, H.C.; Zhou, J.; Liu, Y.; Wang, C.Y.; Wu, Y.C.; He, C.Q. Pulsed electromagnetic fields on postmenopausal osteoporosis in Southwest China: A randomized, active-controlled clinical trial. Bioelectromagnetics 2013, 34, 323–332. [Google Scholar] [CrossRef] [PubMed]
- Bhandari, M.; Tornetta, P., 3rd; Sprague, S.; Najibi, S.; Petrisor, B.; Griffith, L.; Guyatt, G.H. Predictors of reoperation following operative management of fractures of the tibial shaft. J. Orthop. Trauma 2003, 17, 353–361. [Google Scholar] [CrossRef] [PubMed]
- Karladani, A.H.; Granhed, H.; Kärrholm, J.; Styf, J. The influence of fracture etiology and type on fracture healing: A review of 104 consecutive tibial shaft fractures. Arch. Orthop. Trauma Surg. 2001, 121, 325–328. [Google Scholar] [CrossRef] [PubMed]
- Yogesha, S.D.; Khapli, S.M.; Srivastava, R.K.; Mangashetti, L.S.; Pote, S.T.; Mishra, G.C.; Wani, M.R. IL-3 inhibits TNF-alpha-induced bone resorption and prevents inflammatory arthritis. J. Immunol. 2009, 182, 361–370. [Google Scholar] [CrossRef]
- Mizutani, H.; Ishihara, Y.; Izawa, A.; Fujihara, Y.; Kobayashi, S.; Gotou, H.; Okabe, E.; Takeda, H.; Ozawa, Y.; Kamiya, Y.; et al. Lipopolysaccharide of Aggregatibacter actinomycetemcomitans up-regulates inflammatory cytokines, prostaglandin E2 synthesis and osteoclast formation in interleukin-1 receptor antagonist-deficient mice. J. Periodontal Res. 2013, 48, 748–756. [Google Scholar] [CrossRef]
- Vandamme, T.F. Use of rodents as models of human diseases. J. Pharm. Bioallied Sci. 2014, 6, 2–9. [Google Scholar] [CrossRef]
- Yoshii, T.; Magara, S.; Miyai, D.; Nishimura, H.; Kuroki, E.; Furudoi, S.; Komori, T.; Ohbayashi, C. Local levels of interleukin-1beta, -4, -6 and tumor necrosis factor alpha in an experimental model of murine osteomyelitis due to staphylococcus aureus. Cytokine 2002, 19, 59–65. [Google Scholar] [CrossRef]
- Nam, M.H.; Park, H.J.; Seo, Y.K. Reduction of Osteoclastic Differentiation of Raw 264.7 Cells by EMF Exposure through TRPV4 and p-CREB Pathway. Int. J. Mol. Sci. 2023, 24, 3058. [Google Scholar] [CrossRef]
- Park, H.-J.; Choi, J.-H.; Nam, M.-H.; Seo, Y.-K. Induced Neurodifferentiation of hBM-MSCs through Activation of the ERK/CREB Pathway via Pulsed Electromagnetic Fields and Physical Stimulation Promotes Neurogenesis in Cerebral Ischemic Models. Int. J. Mol. Sci. 2022, 23, 1177. [Google Scholar] [CrossRef]
- Wang, Y.; Zhang, L.; Wang, K.; Zhou, H.; Li, G.; Xu, L.; Hu, Z.; Cao, X.; Shi, F.; Zhang, S. Circulating Exosomes from Mice with LPS-Induced Bone Loss Inhibit Osteoblast Differentiation. Calcif. Tissue Int. 2022, 111, 185–195. [Google Scholar] [CrossRef]
- Xu, L.; Sun, X.; Han, X.; Li, H.; Li, X.; Zhu, L.; Wang, X.; Li, J.; Sun, H. Dihydromyricetin ameliorate postmenopausal osteoporosis in ovariectomized mice: Integrative microbiomic and metabolomic analysis. Front. Pharmacol. 2024, 15, 1452921. [Google Scholar] [CrossRef]
- Correa, C.B.; Camargos, G.V.; Chatterjee, M.; Mesquita, M.F.; Del Bel Cury, A.A.; Naert, I.; Duyck, J.; Vandamme, K. Can the alendronate dosage be altered when combined with high-frequency loading in osteoporosis treatment? Osteoporos. Int. 2017, 28, 1287–1293. [Google Scholar] [CrossRef]
- Boonen, S.; Ferrari, S.; Miller, P.D.; Eriksen, E.F.; Sambrook, P.N.; Compston, J.; Reid, I.R.; Vanderschueren, D.; Cosman, F. Postmenopausal osteoporosis treatment with antiresorptives: Effects of discontinuation or long-term continuation on bone turnover and fracture risk—A perspective. J. Bone Miner. Res. 2012, 27, 963–974. [Google Scholar] [CrossRef]
- Ettinger, M.P. Aging bone and osteoporosis: Strategies for preventing fractures in the elderly. Arch. Intern. Med. 2003, 163, 2237–2246. [Google Scholar] [CrossRef]
- Iglesias, J.E.; Salum, F.G.; Figueiredo, M.A.; Cherubini, K. Important aspects concerning alendronate-related osteonecrosis of the jaws: A literature review. Gerodontology 2015, 32, 169–178. [Google Scholar] [CrossRef]
- Bott, K.N.; Feldman, E.; de Souza, R.J.; Comelli, E.M.; Klentrou, P.; Peters, S.J.; Ward, W.E. Lipopolysaccharide-Induced Bone Loss in Rodent Models: A Systematic Review and Meta-Analysis. J. Bone Miner. Res. 2023, 38, 198–213. [Google Scholar] [CrossRef]
- He, Y.Q.; Yang, H.; Shen, Y.; Zhang, J.H.; Zhang, Z.G.; Liu, L.L.; Song, H.T.; Lin, B.; Hsu, H.Y.; Qin, L.P.; et al. Monotropein attenuates ovariectomy and LPS-induced bone loss in mice and decreases inflammatory impairment on osteoblast through blocking activation of NF-κB pathway. Chem. Biol. Interact. 2018, 291, 128–136. [Google Scholar] [CrossRef]
- Xia, G.; Zhao, Y.; Yu, Z.; Tian, Y.; Wang, Y.; Wang, S.; Wang, J.; Xue, C. Phosphorylated Peptides from Antarctic Krill (Euphausia superba) Prevent Estrogen Deficiency Induced Osteoporosis by Inhibiting Bone Resorption in Ovariectomized Rats. J. Agric. Food Chem. 2015, 63, 9550–9557. [Google Scholar] [CrossRef]
- Patwa, C.; Jindani, N.; Afroz, S. Study of serum calcium levels in premenopausal women and postmenopausal women. Med. Pulse Int. J. Physiol. 2017, 4, 14–16. [Google Scholar] [CrossRef]
- Chung, H.J.; Cho, L.; Shin, J.S.; Lee, J.; Ha, I.H.; Park, H.J.; Lee, S.K. Effects of JSOG-6 on protection against bone loss in ovariectomized mice through regulation of osteoblast differentiation and osteoclast formation. BMC Complement. Altern. Med. 2014, 14, 184. [Google Scholar] [CrossRef]
- Mustafa, R.A.; Alfky, N.A.A.; Hijazi, H.H.; Header, E.A.; Azzeh, F.S. Biological effect of calcium and vitamin D dietary supplements against osteoporosis in ovariectomized rats. Prog. Nutr. 2018, 20, 86–93. [Google Scholar]
- Yu, M.; Ding, J.; Zhao, L.; Huang, X.; Ma, K.Z. Possible association between serum alkaline phosphatase concentration and thoracicacute aortic dissection. Int. J. Clin. Exp. Med. 2015, 8, 16737–16740. [Google Scholar]
- Kuo, T.R.; Chen, C.H. Bone biomarker for the clinical assessment of osteoporosis: Recent developments and future perspectives. Biomark. Res. 2017, 5, 18. [Google Scholar] [CrossRef]
- Lei, T.; Li, F.; Liang, Z.; Tang, C.; Xie, K.; Wang, P.; Dong, X.; Shan, S.; Liu, J.; Xu, Q.; et al. Effects of four kinds of electromagnetic fields (EMF) with different frequency spectrum bands on ovariectomized osteoporosis in mice. Sci. Rep. 2017, 7, 553. [Google Scholar] [CrossRef]
- Solberg, L.B.; Brorson, S.H.; Stordalen, G.A.; Bækkevold, E.S.; Andersson, G.; Reinholt, F.P. Increased tartrate-resistant Acid phosphatase expression in osteoblasts and osteocytes in experimental osteoporosis in rats. Calcif. Tissue Int. 2014, 94, 510–521. [Google Scholar] [CrossRef]
- Song, Z.H.; Xie, W.; Zhu, S.Y.; Pan, J.J.; Zhou, L.Y.; He, C.Q. Effects of PEMFs on Osx, Ocn, TRAP, and CTSK gene expression in postmenopausal osteoporosis model mice. Int. J. Clin. Exp. Pathol. 2018, 11, 1784–1790. [Google Scholar]
- Abuohashish, H.M.; Ahmed, M.M.; Al-Rejaie, S.S.; Eltahir, K.E. The antidepressant bupropion exerts alleviating properties in an ovariectomized osteoporotic rat model. Acta Pharmacol. Sin. 2015, 36, 209–220. [Google Scholar] [CrossRef]
- Pi, Y.; Liang, H.; Yu, Q.; Yin, Y.; Xu, H.; Lei, Y.; Han, Z.; Tian, J. Low-frequency pulsed electromagnetic field inhibits RANKL-induced osteoclastic differentiation in RAW264.7 cells by scavenging reactive oxygen species. Mol. Med. Rep. 2019, 19, 4129–4136. [Google Scholar] [CrossRef]
- Hou, G.Q.; Guo, C.; Song, G.H.; Fang, N.; Fan, W.J.; Chen, X.D.; Yuan, L.; Wang, Z.Q. Lipopolysaccharide (LPS) promotes osteoclast differentiation and activation by enhancing the MAPK pathway and COX-2 expression in RAW264.7 cells. Int. J. Mol. Med. 2013, 32, 503–510. [Google Scholar] [CrossRef]
- Dinarello, C.A. Interleukin-1 in the pathogenesis and treatment of inflammatory diseases. Blood 2011, 117, 3720–3732. [Google Scholar] [CrossRef]
- Osta, B.; Benedetti, G.; Miossec, P. Classical and Paradoxical Effects of TNF-α on Bone Homeostasis. Front. Immunol. 2014, 5, 48. [Google Scholar] [CrossRef]
- Kitaura, H.; Kimura, K.; Ishida, M.; Kohara, H.; Yoshimatsu, M.; Takano-Yamamoto, T. Immunological reaction in TNF-α-mediated osteoclast formation and bone resorption in vitro and in vivo. Clin. Dev. Immunol. 2013, 2013, 181849. [Google Scholar] [CrossRef]
- Xu, R.H.; Peck, R.M.; Li, D.S.; Feng, X.; Ludwig, T.; Thomson, J.A. Basic FGF and suppression of BMP signaling sustain undifferentiated proliferation of human ES cells. Nat. Methods 2005, 2, 185–190. [Google Scholar] [CrossRef]
- Liu, S.; Bi, J.; Zhang, Y.; Song, Q.; Yu, M.; Sun, X.; Qu, D.; Liu, S. Preliminary study on the electromagnetic field treatment of osteoporosis in rats. Technol. Health Care 2020, 28, 47–55. [Google Scholar] [CrossRef]
- Inoue, K.; Ng, C.; Xia, Y.; Zhao, B. Regulation of Osteoclastogenesis and Bone Resorption by miRNAs. Front. Cell Dev. Biol. 2021, 9, 651161. [Google Scholar] [CrossRef]
- Da, W.; Tao, L.; Zhu, Y. The Role of Osteoclast Energy Metabolism in the Occurrence and Development of Osteoporosis. Front. Endocrinol. 2021, 12, 675385. [Google Scholar] [CrossRef]
- Ono, T.; Nakashima, T. Recent advances in osteoclast biology. Histochem. Cell Biol. 2018, 149, 325–341. [Google Scholar] [CrossRef]
- Kohara, H.; Kitaura, H.; Fujimura, Y.; Yoshimatsu, M.; Morita, Y.; Eguchi, T.; Masuyama, R.; Yoshida, N. IFN-γ directly inhibits TNF-α-induced osteoclastogenesis in vitro and in vivo and induces apoptosis mediated by Fas/Fas ligand interactions. Immunol. Lett. 2011, 137, 53–61. [Google Scholar] [CrossRef]
- Li, S.; Liu, G.; Hu, S. Osteoporosis: Interferon-gamma-mediated bone remodeling in osteoimmunology. Front. Immunol. 2024, 15, 1396122. [Google Scholar] [CrossRef] [PubMed]
- Evans, K.E.; Fox, S.W. Interleukin-10 inhibits osteoclastogenesis by reducing NFATc1 expression and preventing its translocation to the nucleus. BMC Cell Biol. 2007, 8, 4. [Google Scholar] [CrossRef]
- Houri-Haddad, Y.; Soskolne, W.A.; Halabi, A.; Shapira, L. IL-10 gene transfer attenuates P. gingivalis-induced inflammation. J. Dent. Res. 2007, 86, 560–564. [Google Scholar] [CrossRef] [PubMed]
- Bronte, V.; Pittet, M.J. The spleen in local and systemic regulation of immunity. Immunity 2013, 39, 806–818. [Google Scholar] [CrossRef]
- Sun, D.; Zheng, X.; Chen, Y.; Jia, C.; Xu, S.; Lin, C.; Zhang, P.; Zhang, Z.; Cai, D.; Jin, D.; et al. Enhancement of osteogenesis post-splenectomy does not attenuate bone loss in ovariectomized rats. J. Orthop. Res. 2015, 33, 1356–1363. [Google Scholar] [CrossRef] [PubMed]
- Kavak, N.; Tıkman, M.; Güler, İ.; Balcı, N.; Kavak, R.P.; Sever, M. An experimental study on the effects of splenectomy on bone fracture healing. Signa Vitae 2023, 19, 86–94. [Google Scholar] [CrossRef]
- Kim, J.H.; Kim, M.; Jung, H.S.; Sohn, Y. Leonurus sibiricus L. ethanol extract promotes osteoblast differentiation and inhibits osteoclast formation. Int. J. Mol. Med. 2019, 44, 913–926. [Google Scholar] [CrossRef]
- Kim, B.G.; Kwak, H.B.; Choi, E.Y.; Kim, H.S.; Kim, M.H.; Kim, S.H.; Choi, M.K.; Chun, C.H.; Oh, J.; Kim, J.J. Amorphigenin inhibits Osteoclast differentiation by suppressing c-Fos and nuclear factor of activated T cells. Anat. Cell Biol. 2010, 43, 310–316. [Google Scholar] [CrossRef]
- Chun, K.H.; Jin, H.C.; Kang, K.S.; Chang, T.S.; Hwang, G.S. Poncirin Inhibits Osteoclast Differentiation and Bone Loss through Down-Regulation of NFATc1 In Vitro and In Vivo. Biomol. Ther. 2020, 28, 337–343. [Google Scholar] [CrossRef] [PubMed]
- Zhou, J.; Liao, Y.; Xie, H.; Liao, Y.; Zeng, Y.; Li, N.; Sun, G.; Wu, Q.; Zhou, G. Effects of combined treatment with ibandronate and pulsed electromagnetic field on ovariectomy-induced osteoporosis in rats. Bioelectromagnetics 2017, 38, 31–40. [Google Scholar] [CrossRef]
- Huang, J.; Li, Y.; Wang, L.; He, C. Combined Effects of Low-Frequency Pulsed Electromagnetic Field and Melatonin on Ovariectomy-Induced Bone Loss in Mice. Bioelectromagnetics 2021, 42, 616–628. [Google Scholar] [CrossRef] [PubMed]
- Rentsch, C.; Schneiders, W.; Manthey, S.; Rentsch, B.; Rammelt, S. Comprehensive histological evaluation of bone implants. Biomatter 2014, 4, e27993. [Google Scholar] [CrossRef] [PubMed]





| Genes | (5′-3′) | Sequence |
|---|---|---|
| IL-1β | Forward Reverse | GCA ACT GTT CCT GAA CTC AAC T ATC TTT TGG GGT CCG TCA ACT |
| IL-10 | Forward Reverse | GCT CTT ACT GAC TGG CAT GAG CGC AGC TCT AGG AGC ATG TG |
| TNF-α | Forward Reverse | AGG CGG TGC TTG TTC CTC A AGA CAG AAG AGC GTG GTG GC |
| IFN-γ | Forward Reverse | GAA CTG GCA GAA GAG GCA CT AGA CAG AAG AGC GTG GTG GC |
| BMP-2 | Forward Reverse | AAG AAG CCA TCG AGG ACC TG CAG TTC CAC ATA CAG CAG GC |
| GAPDH | Forward Reverse | AGG TCG GTG TGA ACG GAT TTG TGT AGA CCA TGT AGT TGA GGT CA |
| Days | Body Weight (g) | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| CTRL (n = 10) | LPS (n = 10) | ALN + LPS (n = 10) | PEMF + LPS (n = 10) | |||||||||
| 0 | 31.5 | ± | 1.6 | 32.0 | ± | 1.6 | 32.0 | ± | 1.8 | 31.7 | ± | 1.6 |
| 3 | 33.0 | ± | 1.7 | 29.5 | ± | 2 **** | 30.5 | ± | 1.8 * | 30.7 | ± | 2.0 * |
| 7 | 33.8 | ± | 1.9 | 30.1 | ± | 1.4 **** | 30.6 | ± | 1.7 *** | 30.2 | ± | 1.1 **** |
| 10 | 33.6 | ± | 2.1 | 30.4 | ± | 2.1 *** | 31.3 | ± | 1.3 * | 30.6 | ± | 1.2 ** |
| 14 | 33.7 | ± | 1.8 | 30.6 | ± | 1.9 *** | 31.5 | ± | 1.4 * | 31.5 | ± | 1.0 ** |
| 17 | 32.9 | ± | 2.2 | 30.4 | ± | 1.6 ** | 31.8 | ± | 1.7 | 31.3 | ± | 1.3 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nam, M.-H.; Park, H.-J.; Kim, T.-W.; Lee, I.-H.; Yun, H.-D.; Chen, Z.; Seo, Y.-K. Decreasing Bone Resorption by Inducing Anti-Osteoclastogenic IFN-γ and IL-10 Expression in the Spleen Through an Electromagnetic Field on LPS-Induced Osteoporosis Mice. Bioengineering 2025, 12, 923. https://doi.org/10.3390/bioengineering12090923
Nam M-H, Park H-J, Kim T-W, Lee I-H, Yun H-D, Chen Z, Seo Y-K. Decreasing Bone Resorption by Inducing Anti-Osteoclastogenic IFN-γ and IL-10 Expression in the Spleen Through an Electromagnetic Field on LPS-Induced Osteoporosis Mice. Bioengineering. 2025; 12(9):923. https://doi.org/10.3390/bioengineering12090923
Chicago/Turabian StyleNam, Myeong-Hyun, Hee-Jung Park, Tae-Woo Kim, In-Ho Lee, Hee-Deok Yun, Zuyu Chen, and Young-Kwon Seo. 2025. "Decreasing Bone Resorption by Inducing Anti-Osteoclastogenic IFN-γ and IL-10 Expression in the Spleen Through an Electromagnetic Field on LPS-Induced Osteoporosis Mice" Bioengineering 12, no. 9: 923. https://doi.org/10.3390/bioengineering12090923
APA StyleNam, M.-H., Park, H.-J., Kim, T.-W., Lee, I.-H., Yun, H.-D., Chen, Z., & Seo, Y.-K. (2025). Decreasing Bone Resorption by Inducing Anti-Osteoclastogenic IFN-γ and IL-10 Expression in the Spleen Through an Electromagnetic Field on LPS-Induced Osteoporosis Mice. Bioengineering, 12(9), 923. https://doi.org/10.3390/bioengineering12090923






