Impact of Disinfection and Sterilization on 3D-Printing Resin Performance for Surgical Guides in Cardiac Ablation Surgery
Abstract
1. Introduction
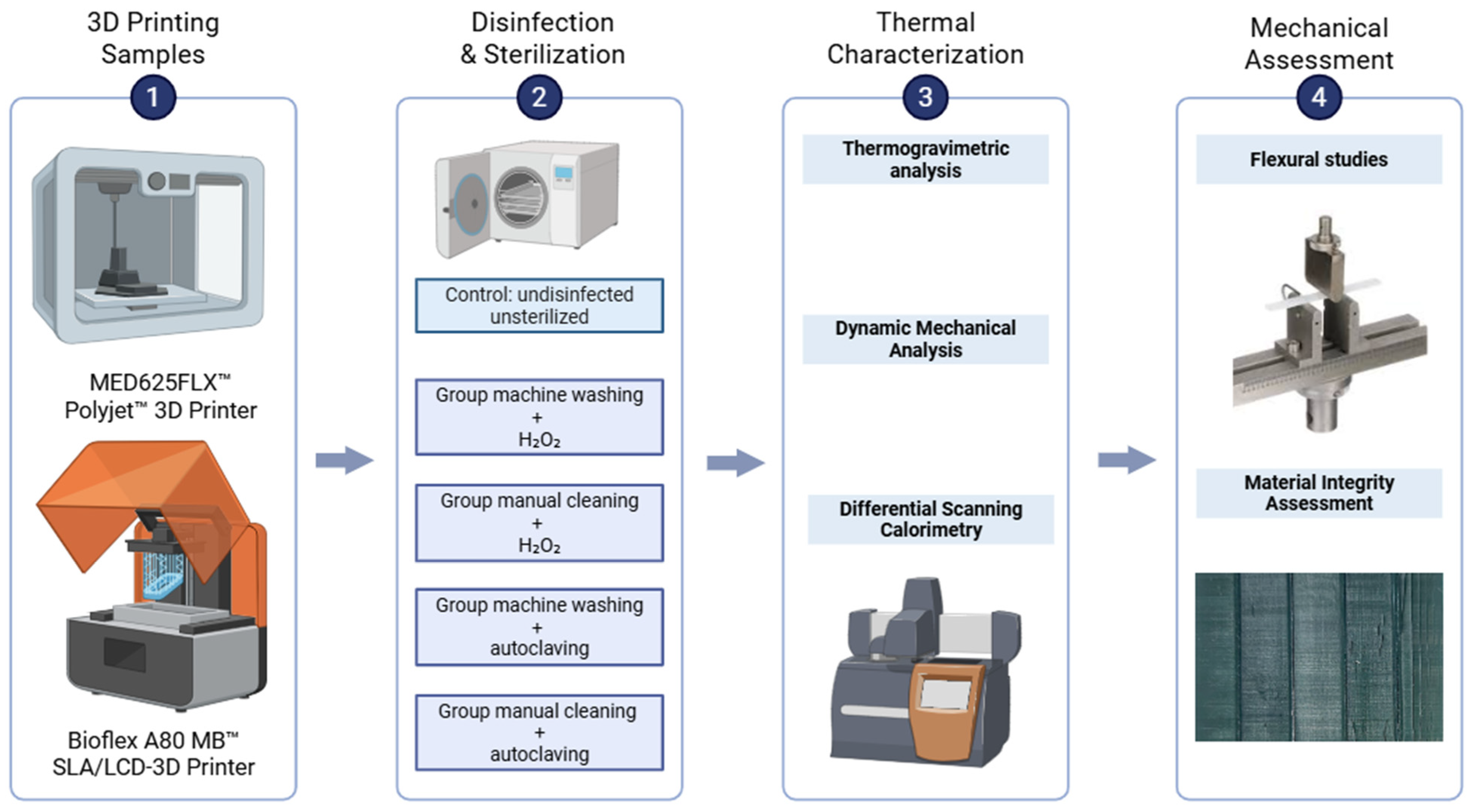
2. Materials and Methods
2.1. Materials and 3D Printing Process
2.1.1. MED625FLX
- 3D Printing Process
- Post-processing
2.1.2. BIOFLEX A80 MB
- 3D Printing Process
- Post-processing
2.2. Disinfection and Sterilization Methods
- Low-Temperature Washing
- High-Temperature Washing
- H2O2 Sterilization
- Autoclaving
2.3. Thermal Characterization
2.4. Flexural Testing
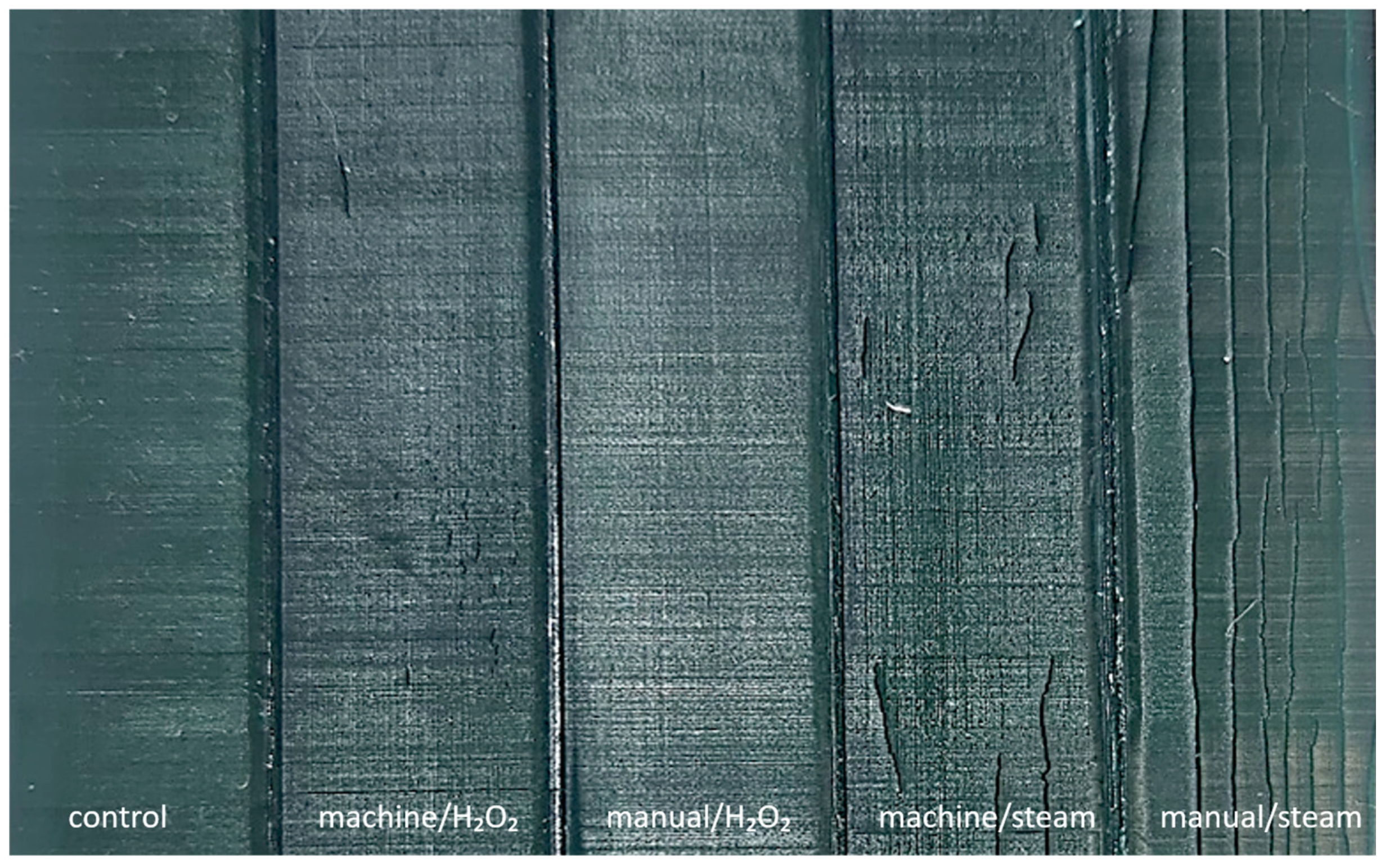
2.5. Material Integrity Assessment
2.6. Statistical Analysis
3. Results
3.1. Material Integrity
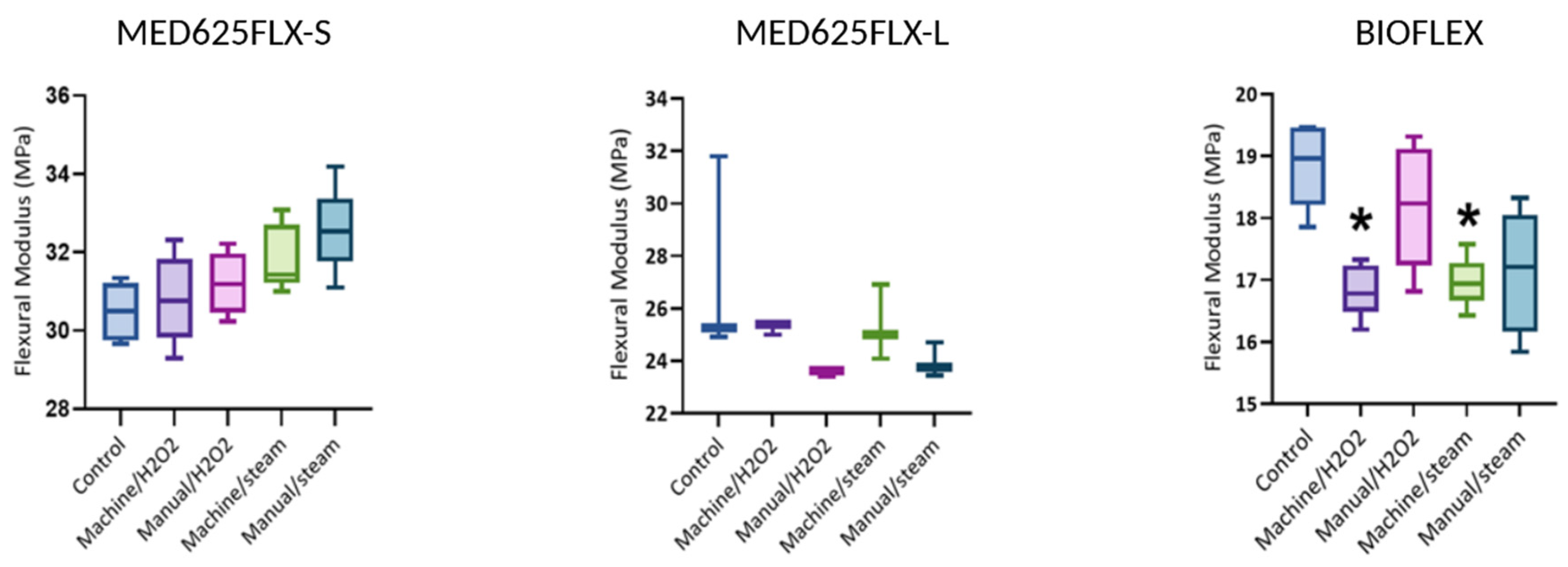
3.2. Flexural Modulus
3.3. Thermal Characterization
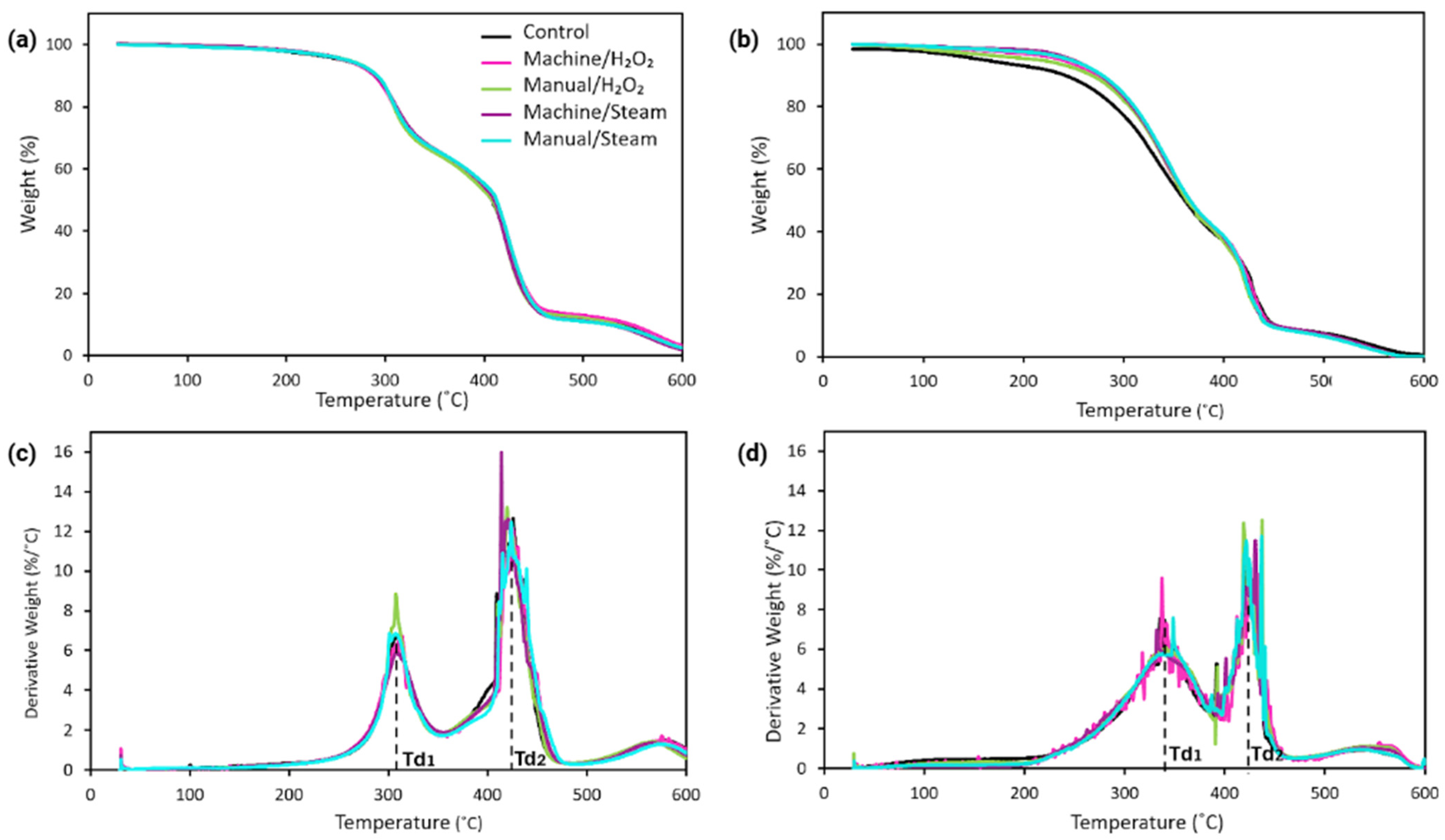
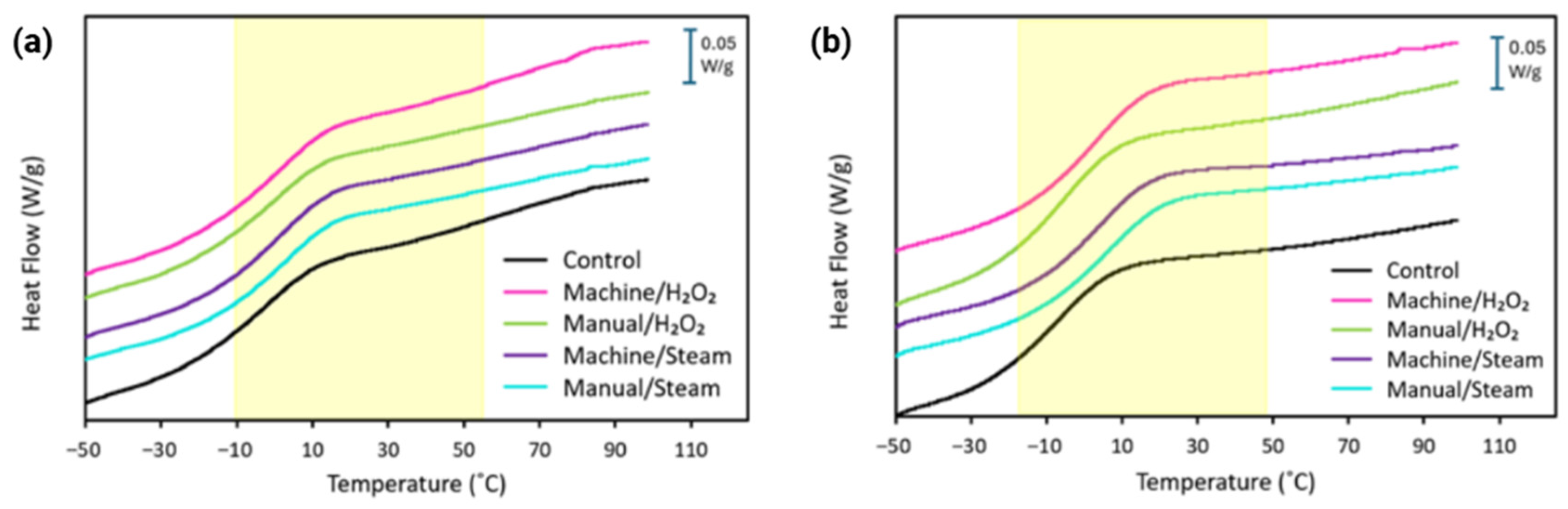
3.3.1. Thermogravimetric Analysis
3.3.2. Differential Scanning Calorimetry
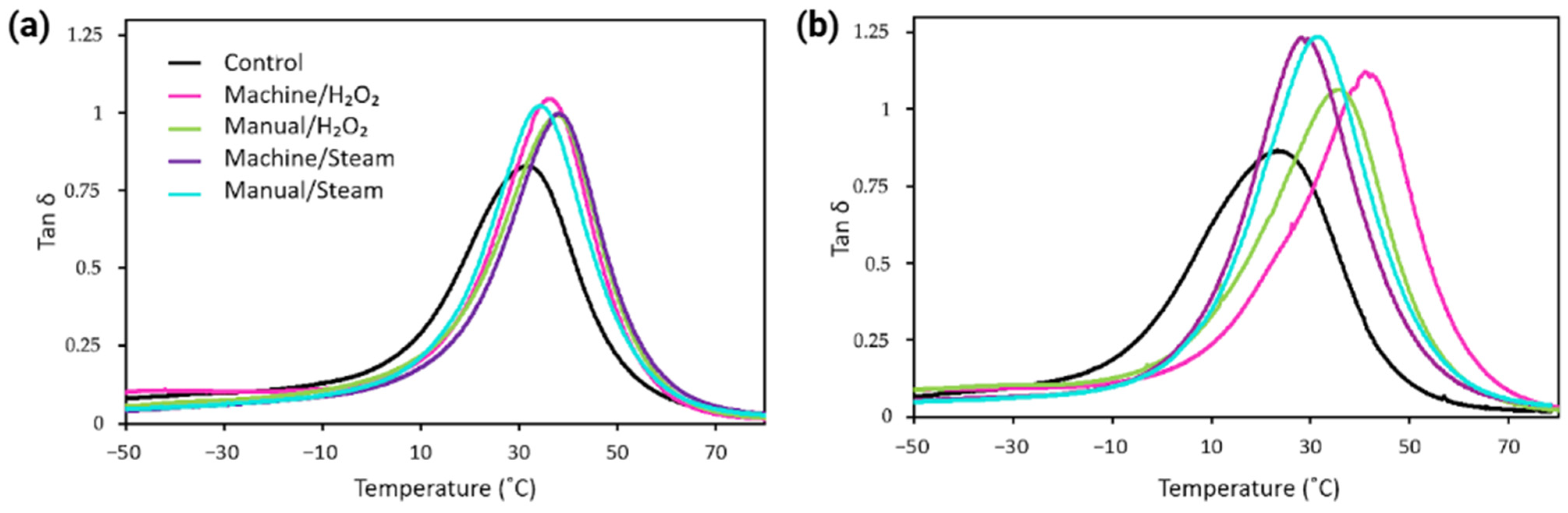
3.3.3. Viscoelastic Behavior
4. Discussion
4.1. Mechanical Integrity
4.2. Flexural Modulus
4.3. Decomposition Behavior
4.4. Glass Transition Temperature
4.5. Additional Considerations
4.6. Limitations and Future Considerations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| 3D | Three-Dimensional |
| CAD | Computer-Aided Design |
| CT | Computed Tomography |
| DMA | Dynamic Mechanical Analysis |
| DSC | Differential Scanning Calorimetry |
| DTG | Derivative Thermogravimetry |
| H2O2 | Hydrogen Peroxide Sterilization |
| LCD | Liquid Crystal Display |
| MDR | Medical Device Regulations |
| SLA | Stereolithography |
| Tg | Glass Transition Temperature |
| TGA | Thermogravimetric Analysis |
| VHP | Vaporized Hydrogen Peroxide |
References
- Pathak, K.; Saikia, R.; Das, A.; Das, D.; Islam, M.A.; Pramanik, P.; Parasar, A.; Borthakur, P.P.; Sarmah, P.; Saikia, M.; et al. 3D printing in biomedicine: Advancing personalized care through additive manufacturing. Explor. Med. 2023, 4, 1135–1167. [Google Scholar] [CrossRef]
- Oleksy, M.; Dynarowicz, K.; Aebisher, D. Rapid Prototyping Technologies: 3D Printing Applied in Medicine. Pharmaceutics 2023, 15, 2169. [Google Scholar] [CrossRef] [PubMed]
- Candelari, M.; Cappello, I.A.; Pannone, L.; Monaco, C.; Talevi, G.; Bori, E.; Ramak, R.; La Meir, M.; Gharaviri, A.; Chierchia, G.B.; et al. A 3D-printed surgical guide for ischemic scar targeting and ablation. Front. Cardiovasc. Med. 2022, 9, 1029816. [Google Scholar] [CrossRef]
- Talevi, G.; Pannone, L.; Monaco, C.; Bori, E.; Cappello, I.A.; Candelari, M.; Ramak, R.; La Meir, M.; Gharaviri, A.; Chierchia, G.B.; et al. Development of a 3D printed surgical guide for Brugada syndrome substrate ablation. Front. Cardiovasc. Med. 2022, 9, 1029685. [Google Scholar] [CrossRef]
- Eltsov, I.; Pannone, L.; Ramak, R.; Monaco, C.; Della Rocca, D.G.; Bala, G.; Kronenberger, R.; Overeinder, I.; Almorad, A.; Stroker, E.; et al. 3D mapping challenges in hybrid video-assisted thoracoscopic surgical ablation of Brugada syndrome. Interdiscip. Cardiovasc. Thorac. Surg. 2023, 37, ivad160. [Google Scholar] [CrossRef]
- Chepelev, L.; Wake, N.; Ryan, J.; Althobaity, W.; Gupta, A.; Arribas, E.; Santiago, L.; Ballard, D.H.; Wang, K.C.; Weadock, W.; et al. Radiological Society of North America (RSNA) 3D printing Special Interest Group (SIG): Guidelines for medical 3D printing and appropriateness for clinical scenarios. 3D Print. Med. 2018, 4, 11. [Google Scholar] [CrossRef]
- Wiseman, J.; Rawther, T.; Langbart, M.; Kernohan, M.; Ngo, Q. Sterilization of bedside 3D-printed devices for use in the operating room. Ann. 3D Print. Med. 2022, 5, 100045. [Google Scholar] [CrossRef]
- Labakoum, B.; Farhan, A.; Taleb, L.B.; Mouhsen, A.; Lyazidi, A. Effects of autoclaving and disinfection on 3D surgical guides using LCD technology for dental implant. 3D Print. Med. 2024, 10, 14. [Google Scholar] [CrossRef]
- Rutala, W.A. Guideline for Disinfection and Sterilization in Healthcare Facilities. 2008. Available online: https://www.cdc.gov/infection-control/hcp/disinfection-and-sterilization/index.html#toc (accessed on 3 January 2023).
- Rynio, P.; Galant, K.; Wójcik, Ł.; Grygorcewicz, B.; Kazimierczak, A.; Falkowski, A.; Gutowski, P.; Dołęgowska, B.; Kawa, M. Effects of Sterilization Methods on Different 3D Printable Materials for Templates of Physician-Modified Aortic Stent Grafts Used in Vascular Surgery—A Preliminary Study. Int. J. Mol. Sci. 2022, 23, 3539. [Google Scholar] [CrossRef]
- CDC. Infection Control. 2024 [cited 31 December 2024]. Bioburden of Surgical Devices. Available online: https://www.cdc.gov/infection-control/hcp/disinfection-sterilization/bioburden-surgical-devices.html (accessed on 31 December 2024).
- Fuentes, J.M.; Arrieta, M.P.; Boronat, T.; Ferrándiz, S. Effects of Steam Heat and Dry Heat Sterilization Processes on 3D Printed Commercial Polymers Printed by Fused Deposition Modeling. Polymers 2022, 14, 855. [Google Scholar] [CrossRef] [PubMed]
- Herczeg, C.K.; Song, J. Sterilization of Polymeric Implants: Challenges and Opportunities. ACS Appl. Bio. Mater. 2022, 5, 5077–5088. [Google Scholar] [CrossRef]
- Tipnis, N.P.; Burgess, D.J. Sterilization of implantable polymer-based medical devices: A review. Int. J. Pharm. 2018, 544, 455–460. [Google Scholar] [CrossRef]
- Guttridge, C.; Shannon, A.; O’Sullivan, A.; O’Sullivan, K.J.; O’Sullivan, L.W. Biocompatible 3D printing resins for medical applications: A review of marketed intended use, biocompatibility certification, and post-processing guidance. Ann. 3D Print. Med. 2022, 5, 100044. [Google Scholar] [CrossRef]
- Pettersson, A.B.V.; Ballardini, R.M.; Mimler, M.; Li, P.; Salmi, M.; Minssen, T.; Gibson, I.; Mäkitie, A. Core Legal Challenges for Medical 3D Printing in the EU. Healthcare 2024, 12, 1114. [Google Scholar] [CrossRef]
- García, R.I.; Jauregui, I.; Del Amo, C.; Gandiaga, A.; Rodriguez, O.; Margallo, L.; Voces, R.; Martin, N.; Gallego, I.; Minguez, R.; et al. Implementation of an In-House 3D Manufacturing Unit in a Public Hospital’s Radiology Department. Healthcare 2022, 10, 1791. [Google Scholar] [CrossRef] [PubMed]
- Sharma, N.; Cao, S.; Msallem, B.; Kunz, C.; Brantner, P.; Honigmann, P.; Thieringer, F.M. Effects of Steam Sterilization on 3D Printed Biocompatible Resin Materials for Surgical Guides—An Accuracy Assessment Study. J. Clin. Med. 2020, 9, 1506. [Google Scholar] [CrossRef] [PubMed]
- Med 625—Polyjet Technology Material—Stratasys. Available online: https://www.stratasys.com/en/materials/materials-catalog/polyjet-materials/flexible-clear-biocompatible-med625flx/ (accessed on 20 May 2024).
- ISO 10993-1:2018; Biological Evaluation of Medical Devices—Part 1: Evaluation and Testing Within a Risk Management Process. ISO: Geneva, Switzerland, 2018. Available online: https://www.iso.org/standard/68936.html (accessed on 29 July 2025).
- Mardis, N.J. Emerging Technology and Applications of 3D Printing in the Medical Field. Mo. Med. 2018, 115, 368–373. [Google Scholar]
- van Dal, V.H.a.M. Effect of Sterilization on 3D Printed Patient-Specific Surgical Guides. 2021. Available online: https://repository.tudelft.nl/record/uuid:58bc3434-251b-4a89-9ff7-5743742616fc (accessed on 27 January 2025).
- ASTM D790-17; Standard Test Methods for Flexural Properties of Unreinforced and Reinforced Plastics and Electrical Insulating Materials. ASTM International: West Conshohocken, PA, USA, 2017. Available online: https://store.astm.org/d0790-17.html (accessed on 29 July 2025).
- The Definitive Guide to ASTM D790 Flexure Testing of Plastics [Internet]. [cited 25 December 2024]. Available online: https://www.instron.com/en/testing-solutions/astm-standards/the-definitive-guide-to-astm-d790 (accessed on 25 December 2024).
- Shuen, C. Effects of Various Sterilization Protocols on the Dimensional Accuracy of 3D Printed Surgical Guides for Dental Implants. Master’s Thesis, University of Connecticut Graduate School, Montville, CT, USA, 2020. [Google Scholar]
- Popescu, D.; Baciu, F.; Amza, C.G.; Cotrut, C.M.; Marinescu, R. The Effect of Disinfectants Absorption and Medical Decontamination on the Mechanical Performance of 3D-Printed ABS Parts. Polymers 2021, 13, 4249. [Google Scholar] [CrossRef]
- Bosc, R.; Tortolano, L.; Hersant, B.; Oudjhani, M.; Leplay, C.; Woerther, P.L.; Aguilar, P.; Leguen, R.; Meningaud, J.-P. Bacteriological and mechanical impact of the Sterrad sterilization method on personalized 3D printed guides for mandibular reconstruction. Sci. Rep. 2021, 11, 581. [Google Scholar] [CrossRef]
- Eveland, R.; Antloga, K.; Meyer, A.; Tuscano, L. Low temperature vaporized hydrogen peroxide sterilization of 3D printed devices. 3D Print. Med. 2024, 10, 6. [Google Scholar] [CrossRef]
- Kirschner, A.; David, S.; Brunello, G.; Keilig, L.; Drescher, D.; Bourauel, C.; Becker, K. Impact of Steam Autoclaving on the Mechanical Properties of 3D-Printed Resins Used for Insertion Guides in Orthodontics and Implant Dentistry. Appl. Sci. 2022, 12, 6195. [Google Scholar] [CrossRef]
- Török, G.; Gombocz, P.; Bognár, E.; Nagy, P.; Dinya, E.; Kispélyi, B.; Hermann, P. Effects of disinfection and sterilization on the dimensional changes and mechanical properties of 3D printed surgical guides for implant therapy—Pilot study. BMC Oral Health 2020, 20, 19. [Google Scholar] [CrossRef] [PubMed]
- Zaborniak, M.; Kluczyński, J.; Stańko, J.; Ślęzak, T. The Influence of the Steam Sterilization Process on Selected Properties of Polymer Samples Produced in MEX and JMT Processes. Materials 2024, 17, 5763. [Google Scholar] [CrossRef]
- Marei, H.F.; Alshaia, A.; Alarifi, S.; Almasoud, N.; Abdelhady, A. Effect of Steam Heat Sterilization on the Accuracy of 3D Printed Surgical Guides. Implant Dent. 2019, 28, 372–377. [Google Scholar] [CrossRef]
- Pop, S.I.; Dudescu, M.; Mihali, S.G.; Păcurar, M.; Bratu, D.C. Effects of Disinfection and Steam Sterilization on the Mechanical Properties of 3D SLA- and DLP-Printed Surgical Guides for Orthodontic Implant Placement. Polymers 2022, 14, 2107. [Google Scholar] [CrossRef] [PubMed]
- Lan, D.; Zhou, Z.; Yang, Y.; Xu, Z.; Man, Y. Influence of Material, Sterilization, and Disinfection on the Accuracy of Three-Dimensional Printed Surgical Templates: An In Vitro Study. Clin. Oral Implant. Res. 2025, 36, 191–201. [Google Scholar] [CrossRef]
- Shaheen, E.; Alhelwani, A.; Van De Casteele, E.; Politis, C.; Jacobs, R. Evaluation of Dimensional Changes of 3D Printed Models After Sterilization: A Pilot Study. Open Dent. J. 2018, 12, 72–79. [Google Scholar] [CrossRef]
- Rindelaub, J.D.; Baird, Z.; Lindner, B.A.; Strantz, A.A. Identifying extractable profiles from 3D printed medical devices. PLoS ONE 2019, 14, e0217137. [Google Scholar] [CrossRef]
- Albert, D.E. Chapter 4—Methods for Verifying Medical Device Cleanliness. In Developments in Surface Contamination and Cleaning; Kohli, R., Mittal, K.L., Eds.; William Andrew Publishing: Oxford, UK, 2015; pp. 109–128. [Google Scholar] [CrossRef]
- Rogers, H.B.; Zhou, L.T.; Kusuhara, A.; Zaniker, E.; Shafaie, S.; Owen, B.C.; Duncan, F.E.; Woodruff, T.K. Dental resins used in 3D printing technologies release ovo-toxic leachates. Chemosphere 2021, 270, 129003. [Google Scholar] [CrossRef]
- Leonhardt, S.; Klare, M.; Scheer, M.; Fischer, T.; Cordes, B.; Eblenkamp, M. Biocompatibility of photopolymers for additive manufacturing. Curr. Dir. Biomed. Eng. 2016, 2, 113–116. [Google Scholar] [CrossRef]
- Rengarajan, V.; Clyde, A.; Pontsler, J.; Valiente, J.; Peel, A.; Huang, Y. Assessing Leachable Cytotoxicity of 3D-Printed Polymers and Facile Detoxification Methods. 3D Print. Addit. Manuf. 2023, 10, 1110–1121. [Google Scholar] [CrossRef] [PubMed]
| Group | Cleaning and Disinfection Method | Sterilization Method with T °C and Cycle Duration |
|---|---|---|
| Control | None | None |
| Machine/H2O2 | Washing machine cleaning with Mediclean Forte, thermal disinfection (~95 °C). Cleaning cycle: 1.5 h | VHP: H2O2 gas plasma at 60 °C (Sterrad System)~60 min |
| Manual/H2O2 | Manual cleaning (Aniosyme XL3, 0.5% (V/V), 25 mL in 5 L water) + 70%(V/V) ethanol ~5 min. Single cycle. | VHP: H2O2 gas plasma at 60 °C (Sterrad System)~60 min |
| Machine/steam | Washing machine cleaning with Mediclean Forte, thermal disinfection (~95 °C). Cleaning cycle: 1.5 h | Autoclave at 134 °C, 4 min plateau, fractionated pre-vacuum~60 min |
| Manual/steam | Manual cleaning (Aniosyme XL3, 0.5% (V/V), 25 mL in 5 L water) + 70% (V/V) ethanol ~5 min. Single cycle. | Autoclave at 134 °C, 4 min plateau, fractionated pre-vacuum~60 min |
| Material | Sample Group | Mass Loss at 100 °C (wt%) | Td5 (°C) | Td1 (°C) | Td2 (°C) |
|---|---|---|---|---|---|
| MED625FLX | Control | 0.67 | 247 ± 8 | 309 ± 1 | 423 ± 2 |
| Machine/H2O2 | 0.66 | 258 ± 2 | 310 ± 3 | 422 ± 5 | |
| Manual/H2O2 | 0.53 | 259 ± 7 | 307 ± 2 | 427 ± 5 | |
| Machine/steam | 0.8 | 256 ± 3 | 309 ± 1 | 423 ± 7 | |
| Manual/steam | 0.53 | 258 ± 2 | 304 ± 2 | 424 ± 1 | |
| Bioflex | Control | 0.42 | 163 ± 7 | 326 ± 6 | 425 ± 3 |
| Machine/H2O2 | 1.04 | 240 ± 5 | 341 ± 3 | 427 ± 4 | |
| Manual/H2O2 | 0.63 | 221 ± 6 | 338 ± 3 | 432 ± 4 | |
| Machine/steam | 0.5 | 252 ± 4 | 341 ± 2 | 430 ± 2 | |
| Manual/steam | 0.9 | 244 ± 2 | 340 ± 4 | 430 ± 4 |
| Material | Group | Tg a (DSC) (°C) | Tg b (DMA) (°C) |
|---|---|---|---|
| MED625FLX | Control | −4 ± 2 | 32 ± 1 |
| Machine/H2O2 | −1 ± 1 | 36 ± 1 | |
| Manual/H2O2 | −2 ± 1 | 36 ± 2 | |
| Machine/steam | 1 ± 1 | 36 ± 1 | |
| Manual/steam | 1 ± 2 | 35 ± 1 | |
| Bioflex | Control | −11 ± 1 | 22 ± 1 |
| Machine/H2O2 | −4 ± 1 | 38 ± 3 | |
| Manual/H2O2 | −9 ± 1 | 35 ± 1 | |
| Machine/steam | −2 ± 1 | 35 ± 1 | |
| Manual/steam | 0 ± 2 | 31 ± 2 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kronenberger, R.; Kazma, R.; Amirabadi, A.; Uribe, L.V.; Talevi, G.; Kaya, G.E.; Van den Brande, N.; Abadi, R.H.; Kalteremidou, K.-A.; Van Hemelrijck, D.; et al. Impact of Disinfection and Sterilization on 3D-Printing Resin Performance for Surgical Guides in Cardiac Ablation Surgery. Bioengineering 2025, 12, 924. https://doi.org/10.3390/bioengineering12090924
Kronenberger R, Kazma R, Amirabadi A, Uribe LV, Talevi G, Kaya GE, Van den Brande N, Abadi RH, Kalteremidou K-A, Van Hemelrijck D, et al. Impact of Disinfection and Sterilization on 3D-Printing Resin Performance for Surgical Guides in Cardiac Ablation Surgery. Bioengineering. 2025; 12(9):924. https://doi.org/10.3390/bioengineering12090924
Chicago/Turabian StyleKronenberger, Rani, Rawan Kazma, Alireza Amirabadi, Leire Viana Uribe, Giacomo Talevi, Görkem Eylül Kaya, Niko Van den Brande, Ramak Hossein Abadi, Kalliopi-Artemi Kalteremidou, Danny Van Hemelrijck, and et al. 2025. "Impact of Disinfection and Sterilization on 3D-Printing Resin Performance for Surgical Guides in Cardiac Ablation Surgery" Bioengineering 12, no. 9: 924. https://doi.org/10.3390/bioengineering12090924
APA StyleKronenberger, R., Kazma, R., Amirabadi, A., Uribe, L. V., Talevi, G., Kaya, G. E., Van den Brande, N., Abadi, R. H., Kalteremidou, K.-A., Van Hemelrijck, D., Baert, K., Hauffman, T., Soete, J., Pannone, L., Paparella, A. M., Eltsov, I., Chierchia, G. B., La Meir, M., Gharaviri, A., & de Asmundis, C. (2025). Impact of Disinfection and Sterilization on 3D-Printing Resin Performance for Surgical Guides in Cardiac Ablation Surgery. Bioengineering, 12(9), 924. https://doi.org/10.3390/bioengineering12090924







