Precision Medicine in Orthobiologics: A Paradigm Shift in Regenerative Therapies
Abstract
1. Introduction
2. Methods
3. Patient Stratification and Phenotyping
4. Biomarkers
4.1. Molecular Biomarkers
4.2. Genetic Biomarkers
4.3. ‘Omics’ Approaches: Proteomics and Metabolomics
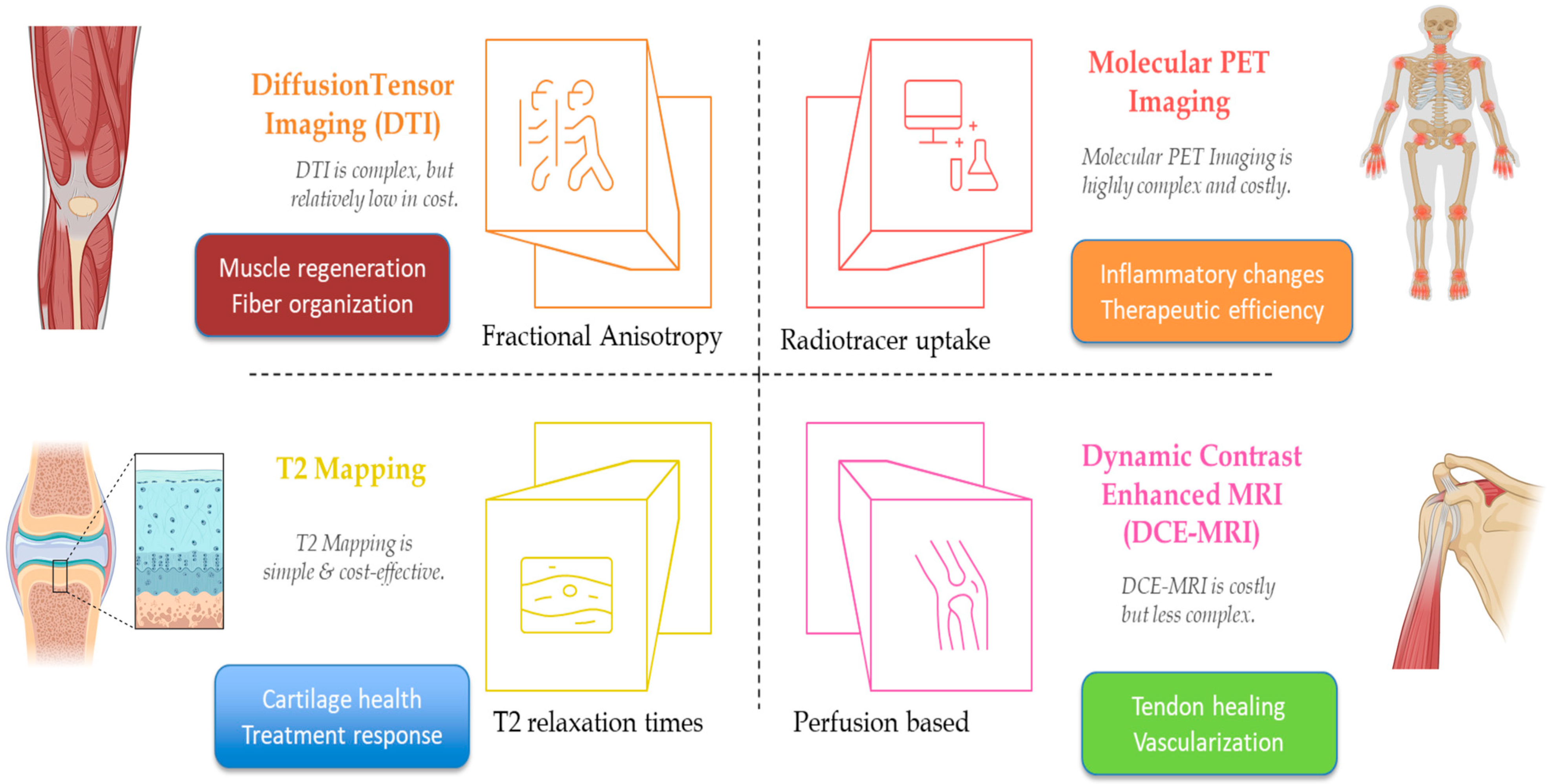
5. Advanced Imaging in Orthobiologics
| Imaging Technique | Musculoskeletal Condition | Orthobiologic Intervention | Measured Parameter | Clinical Correlation | Limitations |
|---|---|---|---|---|---|
| T2 Mapping (MRI) [36] | Knee OA | PRP | T2 Relaxation Times | Increased values correlate with cartilage repair and clinical improvement | Requires specialized protocols; cost and standardization issues |
| DTI (MRI) [40] | Muscle Injury | Growth Factors | Fractional Anisotropy | Increased anisotropy indicates improved tissue organization and regeneration | Complex post-processing; inter-scanner variability |
| DCE-MRI [48] | Rotator Cuff Tear | Growth Factors | Vascularization/Perfusion | Increased perfusion correlates with tendon healing | Contrast use; high cost; protocol variability |
| Molecular PET [45] | Rheumatoid Arthritis | Monitoring (non-specific) | Radiotracer Uptake (Inflammation) | Decreased uptake correlates with reduced inflammatory activity and treatment response | Ionizing radiation; limited availability |
6. Bioengineered Delivery Systems
| Delivery System Type | Orthobiologic Agent Delivered | Musculoskeletal Condition | Reported Therapeutic Benefit | Critical Considerations |
|---|---|---|---|---|
| Injectable Hydrogel [52] | BMP-2/Growth Factors/MSCs | Fracture Healing | Enhanced bone regeneration via sustained release | Control over release kinetics; degradation matching healing timelines |
| Nanoparticles [55] | siRNA (anti-inflammatory) | Knee OA | Reduced cartilage degradation (in vitro/in vivo) | Target specificity; cytotoxicity; manufacturing scalability |
| Scaffolds [64,65] | MSCs/Chondrocytes | Cartilage Regeneration | Enhanced cell survival, integration, and tissue repair | Surgical implantation; vascularization; immune response management |
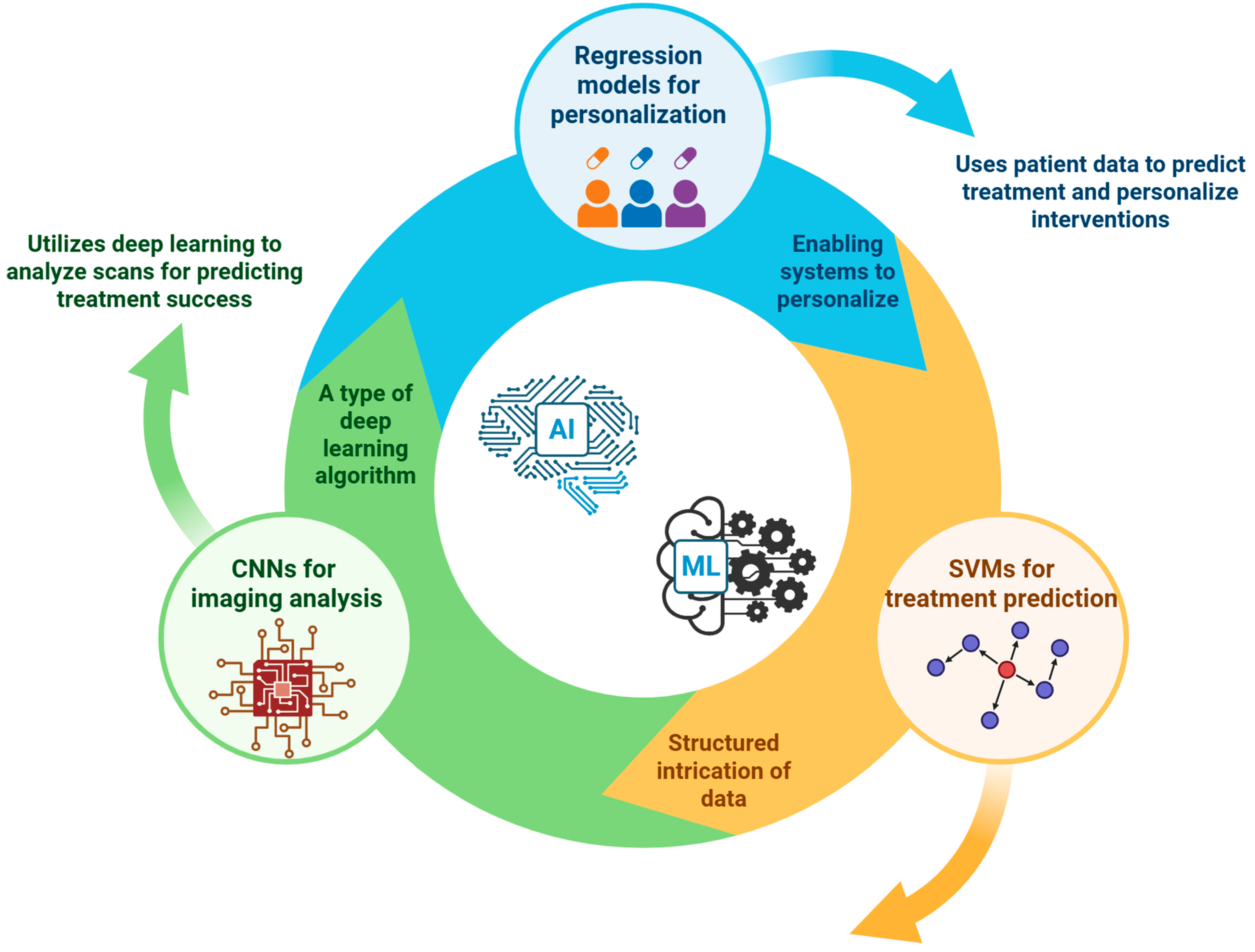
7. Artificial Intelligence and Machine Learning—Transforming Personalized Orthobiologics
8. Challenges, Limitations, and Future Directions
9. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ibrahim, A.; Gupton, M.; Schroeder, F. Regenerative Medicine in Orthopedic Surgery: Expanding Our Toolbox. Cureus 2024, 16, e68487. [Google Scholar] [CrossRef]
- Costa, F.R.; Pires, L.; Martins, R.A.; Santos, M.; Santos, G.S.; Lana, J.V.; Costa, B.R.; Santos, N.; de Macedo, A.P.; Kruel, A.; et al. Orthobiologics Revisited: A Concise Perspective on Regenerative Orthopedics. Curr. Issues Mol. Biol. 2025, 47, 247. [Google Scholar] [CrossRef]
- Molla, G.; Bitew, M. Revolutionizing Personalized Medicine: Synergy with Multi-Omics Data Generation, Main Hurdles, and Future Perspectives. Biomedicines 2024, 12, 2750. [Google Scholar] [CrossRef] [PubMed]
- Rodeo, S.A. Orthobiologics: Current Status in 2023 and Future Outlook. J. Am. Acad. Orthop. Surg. 2023, 31, 604–613. [Google Scholar] [CrossRef] [PubMed]
- Deveza, L.A.; Loeser, R.F. Is Osteoarthritis One Disease or a Collection of Many? Rheumatol. Oxf. Engl. 2018, 57, iv34–iv42. [Google Scholar] [CrossRef] [PubMed]
- Abraham, A.C.; Shah, S.A.; Thomopoulos, S. Targeting Inflammation in Rotator Cuff Tendon Degeneration and Repair. Tech. Shoulder Elb. Surg. 2017, 18, 84–90. [Google Scholar] [CrossRef]
- Duffy, J. Patient Stratification: Leveraging Biomarkers for Precision Medicine. 18 September 2023. Available online: https://oxfordglobal.com/precision-medicine/resources/patient-stratification-leveraging-biomarkers-for-precision-medicine (accessed on 24 April 2025).
- Sokolove, J.; Lepus, C.M. Role of Inflammation in the Pathogenesis of Osteoarthritis: Latest Findings and Interpretations. Ther. Adv. Musculoskelet. Dis. 2013, 5, 77–94. [Google Scholar] [CrossRef]
- Dong, X.; Xiao, T.; Chen, B.; Lu, Y.; Zhou, W. Precision Medicine via the Integration of Phenotype-Genotype Information in Neonatal Genome Project. Fundam. Res. 2022, 2, 873–884. [Google Scholar] [CrossRef]
- Gupta, A.; Jeyaraman, M.; Potty, A.G. Leukocyte-Rich vs. Leukocyte-Poor Platelet-Rich Plasma for the Treatment of Knee Osteoarthritis. Biomedicines 2023, 11, 141. [Google Scholar] [CrossRef]
- Mann, M.; Kumar, C.; Zeng, W.-F.; Strauss, M.T. Artificial Intelligence for Proteomics and Biomarker Discovery. Cell Syst. 2021, 12, 759–770. [Google Scholar] [CrossRef]
- Everts, P.A.; Mazzola, T.; Mautner, K.; Randelli, P.S.; Podesta, L. Modifying Orthobiological PRP Therapies Are Imperative for the Advancement of Treatment Outcomes in Musculoskeletal Pathologies. Biomedicines 2022, 10, 2933. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, A.; Imran, M.; Ahsan, H. Biomarkers as Biomedical Bioindicators: Approaches and Techniques for the Detection, Analysis, and Validation of Novel Biomarkers of Diseases. Pharmaceutics 2023, 15, 1630. [Google Scholar] [CrossRef]
- Huang, K.; Lidbury, B.A.; Thomas, N.; Gooley, P.R.; Armstrong, C.W. Machine Learning and Multi-Omics in Precision Medicine for ME/CFS. J. Transl. Med. 2025, 23, 68. [Google Scholar] [CrossRef]
- Kumavat, R.; Kumar, V.; Malhotra, R.; Pandit, H.; Jones, E.; Ponchel, F.; Biswas, S. Biomarkers of Joint Damage in Osteoarthritis: Current Status and Future Directions. Mediat. Inflamm. 2021, 2021, 5574582. [Google Scholar] [CrossRef]
- Alvand, A.; Rezapoor, M.; Parvizi, J. The Role of Biomarkers for the Diagnosis of Implant-Related Infections in Orthopaedics and Trauma. Adv. Exp. Med. Biol. 2017, 971, 69–79. [Google Scholar] [CrossRef] [PubMed]
- Mariani, E.; Roffi, A.; Cattini, L.; Pulsatelli, L.; Assirelli, E.; Krishnakumar, G.S.; Cenacchi, A.; Kon, E.; Filardo, G. Release Kinetic of Pro- and Anti-Inflammatory Biomolecules from Platelet-Rich Plasma and Functional Study on Osteoarthritis Synovial Fibroblasts. Cytotherapy 2020, 22, 344–353. [Google Scholar] [CrossRef]
- Rathod, V.; Shrivastav, S.; Gharpinde, M.R. Platelet-Rich Plasma Therapy for Rotator Cuff Injuries: A Comprehensive Review of Current Evidence and Future Directions. Cureus 2024, 16, e70042. [Google Scholar] [CrossRef]
- Wang, P.; Shao, W.; Wang, Y.; Wang, B.; Lv, X.; Feng, Y. Angiogenesis of Avascular Necrosis of the Femoral Head: A Classic Treatment Strategy. Biomedicines 2024, 12, 2577. [Google Scholar] [CrossRef]
- Sowers, M.; Karvonen-Gutierrez, C.A.; Yosef, M.; Jannausch, M.; Jiang, Y.; Garnero, P.; Jacobson, J. Longitudinal Changes of Serum COMP and Urinary CTX-II Predict x-Ray Defined Knee Osteoarthritis Severity and Stiffness in Women. Osteoarthr. Cartil. OARS Osteoarthr. Res. Soc. 2009, 17, 1609–1614. [Google Scholar] [CrossRef] [PubMed]
- Sahin, D.; Di Matteo, A.; Emery, P. Biomarkers in the Diagnosis, Prognosis and Management of Rheumatoid Arthritis: A Comprehensive Review. Ann. Clin. Biochem. 2025, 62, 3–21. [Google Scholar] [CrossRef]
- Gibbon, A.; Raleigh, S.M.; Ribbans, W.J.; Posthumus, M.; Collins, M.; September, A.V. Functional COL1A1 Variants Are Associated with the Risk of Acute Musculoskeletal Soft Tissue Injuries. J. Orthop. Res. Off. Publ. Orthop. Res. Soc. 2020, 38, 2290–2298. [Google Scholar] [CrossRef] [PubMed]
- Viganò, M.; Ragni, E.; Marmotti, A.; de Girolamo, L. The Effects of Orthobiologics in the Treatment of Tendon Pathologies: A Systematic Review of Preclinical Evidence. J. Exp. Orthop. 2022, 9, 31. [Google Scholar] [CrossRef] [PubMed]
- Cantisani, R.; Re, V.D.; Toraldo, F.; Cantara, S.; Pozzessere, S.; Marotta, G.; Spreafico, A. HLA Class I Expression on Human Platelets Is Highly Variable and Correlates with Distinct Allele Frequencies: Analysis of HLA Class I Expression and Allele Frequencies in Human Platelets. Blood Transfus. 2024, 22, 440–449. [Google Scholar] [CrossRef]
- Zhou, T.; Yuan, Z.; Weng, J.; Pei, D.; Du, X.; He, C.; Lai, P. Challenges and Advances in Clinical Applications of Mesenchymal Stromal Cells. J. Hematol. Oncol. 2021, 14, 24. [Google Scholar] [CrossRef] [PubMed]
- Colineaux, H.; Ruyssen-Witrand, A.; Cambon-Thomsen, A. Genetic Markers as a Predictive Tool Based on Statistics in Medical Practice: Ethical Considerations through the Analysis of the Use of HLA-B*27 in Rheumatology in France. Front. Genet. 2015, 6, 299. [Google Scholar] [CrossRef]
- Padilla, S.; Sánchez, M.; Vaquerizo, V.; Malanga, G.A.; Fiz, N.; Azofra, J.; Rogers, C.J.; Samitier, G.; Sampson, S.; Seijas, R.; et al. Platelet-Rich Plasma Applications for Achilles Tendon Repair: A Bridge between Biology and Surgery. Int. J. Mol. Sci. 2021, 22, 824. [Google Scholar] [CrossRef]
- García-Sancho, J.; Sánchez, A.; Vega, A.; Noriega, D.C.; Nocito, M. Influence of HLA Matching on the Efficacy of Allogeneic Mesenchymal Stromal Cell Therapies for Osteoarthritis and Degenerative Disc Disease. Transplant. Direct 2017, 3, e205. [Google Scholar] [CrossRef]
- Reiner, A.P.; Wurfel, M.M.; Lange, L.A.; Carlson, C.S.; Nord, A.S.; Carty, C.L.; Rieder, M.J.; Desmarais, C.; Jenny, N.S.; Iribarren, C.; et al. Polymorphisms of the IL1-Receptor Antagonist Gene (IL1RN) Are Associated with Multiple Markers of Systemic Inflammation. Arterioscler. Thromb. Vasc. Biol. 2008, 28, 1407–1412. [Google Scholar] [CrossRef]
- Chu, S.H.; Huang, M.; Kelly, R.S.; Benedetti, E.; Siddiqui, J.K.; Zeleznik, O.A.; Pereira, A.; Herrington, D.; Wheelock, C.E.; Krumsiek, J.; et al. Integration of Metabolomic and Other Omics Data in Population-Based Study Designs: An Epidemiological Perspective. Metabolites 2019, 9, 117. [Google Scholar] [CrossRef]
- Neidhart, M.; Hauser, N.; Paulsson, M.; DiCesare, P.E.; Michel, B.A.; Häuselmann, H.J. Small Fragments of Cartilage Oligomeric Matrix Protein in Synovial Fluid and Serum as Markers for Cartilage Degradation. Br. J. Rheumatol. 1997, 36, 1151–1160. [Google Scholar] [CrossRef]
- Guma, M.; Tiziani, S.; Firestein, G.S. Metabolomics in Rheumatic Diseases: Desperately Seeking Biomarkers. Nat. Rev. Rheumatol. 2016, 12, 269–281. [Google Scholar] [CrossRef]
- Sarsenova, M.; Issabekova, A.; Abisheva, S.; Rutskaya-Moroshan, K.; Ogay, V.; Saparov, A. Mesenchymal Stem Cell-Based Therapy for Rheumatoid Arthritis. Int. J. Mol. Sci. 2021, 22, 11592. [Google Scholar] [CrossRef] [PubMed]
- Lowe, R.; Shirley, N.; Bleackley, M.; Dolan, S.; Shafee, T. Transcriptomics Technologies. PLoS Comput. Biol. 2017, 13, e1005457. [Google Scholar] [CrossRef] [PubMed]
- Haris, M.R.; Gupta, H. Advanced Imaging in Orthopedics. In Orthopedics of the Upper and Lower Limb; Iyer, K.M., Khan, W.S., Eds.; Springer International Publishing: Cham, Switzerland, 2020; pp. 613–634. ISBN 978-3-030-43285-0. [Google Scholar]
- Casula, V.; Kajabi, A.W. Quantitative MRI Methods for the Assessment of Structure, Composition, and Function of Musculoskeletal Tissues in Basic Research and Preclinical Applications. Magn. Reson. Mater. Phys. Biol. Med. 2024, 37, 949–967. [Google Scholar] [CrossRef]
- Cobianchi Bellisari, F.; De Marino, L.; Arrigoni, F.; Mariani, S.; Bruno, F.; Palumbo, P.; De Cataldo, C.; Sgalambro, F.; Catallo, N.; Zugaro, L.; et al. T2-Mapping MRI Evaluation of Patellofemoral Cartilage in Patients Submitted to Intra-Articular Platelet-Rich Plasma (PRP) Injections. Radiol. Med. 2021, 126, 1085–1094. [Google Scholar] [CrossRef]
- Ranzenberger, L.R.; Das, J.M.; Snyder, T. Diffusion Tensor Imaging. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2025. [Google Scholar]
- Hata, J.; Mizuno, S.; Haga, Y.; Shimoda, M.; Kanai, Y.; Chiba, K.; Okano, H.; Nakamura, M.; Horiuchi, K. Semiquantitative Evaluation of Muscle Repair by Diffusion Tensor Imaging in Mice. JBMR Plus 2018, 2, 227–234. [Google Scholar] [CrossRef]
- Yokohama, T.; Iwasaki, M.; Oura, D.; Furuya, S.; Niiya, Y. Increased Muscle Fiber Fractional Anisotropy Value Using Diffusion Tensor Imaging after Compression without Fiber Injury. Acta Radiol. 2023, 64, 139–146. [Google Scholar] [CrossRef]
- Li, X.; Huang, W.; Holmes, J.H. Dynamic Contrast-Enhanced (DCE) MRI. Magn. Reson. Imaging Clin. N. Am. 2024, 32, 47–61. [Google Scholar] [CrossRef] [PubMed]
- Fischer, C.; Miska, M.; Jung, A.; Weber, M.-A.; Saure, D.; Schmidmaier, G.; Weimer, A.; Moghaddam, A.; Doll, J. Posttraumatic Perfusion Analysis of Quadriceps, Patellar, and Achilles Tendon Regeneration With Dynamic Contrast-Enhanced Ultrasound and Dynamic Contrast-Enhanced Magnetic Resonance Imaging. J. Ultrasound Med. 2021, 40, 491–501. [Google Scholar] [CrossRef]
- Kim, S.H.; Lee, H.S.; Kang, B.J.; Song, B.J.; Kim, H.-B.; Lee, H.; Jin, M.-S.; Lee, A. Dynamic Contrast-Enhanced MRI Perfusion Parameters as Imaging Biomarkers of Angiogenesis. PLoS ONE 2016, 11, e0168632. [Google Scholar] [CrossRef]
- Iking, J.; Staniszewska, M.; Kessler, L.; Klose, J.M.; Lückerath, K.; Fendler, W.P.; Herrmann, K.; Rischpler, C. Imaging Inflammation with Positron Emission Tomography. Biomedicines 2021, 9, 212. [Google Scholar] [CrossRef]
- Singh, S.B.; Bhandari, S.; Bhandari, S.; Bhandari, S.; Singh, R.; Raynor, W.Y.; Hess, S.; Werner, T.J.; Alavi, A.; Revheim, M.-E. Role of PET/CT in Diagnosing and Monitoring Disease Activity in Rheumatoid Arthritis: A Review. Ann. Nucl. Med. 2024, 38, 165–175. [Google Scholar] [CrossRef]
- Farabi, B.; Roster, K.; Hirani, R.; Tepper, K.; Atak, M.F.; Safai, B. The Efficacy of Stem Cells in Wound Healing: A Systematic Review. Int. J. Mol. Sci. 2024, 25, 3006. [Google Scholar] [CrossRef]
- Liu, Y.; Wang, J.; Muschler, G.; Wyles, C.; Li, W.; Luo, Y.; Xiong, Z.; Goldberg, A.; Ren, L.; Zhou, S.; et al. BMJ Innovations Roundtable: Innovations That Will Have the Biggest Impact on Orthopaedics over the next Decade. BMJ Innov. 2025, 11, 107–111. [Google Scholar] [CrossRef]
- Sasanuma, H.; Sugimoto, H.; Iijima, Y.; Kanaya, Y.; Saito, T.; Takeshita, K. Blood Flow Evaluation by Dynamic Magnetic Resonance Imaging of Symptomatic Rotator Cuff Tears and Frozen Shoulders. J. Shoulder Elbow Surg. 2018, 27, e372–e379. [Google Scholar] [CrossRef] [PubMed]
- Traditional vs. Novel Drug Delivery Methods | Creative Bioarray. Available online: https://www.creative-bioarray.com/support/traditional-vs-novel-drug-delivery-methods.htm (accessed on 27 April 2025).
- Mansour, A.; Romani, M.; Acharya, A.B.; Rahman, B.; Verron, E.; Badran, Z. Drug Delivery Systems in Regenerative Medicine: An Updated Review. Pharmaceutics 2023, 15, 695. [Google Scholar] [CrossRef] [PubMed]
- Almawash, S.; Osman, S.K.; Mustafa, G.; Hamd, M.A.E. Current and Future Prospective of Injectable Hydrogels—Design Challenges and Limitations. Pharmaceuticals 2022, 15, 371. [Google Scholar] [CrossRef] [PubMed]
- Hwang, H.S.; Lee, C.-S. Recent Progress in Hyaluronic-Acid-Based Hydrogels for Bone Tissue Engineering. Gels 2023, 9, 588. [Google Scholar] [CrossRef]
- Li, J.; Mooney, D.J. Designing Hydrogels for Controlled Drug Delivery. Nat. Rev. Mater. 2016, 1, 16071. [Google Scholar] [CrossRef]
- Ma, C.; Park, M.S.; Alves do Monte, F.; Gokani, V.; Aruwajoye, O.O.; Ren, Y.; Liu, X.; Kim, H.K.W. Local BMP2 Hydrogel Therapy for Robust Bone Regeneration in a Porcine Model of Legg-Calvé-Perthes Disease. NPJ Regen. Med. 2023, 8, 50. [Google Scholar] [CrossRef]
- Moazzam, M.; Zhang, M.; Hussain, A.; Yu, X.; Huang, J.; Huang, Y. The Landscape of Nanoparticle-Based siRNA Delivery and Therapeutic Development. Mol. Ther. 2024, 32, 284–312. [Google Scholar] [CrossRef]
- Hou, X.; Zaks, T.; Langer, R.; Dong, Y. Lipid Nanoparticles for mRNA Delivery. Nat. Rev. Mater. 2021, 6, 1078–1094. [Google Scholar] [CrossRef]
- Li, Y.; Zhao, J.; Guo, S.; He, D. siRNA Therapy in Osteoarthritis: Targeting Cellular Pathways for Advanced Treatment Approaches. Front. Immunol. 2024, 15, 1382689. [Google Scholar] [CrossRef]
- Cheng, X.; Xie, Q.; Sun, Y. Advances in Nanomaterial-Based Targeted Drug Delivery Systems. Front. Bioeng. Biotechnol. 2023, 11, 1177151. [Google Scholar] [CrossRef] [PubMed]
- De Jong, W.H.; Borm, P.J. Drug Delivery and Nanoparticles: Applications and Hazards. Int. J. Nanomed. 2008, 3, 133–149. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X.; Hu, D.A.; Wu, D.; He, F.; Wang, H.; Huang, L.; Shi, D.; Liu, Q.; Ni, N.; Pakvasa, M.; et al. Applications of Biocompatible Scaffold Materials in Stem Cell-Based Cartilage Tissue Engineering. Front. Bioeng. Biotechnol. 2021, 9, 603444. [Google Scholar] [CrossRef]
- Stratton, S.; Shelke, N.B.; Hoshino, K.; Rudraiah, S.; Kumbar, S.G. Bioactive Polymeric Scaffolds for Tissue Engineering. Bioact. Mater. 2016, 1, 93–108. [Google Scholar] [CrossRef]
- Suamte, L.; Tirkey, A.; Barman, J.; Jayasekhar Babu, P. Various Manufacturing Methods and Ideal Properties of Scaffolds for Tissue Engineering Applications. Smart Mater. Manuf. 2023, 1, 100011. [Google Scholar] [CrossRef]
- Sundelacruz, S.; Kaplan, D.L. Stem Cell- and Scaffold-Based Tissue Engineering Approaches to Osteochondral Regenerative Medicine. Semin. Cell Dev. Biol. 2009, 20, 646–655. [Google Scholar] [CrossRef] [PubMed]
- Liang, J.; Liu, P.; Yang, X.; Liu, L.; Zhang, Y.; Wang, Q.; Zhao, H. Biomaterial-Based Scaffolds in Promotion of Cartilage Regeneration: Recent Advances and Emerging Applications. J. Orthop. Transl. 2023, 41, 54–62. [Google Scholar] [CrossRef]
- Zhang, L.; Hu, J.; Athanasiou, K.A. The Role of Tissue Engineering in Articular Cartilage Repair and Regeneration. Crit. Rev. Biomed. Eng. 2009, 37, 1–57. [Google Scholar] [CrossRef] [PubMed]
- Baiguera, S.; Urbani, L.; Del Gaudio, C. Tissue Engineered Scaffolds for an Effective Healing and Regeneration: Reviewing Orthotopic Studies. BioMed Res. Int. 2014, 2014, 398069. [Google Scholar] [CrossRef]
- Schulze, F.; Lang, A.; Schoon, J.; Wassilew, G.I.; Reichert, J. Scaffold Guided Bone Regeneration for the Treatment of Large Segmental Defects in Long Bones. Biomedicines 2023, 11, 325. [Google Scholar] [CrossRef]
- Trucillo, P. Biomaterials for Drug Delivery and Human Applications. Materials 2024, 17, 456. [Google Scholar] [CrossRef]
- Zielińska, A.; Karczewski, J.; Eder, P.; Kolanowski, T.; Szalata, M.; Wielgus, K.; Szalata, M.; Kim, D.; Shin, S.R.; Słomski, R.; et al. Scaffolds for Drug Delivery and Tissue Engineering: The Role of Genetics. J. Control. Release 2023, 359, 207–223. [Google Scholar] [CrossRef]
- Blanco, A.F.; Crecente-Campo, J.; Alonso, M.J. Functionalization of Implantable Systems for Controlled Drug Delivery and Beyond. Regen. Eng. Transl. Med. 2025. [Google Scholar] [CrossRef]
- Kumar, A.; Zhou, L.; Zhi, K.; Raji, B.; Pernell, S.; Tadrous, E.; Kodidela, S.; Nookala, A.; Kochat, H.; Kumar, S. Challenges in Biomaterial-Based Drug Delivery Approach for the Treatment of Neurodegenerative Diseases: Opportunities for Extracellular Vesicles. Int. J. Mol. Sci. 2020, 22, 138. [Google Scholar] [CrossRef] [PubMed]
- Muller, R.H.; Keck, C.M. Challenges and Solutions for the Delivery of Biotech Drugs—A Review of Drug Nanocrystal Technology and Lipid Nanoparticles. J. Biotechnol. 2004, 113, 151–170. [Google Scholar] [CrossRef]
- Santoro, A.; Voto, A.; Fortino, L.; Guida, R.; Laudisio, C.; Cillo, M.; D’Ursi, A.M. Bone Defect Treatment in Regenerative Medicine: Exploring Natural and Synthetic Bone Substitutes. Int. J. Mol. Sci. 2025, 26, 3085. [Google Scholar] [CrossRef] [PubMed]
- Jeyaraman, M.; Jeyaraman, N.; Ramasubramanian, S.; Balaji, S.; Muthu, S. Evidence-Based Orthobiologic Practice: Current Evidence Review and Future Directions. World J. Orthop. 2024, 15, 908–917. [Google Scholar] [CrossRef]
- Vaishya, R.; Dhall, S.; Vaish, A. Artificial Intelligence (AI): A Potential Game Changer in Regenerative Orthopedics—A Scoping Review. Indian J. Orthop. 2024, 58, 1362–1374. [Google Scholar] [CrossRef]
- Sheikh, Z.; Nayak, V.V.; Daood, U.; Kaur, A.; Moussa, H.; Canteenwala, A.; Michaud, P.-L.; de Fátima Balderrama, Í.; de Oliveira Sousa, E.; Tovar, N.; et al. Three-Dimensional Printing Methods for Bioceramic-Based Scaffold Fabrication for Craniomaxillofacial Bone Tissue Engineering. J. Funct. Biomater. 2024, 15, 60. [Google Scholar] [CrossRef]
- Santorsola, M.; Lescai, F. The Promise of Explainable Deep Learning for Omics Data Analysis: Adding New Discovery Tools to AI. New Biotechnol. 2023, 77, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Yetgin, A. Revolutionizing Multi-Omics Analysis with Artificial Intelligence and Data Processing. Quant. Biol. 2025, 13, e70002. [Google Scholar] [CrossRef]
- Micheel, C.M.; Nass, S.J.; Omenn, G.S.; Committee on the Review of Omics-Based Tests for Predicting Patient Outcomes in Clinical Trials. Omics-Based Clinical Discovery: Science, Technology, and Applications. In Evolution of Translational Omics: Lessons Learned and the Path Forward; National Academies Press (US): Washington, DC, USA, 2012. [Google Scholar]
- Dixon, D.; Sattar, H.; Moros, N.; Kesireddy, S.R.; Ahsan, H.; Lakkimsetti, M.; Fatima, M.; Doshi, D.; Sadhu, K.; Junaid Hassan, M. Unveiling the Influence of AI Predictive Analytics on Patient Outcomes: A Comprehensive Narrative Review. Cureus 2024, 16, e59954. [Google Scholar] [CrossRef] [PubMed]
- Khalifa, M.; Albadawy, M. Artificial Intelligence for Clinical Prediction: Exploring Key Domains and Essential Functions. Comput. Methods Programs Biomed. Update 2024, 5, 100148. [Google Scholar] [CrossRef]
- Yamashita, R.; Nishio, M.; Do, R.K.G.; Togashi, K. Convolutional Neural Networks: An Overview and Application in Radiology. Insights Imaging 2018, 9, 611–629. [Google Scholar] [CrossRef]
- Calvert, G.C.; VanBuren Huffmon, G.; Rambo, W.M.; Smith, M.W.; McEntire, B.J.; Bal, B.S. Clinical Outcomes for Lumbar Fusion Using Silicon Nitride versus Other Biomaterials. J. Spine Surg. 2020, 6, 33–48. [Google Scholar] [CrossRef]
- Lieber, D.P. PRP for Lumbar Spinal Stenosis | Regenexx® Pittsburgh Surgery Alternative. Available online: https://regenexxpittsburgh.com/prp-for-lumbar-spinal-stenosis/ (accessed on 27 April 2025).
- Li, M.; Jiang, Y.; Zhang, Y.; Zhu, H. Medical Image Analysis Using Deep Learning Algorithms. Front. Public Health 2023, 11, 1273253. [Google Scholar] [CrossRef]
- Pinto-Coelho, L. How Artificial Intelligence Is Shaping Medical Imaging Technology: A Survey of Innovations and Applications. Bioengineering 2023, 10, 1435. [Google Scholar] [CrossRef]
- Zhou, X.; Li, X.; Zhang, Z.; Han, Q.; Deng, H.; Jiang, Y.; Tang, C.; Yang, L. Support Vector Machine Deep Mining of Electronic Medical Records to Predict the Prognosis of Severe Acute Myocardial Infarction. Front. Physiol. 2022, 13, 991990. [Google Scholar] [CrossRef] [PubMed]
- Guido, R.; Ferrisi, S.; Lofaro, D.; Conforti, D. An Overview on the Advancements of Support Vector Machine Models in Healthcare Applications: A Review. Information 2024, 15, 235. [Google Scholar] [CrossRef]
- Liu, Y.Y.F.; Lu, Y.; Oh, S.; Conduit, G.J. Machine Learning to Predict Mesenchymal Stem Cell Efficacy for Cartilage Repair. PLoS Comput. Biol. 2020, 16, e1008275. [Google Scholar] [CrossRef] [PubMed]
- Houdek, M.T.; Wyles, C.C.; Martin, J.R.; Sierra, R.J. Stem Cell Treatment for Avascular Necrosis of the Femoral Head: Current Perspectives. Stem Cells Cloning Adv. Appl. 2014, 7, 65–70. [Google Scholar] [CrossRef][Green Version]
- Taherdoost, H.; Ghofrani, A. AI’s Role in Revolutionizing Personalized Medicine by Reshaping Pharmacogenomics and Drug Therapy. Intell. Pharm. 2024, 2, 643–650. [Google Scholar] [CrossRef]
- Nair, A.; Alagha, M.A.; Cobb, J.; Jones, G. Assessing the Value of Imaging Data in Machine Learning Models to Predict Patient-Reported Outcome Measures in Knee Osteoarthritis Patients. Bioengineering 2024, 11, 824. [Google Scholar] [CrossRef]
- Fujita, K. Relationship Between Patient-Reported Outcome Measures (Proms) and MRI Abnormalities in Early Knee Osteoarthritis; Kanazawa University Hospital: Kanazawa, Japan, 2025. [Google Scholar]
- Centeno, C.J.; Ghattas, J.R.; Dodson, E.; Steinmetz, N.J.; Murphy, M.B.; Berger, D.R. Establishing Metrics of Clinically Meaningful Change for Treating Knee Osteoarthritis with a Combination of Autologous Orthobiologics. Sci. Rep. 2025, 15, 7244. [Google Scholar] [CrossRef]
- Boffa, A.; Andriolo, L.; Franceschini, M.; Di Martino, A.; Asunis, E.; Grassi, A.; Zaffagnini, S.; Filardo, G. Minimal Clinically Important Difference and Patient Acceptable Symptom State in Patients With Knee Osteoarthritis Treated With PRP Injection. Orthop. J. Sports Med. 2021, 9, 23259671211026242. [Google Scholar] [CrossRef]
- Gong, Y.; Liu, G.; Xue, Y.; Li, R.; Meng, L. A Survey on Dataset Quality in Machine Learning. Inf. Softw. Technol. 2023, 162, 107268. [Google Scholar] [CrossRef]
- Aldoseri, A.; Al-Khalifa, K.N.; Hamouda, A.M. Re-Thinking Data Strategy and Integration for Artificial Intelligence: Concepts, Opportunities, and Challenges. Appl. Sci. 2023, 13, 7082. [Google Scholar] [CrossRef]
- Salvi, M.; Seoni, S.; Campagner, A.; Gertych, A.; Acharya, U.R.; Molinari, F.; Cabitza, F. Explainability and Uncertainty: Two Sides of the Same Coin for Enhancing the Interpretability of Deep Learning Models in Healthcare. Int. J. Med. Inf. 2025, 197, 105846. [Google Scholar] [CrossRef]
- Li, F.; Wang, S.; Gao, Z.; Qing, M.; Pan, S.; Liu, Y.; Hu, C. Harnessing Artificial Intelligence in Sepsis Care: Advances in Early Detection, Personalized Treatment, and Real-Time Monitoring. Front. Med. 2025, 11, 1510792. [Google Scholar] [CrossRef]
- Eche, T.; Schwartz, L.H.; Mokrane, F.-Z.; Dercle, L. Toward Generalizability in the Deployment of Artificial Intelligence in Radiology: Role of Computation Stress Testing to Overcome Underspecification. Radiol. Artif. Intell. 2021, 3, e210097. [Google Scholar] [CrossRef] [PubMed]
- Goetz, L.; Seedat, N.; Vandersluis, R.; van der Schaar, M. Generalization—A Key Challenge for Responsible AI in Patient-Facing Clinical Applications. NPJ Digit. Med. 2024, 7, 126. [Google Scholar] [CrossRef] [PubMed]
- He, Z.; Tang, X.; Yang, X.; Guo, Y.; George, T.J.; Charness, N.; Quan Hem, K.B.; Hogan, W.; Bian, J. Clinical Trial Generalizability Assessment in the Big Data Era: A Review. Clin. Transl. Sci. 2020, 13, 675–684. [Google Scholar] [CrossRef]
- Machine Learning in Healthcare: Uses, Benefits and Pioneers in the Field. Available online: https://eithealth.eu/news-article/machine-learning-in-healthcare-uses-benefits-and-pioneers-in-the-field/ (accessed on 27 April 2025).
- Gómez, L.A.; Escobar, M.; Peñuela, O. Standardization of a Protocol for Obtaining Platelet Rich Plasma from Blood Donors; a Tool for Tissue Regeneration Procedures. Clin. Lab. 2015, 61, 973–980. [Google Scholar] [CrossRef] [PubMed]
- FDA. Artificial Intelligence in Software as a Medical Device. 2025. Available online: https://www.fda.gov/medical-devices/software-medical-device-samd/artificial-intelligence-software-medical-device (accessed on 1 August 2025).
- Liebig, B.E.; Kisiday, J.D.; Bahney, C.S.; Ehrhart, N.P.; Goodrich, L.R. The Platelet-Rich Plasma and Mesenchymal Stem Cell Milieu: A Review of Therapeutic Effects on Bone Healing. J. Orthop. Res. Off. Publ. Orthop. Res. Soc. 2020, 38, 2539–2550. [Google Scholar] [CrossRef]
- Wilson, A.J.; Brown, N.; Rand, E.; Genever, P.G. Attitudes Towards Standardization of Mesenchymal Stromal Cells-A Qualitative Exploration of Expert Views. Stem Cells Transl. Med. 2023, 12, 745–757. [Google Scholar] [CrossRef]

| Biomarker | Musculoskeletal Condition | Orthobiologic Treatment | Sample Type | Observed Correlation | Critical Limitations |
|---|---|---|---|---|---|
| IL-6 [17] | Knee OA | PRP | Synovial Fluid | High baseline levels predict poorer outcomes | Assay variability; invasive sampling |
| TNF-α [18] | Rotator Cuff Tear | PRP | Serum/Tissue | Reduction post-treatment linked with improved outcome | Systemic vs. local measurement discrepancies |
| VEGF [19] | Avascular Necrosis | MSC Therapy | Serum | Reduction post-treatment indicates enhanced repair | Complex angiogenic role; limited causality |
| COMP/CTX-II [20] | Osteoarthritis | Various | Serum/Synovial Fluid | Elevated levels correlate with cartilage degradation | General joint pathology marker; non-specific to treatment |
| Gene | Genetic Variation | Associated Condition | Orthobiologic Therapy | Potential Impact on Outcome | Limitations |
|---|---|---|---|---|---|
| COL1A1 [27] | Specific SNP | Achilles Tendinopathy | PRP | Predisposition to weaker tendon repair | Modest predictive power; gene-environment interactions |
| HLA [28] | Specific Allele | Intervertebral Disk Degeneration | MSC Therapy (Allogeneic) | May influence immune response and cell persistence | Population-specific; high cost |
| IL-1RN [29] | VNTR | Osteoarthritis | Various | Associated with inflammatory risk | Weak clinical validation |
| Technique | Analyte | Musculoskeletal Condition | Orthobiologic Treatment | Correlation with Outcome | Key Challenges |
|---|---|---|---|---|---|
| Proteomics [31] | Collagen fragments, COMP | Osteoarthritis | Hyaluronic Acid, PRP | High levels indicate advanced degeneration | Data complexity: bioinformatics demands |
| Metabolomics [33] | Specific amino acids/lipids | Rheumatoid Arthritis | MSC Therapy | Profiles predict a favorable response | Dietary/medication influences; standardization issues |
| Transcriptomics [34] | mRNA expression profiles | Various | Various | Indicative of active repair pathways | RNA instability; invasive sampling; translation gap |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Navani, A.; Jeyaraman, M.; Jeyaraman, N.; Ramasubramanian, S.; Nallakumarasamy, A.; Azzini, G.; Lana, J.F. Precision Medicine in Orthobiologics: A Paradigm Shift in Regenerative Therapies. Bioengineering 2025, 12, 908. https://doi.org/10.3390/bioengineering12090908
Navani A, Jeyaraman M, Jeyaraman N, Ramasubramanian S, Nallakumarasamy A, Azzini G, Lana JF. Precision Medicine in Orthobiologics: A Paradigm Shift in Regenerative Therapies. Bioengineering. 2025; 12(9):908. https://doi.org/10.3390/bioengineering12090908
Chicago/Turabian StyleNavani, Annu, Madhan Jeyaraman, Naveen Jeyaraman, Swaminathan Ramasubramanian, Arulkumar Nallakumarasamy, Gabriel Azzini, and José Fábio Lana. 2025. "Precision Medicine in Orthobiologics: A Paradigm Shift in Regenerative Therapies" Bioengineering 12, no. 9: 908. https://doi.org/10.3390/bioengineering12090908
APA StyleNavani, A., Jeyaraman, M., Jeyaraman, N., Ramasubramanian, S., Nallakumarasamy, A., Azzini, G., & Lana, J. F. (2025). Precision Medicine in Orthobiologics: A Paradigm Shift in Regenerative Therapies. Bioengineering, 12(9), 908. https://doi.org/10.3390/bioengineering12090908










