The Effects of Calcium Phosphate Bone Cement Preparation Parameters on Injectability and Compressive Strength for Minimally Invasive Surgery
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials and Preparation of CPC
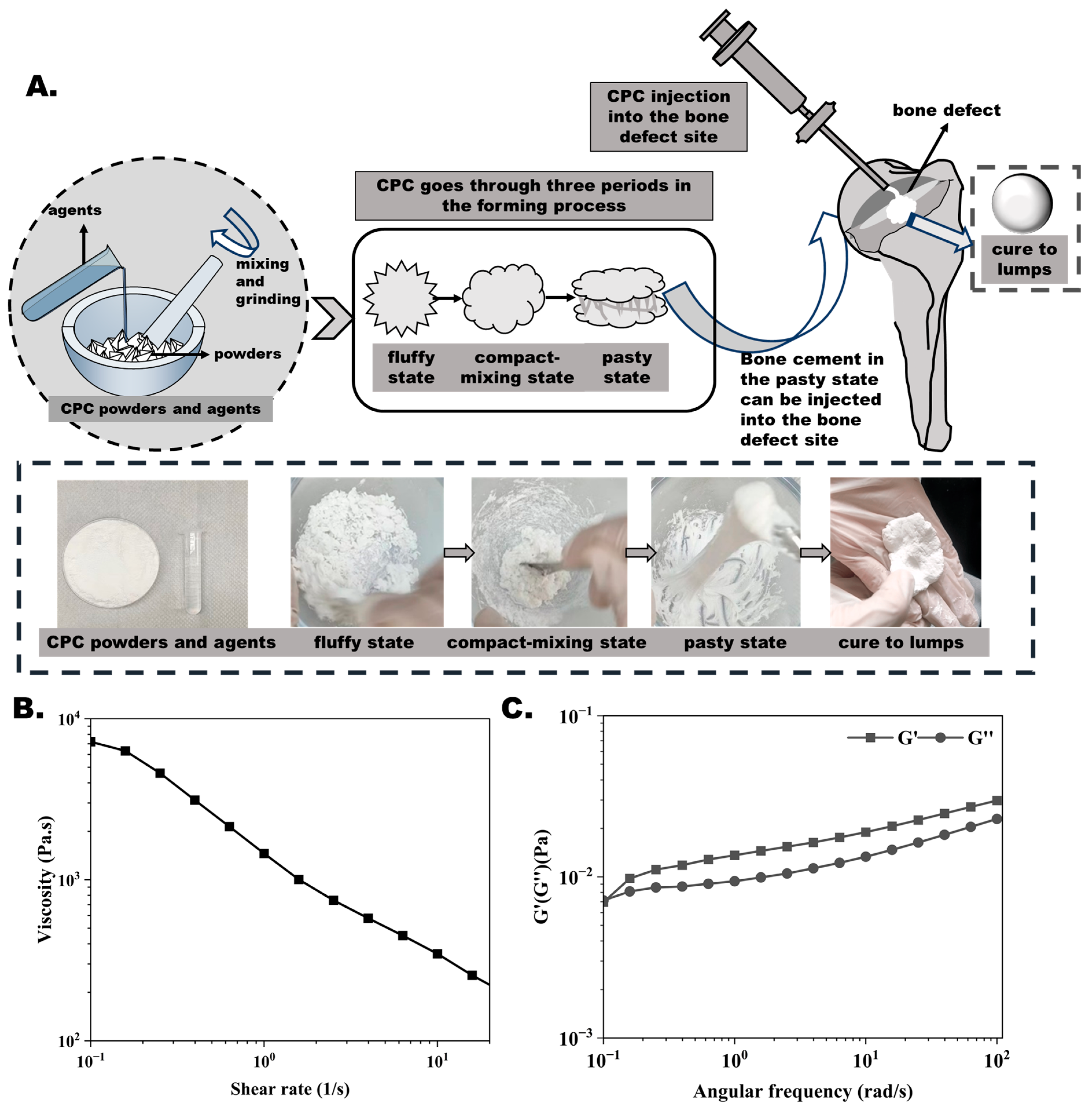
2.2. Rheological Measurement
2.3. Mechanical Property Test
2.4. Scanning Electron Microscopy (SEM)
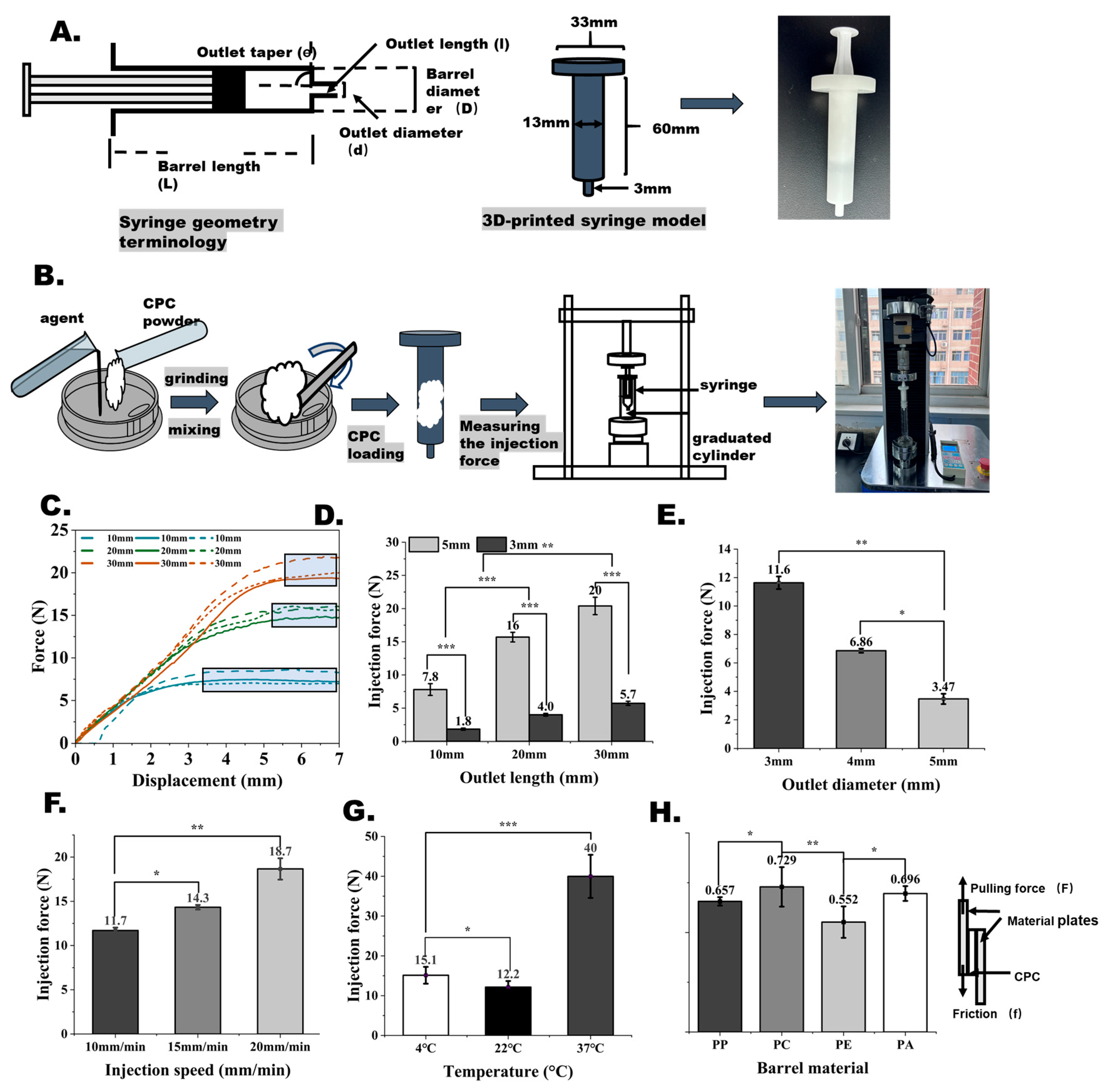
2.5. Injectability of the Bone Cement
2.6. Setting Time
2.7. Collapsibility in Water
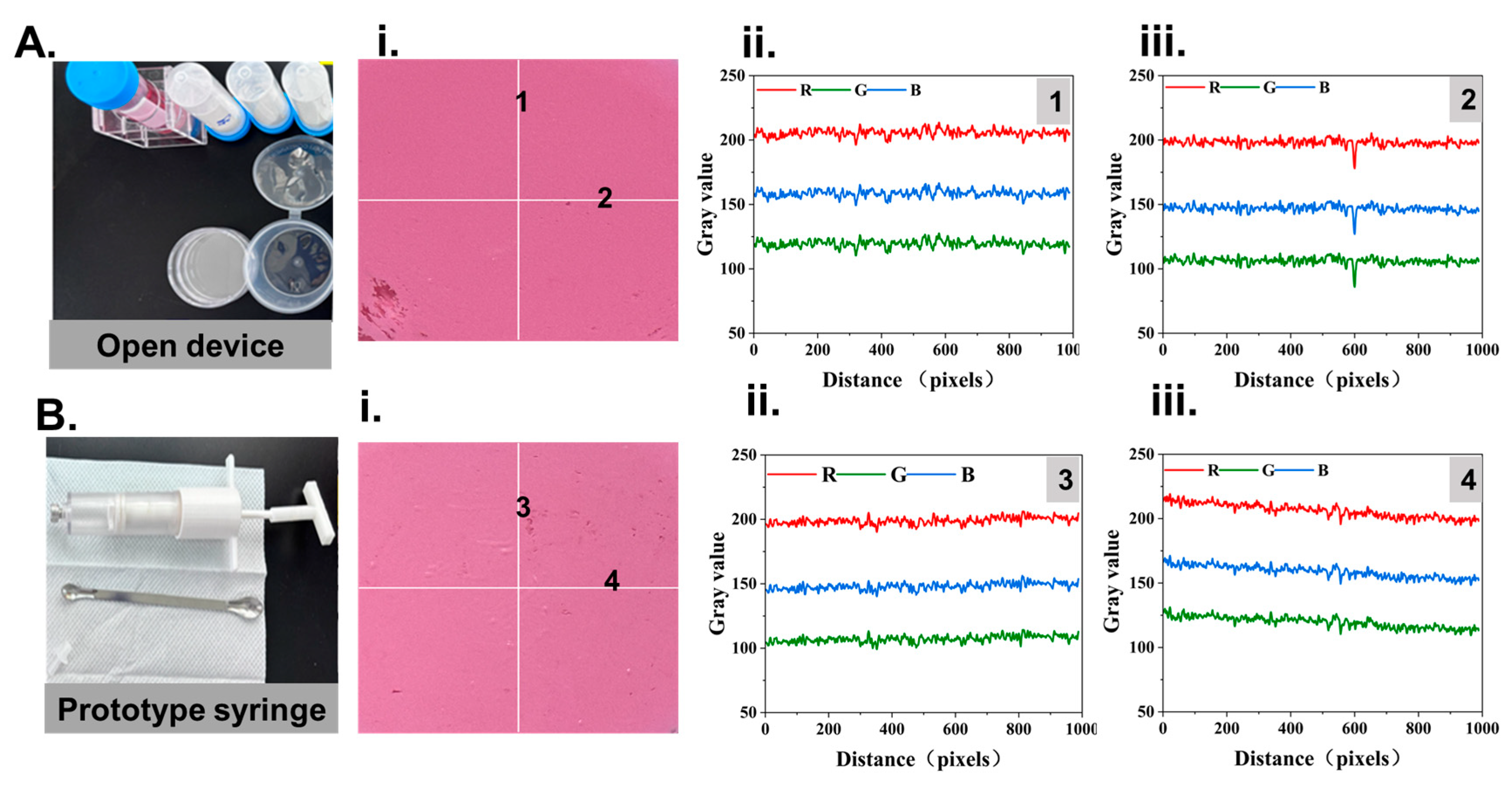
2.8. RGB Colorimetry
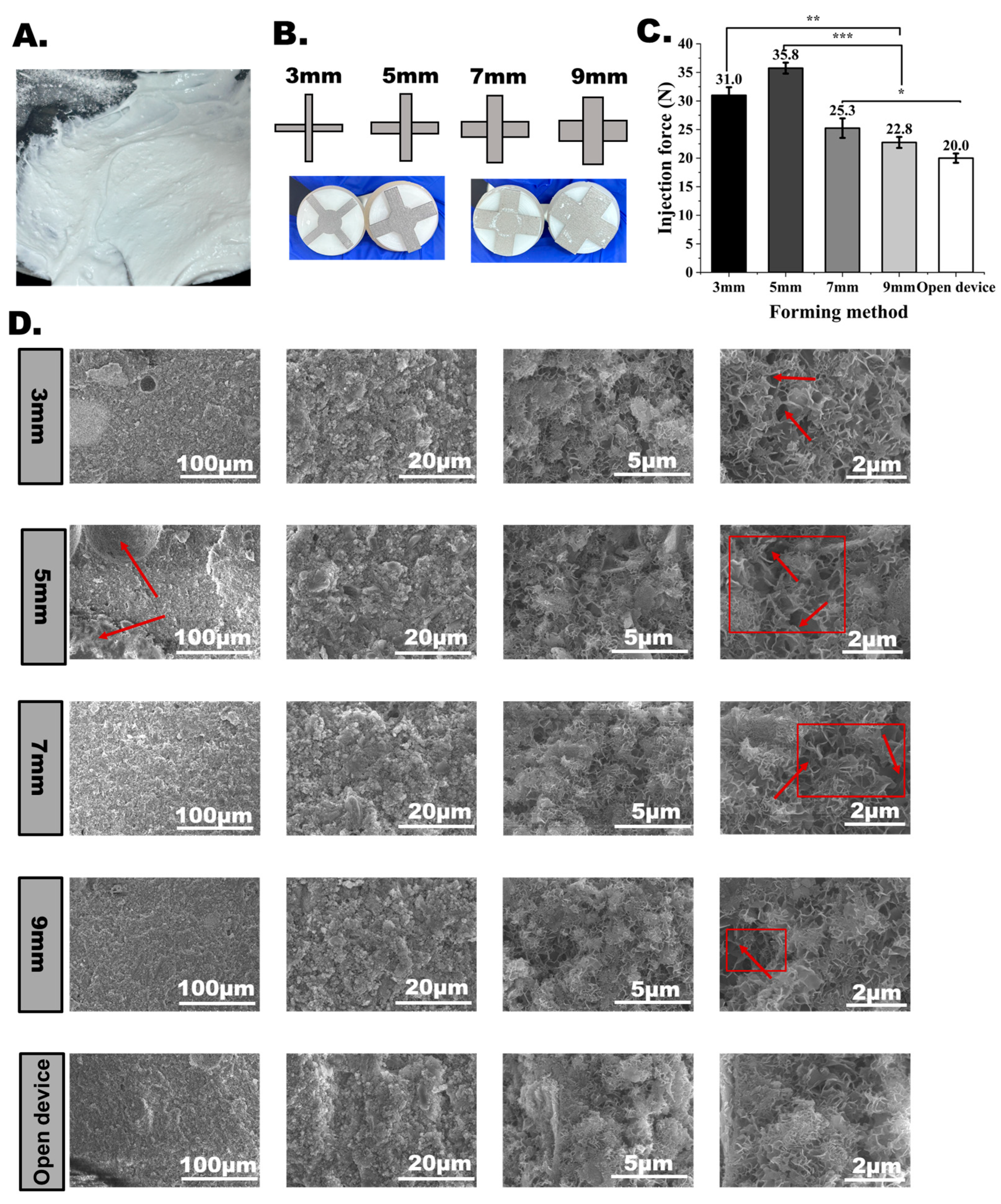
2.9. Design and Fabrication Method
2.10. Statistical Analysis
3. Results and Discussion
3.1. CPC Preparation Procedure and Difficulties
3.2. Strategies of VMBC and NBBSR
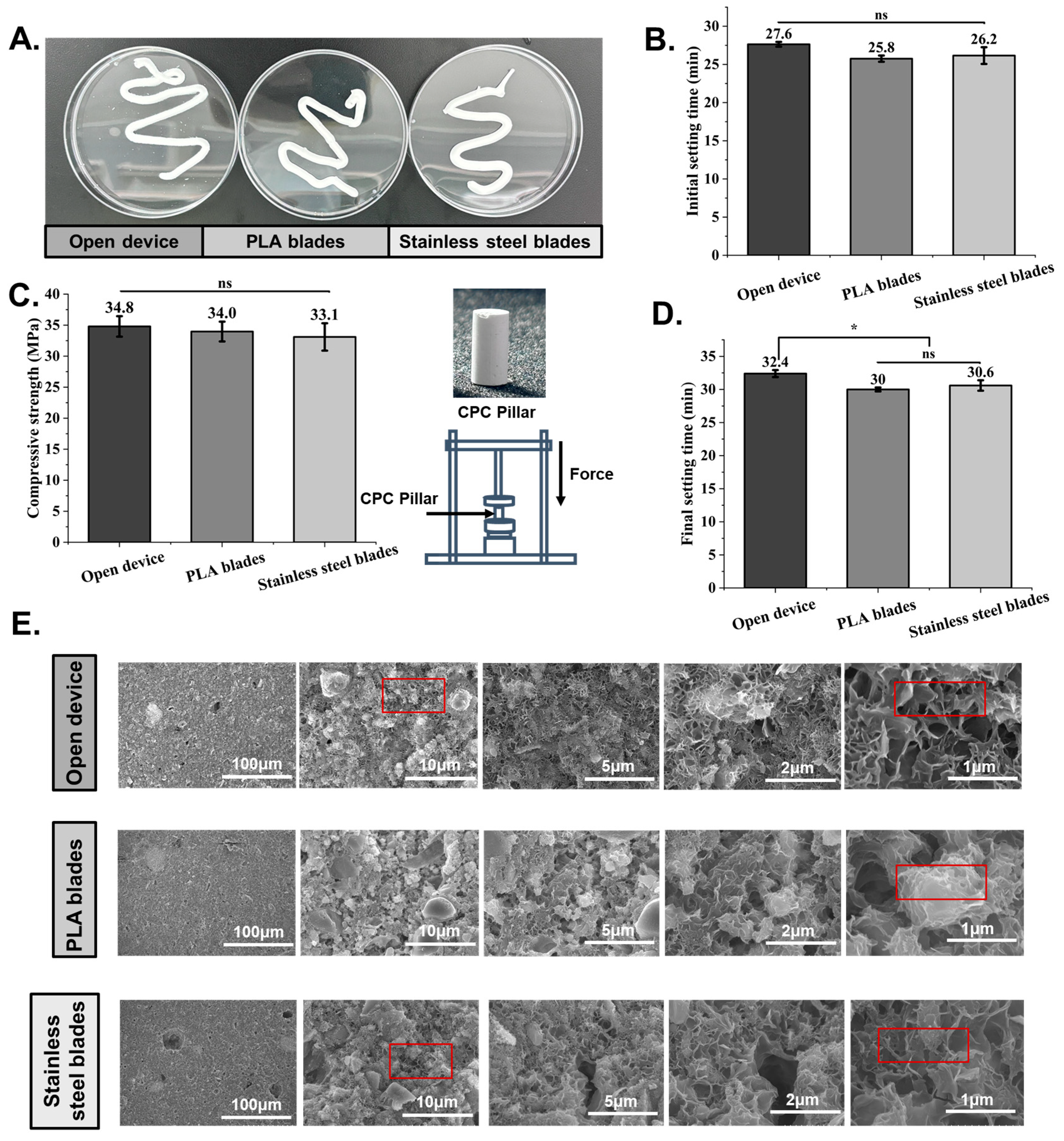
3.3. Study of CPC Mixing Quality Using VMBC and the NBBSR
3.4. Grinding Effects
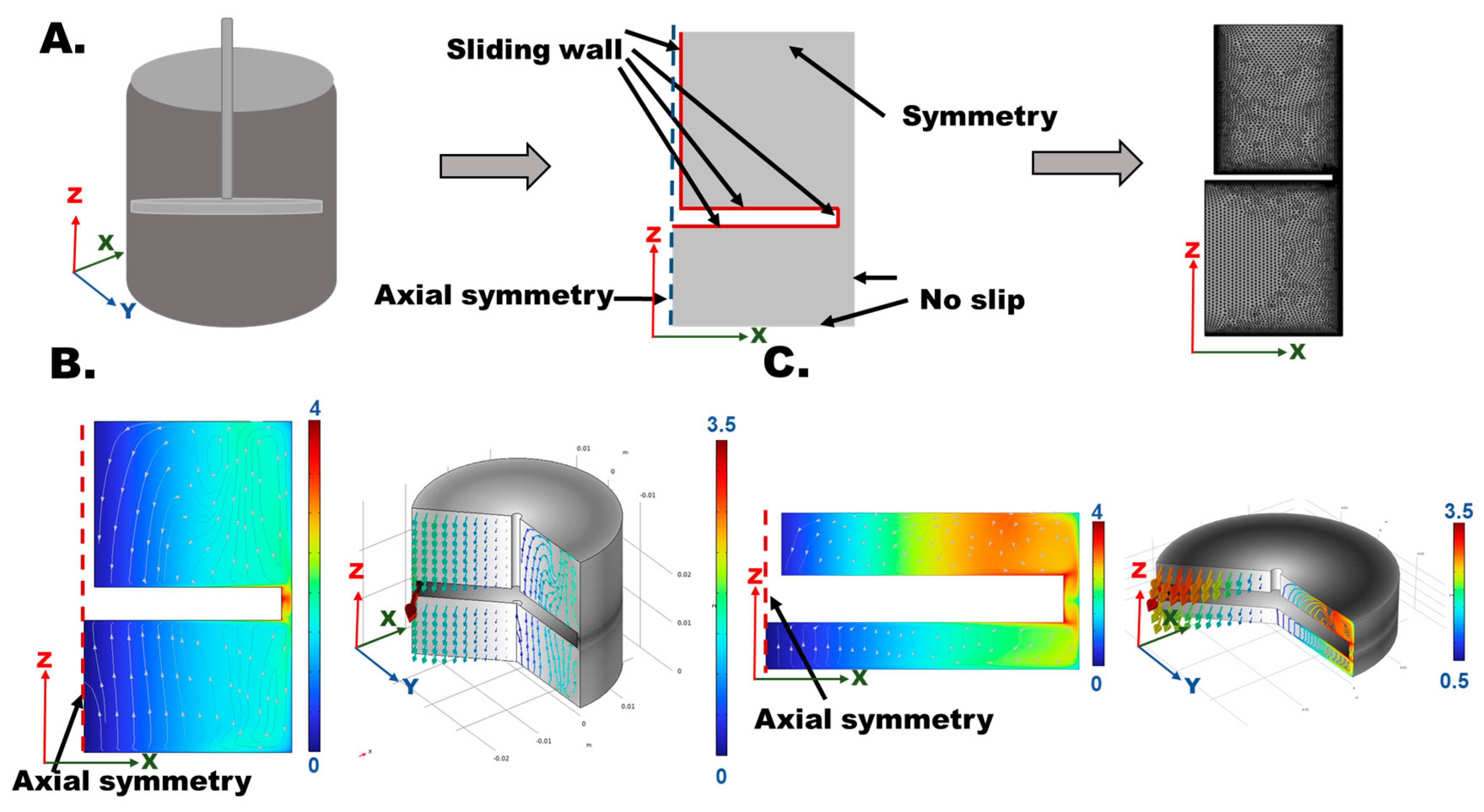
3.5. Effects of Injecting Conditions on CPC Injectability
3.6. Effects of Grinding Performance on CPC Injectability
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Liu, W.; Li, Y.; Zhou, C.; Yu, T. A ternary calcium silicate bone cement with high mechanical properties and good operability. Ceram. Int. 2024, 50, 44017–44030. [Google Scholar] [CrossRef]
- Gamal, S.; Mikhail, M.; Salem, N.; EL-Wakad, M.T.; Abdelbaset, R. Enhanced bone cement for fixation of prosthetic joint utilizing nanoparticles. J. Mater. Sci. Mater. Med. 2025, 36, 10. [Google Scholar] [CrossRef]
- Auran, R.; Movassaghi, K.; Nam, D.; Heckmann, N. Bone cement in adult hip and knee reconstruction: A review of commercially available options and clinical outcomes. J. Am. Acad. Orthop. Surg. 2024, 32, e1057–e1066. [Google Scholar] [CrossRef]
- Arkin, V.H.; Narendrakumar, U.; Madhyastha, H.; Manjubala, I. Characterization and in vitro evaluations of injectable calcium phosphate cement doped with magnesium and strontium. ACS Omega 2021, 6, 2477–2486. [Google Scholar] [CrossRef]
- Belaid, H.; Barou, C.; Collart-Dutilleul, P.-Y.; Desoutter, A.; Kajdan, M.; Bernex, F.; Tétreau, R.; Cuisinier, F.; Barés, J.; Huon, V.; et al. Fabrication of radio-opaque and macroporous injectable calcium phosphate cement. ACS Appl. Bio. Mater. 2022, 5, 3075–3085. [Google Scholar] [CrossRef] [PubMed]
- Galibert, P.; Deramond, H.; Rosat, P.; Le Gars, D. Preliminary note on the treatment of vertebral angioma by percutaneous acrylic vertebroplasty. Neurochirurgie 1987, 33, 166–168. [Google Scholar]
- Kuehn, K.-D.; Ege, W.; Gopp, U. Acrylic bone cements: Composition and properties. Orthop. Clin. N. Am. 2005, 36, 17–28. [Google Scholar] [CrossRef]
- Lu, Q.; Liu, C.; Wang, D.; Liu, H.; Yang, H.; Yang, L. Biomechanical evaluation of calcium phosphate-based nanocomposite versus polymethylmethacrylate cement for percutaneous kyphoplasty. Spine J. 2019, 19, 1871–1884. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.; Yang, D.; Zhuo, C.; Zhou, Z.; Aleem, H.B.; Huang, L.; Chen, H. Comparative analysis of percutaneous vertebroplasty and kyphoplasty in the treatment of Stage III Kummell’s disease without neurological symptoms: A retrospective study. J. Orthop. Surg. Res. 2024, 19, 515. [Google Scholar] [CrossRef]
- Verron, E.; Pissonnier, M.-L.; Lesoeur, J.; Schnitzler, V.; Fellah, B.H.; Pascal-Moussellard, H.; Pilet, P.; Gauthier, O.; Bouler, J.-M. Vertebroplasty using bisphosphonate-loaded calcium phosphate cement in a standardized vertebral body bone defect in an osteoporotic sheep model. Acta Biomater. 2014, 10, 4887–4895. [Google Scholar] [CrossRef]
- Krystyjan, M.; Khachatryan, G.; Grabacka, M.; Krzan, M.; Witczak, M.; Grzyb, J.; Woszczak, L. Physicochemical, bacteriostatic, and biological properties of starch/chitosan polymer composites modified by graphene oxide, designed as new bionanomaterials. Polymers 2021, 13, 2327. [Google Scholar] [CrossRef]
- Wang, X.-H.; Jia, S.-J.; Hao, D.-J. Advances in the modification of injectable calcium-phosphate-based bone cements for clinical application. Chin. Med. J. 2020, 133, 2610–2612. [Google Scholar] [CrossRef] [PubMed]
- Zhang, R.; Liu, H.; Zhou, H.; Liang, C.; Wang, X.; Yang, H.; Bai, Y.; Yang, L. Pregelatinized starch as a cohesion promoter improves mechanical property and surgical performance of calcium phosphate bone cement: The effect of starch type. Mater. Technol. 2022, 37, 3110–3121. [Google Scholar] [CrossRef]
- Ribeiro, N.; Reis, M.; Figueiredo, L.; Pimenta, A.; Santos, L.F.; Branco, A.C.; de Matos, A.A.; Salema-Oom, M.; Almeida, A.; Pereira, M.; et al. Improvement of a commercial calcium phosphate bone cement by means of drug delivery and increased injectability. Ceram. Int. 2022, 48, 33361–33372. [Google Scholar] [CrossRef]
- Guan, J.; Liu, L.; Li, L.; Xie, C.; Khan, M. Mesoscopic model for the fracture of polymethyl methacrylate bone cement. Eng. Fract. Mech. 2024, 303, 110085. [Google Scholar] [CrossRef]
- Ruan, S.; Deng, J.; Yan, L.; Huang, W. Evaluation of the effects of the combination of BMP-2-modified BMSCs and PRP on cartilage defects. Exp. Ther. Med. 2018, 16, 4569–4577. [Google Scholar] [CrossRef]
- Zhang, J.T.; Tancret, F.; Bouler, J.M. Fabrication and mechanical properties of calcium phosphate cements (CPC) for bone substitution. Mater. Sci. Eng. C 2011, 31, 740–747. [Google Scholar] [CrossRef]
- Perrot, A.; Lanos, C.; Melinge, Y.; Estellé, P. Mortar physical properties evolution in extrusion flow. Rheol. Acta 2007, 46, 1065–1073. [Google Scholar] [CrossRef][Green Version]
- Sui, P.; Yu, T.; Sun, S.; Chao, B.; Qin, C.; Wang, J.; Wang, E.; Zheng, C. Advances in materials used for minimally invasive treatment of vertebral compression fractures. Front. Bioeng. Biotechnol. 2023, 11, 1303678. [Google Scholar] [CrossRef]
- Bayfield, M.; Haggett, J.A.; Williamson, M.J.; Zargar, A.; Wilson, D.I. Liquid phase migration in the extrusion of icing sugar pastes. Food Bioprod. Process 1998, 76, 39–46. [Google Scholar] [CrossRef]
- Xu, H.H.; Wang, P.; Wang, L.; Bao, C.; Chen, Q.; Weir, M.D.; Chow, L.C.; Zhao, L.; Zhou, X.; Reynolds, M.A. Calcium phosphate cements for bone engineering and their biological properties. Bone Res. 2017, 5, 17056. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Zhang, Z.; Gao, C.; Bai, Y.; Liu, B.; Wang, W.; Ma, Y.; Saijilafu; Yang, H.; Li, Y.; et al. Enhancing effects of radiopaque agent BaSO4 on mechanical and biocompatibility properties of injectable calcium phosphate composite cement. Mater. Sci. Eng. C 2020, 116, 110904. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Guan, Y.; Wei, D.; Gao, C.; Yang, H.; Yang, L. Reinforcement of injectable calcium phosphate cement by gelatinized starches. J. Biomed. Mater. Res. B Appl. Biomater. 2016, 104, 615–625. [Google Scholar] [CrossRef] [PubMed]
- Sun, H.; Liu, C.; Liu, H.; Bai, Y.; Zhang, Z.; Li, X.; Li, C.; Yang, H.; Yang, L. A novel injectable calcium phosphate-based nanocomposite for the augmentation of cannulated pedicle-screw fixation. Int. J. Nanomedicine 2017, 27, 3395–3406. [Google Scholar] [CrossRef]
- Richter, R.F.; Vater, C.; Korn, M.; Ahlfeld, T.; Rauner, M.; Pradel, W.; Stadlinger, B.; Gelinsky, M.; Lode, A.; Korn, P. Treatment of critical bone defects using calcium phosphate cement and mesoporous bioactive glass providing spatiotemporal drug delivery. Bioact. Mater. 2023, 28, 402–419. [Google Scholar] [CrossRef]
- Zhou, H.; Liang, C.; Wei, Z.; Bai, Y.; Bhaduri, S.B.; Webster, T.J.; Bian, L.; Yang, L. Injectable biomaterials for translational medicine. Mater. Today 2019, 28, 81–97. [Google Scholar] [CrossRef]
- Moussi, H.; Weiss, P.; Le Bideau, J.; Gautier, H.; Charbonnier, B. Injectable macromolecule-based calcium phosphate bone substitutes. Mater. Adv. 2022, 3, 6125–6141. [Google Scholar] [CrossRef]
- Rabideau, B.D.; Moucheront, P.; Bertrand, F.; Rodts, S.; Mélinge, Y.; Lanos, C.; Coussot, P.; Franks, G. Internal flow characteristics of a plastic kaolin suspension during extrusion. J. Am. Ceram. Soc. 2012, 95, 494–501. [Google Scholar] [CrossRef]
- Peng, Y.M.; Unluer, C.; Shi, J.Y. Rheo-viscoelastic behavior and viscosity prediction of calcium sulphoaluminate modified Portland cement pastes. Powder Technol. 2021, 391, 344–352. [Google Scholar] [CrossRef]
- Libos, I.L.S.; Cui, L.; Liu, X. Effect of curing temperature on time-dependent shear behavior and properties of polypropylene fiber-reinforced cemented paste backfill. Constr. Build. Mater. 2021, 311, 125302. [Google Scholar] [CrossRef]
- Lavelle, C.; Tanoto, H.; Wang, Y.; Chen, K.; Milton, E.; Zhou, Y.; Dong, X. Minimally invasive healing of bone implant-cement interfaces by aerogel cement and remote heating. Device 2025, 3, 100680. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Qiao, Q.; Zhao, Q.; Wang, J.; Li, M.; Zhou, H.; Yang, L. The Effects of Calcium Phosphate Bone Cement Preparation Parameters on Injectability and Compressive Strength for Minimally Invasive Surgery. Bioengineering 2025, 12, 834. https://doi.org/10.3390/bioengineering12080834
Qiao Q, Zhao Q, Wang J, Li M, Zhou H, Yang L. The Effects of Calcium Phosphate Bone Cement Preparation Parameters on Injectability and Compressive Strength for Minimally Invasive Surgery. Bioengineering. 2025; 12(8):834. https://doi.org/10.3390/bioengineering12080834
Chicago/Turabian StyleQiao, Qinfeng, Qianbin Zhao, Jinwen Wang, Mingjun Li, Huan Zhou, and Lei Yang. 2025. "The Effects of Calcium Phosphate Bone Cement Preparation Parameters on Injectability and Compressive Strength for Minimally Invasive Surgery" Bioengineering 12, no. 8: 834. https://doi.org/10.3390/bioengineering12080834
APA StyleQiao, Q., Zhao, Q., Wang, J., Li, M., Zhou, H., & Yang, L. (2025). The Effects of Calcium Phosphate Bone Cement Preparation Parameters on Injectability and Compressive Strength for Minimally Invasive Surgery. Bioengineering, 12(8), 834. https://doi.org/10.3390/bioengineering12080834







