Therapeutic Role of Functional Massage in Attenuating Exercise-Induced Neuromuscular Fatigue
Abstract
1. Introduction
2. Methods
2.1. Overall Design and Implementation
2.2. Subjects
2.3. Functional Massage (FM) Protocol
2.4. Electrode Placement
2.5. Incremental Cycle Ergometry
2.6. Determining the PWCFT
2.7. EMG Signal Acquisition and Processing
2.8. Statistical Analysis
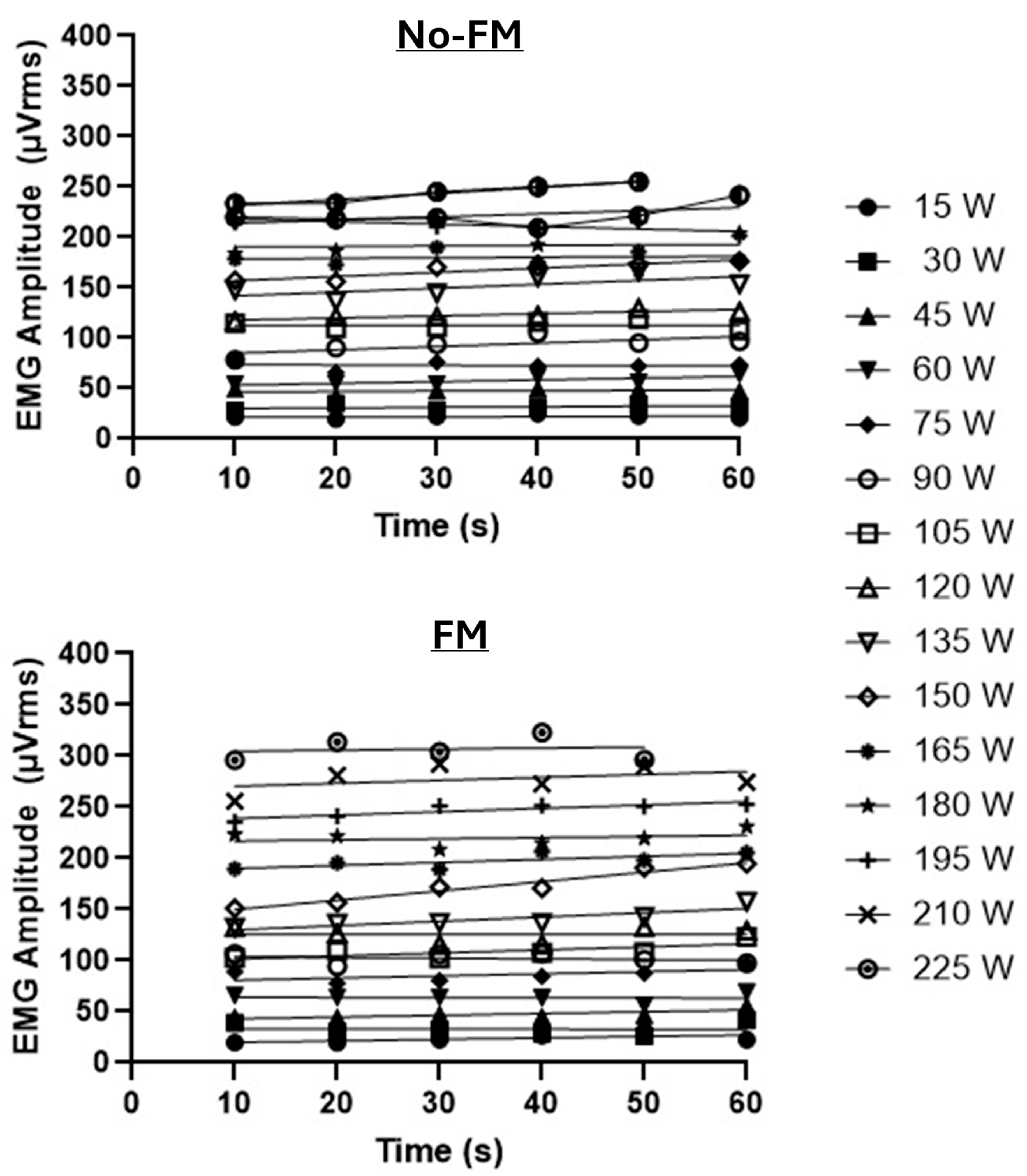
3. Results
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Krauss, J.R.; Creighton, D.S.; Pociask, F.D. Extremity Orthopedics: A Laboratory Manual; Lakeview Media LLC: Rochester Hills, MI, USA, 2004. [Google Scholar]
- Barra-López, M.E.; Lucha-López, M.O.; Castillo-Tomás, S.; López-de-Celis, C.; González-Rueda, V.; Villar-Mateo, E.; Domínguez-Cobo, A. Functional Massage of the Teres Major Muscle in Patients with Subacromial Impingement Syndrome. A Randomized Controlled Case Series Study. Int. J. Med. Pharm. Case Rep. 2016, 8, 1–10. [Google Scholar] [CrossRef]
- Sobeck, G.; Lenk, L.; Knipper, S.; Rhoda, A.; Stickler, L.; Stephenson, P. The effectiveness of functional massage on pain and range of motion measurements in patients with orthopedic impairments of the extremities. Int. Musculo Med. 2016, 38, 21–25. [Google Scholar] [CrossRef]
- Plasencia, R.R.; Van Zant, J.; Charron, S.C.; Manderachia, N.M.; Dickson, J.; Malek, M.H. Massage prior to exercise delays the onset of the physical working capacity at the fatigue threshold (PWCFT). J. Sports Med. Phys. Fit. 2024, 65, 650–656. [Google Scholar] [CrossRef]
- Basmajian, J.V.; De Luca, C.J. Muscles Alive, Their Functions Revealed by Electromyography, 5th ed.; Williams & Wilkins: Baltimore, MD, USA, 1985; pp. 1–200. [Google Scholar]
- Ryan, E.D.; Cramer, J.T.; Housh, T.J.; Beck, T.W.; Herda, T.J.; Hartman, M.J.; Stout, J.R. Inter-individual variability among the mechanomyographic and electromyographic amplitude and mean power frequency responses during isometric ramp muscle actions. Electromyogr. Clin. Neurophysiol. 2007, 47, 161–173. [Google Scholar] [PubMed]
- Herda, T.J.; Housh, T.J.; Weir, J.P.; Ryan, E.D.; Costa, P.B.; Defreitas, J.M.; Walter, A.A.; Stout, J.R.; Beck, T.W.; Cramer, J.T. The consistency of ordinary least-squares and generalized least-squares polynomial regression on characterizing the mechanomyographic amplitude versus torque relationship. Physiol. Meas. 2009, 30, 115–128. [Google Scholar] [CrossRef] [PubMed]
- deVries, H.A.; Brodowicz, G.R.; Robertson, L.D.; Svoboda, M.D.; Schendel, J.S.; Tichy, A.M.; Tichy, M.W. Estimating physical working capacity and training changes in the elderly at the fatigue threshold (PWCft). Ergonomics 1989, 32, 967–977. [Google Scholar] [CrossRef]
- deVries, H.A.; Tichy, M.W.; Housh, T.J.; Smyth, K.D.; Tichy, A.M.; Housh, D.J. A method for estimating physical working capacity at the fatigue threshold (PWCFT). Ergonomics 1987, 30, 1195–1204. [Google Scholar] [CrossRef]
- Housh, T.J.; deVries, H.A.; Johnson, G.O.; Housh, D.J.; Evans, S.A.; Stout, J.R.; Evetovich, T.K.; Bradway, R.M. Electromyographic fatigue thresholds of the superficial muscles of the quadriceps femoris. Eur. J. Appl. Physiol. Occup. Physiol. 1995, 71, 131–136. [Google Scholar] [CrossRef] [PubMed]
- Briscoe, M.J.; Forgach, M.S.; Trifan, E.; Malek, M.H. Validating the EMGFT from a single incremental cycling testing. Int. J. Sports Med. 2014, 35, 566–570. [Google Scholar]
- Camic, C.L.; Housh, T.J.; Johnson, G.O.; Hendrix, C.R.; Zuniga, J.M.; Mielke, M.; Schmidt, R.J. An EMG frequency-based test for estimating the neuromuscular fatigue threshold during cycle ergometry. Eur. J. Appl. Physiol. 2010, 108, 337–345. [Google Scholar] [CrossRef]
- Miller, J.M.; Housh, T.J.; Coburn, J.W.; Cramer, J.T.; Johnson, G.O. A proposed test for determining physical working capacity at the oxygen consumption threshold (PWCVO2). J. Strength Cond. Res. 2004, 18, 618–624. [Google Scholar] [CrossRef]
- Mielke, M.; Housh, T.J.; Hendrix, C.R.; Camic, C.L.; Zuniga, J.M.; Schmidt, R.J.; Johnson, G.O. Oxygen uptake, heart rate, and ratings of perceived exertion at the PWCVo2. J. Strength Cond. Res. 2009, 23, 1292–1299. [Google Scholar] [CrossRef]
- Perry, S.R.; Housh, T.J.; Johnson, G.O.; Ebersole, K.T.; Bull, A.J. Heart rate and ratings of perceived exertion at the physical working capacity at the heart rate threshold. J. Strength Cond. Res. 2001, 15, 225–229. [Google Scholar]
- Sterner, D.A.; Stout, J.R.; Antonio, B.B.; Anderson, A.T.; Fukuda, D.H. A proposed test to determine physical working capacity at pain intensity threshold (PWC(PIT)). Eur. J. Appl. Physiol. 2025, 125, 197–207. [Google Scholar] [CrossRef] [PubMed]
- Evetovich, T.K.; Housh, T.J.; Johnson, G.O.; Evans, S.A.; Stout, J.R.; Bull, A.J.; Smith, D.B.; Evetovich, M.M. Effect of workbout duration on the physical working capacity at fatigue threshold (PWCFT) test. Ergonomics 1996, 39, 314–321. [Google Scholar] [CrossRef] [PubMed]
- Housh, T.J.; deVries, H.A.; Johnson, G.O.; Evans, S.A.; Housh, D.J.; Stout, J.R.; Bradway, R.M.; Evetovich, T.K. Neuromuscular fatigue thresholds of the vastus lateralis, vastus medialis and rectus femoris muscles. Electromyogr. Clin. Neurophysiol. 1996, 36, 247–255. [Google Scholar] [PubMed]
- Feldpausch, J.E.; Blok, A.L.; Frederick, E.L.; Coburn, J.W.; Malek, M.H. The evolution of the physical work capacity at the fatigue threshold test: Past, Present, and Future. J. Strength. Cond. Res. 2021, 35, 3529–3536. [Google Scholar] [CrossRef]
- Housh, T.J.; deVries, H.A.; Johnson, G.O.; Evans, S.A.; Tharp, G.D.; Housh, D.J.; Hughes, R.J. The effect of glycogen depletion and supercompensation on the physical working capacity at the fatigue threshold. Eur. J. Appl. Physiol. Occup. Physiol. 1990, 60, 391–394. [Google Scholar] [CrossRef]
- Housh, T.J.; deVries, H.A.; Johnson, G.O.; Evans, S.A.; McDowell, S. The effect of ammonium chloride and sodium bicarbonate ingestion on the physical working capacity at the fatigue threshold. Eur. J. Appl. Physiol. Occup. Physiol. 1991, 62, 189–192. [Google Scholar] [CrossRef]
- Stout, J.R.; Cramer, J.T.; Zoeller, R.F.; Torok, D.; Costa, P.; Hoffman, J.R.; Harris, R.C.; O’Kroy, J. Effects of beta-alanine supplementation on the onset of neuromuscular fatigue and ventilatory threshold in women. Amino Acids 2007, 32, 381–386. [Google Scholar] [CrossRef]
- Camic, C.L.; Housh, T.J.; Zuniga, J.M.; Hendrix, R.C.; Mielke, M.; Johnson, G.O.; Schmidt, R.J. Effects of arginine-based supplements on the physical working capacity at the fatigue threshold. J. Strength Cond. Res. 2010, 24, 1306–1312. [Google Scholar] [CrossRef]
- Stout, J.R.; Cramer, J.T.; Mielke, M.; O’Kroy, J.; Torok, D.J.; Zoeller, R.F. Effects of twenty-eight days of beta-alanine and creatine monohydrate supplementation on the physical working capacity at neuromuscular fatigue threshold. J. Strength Cond. Res. 2006, 20, 928–931. [Google Scholar] [CrossRef]
- Stout, J.; Eckerson, J.; Ebersole, K.; Moore, G.; Perry, S.; Housh, T.; Bull, A.; Cramer, J.; Batheja, A. Effect of creatine loading on neuromuscular fatigue threshold. J Appl. Physiol. 2000, 88, 109–112. [Google Scholar] [CrossRef]
- Elhaj, H.M.; Imam, O.; Page, B.W.; Vitale, J.M.; Malek, M.H. Perceived consumption of a high dose caffeine drink delays neuromuscular fatigue. J. Strength Cond. Res. 2022, 36, 1185–1190. [Google Scholar] [CrossRef]
- Centala, J.; Pogorel, C.; Pummill, S.W.; Malek, M.H. Listening to fast-temp music delays the onset of neuromuscular fatigue. J. Strength Cond. Res. 2020, 34, 617–622. [Google Scholar] [CrossRef]
- Hermens, H.J.; Freriks, B.; Disselhorst-Klug, C.; Rau, G. Development of recommendations for SEMG sensors and sensor placement procedures. J. Electromyogr. Kinesiol. Off. J. Int. Soc. Electrophysiol. Kinesiol. 2000, 10, 361–374. [Google Scholar] [CrossRef] [PubMed]
- Sjogaard, G. Force–velocity curve for bicycle work. In Biomechanics VI-A; Asmussen, E., Jørgensen, K., Eds.; University Park Press: Baltimore, MD, USA, 1978; pp. 93–99. [Google Scholar]
- Robbins, D.W. Postactivation potentiation and its practical applicability: A brief review. J. Strength Cond. Res. 2005, 19, 453–458. [Google Scholar] [CrossRef] [PubMed]
- Sale, D.G. Postactivation potentiation: Role in human performance. Exerc. Sport. Sci. Rev. 2002, 30, 138–143. [Google Scholar] [CrossRef]
- Franco, B.L.; Signorelli, G.R.; Trajano, G.S.; Costa, P.B.; de Oliveira, C.G. Acute effects of three different stretching protocols on the wingate test performance. J. Sports Sci. Med. 2012, 11, 1–7. [Google Scholar] [PubMed]
- Li, T.; Liang, Y. The effects of different post-activation potentiation strategies on the performance of elite female track cyclists in position 1 of team sprint. Sci. Rep. 2024, 14, 24604. [Google Scholar] [CrossRef]
- Hamada, T.; Sale, D.G.; Macdougall, J.D. Postactivation potentiation in endurance-trained male athletes. Med. Sci. Sports Exerc. 2000, 32, 403–411. [Google Scholar] [CrossRef] [PubMed]
- Bremer, N.; Peoples, G.; Hasler, B.; Litzenburg, R.; Johnson, A.; Malek, M.H. Repeated Incremental Workbouts Separated by 1 Hour Increase the Electromyographic Fatigue Threshold. J. Strength. Cond. Res. 2021, 35, 1397–1402. [Google Scholar] [CrossRef] [PubMed]
| Experimental Conditions | |||||
|---|---|---|---|---|---|
| Outcome Variable | No-FM | FM | t-Statistic | p-Value | Cohen’s d |
| Maximal Power Output (W) | 213 ± 13 | 218 ± 12 | 1.483 | 0.166 | 0.11 |
| PWCFT (W) | 120 ± 8 | 145 ± 11 | 4.43 | 0.001 | 0.74 |
| PWCFT (%max) | 57 ± 2 | 67 ± 3 | 3.21 | 0.008 | 1.04 |
| End-exercise heart rate (bpm) | 184 ± 3 | 182 ± 4 | 1.02 | 0.332 | 0.15 |
| End-exercise heart rate (%max) | 95 ± 2 | 93 ± 2 | 1.01 | 0.333 | 0.16 |
| End-exercise RPE | 10 ± 0 | 10 ± 0 | 1.15 | 0.275 | 0.36 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Darwich, Z.; Issa, A.; Parkin, E.; Young, J.; Pepin, M.E.; Malek, M.H. Therapeutic Role of Functional Massage in Attenuating Exercise-Induced Neuromuscular Fatigue. Bioengineering 2025, 12, 880. https://doi.org/10.3390/bioengineering12080880
Darwich Z, Issa A, Parkin E, Young J, Pepin ME, Malek MH. Therapeutic Role of Functional Massage in Attenuating Exercise-Induced Neuromuscular Fatigue. Bioengineering. 2025; 12(8):880. https://doi.org/10.3390/bioengineering12080880
Chicago/Turabian StyleDarwich, Zahraa, Alaa Issa, Emma Parkin, Jada Young, Marie Eve Pepin, and Moh H. Malek. 2025. "Therapeutic Role of Functional Massage in Attenuating Exercise-Induced Neuromuscular Fatigue" Bioengineering 12, no. 8: 880. https://doi.org/10.3390/bioengineering12080880
APA StyleDarwich, Z., Issa, A., Parkin, E., Young, J., Pepin, M. E., & Malek, M. H. (2025). Therapeutic Role of Functional Massage in Attenuating Exercise-Induced Neuromuscular Fatigue. Bioengineering, 12(8), 880. https://doi.org/10.3390/bioengineering12080880








