Automatic 3D Tracking of Liver Metastases: Follow-Up Assessment of Cancer Patients in Contrast-Enhanced MRI
Abstract
1. Introduction
2. Materials and Methods
2.1. Patient Cohorts
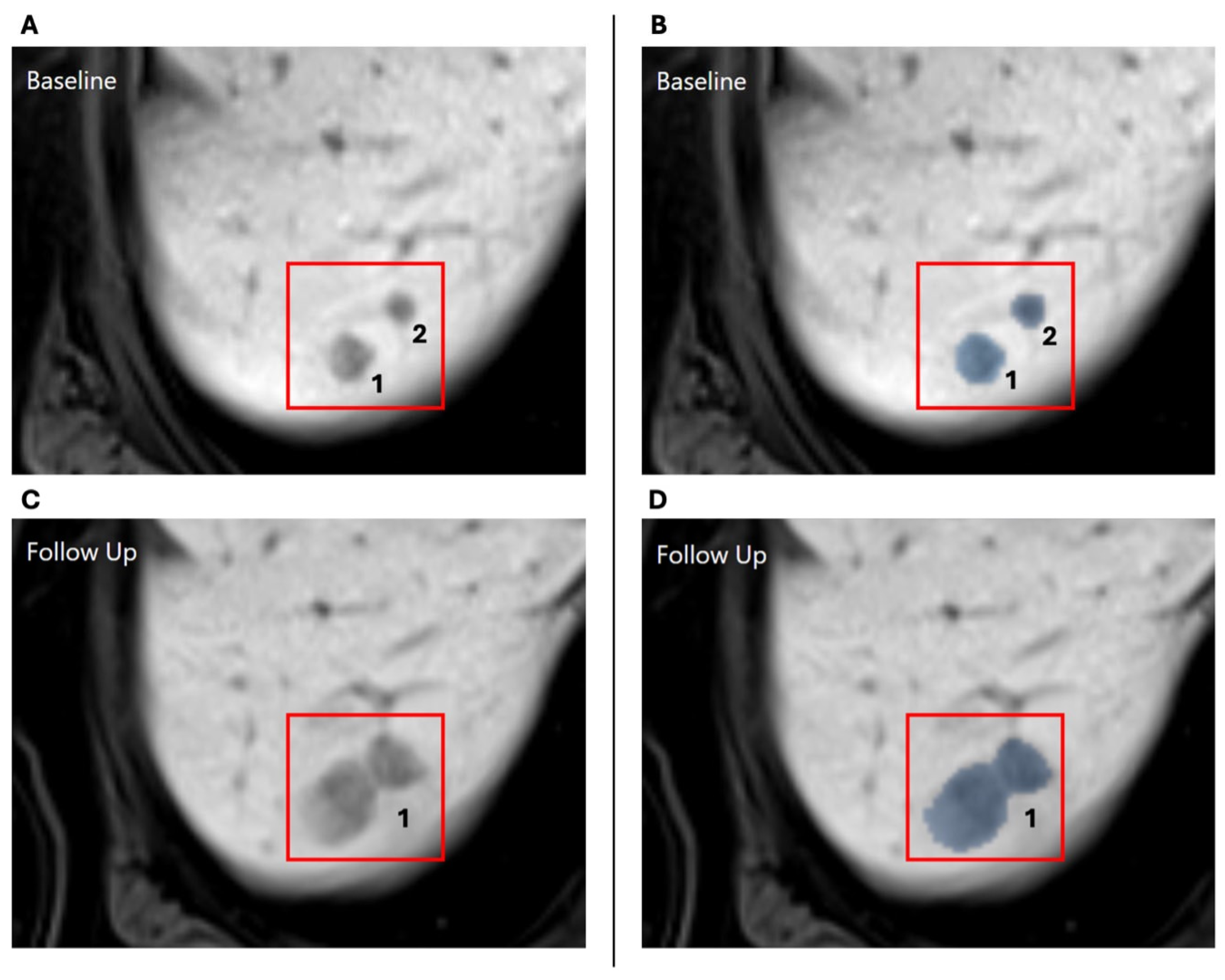
2.2. Automatic Lesion Detection and 3D Tracking
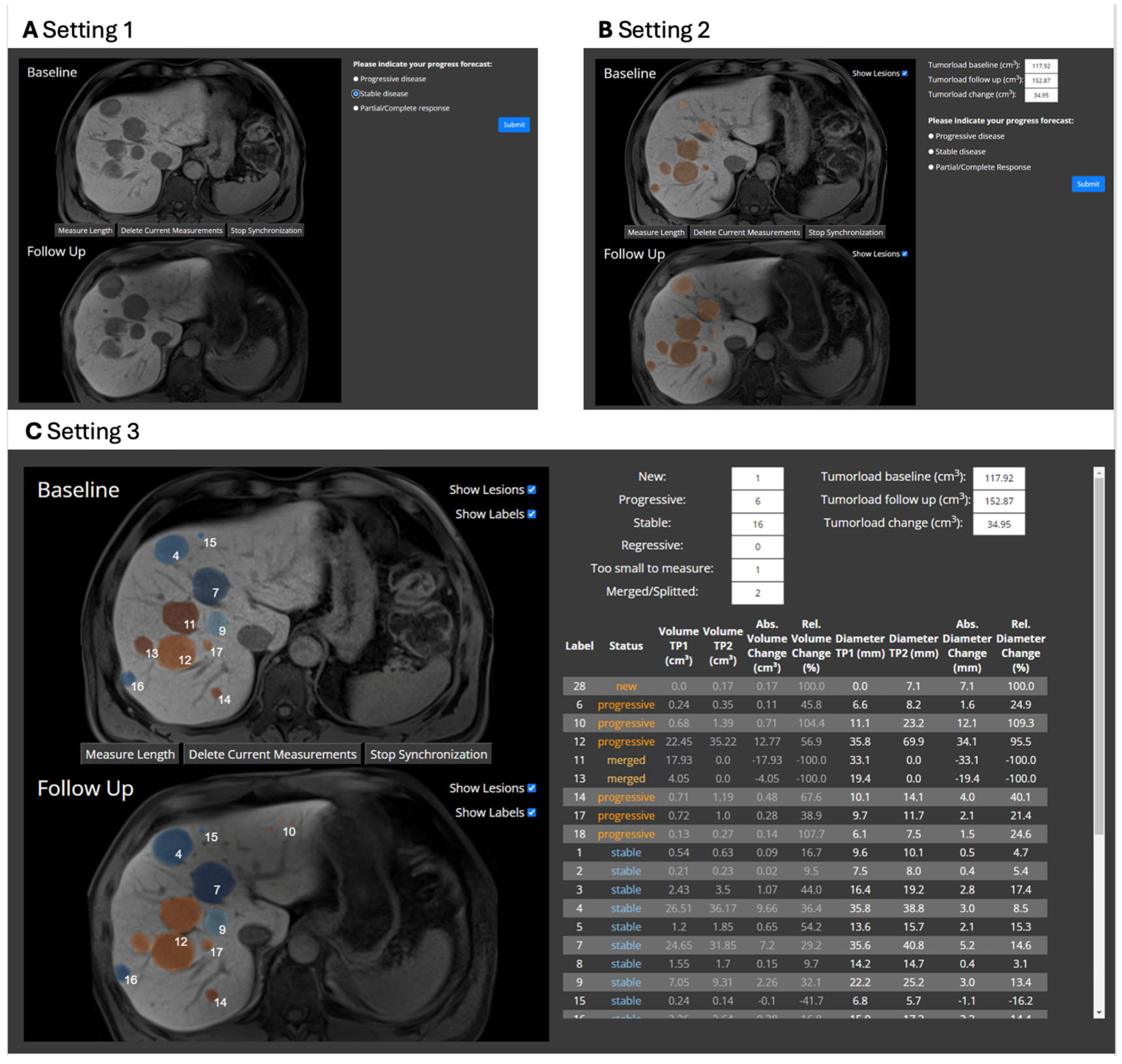
2.3. Evaluation Study
2.4. Statistical Analyses
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| FS | Fat saturation |
| Gd-EOB | Gadoxetic acid |
| GRE | Gradient echo |
| HBP | Hepatobiliary contrast phase |
| MTB | Multidisciplinary Tumor Board |
| MRI | Magnetic resonance imaging |
| NELM | Neuroendocrine liver metastasis |
| RECIST | Response evaluation criteria in solid tumors |
| SD | Standard deviation |
| VIBE | Volumetric interpolated breath-hold examination |
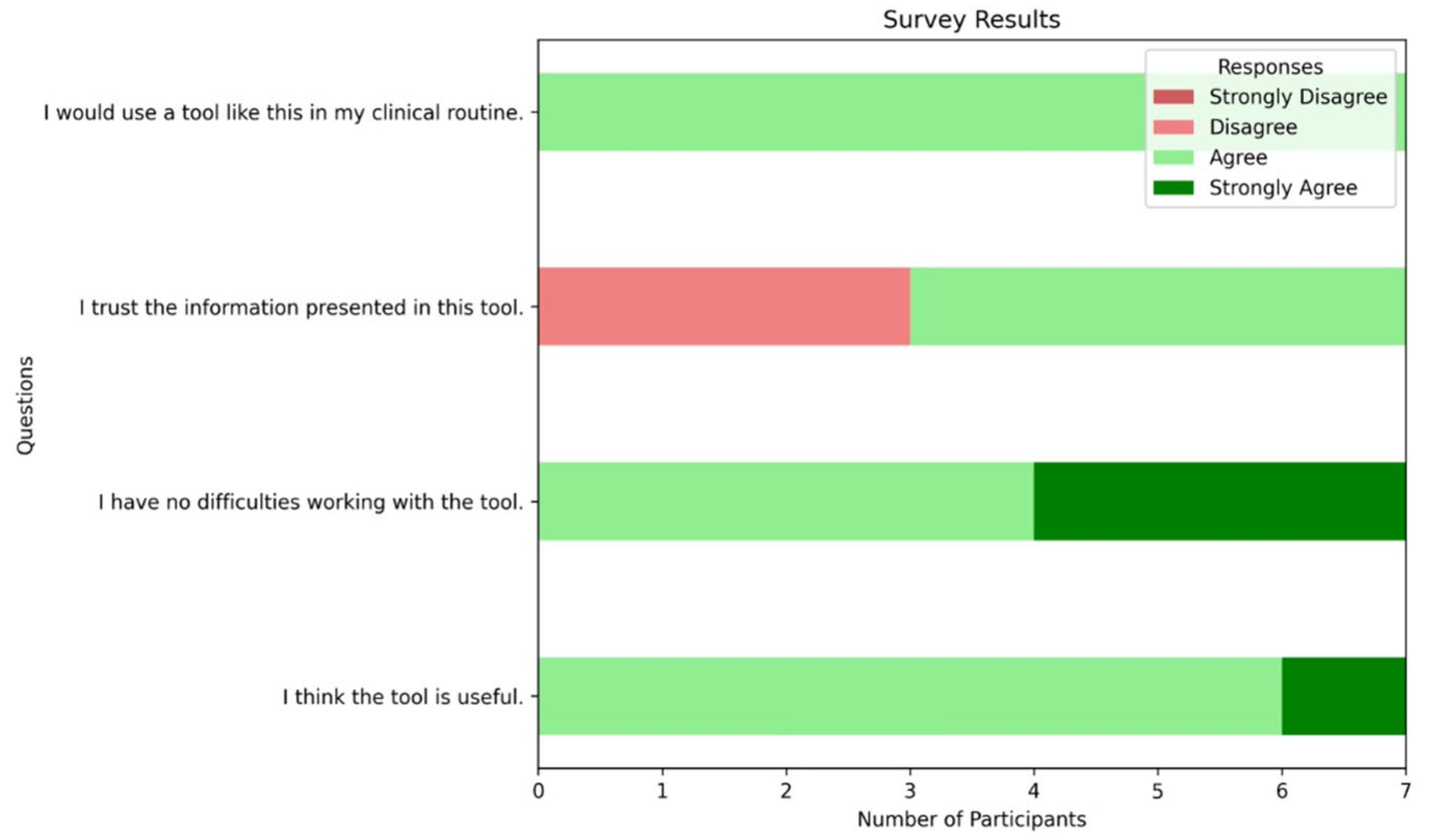
Appendix A. Survey Results
Appendix A.1. Survey Questions
- I think the tool is useful.
- I have no difficulties working with the tool.
- I trust the information presented in this tool.
- I would use a tool like this in my clinical routine.
- -
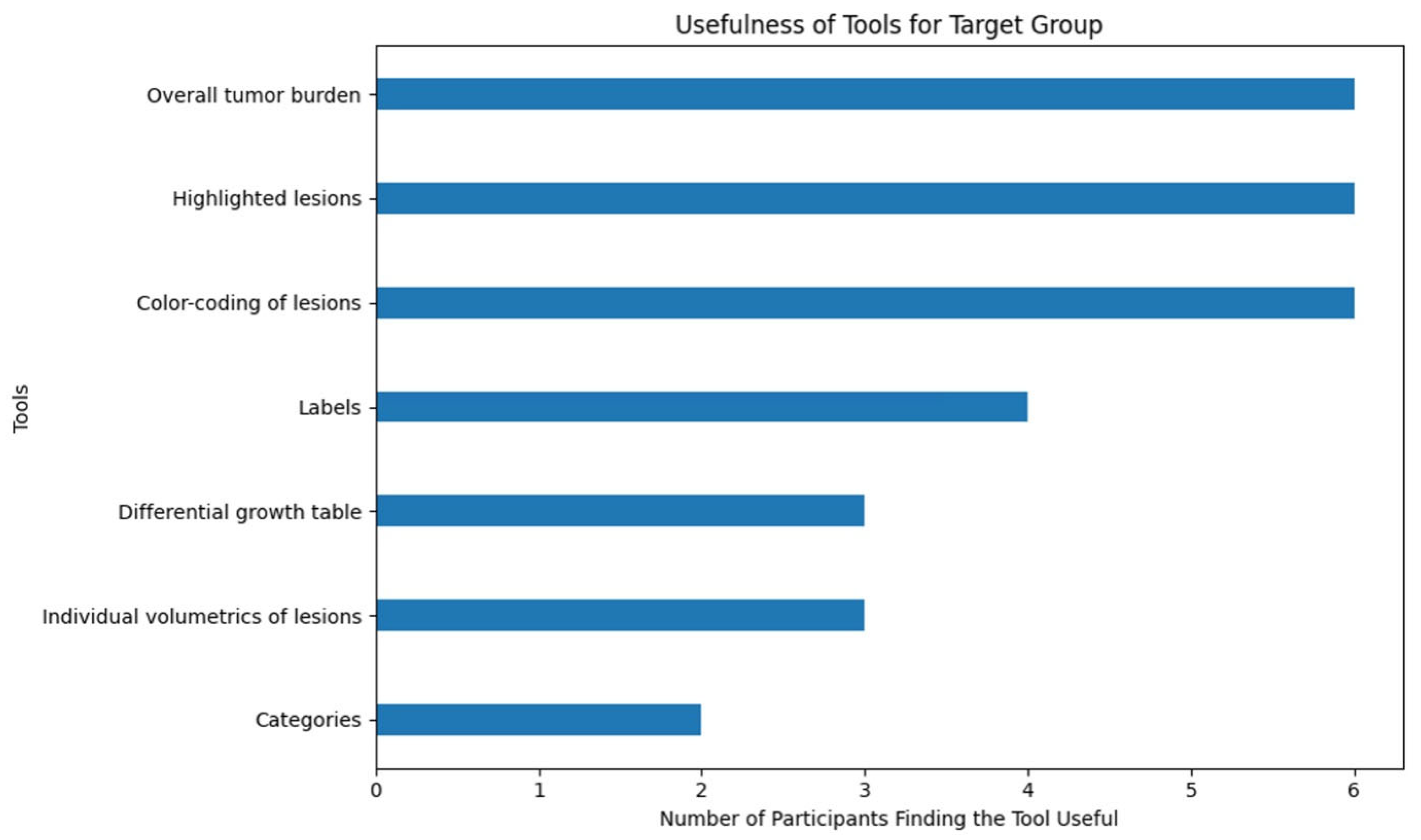
- Overall tumor burden
- -
- Highlighted lesions
- -
- Color-coding of lesions
- -
- Showing the unique labels
- -
- Differential growth table
- -
- Individual volumetrics of lesions
- -
- Categories
Appendix A.2. Survey Results
References
- Dudjak, L.A. Cancer metastasis. Semin. Oncol. Nurs. 1992, 8, 40–50. [Google Scholar] [CrossRef] [PubMed]
- Sethi, N.; Kang, Y. Unravelling the complexity of metastasis–molecular understanding and targeted therapies. Nat. Rev. Cancer 2011, 11, 735–748. [Google Scholar] [CrossRef] [PubMed]
- Frilling, A.; Modlin, I.M.; Kidd, M.; Russell, C.; Breitenstein, S.; Salem, R.; Kwekkeboom, D.; Lau, W.-Y.; Klersy, C.; Vilgrain, V.; et al. Recommendations for management of patients with neuroendocrine liver metastases. Lancet. Oncol. 2014, 15, e8–e21. [Google Scholar] [CrossRef] [PubMed]
- Pape, U.F.; Berndt, U.; Müller-Nordhorn, J.; Böhmig, M.; Roll, S.; Koch, M.; Willich, S.N.; Wiedenman, B. Prognostic factors of long-term outcome in gastroenteropancreatic neuroendocrine tumours. Endocr. Relat. Cancer 2008, 15, 1083–1097. (In English) [Google Scholar] [CrossRef]
- Frilling, A.; Clift, A.K. Therapeutic strategies for neuroendocrine liver metastases. Cancer 2015, 121, 1172–1186. [Google Scholar] [CrossRef]
- Zhou, J.W.; Li, Q.F.; Cao, Y.G. Spatiotemporal Heterogeneity across Metastases and Organ-Specific Response Informs Drug Efficacy and Patient Survival in Colorectal Cancer. Cancer Res. 2021, 81, 2522–2533. (In English) [Google Scholar] [CrossRef]
- Rindi, G.; D’Adda, T.; Froio, E.; Fellegara, G.; Bordi, C. Prognostic factors in gastrointestinal endocrine tumors. Endocr. Pathol. 2007, 18, 145–149. [Google Scholar] [CrossRef]
- Eisenhauer, E.A.; Therasse, P.; Bogaerts, J.; Schwartz, L.H.; Sargent, D.; Ford, R.; Dancey, J.; Arbuck, S.; Gwyther, S.; Mooney, M.; et al. New response evaluation criteria in solid tumours: Revised RECIST guideline (version 1.1). Eur. J. Cancer 2009, 45, 228–247. [Google Scholar] [CrossRef]
- Fournier, L.; de Geus-Oei, L.F.; Regge, D.; Oprea-Lager, D.E.; D’Anastasi, M.; Bidaut, L.; Bäuerle, T.; Lopci, E.; Cappello, G.; Lecouvet, F.; et al. Twenty Years On: RECIST as a Biomarker of Response in Solid Tumours an EORTC Imaging Group-ESOI Joint Paper. Front. Oncol. 2022, 11, 800547. (In English) [Google Scholar] [CrossRef]
- Vreugdenburg, T.D.; Ma, N.; Duncan, J.K.; Riitano, D.; Cameron, A.L.; Maddern, G.J. Comparative diagnostic accuracy of hepatocyte-specific gadoxetic acid (Gd-EOB-DTPA) enhanced MR imaging and contrast enhanced CT for the detection of liver metastases: A systematic review and meta-analysis. Int. J. Color. Dis. 2016, 31, 1739–1749. [Google Scholar] [CrossRef]
- Zhang, L.; Yu, X.; Huo, L.; Lu, L.; Pan, X.; Jia, N.; Fan, X.; Morana, G.; Grazioli, L.; Schneider, G. Detection of liver metastases on gadobenate dimeglumine-enhanced MRI: Systematic review, meta-analysis, and similarities with gadoxetate-enhanced MRI. Eur. Radiol. 2019, 29, 5205–5216. [Google Scholar] [CrossRef]
- Fehrenbach, U.; Xin, S.; Hartenstein, A.; Auer, T.A.; Dräger, F.; Froböse, K.; Jann, H.; Mogl, M.; Amthauer, H.; Geisel, D.; et al. Automatized Hepatic Tumor Volume Analysis of Neuroendocrine Liver Metastases by Gd-EOB MRI-A Deep-Learning Model to Support Multidisciplinary Cancer Conference Decision-Making. Cancers 2021, 13, 2726. [Google Scholar] [CrossRef] [PubMed]
- Van Kessel, C.S.; Veldhuis, W.B.; van den Bosch, M.A.A.J.; van Leeuwen, M.S. MR liver imaging with Gd-EOB-DTPA: A delay time of 10 minutes is sufficient for lesion characterization. Eur. Radiol. 2012, 22, 2153–2160. (In English) [Google Scholar] [CrossRef] [PubMed]
- Isensee, F.; Jaeger, P.F.; Kohl, S.A.A.; Petersen, J.; Maier-Hein, K.H. nnU-Net: A self-configuring method for deep learning-based biomedical image segmentation. Nat. Methods 2021, 18, 203–211. [Google Scholar] [CrossRef] [PubMed]
- Dice, L.R. Measures of the Amount of Ecologic Association between Species. Ecology 1945, 26, 297–302. (In English) [Google Scholar] [CrossRef]
- Shapiro, S.S.; Wilk, M.B. An Analysis of Variance Test for Normality (Complete Samples). Biometrika 1965, 52, 591. (In English) [Google Scholar] [CrossRef]
- Friedman, M. The use of ranks to avoid the assumption of normality implicit in the analysis of variance. J. Am. Stat. Assoc. 1937, 32, 675. [Google Scholar] [CrossRef]
- Wilcoxon, F. Individual Comparisons by Ranking Methods. Biom. Bull. 1945, 1, 80–83. (In English) [Google Scholar] [CrossRef]
- Prasad, S.R.; Jhaveri, K.S.; Saini, S.; Hahn, P.F.; Halpern, E.F.; Sumner, J.E. CT tumor measurement for therapeutic response assessment: Comparison of unidimensional, bidimensional, and volumetric techniques-Initial observations. Radiology 2002, 225, 416–419. (In English) [Google Scholar] [CrossRef]
- Tran, L.N.; Brown, M.S.; Goldin, J.G.; Yan, X.; Pais, R.C.; McNitt-Gray, M.F.; Gjertson, D.; Rogers, S.R.; Aberle, D.R. Comparison of treatment response classifications between unidimensional, bidimensional, and volumetric measurements of metastatic lung lesions on chest computed tomography. Acad. Radiol. 2004, 11, 1355–1360. (In English) [Google Scholar] [CrossRef]
- Warren, K.E.; Patronas, N.; Aikin, A.A.; Albert, P.S.; Balis, F.M. Comparison of one-, two-, and three-dimensional measurements of childhood brain tumors. J. Natl. Cancer I. 2001, 93, 1401–1405. (In English) [Google Scholar] [CrossRef] [PubMed]
- Suter, Y.; Notter, M.; Meier, R.; Loosli, T.; Schucht, P.; Wiest, R.; Reyes, M.; Knecht, U. Evaluating automated longitudinal tumor measurements for glioblastoma response assessment. Front. Radiol. 2023, 3, 1211859. (In English) [Google Scholar] [CrossRef]
- Machura, B.; Kucharski, D.; Bozek, O.; Eksner, B.; Kokoszka, B.; Pekala, T.; Radom, M.; Strzelczak, M.; Zarudzki, L.; Gutiérrez-Becker, B.; et al. Deep learning ensembles for detecting brain metastases in longitudinal multi-modal MRI studies. Comput. Med. Imaging Graph. 2024, 116, 102401. [Google Scholar] [CrossRef]
- Nishino, M.; Wakai, S.; Hida, T.; Dahlberg, S.E.; Ozaki, M.; Hatabu, H.; Tachizaki, H.; Johnson, B.E. Automated image analysis tool for tumor volume growth rate to guide precision cancer therapy: EGFR-mutant non-small-cell lung cancer as a paradigm. Eur. J. Radiol. 2018, 109, 68–76. (In English) [Google Scholar] [CrossRef]
- Kohler, C.; Wahl, H.; Ziemssen, T.; Linn, J.; Kitzler, H.H. Exploring individual multiple sclerosis lesion volume change over time: Development of an algorithm for the analyses of longitudinal quantitative MRI measures. Neuroimage Clin. 2019, 21, 101623. [Google Scholar] [CrossRef]
- Hsu, D.G.; Ballangrud, Å.; Prezelski, K.; Swinburne, N.C.; Young, R.; Beal, K.; Deasy, J.O.; Cerviño, L.; Aristophanous, M. Automatically tracking brain metastases after stereotactic radiosurgery. Phys. Imaging Radiat. Oncol. 2023, 27, 100452. [Google Scholar] [CrossRef]




| Name | Definition |
|---|---|
| Progressive | The diameter increased by at least 20% |
| Stable | The diameter change is between 20% increase and 30% decrease |
| Regressive | The diameter decreased by at least 30% |
| New | The lesion only appears in the follow-up MRI |
| Merged | The lesion grew together with another lesion |
| Too small to measure | The lesion has a diameter smaller than 5 mm at both timepoints |
| Metric | Setting 1 | Setting 2 | Setting 3 | p-Value |
|---|---|---|---|---|
| Median decision time in s (IQR) | 13.8 (9.2–21.8) | 14.4 (10.3–24.0) | 23.8 (14.2–42.8) | <0.001 |
| Accuracy in % (SD, range) | 88.7 (SD 11.0, range 67–97) | 90.6 (SD 8.7, range 73–97) | 90.1 (SD 6.1, range 80–97) | 0.72 |
| Precision in % (SD, range) | 81.6 (SD 9.5, range 63–89) | 83.6 (SD 6.4, range 72–89) | 83.4 (SD 5.8, range 73–89) | 0.72 |
| Recall in % (SD, range) | 91.9 (SD 8.7, range 74–98) | 92.9 (SD 7.2, range 78–98) | 90.7 (SD 9.4, range 71–98) | 0.30 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Schulze-Weddige, S.; Fehrenbach, U.; Kolck, J.; Ruppel, R.; Baumgärtner, G.L.; Lindholz, M.; Schobert, I.T.; Haack, A.-M.; Jann, H.; Mogl, M.; et al. Automatic 3D Tracking of Liver Metastases: Follow-Up Assessment of Cancer Patients in Contrast-Enhanced MRI. Bioengineering 2025, 12, 874. https://doi.org/10.3390/bioengineering12080874
Schulze-Weddige S, Fehrenbach U, Kolck J, Ruppel R, Baumgärtner GL, Lindholz M, Schobert IT, Haack A-M, Jann H, Mogl M, et al. Automatic 3D Tracking of Liver Metastases: Follow-Up Assessment of Cancer Patients in Contrast-Enhanced MRI. Bioengineering. 2025; 12(8):874. https://doi.org/10.3390/bioengineering12080874
Chicago/Turabian StyleSchulze-Weddige, Sophia, Uli Fehrenbach, Johannes Kolck, Richard Ruppel, Georg Lukas Baumgärtner, Maximilian Lindholz, Isabel Theresa Schobert, Anna-Maria Haack, Henning Jann, Martina Mogl, and et al. 2025. "Automatic 3D Tracking of Liver Metastases: Follow-Up Assessment of Cancer Patients in Contrast-Enhanced MRI" Bioengineering 12, no. 8: 874. https://doi.org/10.3390/bioengineering12080874
APA StyleSchulze-Weddige, S., Fehrenbach, U., Kolck, J., Ruppel, R., Baumgärtner, G. L., Lindholz, M., Schobert, I. T., Haack, A.-M., Jann, H., Mogl, M., Geisel, D., Wiedenmann, B., & Penzkofer, T. (2025). Automatic 3D Tracking of Liver Metastases: Follow-Up Assessment of Cancer Patients in Contrast-Enhanced MRI. Bioengineering, 12(8), 874. https://doi.org/10.3390/bioengineering12080874








