A Deep-Learning-Based Diffusion Tensor Imaging Pathological Auto-Analysis Method for Cervical Spondylotic Myelopathy
Abstract
1. Introduction
- The current analysis process relies heavily on manual methods, which are time-consuming and laborious and require substantial expertise. On the other hand, the analysis process requires regions of interest (ROIs) in the spinal cord to be drawn manually, which is generally believed to be susceptible to human experience and expertise [16,17]. Therefore, the conventional manual approach is inherently limited by subjectivity and variability, resulting in inconsistent outcomes.
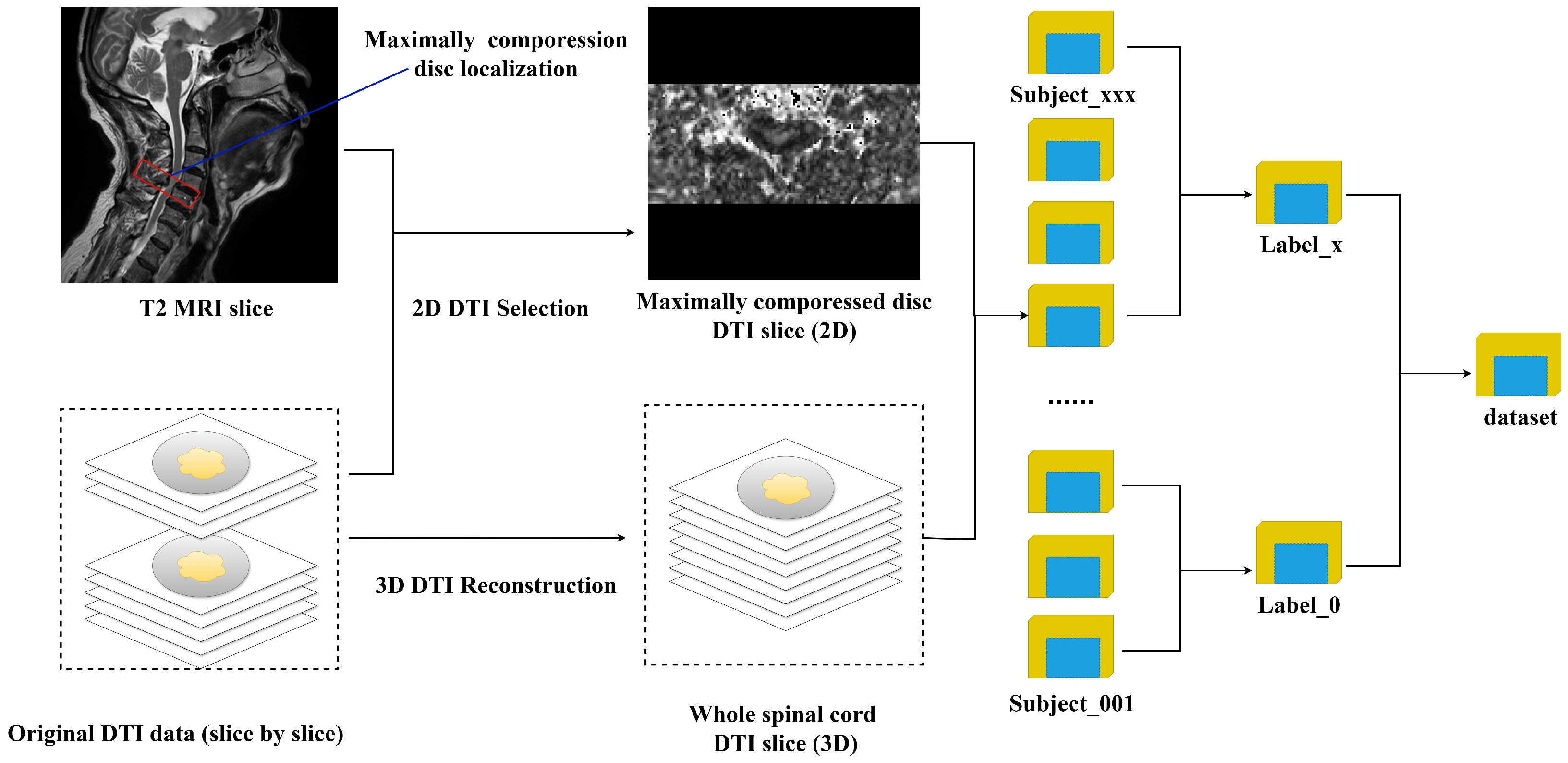
- The selection of spatial features in DTI remains underexplored. In the current research, the ROIs are often manually drawn onto DTI slices that cover the most compressed disc in the spinal cord [9,18]. While this approach has provided valuable insights, it neglects the impact of the compression that occurs in other discs of the spinal cord, which could have a synergistic effect on pathological changes. Consequently, these limitations hinder the scalability of DTI-assisted pathological analyses and restrict their clinical applicability.
- To alleviate the effect of the manual method, the feasibility of deep-learning-based DTI automatic analysis was investigated. The preliminary results demonstrated the effectiveness of an end-to-end pathological analysis, focusing on DTI (the FA values in an image) on both the most compressed spinal disc and the entire spinal cord. This represents the first study to validate the potential of such a deep learning approach in this application field.
- To investigate the utilization of the spatial information from DTI further, this study designed a multi-dimensional feature fusion mechanism enhanced model, referred to as DCSANet-MD (DTI-Based CSM Severity Assessment Network-Multi-Dimensional). By integrating features from DTI of the maximally compressed cervical disc (2D) and the whole spinal cord (3D), our approach provides an enlarged decision framework for CSM pathological automatic assessment and promotes DTI-assisted clinical management.
2. Materials and Methods
2.1. Dataset Description
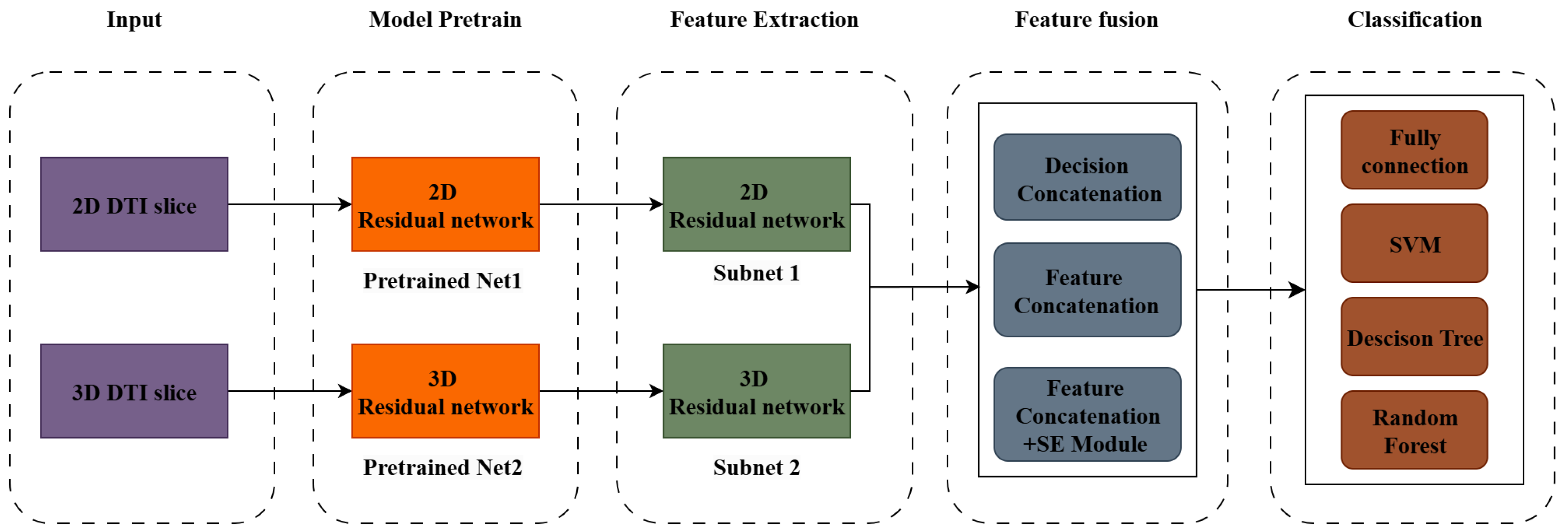
2.2. The Model Structure
2.2.1. Feature Extraction
2.2.2. The Feature Integration Mechanism
- Decision fusion is a method that combines the outputs (predictions) of multiple classifiers or models to make a final decision, which simplifies the integration of pre-trained models into an ensemble with minimum further computation, which is beneficial for reducing the model’s potential complexity.
- Feature fusion directly combines the feature representations extracted from different sources of models into a unified feature space; thus, this mechanism is designed to optimize the fusion process, enabling end-to-end learning of the optimal feature representations. After fusion, the combined feature vector is fed into a classifier for the final decision.
- Attention-enhanced feature fusion builds on feature fusion by incorporating attention mechanisms to focus on the most relevant features during the fusion process. In multi-dimensional classification like that in our research, since the multi-source features are noisy and redundant, this mechanism is thought to be especially beneficial. Attention assigns weights to features based on their importance to the classification task, helping the model prioritize critical information while suppressing irrelevant or noisy features, where 2-dimensional modality (2D and 3D) features may have varying importance depending on the pathological context.
2.3. The Experimental Setting
2.3.1. Data Pre-Processing
2.3.2. Model Training Setting
2.3.3. Comparison Models and the Baseline
2.3.4. Hierarchical Classification (H-Classification)
3. Results
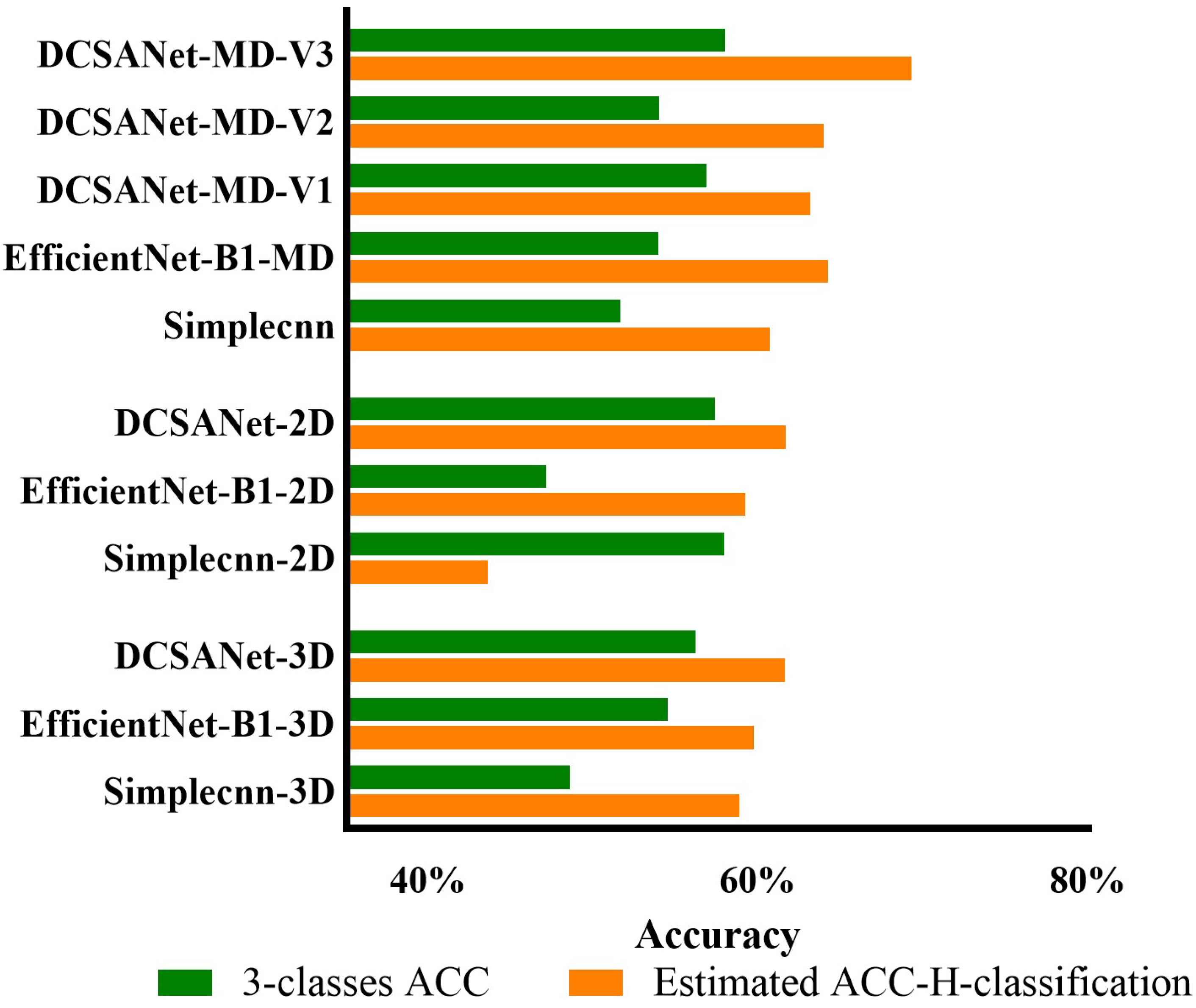
3.1. Comparison of Different Input Sources
3.2. The Effectiveness of DCSANet in Predicting the Three-Class Severity Categories
4. Discussion
4.1. The Effectiveness of Deep-Learning-Based Pathological Severity Predictions Using DTI in CSM
4.2. Analysis of the Model Performance in Two-Class Categorization
4.3. The Effectiveness of the Feature Fusion Mechanism
4.4. The Effectiveness of the Machine Learning Classifier
4.5. Analysis of the Model Performance in Three-Class Categorization
4.6. Analysis-Associated Factors May Interfere with DTI Assessments
- The Segmentation Process for DTI: In this study, the entire DTI slice was used as a feature matrix to identify CSM’s severity. Although this design aims to realize the primary objective of providing an end-to-end method, in manual methods, a segmentation process is typically applied to extract anatomical-level structures from the background [33]. The absence of segmentation in our approach may have affected the analysis and the interpretation of DTI features at the anatomical structure level.
- Unexplored Relationships Between Multiple Compressed Discs: As described in Section 1, cervical compression frequently affects multiple discs. However, due to manpower and time constraints, most existing studies have focused only on the maximally compressed cervical disc (MCCD) or the entire cervical spinal cord [14,16]. While our method innovatively integrates both into the analysis, the relationship among the number and location of affected cervical discs (rather than the entire spinal cord) and the severity of CSM has not been thoroughly investigated. This limitation may have impacted the precision of the model’s performance.
- Selection of DTI Diffusive Features: While DTI provides several diffusive features, besides the FA value, other features such as the Mean Diffusivity (MD), Axial Diffusivity (AD), and Radial Diffusivity (RD) can also be used for analysis [36]. This study primarily utilized FA as the target feature because it is the most commonly used index and represents a combination of the other three metrics. However, the relationships among MD, AD, and RD in CSM analysis remain unclear. A more comprehensive study is needed to explore these relationships and develop synthesis methods for integrating these indices into the analysis.
4.7. Clinical Relevance and Research Value
4.8. Limitations and Future Direction
4.8.1. Limitations
4.8.2. Future Directions
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| CSM | cervical spondylotic myelopathy |
| DTI | Diffusion Tensor Imaging |
| FA | Fractional Anisotropy |
| DL | deep learning |
| DCSANet | DTI-Based CSM Severity Assessment Network |
| MCCD | maximally compressed cervical disc |
References
- Zhang, J.K.; Sun, P.; Jayasekera, D.; Greenberg, J.K.; Javeed, S.; Dibble, C.F.; Blum, J.; Song, C.; Song, S.K.; Ray, W.Z. Utility of diffusion basis spectrum imaging in quantifying baseline disease severity and prognosis of cervical spondylotic myelopathy. Spine 2022, 47, 1687–1693. [Google Scholar] [CrossRef]
- Badhiwala, J.H.; Ahuja, C.S.; Akbar, M.A.; Witiw, C.D.; Nassiri, F.; Furlan, J.C.; Curt, A.; Wilson, J.R.; Fehlings, M.G. Degenerative cervical myelopathy—Update and future directions. Nat. Rev. Neurol. 2020, 16, 108–124. [Google Scholar] [CrossRef]
- Kalsi-Ryan, S.; Karadimas, S.K.; Fehlings, M.G. Cervical spondylotic myelopathy: The clinical phenomenon and the current pathobiology of an increasingly prevalent and devastating disorder. Neuroscientist 2013, 19, 409–421. [Google Scholar] [CrossRef]
- McCormick, J.R.; Sama, A.J.; Schiller, N.C.; Butler, A.J.; Donnally, C.J. Cervical spondylotic myelopathy: A guide to diagnosis and management. J. Am. Board Fam. Med. 2020, 33, 303–313. [Google Scholar] [CrossRef]
- Guo, X.; Hu, J.; Feng, S.; Gao, X.; Sun, C.; Ao, Q.; Chen, L.; Chen, L.; Zhang, P.; Dai, Y.; et al. Clinical neurorestorative treatment guidelines for neurological dysfunctions of sequels from vertebral and spinal cord lesions (CANR 2023 version). J. Neurorestoratol. 2023, 11, 100070. [Google Scholar] [CrossRef]
- Troxell, D.A.; Bach, J.R.; Nilsestuen, J.O. History, evolution, and graphic analyses of mechanical insufflation exsufflation for treatment of neurological disorders. J. Neurorestoratol. 2023, 11, 100080. [Google Scholar] [CrossRef]
- Yekaninejad, M.S.; Derakhshanrad, N.; Kazemi, E.; Derakhshanrad, A.; Saberi, H. Influential factors for final neurorehabilitation outcome scores in patients with spinal cord injury: A longitudinal cohort study. J. Neurorestoratol. 2024, 12, 100117. [Google Scholar] [CrossRef]
- Mattei, T.A.; Goulart, C.R.; Milano, J.B.; Dutra, L.P.F.; Fasset, D.R. Cervical spondylotic myelopathy: Pathophysiology, diagnosis, and surgical techniques. Int. Sch. Res. Not. 2011, 2011, 463729. [Google Scholar] [CrossRef]
- Lebl, D.R.; Hughes, A.; Cammisa, F.P., Jr.; O’leary, P.F. Cervical spondylotic myelopathy: Pathophysiology, clinical presentation, and treatment. HSS J. 2011, 7, 170–178. [Google Scholar] [CrossRef]
- Al-Shaari, H.; Fulford, J.; Heales, C. Diffusion tensor imaging within the healthy cervical spinal cord: Within-participants reliability and measurement error. Magn. Reson. Imaging 2024, 109, 56–66. [Google Scholar] [CrossRef]
- Basser, P.J.; Jones, D.K. Diffusion-tensor MRI: Theory, experimental design and data analysis–a technical review. NMR Biomed. Int. J. Devoted Dev. Appl. Magn. Reson. Vivo 2002, 15, 456–467. [Google Scholar] [CrossRef]
- Wen, C.Y.; Cui, J.L.; Liu, H.S.; Mak, K.C.; Cheung, W.Y.; Luk, K.D.; Hu, Y. Is diffusion anisotropy a biomarker for disease severity and surgical prognosis of cervical spondylotic myelopathy? Radiology 2014, 270, 197–204. [Google Scholar] [CrossRef]
- Kara, B.; Celik, A.; Karadereler, S.; Ulusoy, L.; Ganiyusufoglu, K.; Onat, L.; Mutlu, A.; Ornek, I.; Sirvanci, M.; Hamzaoglu, A. The role of DTI in early detection of cervical spondylotic myelopathy: A preliminary study with 3-T MRI. Neuroradiology 2011, 53, 609–616. [Google Scholar] [CrossRef]
- Shabani, S.; Kaushal, M.; Budde, M.D.; Wang, M.C.; Kurpad, S.N. Diffusion tensor imaging in cervical spondylotic myelopathy: A review. J. Neurosurg. Spine 2020, 33, 65–72. [Google Scholar]
- DeBoy, C.A.; Zhang, J.; Dike, S.; Shats, I.; Jones, M.; Reich, D.S.; Mori, S.; Nguyen, T.; Rothstein, B.; Miller, R.H.; et al. High resolution diffusion tensor imaging of axonal damage in focal inflammatory and demyelinating lesions in rat spinal cord. Brain 2007, 130, 2199–2210. [Google Scholar] [CrossRef]
- Guan, X.; Fan, G.; Wu, X.; Gu, G.; Gu, X.; Zhang, H.; He, S. Diffusion tensor imaging studies of cervical spondylotic myelopathy: A systemic review and meta-analysis. PLoS ONE 2015, 10, e0117707. [Google Scholar] [CrossRef]
- Rajasekaran, S.; Yerramshetty, J.S.; Chittode, V.S.; Kanna, R.M.; Balamurali, G.; Shetty, A.P. The assessment of neuronal status in normal and cervical spondylotic myelopathy using diffusion tensor imaging. Spine 2014, 39, 1183–1189. [Google Scholar] [CrossRef]
- Jin, R.; Hu, Y. Effect of segmentation from different diffusive metric maps on diffusion tensor imaging analysis of the cervical spinal cord. Quant. Imaging Med. Surg. 2019, 9, 292. [Google Scholar] [CrossRef]
- Shen, D.; Wu, G.; Suk, H.I. Deep learning in medical image analysis. Annu. Rev. Biomed. Eng. 2017, 19, 221–248. [Google Scholar] [CrossRef]
- Behrad, F.; Abadeh, M.S. An overview of deep learning methods for multimodal medical data mining. Expert Syst. Appl. 2022, 200, 117006. [Google Scholar] [CrossRef]
- Machino, M.; Imagama, S.; Ando, K.; Kobayashi, K.; Ito, K.; Tsushima, M.; Matsumoto, A.; Morozumi, M.; Tanaka, S.; Ito, K.; et al. Image diagnostic classification of magnetic resonance T2 increased signal intensity in cervical spondylotic myelopathy: Clinical evaluation using quantitative and objective assessment. Spine 2018, 43, 420–426. [Google Scholar] [CrossRef]
- Li, X.; Fei, N.; Wan, K.; Cheung, J.P.Y.; Hu, Y. A deep learning-based hand motion classification for hand dysfunction assessment in cervical spondylotic myelopathy. Biomed. Signal Process. Control 2025, 99, 106884. [Google Scholar] [CrossRef]
- Kato, S.; Oshima, Y.; Oka, H.; Chikuda, H.; Takeshita, Y.; Miyoshi, K.; Kawamura, N.; Masuda, K.; Kunogi, J.; Okazaki, R.; et al. Comparison of the Japanese Orthopaedic Association (JOA) score and modified JOA (mJOA) score for the assessment of cervical myelopathy: A multicenter observational study. PLoS ONE 2015, 10, e0123022. [Google Scholar] [CrossRef]
- Chan, T.Y.; Li, X.; Mak, K.C.; Cheung, J.P.Y.; Luk, K.D.K.; Hu, Y. Normal values of cervical spinal cord diffusion tensor in young and middle-aged healthy Chinese. Eur. Spine J. 2015, 24, 2991–2998. [Google Scholar] [CrossRef]
- De Leener, B.; Lévy, S.; Dupont, S.M.; Fonov, V.S.; Stikov, N.; Collins, D.L.; Callot, V.; Cohen-Adad, J. SCT: Spinal Cord Toolbox, an open-source software for processing spinal cord MRI data. Neuroimage 2017, 145, 24–43. [Google Scholar] [CrossRef]
- Cui, J.L.; Wen, C.Y.; Hu, Y.; Mak, K.C.; Mak, K.H.H.; Luk, K.D.K. Orientation entropy analysis of diffusion tensor in healthy and myelopathic spinal cord. Neuroimage 2011, 58, 1028–1033. [Google Scholar] [CrossRef]
- Matsunaga, S.; Komiya, S.; Toyama, Y. Risk factors for development of myelopathy in patients with cervical spondylotic cord compression. Eur. Spine J. 2015, 24, 142–149. [Google Scholar] [CrossRef]
- Kim, T.H.; Ha, Y.; Shin, J.J.; Cho, Y.E.; Lee, J.H.; Cho, W.H. Signal intensity ratio on magnetic resonance imaging as a prognostic factor in patients with cervical compressive myelopathy. Medicine 2016, 95, e4649. [Google Scholar] [CrossRef]
- Hu, J.; Shen, L.; Sun, G. Squeeze-and-excitation networks. In Proceedings of the IEEE Conference on Computer Vision and Pattern Recognition, Salt Lake City, UT, USA, 18–22 June 2018; pp. 7132–7141. [Google Scholar]
- He, K.; Zhang, X.; Ren, S.; Sun, J. Deep residual learning for image recognition. In Proceedings of the IEEE Conference on Computer Vision and Pattern Recognition, Las Vegas, NV, USA, 26 June–1 July 2016; pp. 770–778. [Google Scholar]
- Tan, M.; Le, Q. Efficientnet: Rethinking model scaling for convolutional neural networks. In Proceedings of the International Conference on Machine Learning, Long Beach, CA, USA, 9–15 June 2019; pp. 6105–6114. [Google Scholar]
- Silla, C.N.; Freitas, A.A. A survey of hierarchical classification across different application domains. Data Min. Knowl. Discov. 2011, 22, 31–72. [Google Scholar] [CrossRef]
- Fei, N.; Li, G.; Wang, X.; Li, J.; Hu, X.; Hu, Y. Deep learning-based auto-segmentation of spinal cord internal structure of diffusion tensor imaging in cervical spondylotic myelopathy. Diagnostics 2023, 13, 817. [Google Scholar] [CrossRef]
- Xu, W.; Fu, Y.L.; Zhu, D. ResNet and its application to medical image processing: Research progress and challenges. Comput. Methods Programs Biomed. 2023, 240, 107660. [Google Scholar] [CrossRef]
- LeCun, Y.; Bengio, Y.; Hinton, G. Deep learning. Nature 2015, 521, 436–444. [Google Scholar] [CrossRef]
- Geraldo, A.F.; Pereira, J.; Nunes, P.; Reimão, S.; Sousa, R.; Castelo-Branco, M.; Pinto, S.; Campos, J.G.; de Carvalho, M. Beyond fractional anisotropy in amyotrophic lateral sclerosis: The value of mean, axial, and radial diffusivity and its correlation with electrophysiological conductivity changes. Neuroradiology 2018, 60, 505–515. [Google Scholar] [CrossRef]
- Li, X.; Cui, J.L.; Mak, K.C.; Luk, K.D.K.; Hu, Y. Potential use of diffusion tensor imaging in level diagnosis of multilevel cervical spondylotic myelopathy. Spine 2014, 39, E615–E622. [Google Scholar] [CrossRef]
- Chakraborty, S.; Tomsett, R.; Raghavendra, R.; Harborne, D.; Alzantot, M.; Cerutti, F.; Srivastava, M.; Preece, A.; Julier, S.; Rao, R.M.; et al. Interpretability of deep learning models: A survey of results. In Proceedings of the 2017 IEEE Smartworld, Ubiquitous Intelligence & Computing, Advanced & Trusted Computed, Scalable Computing & Communications, Cloud & Big Data Computing, Internet of People and Smart City Innovation (Smartworld/SCALCOM/UIC/ATC/CBDcom/IOP/SCI), San Francisco, CA, USA, 4–8 August 2017; pp. 1–6. [Google Scholar]
- Li, X.; Xiong, H.; Li, X.; Wu, X.; Zhang, X.; Liu, J.; Bian, J.; Dou, D. Interpretable deep learning: Interpretation, interpretability, trustworthiness, and beyond. Knowl. Inf. Syst. 2022, 64, 3197–3234. [Google Scholar] [CrossRef]
- Han, K.; Wang, Y.; Chen, H.; Chen, X.; Guo, J.; Liu, Z.; Tang, Y.; Xiao, A.; Xu, C.; Xu, Y.; et al. A survey on vision transformer. IEEE Trans. Pattern Anal. Mach. Intell. 2022, 45, 87–110. [Google Scholar] [CrossRef]
- Achiam, J.; Adler, S.; Agarwal, S.; Ahmad, L.; Akkaya, I.; Aleman, F.L.; Almeida, D.; Altenschmidt, J.; Altman, S.; Anadkat, S.; et al. Gpt-4 technical report. arXiv 2023, arXiv:2303.08774. [Google Scholar] [CrossRef]
- Teng, Q.; Liu, Z.; Song, Y.; Han, K.; Lu, Y. A survey on the interpretability of deep learning in medical diagnosis. Multimed. Syst. 2022, 28, 2335–2355. [Google Scholar] [CrossRef]
- ŞAHiN, E.; Arslan, N.N.; Özdemir, D. Unlocking the black box: An in-depth review on interpretability, explainability, and reliability in deep learning. Neural Comput. Appl. 2025, 37, 859–965. [Google Scholar] [CrossRef]
- Yan, L.; Liang, Z.; Zhang, H.; Zhang, G.; Zheng, W.; Han, C.; Yu, D.; Zhang, H.; Xie, X.; Liu, C.; et al. A domain knowledge-based interpretable deep learning system for improving clinical breast ultrasound diagnosis. Commun. Med. 2024, 4, 90. [Google Scholar] [CrossRef]
- Azam, M.A.; Khan, K.B.; Salahuddin, S.; Rehman, E.; Khan, S.A.; Khan, M.A.; Kadry, S.; Gandomi, A.H. A review on multimodal medical image fusion: Compendious analysis of medical modalities, multimodal databases, fusion techniques and quality metrics. Comput. Biol. Med. 2022, 144, 105253. [Google Scholar] [CrossRef]
- Nouri, A.; Martin, A.R.; Kato, S.; Reihani-Kermani, H.; Riehm, L.E.; Fehlings, M.G. The relationship between MRI signal intensity changes, clinical presentation, and surgical outcome in degenerative cervical myelopathy: Analysis of a global cohort. Spine 2017, 42, 1851–1858. [Google Scholar] [CrossRef]
- Funaba, M.; Imajo, Y.; Suzuki, H.; Nishida, N.; Nagao, Y.; Sakamoto, T.; Fujimoto, K.; Sakai, T. Impact of various MRI signal intensity changes on radiological parameters, the neurological status, and surgical outcomes in degenerative cervical myelopathy. Clin. Neurol. Neurosurg. 2021, 207, 106802. [Google Scholar] [CrossRef]

| Two-Class Categorization | Two-Class Severity Level | Three-Class Categorization | Three-Class Severity Level |
|---|---|---|---|
| Severe | Severe | ||
| Mild | Moderate | ||
| N/A | N/A | Mild |
| Hyperparameter Item | Parameter Space | Choice |
|---|---|---|
| Optimizer | [Adam, SGD] | Adam |
| Learning rate | [1 × 10−4, 3 × 10−4, 1 × 10−3, 3 × 10−3] | 1 × 10−4 |
| Batch size | [8, 16, 32] | 8 |
| Training epoch | [30, 50, 100] | 50 |
| Loss | [Focal loss, CE loss] | Focal loss |
| Classification rebalance weight-2 classes | [1:1, 1:3] | 1:3 |
| Classification rebalance weight-3 classes | [1:2:1, 1:1:1] | 1:2:1 |
| Input Source | Model Name | ACC 2-Class Categorization | F1 2-Class Categorization |
|---|---|---|---|
| 2D | resnet-18-2D | 71.59% | 0.6997 |
| EfficientNet-B1-2D | 56.73% | 0.5883 | |
| Simplecnn-2D | 81.24% | 0.7878 | |
| DCSANet-2D | 80.68% | 0.7966 | |
| 3D | resnet-18-3D | 68.17% | 0.6696 |
| EfficientNet-B1-3D | 74.98% | 0.7330 | |
| Simplecnn-3D | 76.13% | 0.7259 | |
| DCSANet-2D | 76.10% | 0.7371 | |
| 2D-3D | resnet-18-MD | 72.70% | 0.7231 |
| EfficientNet-B1-MD | 78.43% | 0.7756 | |
| Simplecnn-MD | 78.41% | 0.7643 | |
| DCSANet-MD-V1 | 79.54% | 0.7771 |
| Model | ACC 2-Class Categorization | F1 2-Class Categorization |
|---|---|---|
| DCSANet-MD-V1 | 79.54 % | 0.7771 |
| DCSANet-MD-V2 | 79.54 % | 0.7724 |
| DCSANet-MD-V3 | 82.95 % | 0.8135 |
| DCSANet-MD-V3-SVM | 76.71 % | 0.7238 |
| DCSANet-MD-V3-RF | 77.27 % | 0.7372 |
| DCSANet-MD-V3-DT | 75.03 % | 0.7575 |
| Input | Model | H-CLASS-2nd Level | 3-CLASS | ||
|---|---|---|---|---|---|
| ACC | F1 | ACC | F1 | ||
| 2D | Simplecnn-2D | 65.30% | 57.34% | 57.94% | 50.58% |
| EfficientNet-B1-2D | 59.31% | 59.37% | 47.17% | 45.99% | |
| DCSANet-2D | 64.62% | 53.74% | 57.37% | 52.67% | |
| 3D | Simplecnn-3D | 67.70% | 58.61% | 48.57% | 47.99% |
| EfficientNet-B1-3D | 67.64% | 57.68% | 54.52% | 50.68% | |
| DCSANet-3D | 71.50% | 67.19% | 56.22% | 49.58% | |
| 2D-3D | Simplecnn-MD | 66.13% | 60.85% | 51.67% | 45.63% |
| EfficientNet-B1-MD | 72.89% | 71.33% | 53.97% | 51.18% | |
| DCSANet-MD-V1 | 69.12% | 63.03% | 56.84% | 52.16% | |
| DCSANet-MD-V2 | 70.60% | 64.37% | 53.98% | 46.17% | |
| DCSANet-MD-V3 | 75.29% | 71.86% | 57.98% | 54.81% | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, S.; Li, J.; Fei, N.; Li, G.; Hu, Y. A Deep-Learning-Based Diffusion Tensor Imaging Pathological Auto-Analysis Method for Cervical Spondylotic Myelopathy. Bioengineering 2025, 12, 806. https://doi.org/10.3390/bioengineering12080806
Yang S, Li J, Fei N, Li G, Hu Y. A Deep-Learning-Based Diffusion Tensor Imaging Pathological Auto-Analysis Method for Cervical Spondylotic Myelopathy. Bioengineering. 2025; 12(8):806. https://doi.org/10.3390/bioengineering12080806
Chicago/Turabian StyleYang, Shuoheng, Junpeng Li, Ningbo Fei, Guangsheng Li, and Yong Hu. 2025. "A Deep-Learning-Based Diffusion Tensor Imaging Pathological Auto-Analysis Method for Cervical Spondylotic Myelopathy" Bioengineering 12, no. 8: 806. https://doi.org/10.3390/bioengineering12080806
APA StyleYang, S., Li, J., Fei, N., Li, G., & Hu, Y. (2025). A Deep-Learning-Based Diffusion Tensor Imaging Pathological Auto-Analysis Method for Cervical Spondylotic Myelopathy. Bioengineering, 12(8), 806. https://doi.org/10.3390/bioengineering12080806






