AI in Cervical Cancer Cytology Diagnostics: A Narrative Review of Cutting-Edge Studies
Abstract
1. Introduction
1.1. Historical Foundations of Cervical Cancer Diagnostics
1.2. The Impact of Digitalization and Artificial Intelligence on Cervical Cancer Diagnostics
1.3. Purpose
- Evolution of Scientific Output: Examining recent publications on AI’s role in cervical cancer diagnostics, identifying major research milestones and advancements.
- Key Topics and Categorization: Focusing on key themes in AI applications, such as automated cell analysis, image recognition, and diagnostic enhancement.
- Opportunities and Challenges: Analyzing AI’s potential to optimize workflows, while also addressing ongoing challenges, like data biases, model accuracy, and regulatory barriers.
2. Methods
3. Results
3.1. Bibliometric Trends
| ((Cervix cancer[Title/Abstract]) OR (Cervix carcinoma[Title/Abstract]) OR (Cervix dysplasia[Title/Abstract]) OR (Cervical intraepithelial neoplasia [Title/Abstract]) OR (Cervical cancer pathophysiology[Title/Abstract]) OR (Cervix HPV infection[Title/Abstract]) OR (Cervix biopsy and cancer diagnosis[Title/Abstract]) OR (Cervix cancer staging[Title/Abstract]) OR (Cervix cancer early detection[Title/Abstract]) OR (Cervix cancer metastasis[Title/Abstract]) OR (Cervical cancer[Title/Abstract]) OR (Cervical carcinoma[Title/Abstract]) OR (Human papillomavirus (HPV) and cervical cancer[Title/Abstract]) OR (Cervical cancer screening[Title/Abstract]) OR (Cervical dysplasia[Title/Abstract]) OR (Cervical cancer prevention[Title/Abstract]) OR (HPV vaccination and cervical cancer[Title/Abstract]) OR (Cervical cancer treatment[Title/Abstract]) OR (Risk factors for cervical cancer[Title/Abstract]) OR (Cervical cancer stages[Title/Abstract]) OR (Cervical cancer survival rates[Title/Abstract]) OR (Cervical cancer prognosis[Title/Abstract]) OR (Cervical cancer immunotherapy[Title/Abstract]) OR (Cervical cancer biomarkers[Title/Abstract]) OR (Early detection of cervical cancer[Title/Abstract]))AND ((Machine learning[Title/Abstract] OR Deep learning[Title/Abstract]) OR (Neural networks[Title/Abstract] OR Predictive modeling[Title/Abstract]) OR (Natural language[Title/Abstract] OR Data science[Title/Abstract]) OR (AI applications[Title/Abstract] OR Reinforcement learning[Title/Abstract]) OR (Supervised learning[Title/Abstract] OR AI models[Title/Abstract])) AND ((Cytology[Title/Abstract]) OR (Cytopathology[Title/Abstract])) 1 |
| ((Machine learning[Title/Abstract] OR Deep learning[Title/Abstract] OR Neural networks[Title/Abstract] OR Predictive modeling[Title/Abstract] OR Natural language[Title/Abstract] OR Data science[Title/Abstract] OR AI applications[Title/Abstract] OR Reinforcement learning[Title/Abstract] OR Supervised learning[Title/Abstract] OR AI models[Title/Abstract])) AND ((Cytology[Title/Abstract]) OR (Cytopathology[Title/Abstract])) |
| 1 Note: While several terms in the search string may appear semantically similar (e.g., “Cervix cancer” vs. “Cervical cancer”, or “Cervix dysplasia” vs. “Cervical dysplasia”), they are not redundant. Rather, their inclusion reflects intentional variation to accommodate differences in how concepts are labeled across scientific literature. The use of both noun-based and adjective-based terms (e.g., “cervix” vs. “cervical”) broadens the scope of the search and enhances its sensitivity, ensuring that relevant articles are not missed due to terminological differences. |
3.2. Themes and Categorization
3.3. Emerging Opportunities and Challenges
3.3.1. Opportunities in AI Integration for Cervical Cancer Diagnostics
3.3.2. Challenges in AI Integration for Cervical Cancer Diagnostics
4. Discussion
4.1. Added Values and Highlights
4.1.1. Added Value
4.1.2. Highlights
4.2. Overcoming Challenges and Accelerating Growth: Integrating AI into Cervical Cancer Diagnostics and Cytology
- Generalizability: Many algorithms are trained on narrow, homogeneous datasets, limiting their applicability to diverse populations and imaging systems, which can lead to biased predictions or poor real-world performance if not validated externally.
- Model Explainability and Transparency: AI models often act as “black boxes,” making it hard for clinicians to understand or trust the rationale behind the outputs, which hinders adoption and raises safety concerns.
- Regulatory and Legal Challenges: Most AI tools in cervical cancer diagnostics are in early stages, with few receiving regulatory approval. Issues like data privacy, liability in case of misdiagnosis, and a lack of clear clinical guidelines remain unresolved.
- Cultural and Infrastructural Integration: Implementing AI requires substantial changes in clinical workflows, including training healthcare providers and fostering collaboration between clinicians, data scientists, and public health authorities. Without these efforts, AI tools may not improve patient outcomes.
- Socio-Technical Barriers: Digital infrastructure disparities, resistance to automation, and insufficient funding, particularly in under-resourced areas, can slow down the implementation of AI.
4.2.1. Market Growth and Expanding Applications of AI in Cervical Cytology
4.2.2. Towards Sustainable AI Integration: Addressing Technical, Operational, and Socio-Technical Challenges for Broader and Targeted Applications in Healthcare
4.3. Innovative AI and Automated Platforms for Cervical Cancer Screening and Diagnosis
4.4. Limitations
5. Bridging the Gap: AI as the Future of Cervical Cancer Cytology
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Safaeian, M.; Solomon, D.; Castle, P.E. Cervical cancer prevention–cervical screening: Science in evolution. Obstet. Gynecol. Clin. N. Am. 2007, 34, 739–760. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Cytology Automation: An Overview. Available online: https://scispace.com/pdf/cytology-automation-an-overview-efultv68k5.pdf (accessed on 3 June 2025).
- Cooper, D.B.; Dunton, C.J. Colposcopy. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2025. Available online: https://www.ncbi.nlm.nih.gov/books/NBK564514/ (accessed on 3 June 2025).
- Pangarkar, M.A. The Bethesda System for reporting cervical cytology. Cytojournal 2022, 19, 28. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Cox, J.T. History of the use of HPV testing in cervical screening and in the management of abnormal cervical screening results. J. Clin. Virol. 2009, 45 (Suppl. S1), S3–S12, Erratum in J. Clin. Virol. 2010, 47, 299. [Google Scholar] [CrossRef] [PubMed]
- Bedell, S.L.; Goldstein, L.S.; Goldstein, A.R.; Goldstein, A.T. Cervical Cancer Screening: Past, Present, and Future. Sex. Med. Rev. 2020, 8, 28–37. [Google Scholar] [CrossRef] [PubMed]
- Sturgis, C.D.; Isoe, C.; McNeal, N.E.; Yu, G.H.; DeFrias, D.V. PAPNET computer-aided rescreening for detection of benign and malignant glandular elements in cervicovaginal smears: A review of 61 cases. Diagn. Cytopathol. 1998, 18, 307–311. [Google Scholar] [CrossRef] [PubMed]
- Saraiya, M.; Steben, M.; Watson, M.; Markowitz, L. Evolution of cervical cancer screening and prevention in United States and Canada: Implications for public health practitioners and clinicians. Prev. Med. 2013, 57, 426–433. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Saini, T.; Bansal, B.; Dey, P. Digital cytology: Current status and future prospects. Diagn. Cytopathol. 2023, 51, 211–218. [Google Scholar] [CrossRef] [PubMed]
- Yamashiro, K.; Kawamura, N.; Matsubayashi, S.; Dota, K.; Suzuki, H.; Mizushima, H.; Wakao, F.; Azumi, N. Telecytology in Hokkaido Island, Japan: Results of primary telecytodiagnosis of routine cases. Cytopathology 2004, 15, 221–227. [Google Scholar] [CrossRef] [PubMed]
- Harangi, B.; Bogacsovics, G.; Toth, J.; Kovacs, I.; Dani, E.; Hajdu, A. Pixel-wise segmentation of cells in digitized Pap smear images. Sci. Data 2024, 11, 733. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Cervical Cancer Screening (PDQ®)–Health Professional Version. Available online: https://www.cancer.gov/types/cervical/hp/cervical-screening-pdq (accessed on 3 June 2025).
- Bao, H.; Bi, H.; Zhang, X.; Zhao, Y.; Dong, Y.; Luo, X.; Zhou, D.; You, Z.; Wu, Y.; Liu, Z.; et al. Artificial intelligence-assisted cytology for detection of cervical intraepithelial neoplasia or invasive cancer: A multicenter, clinical-based, observational study. Gynecol. Oncol. 2020, 159, 171–178. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Liu, J.; Su, Q.; Chu, Y.; Xia, H.; Xu, R. Performance of artificial intelligence for diagnosing cervical intraepithelial neoplasia and cervical cancer: A systematic review and meta-analysis. EClinicalMedicine 2024, 80, 102992. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Wang, J.; Wang, T.; Han, R.; Shi, D.; Chen, B. Artificial intelligence in cancer pathology: Applications, challenges, and future directions. Cytojournal 2025, 22, 45. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Ouh, Y.-T.; Kim, T.J.; Ju, W.; Kim, S.W.; Jeon, S.; Kim, S.-N.; Kim, K.G.; Lee, J.-K. Development and validation of artificial intelligence-based analysis software to support screening system of cervical intraepithelial neoplasia. Sci. Rep. 2024, 14, 1957. [Google Scholar] [CrossRef] [PubMed]
- Dellino, M.; Cerbone, M.; d’Amati, A.; Bochicchio, M.; Laganà, A.S.; Etrusco, A.; Malvasi, A.; Vitagliano, A.; Pinto, V.; Cicinelli, E.; et al. Artificial Intelligence in Cervical Cancer Screening: Opportunities and Challenges. AI 2024, 5, 2984–3000. [Google Scholar] [CrossRef]
- Akazawa, M.; Hashimoto, K. Artificial intelligence in gynecologic cancers: Current status and future challenges—A systematic review. Artif. Intell. Med. 2021, 120, 102164. [Google Scholar] [CrossRef] [PubMed]
- FDA Clears AI-Powered Digital Cytology Platform for Cervical Cancer. Available online: https://www.diagnosticimaging.com/view/fda-clears-ai-powered-digital-cytology-platform-for-cervical-cancer (accessed on 3 June 2025).
- Evaluation of Automatic Class III Designation for GeniusTM Digital Diagnostics System with the Genius TM Cervical Al Algorithm. Available online: https://www.accessdata.fda.gov/cdrh_docs/reviews/DEN210035.pdf (accessed on 3 June 2025).
- Xue, P.; Dang, L.; Kong, L.H.; Tang, H.P.; Xu, H.M.; Weng, H.Y.; Wang, Z.; Wei, R.G.; Xu, L.; Li, H.X.; et al. Deep learning enabled liquid-based cytologmodel for cervical precancer and cancer detection. Nat. Commun. 2025, 16, 3506. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Taghados, Z.; Azimifar, Z.; Monsefi, M.; Jahromi, M.A. CausalCervixNet: Convolutional neural networks with causal insight (CICNN) in cervical cancer cell classification-leveraging deep learning models for enhanced diagnostic accuracy. BMC Cancer 2025, 25, 607. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Himabindu, D.D.; Lydia, E.L.; Rajesh, M.V.; Ahmed, M.A.; Ishak, M.K. Leveraging swin transformer with ensemble of deep learning model for cervical cancer screening using colposcopy images. Sci. Rep. 2025, 15, 7900. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Yang, J.; Chen, S.; Liu, Y.; Wang, P.; Zhao, J.; Yi, J.; Wei, J.; Wang, R. Identification of a novel hypermethylation marker, ZSCAN18, and construction of a diagnostic model in cervical cancer. Clin. Transl. Oncol. 2025; Epub ahead of print. [Google Scholar] [CrossRef] [PubMed]
- Dong, B.; Lu, Z.; Yang, T.; Wang, J.; Zhang, Y.; Tuo, X.; Wang, J.; Lin, S.; Cai, H.; Cheng, H.; et al. Development, validation, and clinical application of a machine learning model for risk stratification and management of cervical cancer screening based on full-genotyping hrHPV test (SMART-HPV): A modelling study. Lancet Reg. Health West. Pac. 2025, 55, 101480. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Pang, W.; Ma, Y.; Jiang, H.; Yu, Q. Cells Grouping Detection and Confusing Labels Correction on Cervical Pathology Images. Bioengineering 2024, 12, 23. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Zhang, X.; Ji, J.; Zhang, Q.; Zheng, X.; Ge, K.; Hua, M.; Cao, L.; Wang, L. A large annotated cervical cytology images dataset for AI models to aid cervical cancer screening. Sci. Data 2025, 12, 23. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Chun, J.; Yu, A.; Ko, S.; Chong, G.; Park, J.; Han, H.; Park, N.J.; Cho, J. Automated Screening of Precancerous Cervical Cells Through Contrastive Self-Supervised Learning. Life 2024, 14, 1565. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Pang, W.; Qiu, Y.; Jin, S.; Jiang, H.; Ma, Y. Label credibility correction based on cell morphological differences for cervical cells classification. Sci. Rep. 2025, 15, 2. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Welch, E.C.; Lu, C.; Sung, C.J.; Zhang, C.; Tripathi, A.; Ou, J. BMT: A Cross-Validated ThinPrep Pap Cervical Cytology Dataset for Machine Learning Model Training and Validation. Sci. Data 2024, 11, 1444. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Lim, C.; Seo, Y.J.; Lee, J.Y.; Jung, E.S.; Lee, S.; Kim, H.; Kim, K.; Kim, J.M. Evaluation of the cervical liquid-based cytology sample as a microbiome resource for dual diagnosis. PLoS ONE 2024, 19, e0308985. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Muksimova, S.; Umirzakova, S.; Shoraimov, K.; Baltayev, J.; Cho, Y.I. Novelty Classification Model Use in Reinforcement Learning for Cervical Cancer. Cancers 2024, 16, 3782. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Hays, P. Artificial intelligence in cytopathological applications for cancer: A review of accuracy and analytic validity. Eur. J. Med. Res. 2024, 29, 553. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Goldstein, A.; Gersh, M.; Skovronsky, G.; Moss, C. The Future of Cervical Cancer Screening. Int. J. Women’s Health 2024, 16, 1715–1731. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Zhang, L.; Tian, P.; Li, B.; Xu, L.; Qiu, L.; Bi, Z.; Chen, L.; Sui, L. Risk-stratified management of cervical high-grade squamous intraepithelial lesion based on machine learning. J. Med. Virol. 2024, 96, e70016. [Google Scholar] [CrossRef] [PubMed]
- Liu, D.; Chu, J. Analysis of effectiveness in an artificial intelligent film reading system combined with liquid based cytology examination for cervical cancer screening. Am. J. Transl. Res. 2024, 16, 4979–4987. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Sood, T.; Khandnor, P.; Bhatia, R. Enhancing pap smear image classification: Integrating transfer learning and attention mechanisms for improved detection of cervical abnormalities. Biomed. Phys. Eng. Express 2024, 10, 065031. [Google Scholar] [CrossRef] [PubMed]
- Wu, T.; Lucas, E.; Zhao, F.; Basu, P.; Qiao, Y. Artificial intelligence strengthens cervical cancer screening—Present and future. Cancer Biol. Med. 2024, 21, 864–879. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Song, H.; Lee, H.Y.; Oh, S.A.; Seong, J.; Hur, S.Y.; Choi, Y.J. Application of Machine Learning Algorithms for Risk Stratification and Efficacy Evaluation in Cervical Cancer Screening among the ASCUS/LSIL Population: Evidence from the Korean HPV Cohort Study. Cancer Res. Treat. 2025, 57, 547–557. [Google Scholar] [CrossRef] [PubMed]
- Qin, J.; He, Y.; Liang, Y.; Kang, L.; Zhao, J.; Ding, B. Cell comparative learning: A cervical cytopathology whole slide image classification method using normal and abnormal cells. Comput. Med. Imaging Graph. 2024, 117, 102427. [Google Scholar] [CrossRef] [PubMed]
- Pereira, A.R.; Redzic, N.; Van Vooren, S.; Pelak, K.; Broekmans, A.; Desloovere, G.; Vanden Broeck, D.; Kehoe, K.; Bogers, J.; Coppens, A.; et al. Development, Validation, and Implementation of an Augmented Multiwell, Multitarget Quantitative PCR for the Analysis of Human Papillomavirus Genotyping through Software Automation, Data Science, and Artificial Intelligence. J. Mol. Diagn. 2024, 26, 781–791. [Google Scholar] [CrossRef] [PubMed]
- Ando, Y.; Cho, J.; Park, N.J.; Ko, S.; Han, H. Toward Interpretable Cell Image Representation and Abnormality Scoring for Cervical Cancer Screening Using Pap Smears. Bioengineering 2024, 11, 567. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Cai, D.; Chen, J.; Zhao, J.; Xue, Y.; Yang, S.; Yuan, W.; Feng, M.; Weng, H.; Liu, S.; Peng, Y.; et al. HiCervix: An ExtensiveHierarchical Dataset and Benchmark for Cervical Cytology Classification. IEEE Trans. Med. Imaging 2024, 43, 4344–4355. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Yu, Y.; Tan, Y.; Wan, H.; Zheng, N.; He, Z.; Mao, L.; Ren, W.; Chen, K.; Lin, Z.; et al. Artificial intelligence enables precision diagnosis of cervical cytology grades and cervical cancer. Nat. Commun. 2024, 15, 4369. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Mahajan, P.; Kaur, P. Improving cervical cancer classification in PAP smear images with enhanced segmentation and deep progressive learning-based techniques. Diagn. Cytopathol. 2024, 52, 313–324. [Google Scholar] [CrossRef] [PubMed]
- Civit-Masot, J.; Luna-Perejon, F.; Muñoz-Saavedra, L.; Domínguez-Morales, M.; Civit, A. A lightweight xAI approach to cervical cancer classification. Med. Biol. Eng. Comput. 2024, 62, 2281–2304. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Zhang, Z.; Yao, P.; Chen, M.; Zeng, L.; Shao, P.; Shen, S.; Xu, R.X. SCAC: A Semi-Supervised Learning Approach for Cervical Abnormal Cell Detection. IEEE J. Biomed. Health Inform. 2024, 28, 3501–3512. [Google Scholar] [CrossRef] [PubMed]
- Tang, J.; Zhang, T.; Gong, Z.; Huang, X. High Precision Cervical Precancerous Lesion Classification Method Based on ConvNeXt. Bioengineering 2023, 10, 1424. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Stegmüller, T.; Abbet, C.; Bozorgtabar, B.; Clarke, H.; Petignat, P.; Vassilakos, P.; Thiran, J.P. Self-supervised learning-based cervical cytology for the triage of HPV-positive women in resource-limited settings and low-data regime. Comput. Biol. Med. 2024, 169, 107809. [Google Scholar] [CrossRef] [PubMed]
- Rutili de Lima, C.; Khan, S.G.; Shah, S.H.; Ferri, L. Mask region-based CNNs for cervical cancer progression diagnosis on pap smear examinations. Heliyon 2023, 9, e21388. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Thakur, N.; Alam, M.R.; Abdul-Ghafar, J.; Chong, Y. Recent Application of Artificial Intelligence in Non-Gynecological Cancer Cytopathology: A Systematic Review. Cancers 2022, 14, 3529. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Cervical Cancer Screening Market. Available online: https://www.futuremarketinsights.com/reports/cervical-cancer-screening-market (accessed on 3 June 2025).
- Huang, Q.; Su, W.; Li, S.; Lin, Y.; Cheng, Z.; Chen, Y.; Mo, Y. A bibliometric analysis of artificial intelligence applied to cervical cancer. Front. Med. 2025, 12, 1562818. [Google Scholar] [CrossRef]
- Hou, X.; Shen, G.; Zhou, L.; Li, Y.; Wang, T.; Ma, X. Artificial Intelligence in Cervical Cancer Screening and Diagnosis. Front. Oncol. 2022, 12, 851367. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Giansanti, D. AI in Cytopathology: A Narrative Umbrella Review on Innovations, Challenges, and Future Directions. J. Clin. Med. 2024, 13, 6745. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Lastrucci, A.; Giarnieri, E.; Carico, E.; Giansanti, D. Revolutionizing Cytology and Cytopathology with Natural Language Processing and Chatbot Technologies: A Narrative Review on Current Trends and Future Directions. Bioengineering 2024, 11, 1134. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Kim, D.; Sundling, K.E.; Virk, R.; Thrall, M.J.; Alperstein, S.; Bui, M.M.; Chen-Yost, H.; Donnelly, A.D.; Lin, O.; Liu, X.; et al. Digital cytology part 2: Artificial intelligence in cytology: A concept paper with review and recommendations from the American So-ciety of Cytopathology Digital Cytology Task Force. J. Am. Soc. Cytopathol. 2024, 13, 97–110. [Google Scholar] [CrossRef]
- Kim, D.; Sundling, K.E.; Virk, R.; Thrall, M.J.; Alperstein, S.; Bui, M.M.; Chen-Yost, H.; Donnelly, A.D.; Lin, O.; Liu, X.; et al. Digital cytology part 1: Digital cytology implementation for practice: A concept paper with review and recommendations from the American Society of Cytopathology Digital Cytology Task Force. J. Am. Soc. Cytopathol. 2024, 13, 86–96. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.; Thrall, M.J.; Michelow, P.; Schmitt, F.C.; Vielh, P.R.; Siddiqui, M.T.; Sundling, K.E.; Virk, R.; Alperstein, S.; Bui, M.M.; et al. The Current State of Digital Cy-tology and Artificial Intelligence (AI): Global Survey Results from the American Society of Cytopathology Digital Cytology Task Force. J. Am. Soc. Cytopathol. 2024, 13, 319–328. [Google Scholar] [CrossRef]
- Giansanti, D.; Carico, E.; Lastrucci, A.; Giarnieri, E. Surveying the Digital Cytology Workflow in Italy: An Initial Report on AI Integration Across Key Professional Roles. Healthcare 2025, 13, 903. [Google Scholar] [CrossRef]
- Harsono, A.B.; Susiarno, H.; Suardi, D.; Mantilidewi, K.I.; Wibowo, V.D.; Hidayat, Y.M. Results comparison of cervical cancer early detection using cerviray ® with VIA test. BMC Res. Notes 2025, 18, 30. [Google Scholar] [CrossRef]
- CellPrep Plus. Available online: https://biodyne.en.ec21.com/CellPrep_Plus--8770069_8770070.html (accessed on 3 June 2025).
- ThinPrep® Operator’s Manual Imaging System Image Processor. Available online: https://www.hologic.com/sites/default/files/2017-12/MAN-04199-001_002_02.pdf (accessed on 3 June 2025).
- Genius™ Digital Diagnostics System. Available online: https://www.hologic.com/hologic-products/cytology/genius-digital-diagnostics-system (accessed on 3 June 2025).
- CytoProcessor®. Available online: https://datexim.ai/cytoprocessor/ (accessed on 3 June 2025).
- ScanAI Digital Pathology Platform. Available online: https://scanome.com/services/scanai-digital-pathology-platform/ (accessed on 3 June 2025).
- EVApro. Available online: https://www.mobileodt.com/products/eva-pro/ (accessed on 3 June 2025).
- Paige.ai. Available online: https://www.paige.ai/ (accessed on 3 June 2025).
- CytoSiA—To Advance Cytology Screening Through Unmatched Image Quality and Artificial Intelligence Based Image Analysis Solution. Available online: https://www.optrascan.com/blogs/cytosia-to-advance-cytology-screening-through-unmatched-image-quality-and-artificial-intelligence-based-image-analysis-solution (accessed on 3 June 2025).
- BD FocalPoint™ GS Imaging System. Available online: https://www.bd.com/en-us/products-and-solutions/products/product-families/bd-focalpoint-gs-imaging-system (accessed on 3 June 2025).
- Lahrmann, B.; Keil, A.; Ruiz, F.M.; Clarke, M.A.; Egemen, D.; Grewal, K.K.; Grabe, F.P.; Bartels, L.; Krauthoff, A.; Ströbel, P.; et al. Closing the Automation Gap: Robust AI for Dual-Stain Cervical Cancer Screening Triage. Res. Sq. [Preprint] 2025, rs.3.rs-5985837. [Google Scholar] [CrossRef] [PubMed]

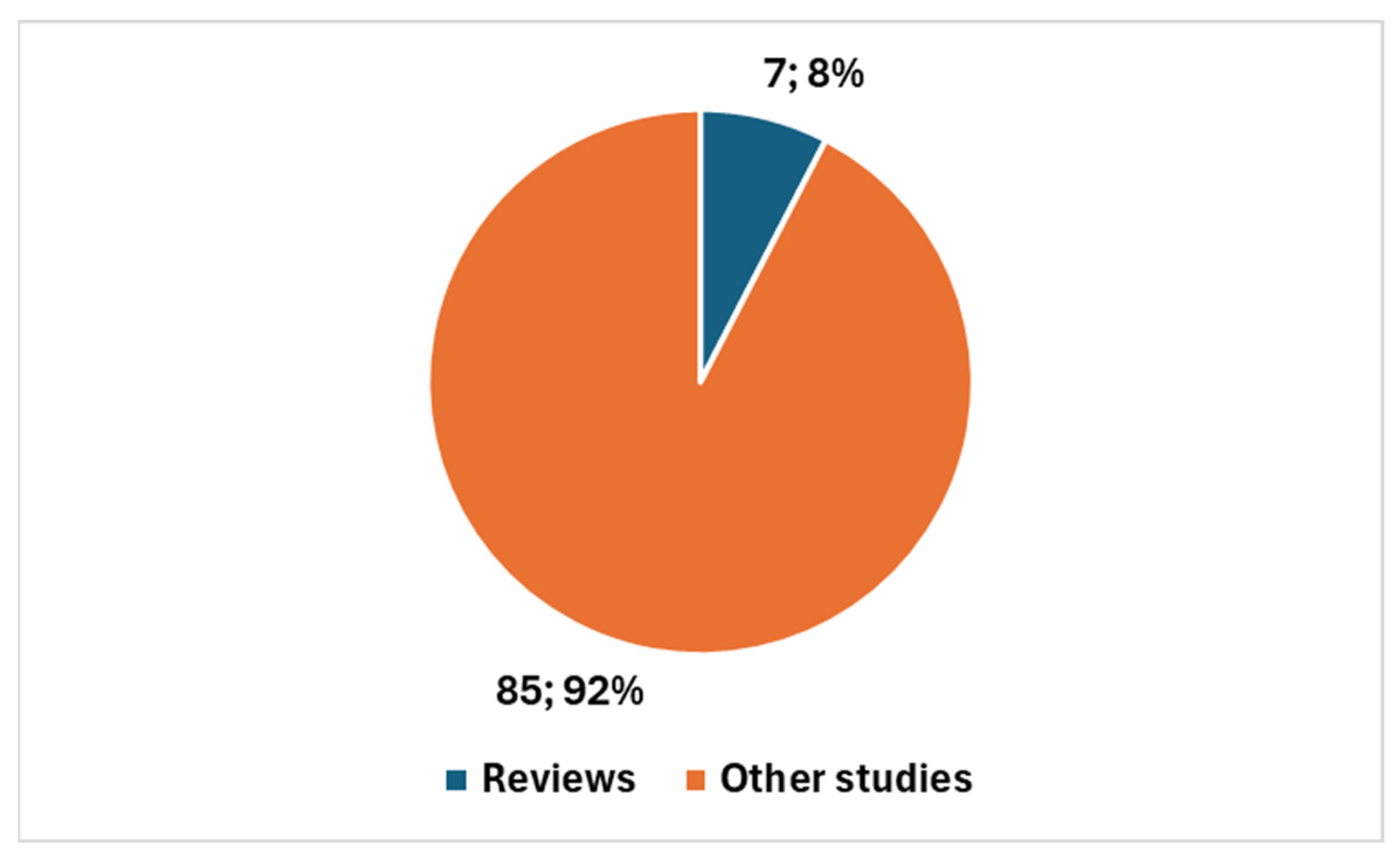
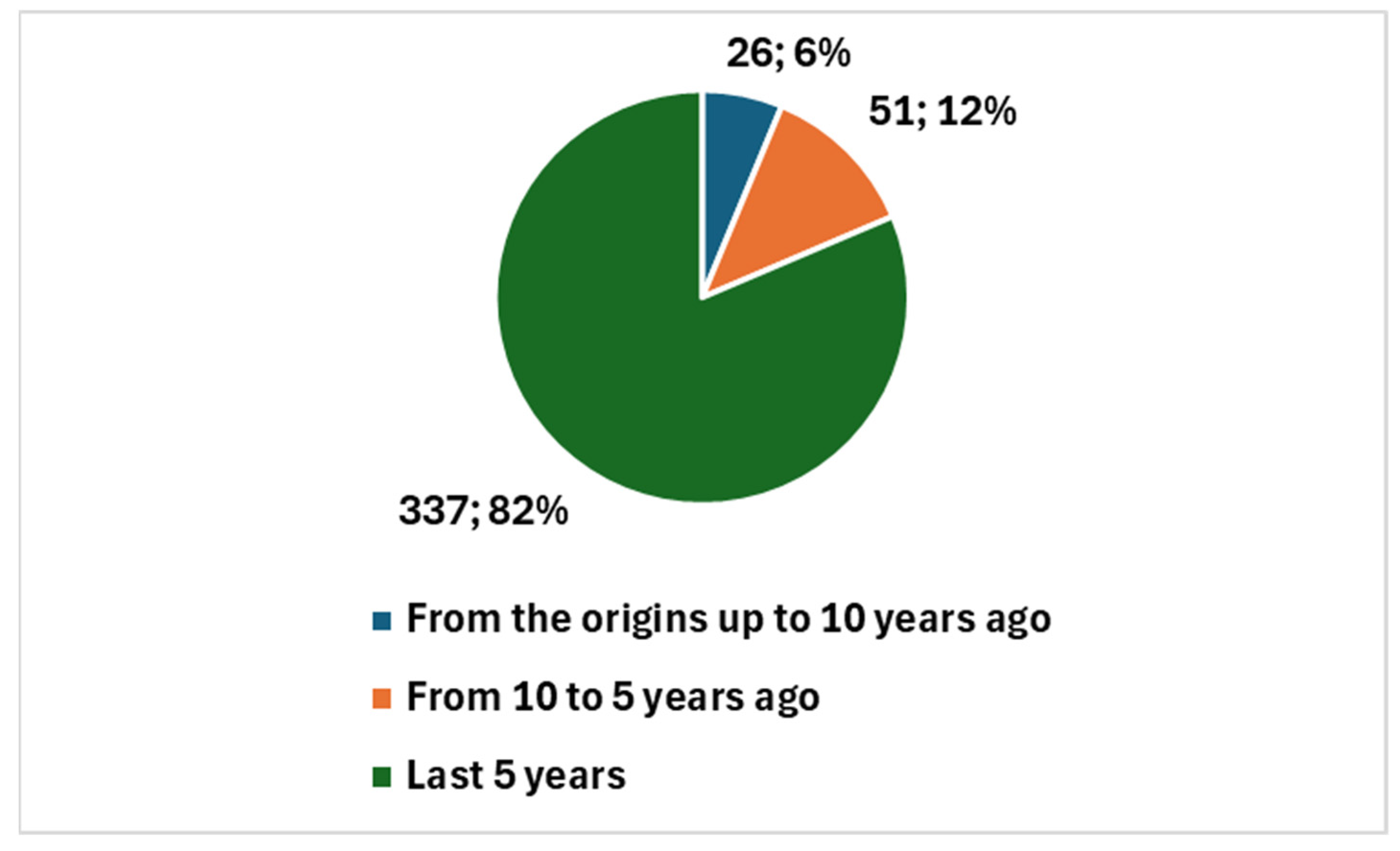


| Reference | Brief Summary | Focus | Application of AI | Details on AI |
|---|---|---|---|---|
| Xue et al. (2025) [21] | This study presents a deep learning-based model for detecting cervical cancer and precancerous lesions from liquid-based cytology samples. The AI model outperforms traditional methods in accuracy, sensitivity, and specificity, leading to better early detection of cervical cancer. | AI in cervical cancer detection | The AI model automates the detection process, enabling more accurate and faster identification of cervical cancer at early stages, improving diagnostic outcomes. | Deep learning model applied to whole-slide liquid-based cytology images for detection and triage. Validated on >28,000 cases across nine hospitals, showing improved sensitivity and reduced reading time vs. cytopathologists. |
| Taghados et al. (2025) [22] | The paper introduces CausalCervixNet, a deep learning model designed to classify cervical cancer cells using a causal inference framework. By leveraging causal insights, the model improves the interpretability and accuracy of cervical cancer diagnoses, offering a new dimension for AI in medical imaging. | Deep learning in cervical cell classification | The AI-driven model provides more accurate and interpretable classifications of cervical cancer cells, enhancing clinicians’ diagnostic accuracy and treatment decisions. | CausalCervixNet employs a convolutional neural network architecture enhanced with causal inference techniques to model and identify underlying causal relationships in cervical cell images, moving beyond traditional correlation-based DL models. This integration allows the AI to provide more interpretable and explainable diagnostic outputs by uncovering latent causal factors that drive accurate cell classification. |
| Himabindu et al. (2025) [23] | The study introduces a hybrid deep learning model that integrates a Swin Transformer for analyzing colposcopy images. The model aims to detect cervical cancer and precancerous lesions with high accuracy, providing an automated tool to assist in the interpretation of colposcopic images, discussing their complementary role beyond cytology. | AI in colposcopy image analysis as a complimentary solution beyond the cytology | AI enhances the accuracy of cervical cancer detection by automating the analysis of colposcopy images, allowing clinicians to detect abnormalities that might be overlooked in manual examination. | The AI system uses Wiener filtering for image denoising, followed by a Swin Transformer to extract detailed features from colposcopy images. It combines three deep learning models—autoencoder, bidirectional GRU, and deep belief network—in an ensemble to improve classification accuracy. Hyperparameters are optimized via the Pelican Optimization Algorithm. This integrated approach achieves 99.44% accuracy in cervical cancer screening. |
| Yang et al. (2025) [24] | This paper identifies a hypermethylation marker (ZSCAN18) for cervical cancer and combines it with an AI-driven diagnostic model. By integrating molecular biomarkers with imaging data, the model aims to improve diagnostic precision and identify high-risk patients for early intervention. | Biomarker-based diagnostics | AI integrates molecular biomarkers like ZSCAN18 with traditional cytological methods to create a more accurate diagnostic tool for cervical cancer, aiding in early detection and personalized treatment. | This study integrated machine learning with bioinformatics to analyze methylation and gene expression data from large cervical cancer cohorts. Using feature selection and ridge regression, a diagnostic model was developed based on methylation biomarkers, notably in the ZSCAN18 promoter region. The model achieved high diagnostic accuracy (AUC 0.9421) in validation. Machine learning enabled effective identification and prioritization of methylation markers correlated with disease severity, supporting both diagnosis and potential therapeutic targeting. |
| Dong et al. (2025) [25] | The research presents SMART-HPV, a machine learning model for HPV genotyping that aims to enhance risk stratification in cervical cancer screening. By analyzing multiple factors, the AI model helps identify patients at higher risk for cervical cancer, improving the efficiency of screening protocols. | AI for risk stratification in HPV testing | SMART-HPV uses AI to analyze HPV genotyping data, allowing for more accurate identification of high-risk individuals and ensuring that resources are focused on patients who require immediate attention. | This study developed and validated four machine learning models—XGBoost, SVM, Random Forest, and Naïve Bayes—using hrHPV full genotyping data from over 1.1 million women to predict cervical precancerous lesions (CIN2+ and CIN3+). The optimized XGBoost model demonstrated high discrimination (AUROC up to 0.989) and reliable calibration across multiple large external validation cohorts. It incorporated clinical features and HPV genotypes to stratify risk and guide colposcopy referrals, especially useful in low screening rate settings. Decision curve analysis confirmed its clinical utility for improving referral decisions. |
| Pang et al. (2024) [26] | This study focuses on using AI to correct mislabeled cervical pathology images and accurately detect abnormal cell clusters. By improving image labeling accuracy, the AI system increases the reliability of automated cervical cancer screenings, particularly in large-scale public health settings. | Image correction and cell group detection | AI addresses mislabeled pathology images and enhances the ability to detect abnormal cell groups, improving classification accuracy and contributing to more reliable cervical cancer diagnostics. | This study presents PGCC-Net, a deep learning network for cervical cell detection that integrates clinical prior knowledge and corrects ambiguous labels. The method decomposes detection into sub-tasks for grouping cells, improving learning of cell structures. To tackle label ambiguity caused by complex Bethesda system categories and pathologist variability, a label correction module uses feature similarity and cluster centers to refine annotations. Validated on public (7410 images) and private (13,526 images) datasets, PGCC-Net outperforms existing cervical cell detection methods, enhancing diagnostic accuracy and efficiency. |
| Zhang et al. (2025) [27] | This research develops a dataset of annotated cervical cytology images to train AI models for cervical cancer screening. The dataset enables the creation of more accurate AI models that can automate the screening process, providing more reliable results for early-stage cancer detection. | AI model training using annotated datasets | AI leverages large, annotated datasets to train models for cervical cancer screening, which in turn improves the accuracy of automated diagnostic tools and speeds up the screening process. | The study implements deep learning-based models trained on a large annotated dataset of 8037 cervical cytology images from ThinPrep slides to detect abnormal cervical cells. The models leverage convolutional neural networks (CNNs) optimized for cytology image features to improve detection accuracy and robustness despite limited prior datasets. |
| Chun et al. (2024) [28] | This paper explores the use of contrastive self-supervised learning to automate the screening of precancerous cervical cells. The method allows AI models to learn from large datasets without the need for labeled images, making it a cost-effective solution for large-scale screening efforts. | Self-supervised learning in cancer detection | AI uses self-supervised learning to detect precancerous cells in cervical cancer screening, providing an efficient, cost-effective alternative to traditional manual methods. | The AI framework leverages contrastive self-supervised learning (SSL) enhanced with color distribution augmentations to train the model solely on normal cervical cell images, enabling it to learn robust feature representations without requiring extensive abnormal cell annotations. This distribution-augmented SSL approach enhances the model’s sensitivity to subtle morphological and color variations typical of high-grade lesions (HSIL and ASC-H), while kernel density estimation (KDE) is used post-training to model and detect deviations in cell distributions, enabling precise abnormality detection despite variability in staining and imaging conditions. |
| Pang et al. (2025) [29] | The study discusses the application of AI for correcting label credibility errors in cervical cancer screening images. By addressing inconsistencies in image labeling, the AI model improves the overall accuracy and reliability of cervical cancer diagnostic systems. | Image labeling improvement for classification | AI enhances classification accuracy by correcting label errors in cytology images, which contributes to more reliable cervical cancer diagnoses, reducing human error in the screening process. | The method uses contrastive learning to extract discriminative features and applies unsupervised clustering to correct noisy labels in cervical cell images. Label credibility is analyzed by comparing cluster features to class centers, improving multi-class classification stability via a synergistic grouping loss with momentum. This approach achieved over 92% accuracy on a large, multi-hospital dataset. |
| Welch et al. (2024) [30] | This paper introduces a cross-validated cervical cytology dataset for training AI models aimed at improving cervical cancer detection. By providing a standardized set of images, the dataset allows AI systems to achieve higher levels of accuracy and consistency in screening. | Dataset development for AI model training | AI models trained on high-quality, cross-validated datasets improve cervical cancer detection by offering more reliable and standardized results across different diagnostic settings. | The BMT dataset supports AI training by providing multicellular ThinPrep® images that reflect real screening variability. AI models trained on this dataset can better handle visual differences from varying slide preparations, improving generalization. This enables more accurate classification of cervical cell abnormalities in diverse clinical settings. |
| Lim et al. (2024) [31] | The research integrates microbiome analysis with liquid-based cytology to enhance cervical cancer diagnostics. By incorporating microbiome data into AI-driven models, the study aims to identify new biomarkers that could improve the accuracy of cervical cancer screening. | Microbiome and AI integration | AI models combine microbiome data with cytology results to enhance cervical cancer detection, offering more precise diagnostic capabilities and uncovering new biomarkers for early-stage diagnosis. | Supervised machine learning was applied to microbiome data from LBC and SWAB samples to distinguish sampling methods based on microbial features. The AI analysis highlighted challenges in classification due to high similarity between sample types. Network analysis and weighted co-expression methods further demonstrated weak correlations between microbial clusters, sampling techniques, and clinical data, underscoring the robustness of microbiome profiles across methods and the potential for integrated diagnostic approaches. |
| Muksimova et al. (2024) [32] | This study explores the use of reinforcement learning for classifying cervical cancer images. The model adapts itself based on feedback, improving its diagnostic performance over time and offering a more flexible approach to cervical cancer detection. | Reinforcement learning in cervical cancer diagnosis | AI models powered by reinforcement learning dynamically improve their ability to classify cervical cancer images, allowing for more adaptive and accurate detection as they process more data. | The proposed RL-CancerNet model combines EfficientNetV2 and Vision Transformers within a reinforcement learning framework to enhance cervical cancer diagnosis. EfficientNetV2 extracts local features, while ViTs capture global image dependencies. A reinforcement learning agent dynamically mitigates class imbalance by focusing on minority classes. A Supporter Module with Conv3D, BiLSTM, and attention mechanisms further improves contextual feature learning. Evaluated on benchmark datasets, RL-CancerNet achieved 99.7% accuracy, outperforming state-of-the-art methods and demonstrating robust detection of subtle diagnostic patterns in cervical cytology images. |
| Hays (2024) [33] | This review examines the growing role of AI in cytopathology, focusing on its application in cervical cancer detection. The paper highlights the advancements in AI systems that offer higher accuracy and faster diagnosis compared to traditional methods. | AI in cytopathology | AI improves the accuracy and efficiency of cytopathological diagnoses, including cervical cancer, by automating the interpretation of complex cytological images. | This review emphasizes the role of AI—especially machine learning and deep learning—in cytopathological diagnosis. AI models extract features from cytology images, improving classification accuracy, sensitivity, and specificity in detecting cervical and other cancers. These models reduce human error and interobserver variability by learning from large labeled datasets. Both standalone AI and human-AI hybrid approaches show promising diagnostic performance. The study highlights the increasing deployment of AI-assisted tools in clinical cytopathology to enhance diagnostic precision and workflow efficiency. |
| Goldstein et al. (2024) [34] | This article discusses the future of cervical cancer screening with AI, outlining its potential to reduce false negatives and improve early detection. The paper predicts that AI-driven technologies will become integral to cervical cancer prevention efforts worldwide. | Future trends in cervical cancer screening | AI is expected to transform cervical cancer screening by providing more accurate, cost-effective, and accessible diagnostic solutions, which could ultimately reduce cervical cancer incidence rates globally. | This study emphasizes AI applications in cervical cancer screening using deep learning architectures for image analysis. AI models utilize convolutional neural networks (CNNs) to extract detailed features from digital colposcopy and cytology images. These models perform automated classification and segmentation of HPV-related lesions and cervical dysplasia. Advanced AI techniques, like transfer learning, attention mechanisms, and ensemble models, improve diagnostic accuracy and robustness. Additionally, AI algorithms integrate data from rapid HPV tests and methylation assays to enhance risk prediction and screening efficiency, enabling scalable and cost-effective deployment in resource-limited environments. |
| Zhang et al. (2024) [35] | This study explores machine learning techniques for risk-stratifying women with high-grade squamous intraepithelial lesions (HSIL) in cervical cancer screening. The AI model helps identify women at greater risk for progression to cervical cancer, guiding treatment decisions. | Machine learning in risk stratification | AI improves cervical cancer screening by using machine learning to identify patients with high-grade lesions who are at increased risk, enabling personalized and timely interventions. | The study employed machine learning to predict pathological outcomes after conization in cervical HSIL patients. Among six models tested, the back propagation (BP) neural network demonstrated the highest predictive accuracy and robustness, outperforming logistic regression with an AUC of 0.91 on external validation. This AI model integrates multiple clinical and pathological variables to stratify patient risk effectively. A web-based tool based on the BP neural network was developed to support clinical decision-making, showcasing AI’s potential in enhancing personalized cervical cancer management. |
| Liu et al. (2024) [36] | This paper presents an AI-assisted film reading system combined with liquid-based cytology for cervical cancer screening. The system helps clinicians review and analyze cytology slides with greater speed and accuracy, improving the quality of screening programs. | AI and liquid-based cytology | AI-driven film reading systems enhance cervical cancer detection by automating the analysis of cytology slides, reducing human error and speeding up the screening process. | Combines automated image analysis with liquid-based cytology examination. The system uses AI algorithms for automated interpretation of cervical cytology images, improving detection accuracy through integration of image processing and pattern recognition. |
| Sood et al. (2024) [37] | The study applies transfer learning and attention mechanisms in AI to improve the detection of cervical abnormalities in Pap smears. This approach allows AI to focus on the most relevant features of the image, increasing diagnostic precision. | AI for Pap smear analysis | AI helps detect cervical abnormalities by applying advanced machine learning techniques, offering enhanced diagnostic accuracy for Pap smear analysis and early cancer detection. | Uses pre-trained convolutional neural networks (CNNs) fine-tuned with pap smear image data (transfer learning) to leverage learned features from large datasets. Attention mechanisms are integrated to enhance focus on relevant image regions, improving detection of cervical abnormalities. |
| Wu et al. (2024) [38] | This research investigates how AI can enhance both detection and triage in cervical cancer screening. The model identifies abnormalities in screening images and helps prioritize cases for further examination or biopsy, improving overall clinical workflow. | Enhancing screening with AI | AI improves cervical cancer screening by automating detection and triaging patients based on the severity of abnormalities, leading to quicker and more accurate diagnoses. | Discusses various AI approaches, such as machine learning classifiers, deep learning (including CNNs), and ensemble learning applied to image-based cervical cancer screening. Emphasizes current AI frameworks that support image analysis, risk stratification, and prediction for screening optimization. |
| Song et al. (2025) [39] | The study applies machine learning techniques to evaluate risk and efficacy in cervical cancer screening. By analyzing a wide range of patient data, the AI model helps determine the most effective screening protocols for different risk groups. | Risk stratification with machine learning | AI assists in risk assessment for cervical cancer by analyzing patient data and determining personalized screening and treatment protocols, improving clinical outcomes. | The study applies classical machine learning models (Random Forest, SVM) to integrate clinical and molecular data for risk stratification in cervical screening. Emphasis on model interpretability and validation with a large HPV cohort enhances clinical utility. |
| Qin et al. (2024) [40] | This research presents a comparative learning method to classify cervical cytology images using whole-slide scanning technology. The model aims to improve the detection of abnormal cells with high sensitivity and specificity. | Whole-slide image classification | AI enhances the detection of cervical cancer by analyzing whole-slide images, which provides a more comprehensive view of cytological samples for more accurate diagnoses. | Uses comparative deep learning on whole slide cytology images, employing convolutional neural networks with attention modules to distinguish normal vs. abnormal cells. Improves feature learning from large, high-resolution datasets. |
| Pereira et al. (2024) [41] | This study discusses the development of an AI-enhanced PCR method for HPV genotyping, which improves the accuracy of cervical cancer risk detection. The model helps identify high-risk HPV strains that are most likely to lead to cancer. | AI-driven HPV genotyping | AI models applied to HPV genotyping improve the accuracy and speed of cervical cancer risk assessment by identifying high-risk HPV types, enabling early intervention. | AI automates multiplex qPCR data analysis by processing fluorescence signals and classifying HPV genotypes. Machine learning models increase sensitivity and specificity, supporting scalable molecular diagnostics. |
| Ando et al. (2024) [42] | The paper presents an AI model for interpreting cell images and scoring abnormalities in Pap smears, aiming to automate and standardize the screening process for cervical cancer. | AI for image interpretation and scoring | AI automates the scoring of cervical cell abnormalities in Pap smears, improving the efficiency and consistency of cervical cancer screening, reducing the workload for pathologists. | This study proposes an interpretable AI framework for cervical cancer screening by developing cell image representations and abnormality scoring based on Pap smear images. The model emphasizes explainability, helping pathologists understand AI-driven decisions and improving trust in automated screening. |
| Cai et al. (2024) [43] | The HiCervix dataset is introduced, a comprehensive collection of cervical cytology images for AI model training. The dataset includes various types of abnormal cells, providing a robust resource for improving AI-based screening tools. | Large dataset for AI classification | AI models trained on the HiCervix dataset improve cervical cancer detection by offering higher accuracy in classifying cytological abnormalities, supporting early diagnosis. | HiCervix introduces a large, hierarchical dataset and benchmark for cervical cytology classification using deep learning. The paper presents state-of-the-art convolutional neural networks trained on multi-level annotations to enhance accuracy and robustness in cell-level classification. |
| Wang et al. (2024) [44] | AI is applied to develop a precision diagnostic tool that grades cervical cytology images and stages cervical cancer. The model helps clinicians identify cancer stages more accurately, improving treatment planning. | Precision diagnosis with AI | AI models enhance the staging of cervical cancer by accurately grading cytology images, supporting better treatment decisions and personalized care. | This paper describes a precision AI system that diagnoses cervical cytology grades and cancer with high accuracy. The model leverages ensemble deep learning architectures to improve diagnostic performance and supports clinical decision-making by providing detailed grading. |
| Mahajan et al. (2024) [45] | The study uses AI-enhanced deep learning techniques to improve cervical cancer classification in Pap smear images. The model achieves high accuracy, providing an automated and reliable tool for clinical diagnosis. | AI for improved Pap smear analysis | AI automates the analysis of Pap smear images, improving classification accuracy and providing clinicians with a more reliable tool for cervical cancer screening. | The authors enhance cervical cancer classification in Pap smear images through advanced segmentation techniques combined with deep progressive learning. Their AI pipeline improves feature extraction and classification accuracy by iteratively refining the model’s learning process. |
| Civit-Masot et al. (2024) [46] | This paper introduces a lightweight explainable AI (xAI) model for cervical cancer classification, offering transparency in decision-making. The model provides clinicians with understandable reasons for predictions, enhancing trust in AI-based diagnostic systems. | Explainable AI in cervical cancer detection | AI offers transparency in its decision-making process, which helps clinicians trust AI-based systems for cervical cancer detection, making them more likely to adopt these technologies in clinical practice. | This work presents a lightweight explainable AI (xAI) approach tailored for cervical cancer classification. The model balances computational efficiency and interpretability, enabling deployment in low-resource settings while providing insights into the AI decision process. |
| Zhang et al. (2024) [47] | The study develops a semi-supervised learning approach called SCAC for detecting abnormal cervical cells in cytology images. This model allows for accurate detection with limited labeled data, making it an efficient tool for large-scale screenings. | Semi-supervised learning for abnormal cell detection | AI uses semi-supervised learning to improve cervical cancer detection in cytology images, achieving high accuracy with fewer labeled data and making it scalable for large datasets. | SCAC proposes a semi-supervised learning algorithm for detecting cervical abnormal cells. By leveraging both labeled and unlabeled data, the method reduces annotation needs while maintaining high detection sensitivity and specificity. |
| Tang et al. (2023) [48] | The study applies AI-based ConvNeXt models to classify cervical precancerous lesions, offering enhanced detection accuracy. This approach allows for better identification of early-stage cervical abnormalities. | AI-based classification of precancerous lesions | AI models like ConvNeXt improve the classification of cervical lesions, leading to earlier detection and better patient outcomes. | The study uses ConvNeXt, a cutting-edge convolutional neural network, for high-precision classification of cervical precancerous lesions. The model achieves superior performance by integrating advanced feature extraction with fine-tuned network architectures. |
| Stegmüller et al. (2024) [49] | This research investigates the use of AI to triage HPV-positive women in low-resource settings, aiding in cervical cancer screening. The system prioritizes women based on risk, providing quicker and more efficient screenings. | Self-supervised learning for triage in low-resource settings | AI triages HPV-positive women in low-resource settings, improving access to cervical cancer screening by prioritizing high-risk cases for further diagnostic testing. | This paper develops a self-supervised learning framework for cervical cytology triage, designed for HPV-positive women in low-resource settings. The approach effectively learns from limited labeled data, improving screening accessibility and accuracy where data are scarce. |
| Rutili de Lima et al. (2024) [50] | This study proposes a deep learning approach using Mask RCNN for detecting and segmenting cervical cancer cells in tissue images. It achieves high detection accuracy and generates automatic reports for medical consultants. | Cervical cancer detection and segmentation | The AI model uses a Mask RCNN-based deep learning architecture to detect and segment cervical cancer cells, automatically generating reports to help medical professionals identify cancerous areas efficiently. | The authors apply Mask Region-based Convolutional Neural Networks (Mask R-CNN) to diagnose cervical cancer progression from Pap smear images. The method excels in precise localization and classification of lesion regions, aiding detailed pathological assessment. |
| Device Name | Manufacturer | Focus Area | Reference |
|---|---|---|---|
| Cerviray AI | Cerviray (Seoul, Republic of Korea) | Colposcopy +AI | [61] |
| CellPrep Plus | Biodyne (Seoul, Republic of Korea) | Cervical cytology | [62] |
| ThinPrep Imaging System | Hologic (Marlborough, MA, USA) | General cytology, applicable to cervical | [63] |
| Genius™ Digital Diagnostics System | Hologic (Marlborough, MA, USA) | Cervical cytology + AI | [64] |
| CytoProcessor® | Datexim (Caen, France) | Cervical cytology + AI | [65] |
| ScanAI | Scanome (Warszawa, Poland ) | Cervical cytology + AI | [66] |
| EVA® Digital Colposcope | MobileODT (Tel Aviv, Israel) | Colposcopy + AI | [67] |
| Paige AI | Paige AI (New York, NY, USA) | General cytology/pathology | [68] |
| CytoSiA | OptraSCAN (San Jose, CA, USA) | General cytology + AI | [69] |
| BD FocalPoint Slide Profiler | BD (Franklin Lakes, NJ, USA) | Cervical cytology + algorithmic support | [70] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Giansanti, D.; Lastrucci, A.; Pirrera, A.; Villani, S.; Carico, E.; Giarnieri, E. AI in Cervical Cancer Cytology Diagnostics: A Narrative Review of Cutting-Edge Studies. Bioengineering 2025, 12, 769. https://doi.org/10.3390/bioengineering12070769
Giansanti D, Lastrucci A, Pirrera A, Villani S, Carico E, Giarnieri E. AI in Cervical Cancer Cytology Diagnostics: A Narrative Review of Cutting-Edge Studies. Bioengineering. 2025; 12(7):769. https://doi.org/10.3390/bioengineering12070769
Chicago/Turabian StyleGiansanti, Daniele, Andrea Lastrucci, Antonia Pirrera, Sandra Villani, Elisabetta Carico, and Enrico Giarnieri. 2025. "AI in Cervical Cancer Cytology Diagnostics: A Narrative Review of Cutting-Edge Studies" Bioengineering 12, no. 7: 769. https://doi.org/10.3390/bioengineering12070769
APA StyleGiansanti, D., Lastrucci, A., Pirrera, A., Villani, S., Carico, E., & Giarnieri, E. (2025). AI in Cervical Cancer Cytology Diagnostics: A Narrative Review of Cutting-Edge Studies. Bioengineering, 12(7), 769. https://doi.org/10.3390/bioengineering12070769







