Abstract
Medical simulators have revolutionized clinical training, particularly in teaching skills such as cardiac auscultation. This review synthesizes recent advances in the technological design and implementation of cardiac simulators for medical education, alongside scientometric and patentometric analyses. The focus is on innovations enhancing efficacy, safety, and accessibility. Analyses included 69 patents published over the past five years, sourced from Google Patents, Patentscope, Espacenet, and The Lens. A bibliometric analysis was performed using 52 scientific reports from PubMed, ScienceDirect, and The Lens within the same timeframe. Key findings indicate an 8% increase in AI-integrated cardiac auscultation devices compared to conventional equipment. Furthermore, 85% of the studies reported compliance with applicable regulations of at least 90%, reflecting improved regulatory alignment. This analysis provides a foundation for future research and the development of more accurate and accessible educational tools for cardiac auscultation training.
1. Introduction
In recent decades, technological advances have revolutionized the field of cardiovascular medicine, driven mainly by three fundamental factors: the increase in the prevalence of heart disease, the need to improve diagnostic accuracy, and the growing demand for more effective educational tools in medical training. According to the World Health Organization (WHO), cardiovascular disease (CVD) is the leading cause of death worldwide. An estimated 17.9 million people died in 2019 because of CVD, representing 32% of all deaths worldwide [1].
In Mexico, this reality is no different. According to data from the National Institute of Statistics and Geography (INEGI), during the period of January–June 2022, deaths due to heart disease were the leading cause of death nationwide, with 105,864 cases [2]. Timely attention is essential to avoid serious, irreversible sequelae and even death due to cardiovascular diseases, since up to 90% of infarcted persons survive when treated in time, according to the National Institute of Cardiology “Ignacio Chávez” [3]. Timely treatment is the action that follows as soon as the disease is diagnosed. It is important to understand that treatment is most effective if it is started as quickly as possible [4].
In this context, cardiac auscultation continues to be a fundamental diagnostic tool in clinical practice, allowing early detection of various cardiovascular pathologies. However, mastering this skill poses a significant challenge in medical education, requiring extensive practice and exposure to a wide range of cardiac sounds, both normal and pathological. Traditional teaching of cardiac auscultation faces significant limitations, including reduced availability of patients with specific conditions, variability in the presentation of clinical signs, and difficulty in repeated exposure to heart sounds [5]. An electrocardiogram (ECG) is a signal derived from the electrical activity of the heart, while a phonocardiogram (PCG) is a recording of the sounds that are produced during its activity [6]. The combination of ECG and PCG leads to higher performance of the evaluation of the cardiac condition than a diagnosis based only on PCG recordings, according to results such as accuracy: 92.5% vs. 82.5%, AUC: 0.9505 vs. 0.9066, sensitivity: 92.31% vs. 76.92%, and specificity: 92.86% for both [7]. (see Figure 1). The first heart sound (S1) is produced when the atrioventricular valves (mitral and tricuspid) close at the beginning of systole, while the second heart sound (S2) is generated by the closure of the semilunar valves (aortic and pulmonary) after systole. R peaks refer to sharp upward deflections in an ECG signal, representing ventricular depolarization.
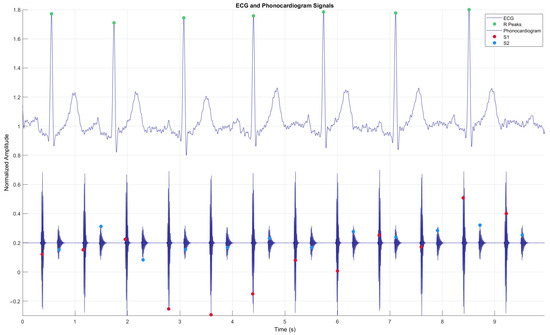
Figure 1.
Simulated simultaneous signals from a phonocardiogram (PCG) and an electrocardiogram (ECG), including the R-peaks of the ECG, showing the four states of the PCG: S1—Systole, S2—Diastole.
The applications of simulation in healthcare can be categorized into 11 dimensions: goals and purposes of the simulation activity; unit of participation; level of experience of participants; scope of healthcare; professional discipline of participants; type of knowledge, skills, attitudes, or behaviors addressed; age of the simulated patient; technology applicable or needed; location of simulation; degree of direct participation; and method of feedback used. Using simulation to improve safety will require the full integration of its applications into routine healthcare structures and practices [8]. One particular type of simulation is based on high-fidelity mannequins, which reproduce many of the characteristics of patients and make it possible to recreate highly realistic scenarios [9].
A simple classification of cardiac simulators is divided into those based on physical models, those that use computers to create illusions of reality, and those that combine the two models [10]. The initial step in these simulations is provided by mannequins that, in addition to anatomical reproduction, are equipped with software that reproduces the function of some organs and allow the student to apply their knowledge; the most obvious example is the equipment in which a thorax reproduces cardiac and pulmonary semiology with enough realism. The student can identify and distinguish between different options and recognize the syndrome to which they are linked. The second link is provided by complex interactive robotics, where a complete human body is replicated using software that equips the doll with all the cardiac, vascular, and pulmonary functions. This allows for the design of complete clinical syndromes or cases: the student must explore the robot, arrive at a clinical orientation, and initiate a set of basic skills if the situation requires it. From here, the level of complexity can be increased [11].
This work is organized into five sections that allow a clear and orderly understanding of the development of the study. Section 2 reviews the methodology based on some aspects suggested by the PRISMA framework. Section 3 presents the results obtained from the analysis of the data, presenting the information objectively by means of tables, graphs, and descriptions. Section 4 corresponds to the discussion, where the results are interpreted, highlighting relevant findings. Finally, Section 5 presents the conclusions, in which the main contributions of the research are synthesized.
2. Review Methodology
The search strategy was divided into two segments: (1) acquisition of patents and (2) acquisition of scientific communications. In both cases, search engines were used, as well as the arrangement of keywords, and Boolean operators were used (cardiac or (medical)) and (simulators or (auscultation)). A 5-year search period was considered, that is, from 2020 to 2024, as the first filter (Filter 1). Subsequently, the collected data were processed for analysis using three filters, which are explained in Section 2.1 and Section 2.2 In addition, duplicate documents were excluded from the dataset. The detailed checklist is available in the Supplementary Materials (Table S1).
2.1. Patent Search
Four search engines were used (Google Patents, Patentscope, Espacenet, and The Lens), in which the arrangement of keywords and Boolean operators mentioned in the previous subsection shows a constant in the registration of patents for patient simulators for cardiac auscultation in recent years (see Figure 2).
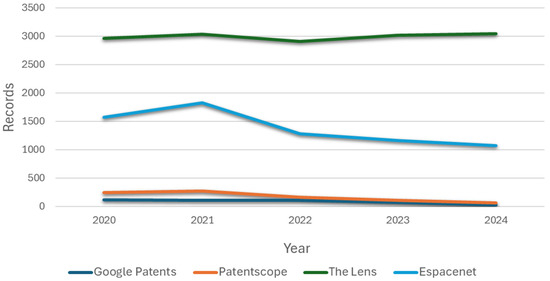
Figure 2.
Documents published by year.
Subsequently, a filter called Filter 2 related to the international patent classification (IPC) A61B 5/00 and A61B 5/024 was used. These patents belong to the group of simulators specifically designed to teach diagnostic skills, such as auscultation, by simulating heart sounds and other vital signs. This filter was applied directly in the corresponding search engine, since the objective is to determine the prior art of the patient simulator, and this classification ensures relevance by focusing on inventions within the appropriate technological scope.
Filter 3 pertains to including the word “simulator”, which must appear in the patent title. Finally, after obtaining the results from the databases by applying the three filters, a single record was integrated, and “data cleaning” was performed using the free Open Refine® (Version 3.8.7) https://openrefine.org/ (accessed on 17 February 2025). Subsequently, the data were disaggregated, and a manual review of each record was performed, applying criteria for restriction and selection of patents of interest. This action constitutes Filter 4.
Filter 4 was implemented with the aim of ensuring the relevance and timeliness of the patents included in the analysis, focusing exclusively on simulators that integrate cardiac auscultation systems.
The specialized nature of the study required a rigorous filtering of the records identified in the databases, thus enabling a more precise approach. Inclusion criteria were established to identify patents containing the technical features essential to the purpose of the study. Conversely, the exclusion criteria allowed for the removal of records that, although related to medical simulation, did not directly contribute to the specific focus of the study. Taken together, these criteria ensured a rigorous and targeted selection process, thereby enhancing the validity and relevance of the patentometric analysis results.
The following section outlines the inclusion and exclusion criteria applied to the patent selection process.
- A.
- Inclusion criteria:
- Covers simulators that specifically incorporate a cardiac auscultation system.
- Describes mechanisms for reproducing normal or pathological heart sounds.The patent may be registered in any patent office of any country.
- Details the design or manufacturing method of simulators with defined anatomical landmarks for auscultation.
- May be registered in any national or international patent office.
- B.
- Exclusion criteria:
- Refers to general-purpose simulators without explicit mention of cardiac auscultation.
- Focused exclusively on other physiological systems.
- Duplicates across databases—only the first occurrence was retained to avoid redundancy.
- Filed before 2020, given the typically slow pace of development and dissemination.
Filter 4 consisted of a manual review of the articles that passed the previous stages to verify that they met all the previously established inclusion criteria. This review was carried out independently by two evaluators (C.R.-M. and L.A.Z.-A.). To ensure consistency and standardization of the process, a review guide was developed that included clear operational definitions of each inclusion and exclusion criterion.
During the process, each evaluator independently recorded their decision to include or exclude. In cases where there were discrepancies between the evaluators, a discussion was held to reach a consensus. If agreement could not be reached after the discussion, a third evaluator (J.J.R.-L.) acted as an arbitrator to make the final decision. In order to assess inter-rater reliability, Cohen’s kappa coefficient was calculated. The value obtained was κ = 0.82, indicating a high degree of agreement according to commonly accepted standards. This reliability analysis supports the robustness of the manual selection process and minimizes selection bias.
The discrimination of Filter 4 involved adding all the documents obtained after applying Filter 3. A total of 263 documents were evaluated using the inclusion and exclusion criteria, ultimately identifying 69 documents that contained information about the inventor, registration office, year of publication, patent title, registration key, and international classification of the patent. Table 1 illustrates the effect of reducing records when applying each filter.

Table 1.
Results of the patent search across various search engines.
2.2. Search for Scientific Communications
Three search engines (PubMed, ScienceDirect, and The Lens) were used to analyze the literature, in which the arrangement of keywords and Boolean operators was applied, as well as the period of 5 years as a filter (Filter 1). The number of articles related to cardiac simulators used in medical education per year is shown in Figure 3, where an increasing trend is observed in the first years. In recent years, a decreasing trend has been observed in the case of the PubMed search engine. This contrasts with the patterns seen in ScienceDirect and The Lens, where no clear or consistent trend is evident. The lack of a marked increase or decrease in these platforms may be attributed to their unified systems, which compile articles from broader, indexed databases. Regarding the second filter, Filter 2 is related to including the word “simulator” to more specifically select the relevant articles.
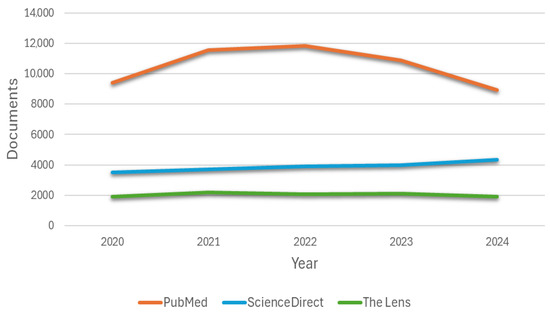
Figure 3.
Annual production of articles found related to cardiac simulators used in medical education by three databases.
Subsequently, a third filter (Filter 3) was applied to the results from each search engine, using inclusion and exclusion criteria to refine and identify the most relevant information on the topic:
- A.
- Inclusion criteria:
- Focused on the design of patient simulators for cardiac auscultation.
- Incorporating clinical cases involving auscultation.
- Addressing testing and evaluation metrics for cardiac auscultation simulators.
- Including feedback mechanisms for auscultation techniques.
- Involving the development of patient simulators for cardiac auscultation.
- Describing fabrication methods for mannequin simulators.
- B.
- Exclusion Criteria:
- Generalist simulators without specific reference to cardiac auscultation.
- Devices are limited to sound recording with no educational component.
- Simulators dedicated solely to arrhythmia or ECG.
- Devices focus exclusively on vital sign monitoring.
- Duplicate records.
- Items published before 2020.
- Simulators targeting systems other than the cardiovascular system.
Table 2 shows the effect of reducing records when applying the inclusion and exclusion criteria. A total of 52 records were obtained by performing the bibliometric analysis using free Open Refine® software.

Table 2.
Total number of documents collected from search engine queries.
3. Results
A total of 115,199 records were found in the database searches. After data cleaning, we examined 2335 records, of which we reviewed 378 full-text papers and finally included 121 [11,12,13,14,15,16,17,18,19,20,21,22,23,24,25,26,27,28,29,30,31,32,33,34,35,36,37,38,39,40,41,42,43,44,45,46,47,48,49,50,51,52,53,54,55,56,57,58,59,60,61,62,63,64,65,66,67,68,69,70,71,72,73,74,75,76,77,78,79,80,81,82,83,84,85,86,87,88,89,90,91,92,93,94,95,96,97,98,99,100,101,102,103,104,105,106,107,108,109,110,111,112,113,114,115,116,117,118,119,120,121,122,123,124,125,126,127,128,129,130,131]. Subsequently, we searched for papers that cited any of the initially included studies, as well as their references. However, no additional articles meeting the criteria were found in these searches (see Figure 4).
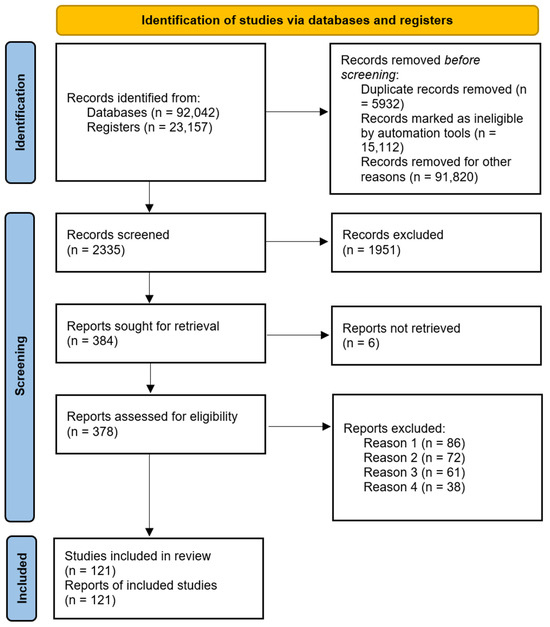
Figure 4.
Flowchart corresponding to the Identification of studies via databases and registers.
3.1. Patentometric Analysis
After filtering the documents, 69 patents related to medical simulation devices were selected; the results are shown in Table 3. The main offices from which the patents were registered are China (CN, 27 patents), the United States (US, 26 patents), the International Office (WO, 10), the European Office (EU, 3 patents), the United Kingdom (GB, 1 patent), Moldova (MD, 1 patent), and Canada (CA, 1 patent). As Figure 5 shows, China has the highest number of patents, although it is not far behind the United States, which has the second highest number of patents.

Table 3.
Cardiac simulation device patents.
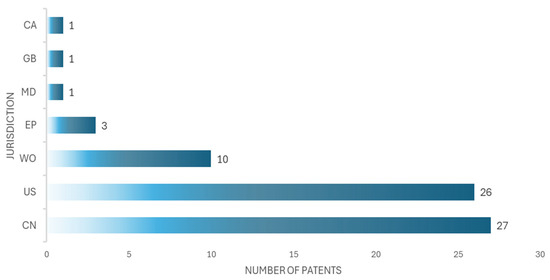
Figure 5.
The number of patents in seven jurisdictions is shown, corresponding to China (CN), the United States (US), the Worldwide Office (WO), the European Office (EU), the United Kingdom (GB), Moldova (MD), and Canada (CA).
The applicant with the highest number of patents is Xu Liugen, an inventor, who in this case has three patents, from 2020 to 2024. Figure 6 shows the principal inventors of patient simulators for cardiac auscultation, while Figure 7 shows the principal applicants of patient simulators for cardiac auscultation.
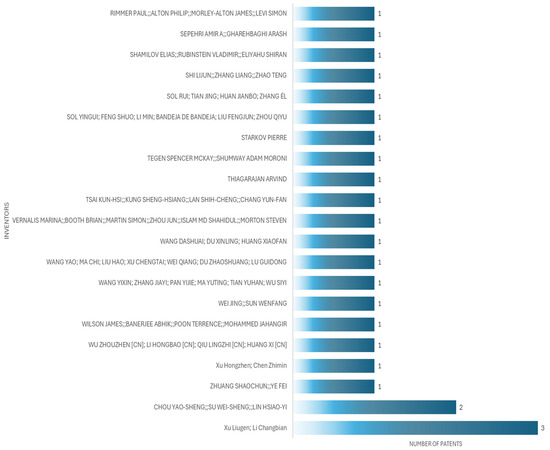
Figure 6.
Top patent inventors for patient simulators for cardiac auscultation.
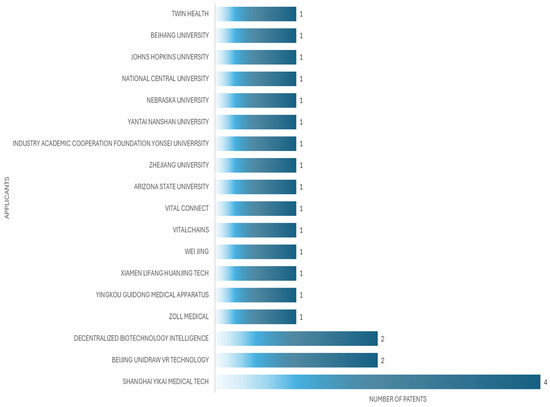
Figure 7.
Top patent applicants for patient simulators for cardiac auscultation.
In the analysis of the patents, we found that 27 documents are related to devices for auscultation exclusively of the heart, and 42 documents are related to devices for auscultation of the heart and another anatomical part, as seen in Figure 8.
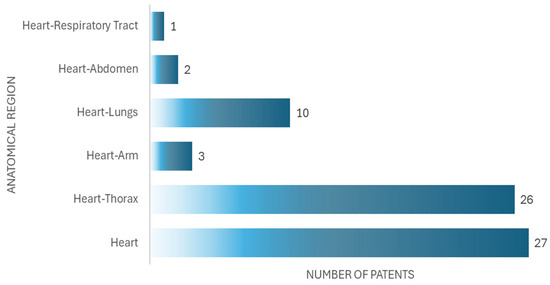
Figure 8.
Number of patents by anatomical region.
Among the 69 results obtained, 25 correspond to “mannequin simulator”, 20 to monitoring device, 6 to specialized stethoscope, 6 to simulation device, 3 to software, and 1 to detection system. In addition, eight results are associated with methodologies, which can be design methods or application methods. Figure 9 shows the resulting patents and the distribution of the body region they represent.
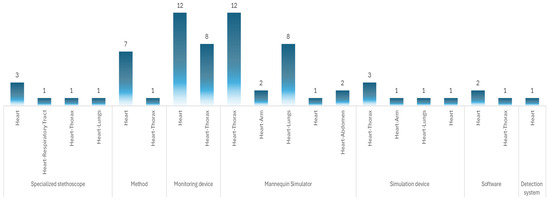
Figure 9.
Description of the patents and primary coverage.
From the patent data, 55 patents refer to patents related only to the thoracic region and physical devices, leaving out methods. In 31 patents, the predominant technology is electronic, using sensors of different characteristics, microprocessors, and controllers as the main elements to simulate cardiac tones. In eight patents, the predominant technology is electromechanical–electronic, using sensors, actuators, and motors with which tones that emulate the heart are made. In seven patents, the technology used is simulation, in which the auscultation process is performed using 3D models, augmented reality, virtual reality, and mixed reality. In four patents, the technology used is artificial intelligence, where, in addition to a physical model, it uses neural networks for continuous learning of the software. In the four patents, the technology used is mechanical, which uses connecting rods, springs, and threaded mechanisms to produce heart tones. For one patent, the technology used is machine learning, supported by a mannequin simulator.
Devices based on electronic technology are not only the most prevalent, 56.3% of the patents analyzed, but also represent a functionally adequate solution for simulating heart sounds.
This technology allows for the easy integration of speakers, sensors, and control systems, facilitating the auditory reproduction of normal and pathological conditions. However, when compared to more advanced technologies, such as artificial intelligence systems, there is a significant difference in terms of educational capabilities: while electronic devices allow for passive and repetitive training, AI-based systems can offer personalized feedback and automatic analysis of user performance. Therefore, although electronics remain the predominant option due to their technical and economic viability, their educational potential is more limited compared to emerging technologies.
Table 3 shows the selected patents, the technology used, the type of device, and the anatomical region it simulates. Figure 10 shows the technology used in the patents and the main elements with which they implement the functionality of the simulation device.
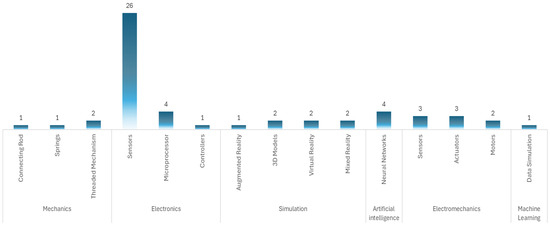
Figure 10.
Type of technology used in cardiac simulation.
3.2. Scientometric Analysis
For the articles found in Section 2.2, an analysis was carried out, and the following results were obtained. Specifically, the most recurrent keywords were identified, including: simulation (frequency = 19 articles), training (frequency = 18 articles), simulator (frequency = 17 articles), education (frequency = 17 articles), medical (frequency = 16 articles), virtual reality (frequency = 15 articles), surgery learning (frequency = 11 articles), heart care (frequency = 10 articles), robotic patient (frequency = 6 articles), auscultation (frequency = 3 articles), surgical (frequency = 2 articles), validity (frequency = 2 articles), and assisted (frequency = 1 article).
The keywords identified also allow us to infer relevant aspects of the technological development of the devices. For example, the high frequency of terms such as “virtual reality” and “simulation” suggests a growing incorporation of immersive technologies in the design of medical simulators. The appearance of the term “robotic patient” reflects interest in high-fidelity mannequins with components capable of reproducing physiological functions such as heart sounds, chest movements, or responses to auscultation. Likewise, words such as “training” and “education” indicate that the design of these devices is oriented not only toward anatomical representation but also toward improving clinical skills through repetitive practice and feedback. Therefore, the scientometric trends observed are directly linked to the type of technologies integrated into the simulators and the pedagogical objectives they seek to achieve.
Regarding the authors, it was found that some have the same publications: Yutaka Kagaya, Leijte E., Pinto L., Fu Y., and Qi Z.; however, Ock J. is the most cited author in this field related to cardiac simulators used in medical education (see Table 4 and Figure 11).

Table 4.
Publication trends of leading authors across the years.
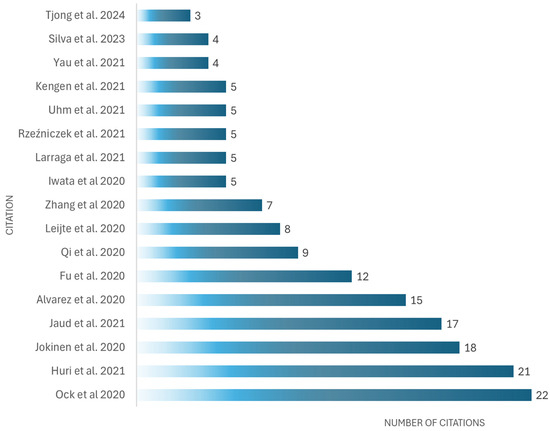
Figure 11.
Most cited documents related to cardiac simulators used in medical education, as cited in The Lens and Google Scholar, February 2025 [83,84,85,93,94,97,98,105,107,108,115,118,121,124,126,128,130].
The United States is the most productive country (six documents), followed by the Japan (five documents), China (four documents), and the Netherlands (four documents) (see Figure 12).
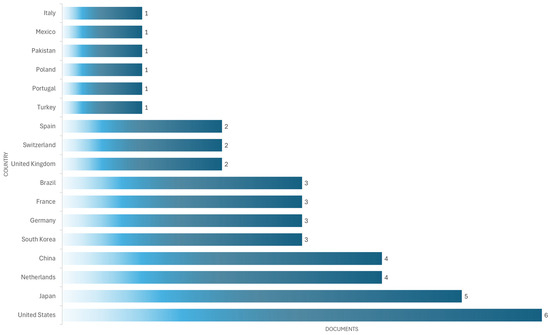
Figure 12.
The most productive countries in developing simulator devices for cardiac auscultation.
As shown in Figure 12, most studies come from high-income countries such as the United States, Japan, and the Netherlands, suggesting greater adoption and investment in these technologies. In contrast, middle- and low-income countries such as Mexico, Pakistan, and Turkey have a much smaller share, reflected in limited output with only one document per country. This disparity can be attributed to multiple barriers: the high initial investment required to acquire high-fidelity simulators, limited technological infrastructure, and a lack of specialized training programs. Furthermore, in some contexts, medical curricula do not yet systematically integrate simulation as a pedagogical strategy. In contrast, institutions in countries with greater resources tend to have fully equipped simulation laboratories and educational policies that promote their use. These regional differences must be considered when designing global medical education policies, promoting strategies for collaboration, technology transfer, and the development of low-cost simulators for contexts with limited resources.
Table 5 shows the selected articles used in the scientometric analysis employed, database results, and selection criteria. The presentation of the performance parameters of each selected article is essential, as it allows us to identify the criteria used to evaluate the effectiveness of medical simulators in educational and clinical settings. This information not only facilitates comparison between different types of simulators, it also helps to detect trends and limitations in the validation methods applied.

Table 5.
Articles analyzed about simulator devices for cardiac auscultation.
4. Discussion
In this review of 69 patents and 52 scientific articles, different analyses have been used to find similarities to guide new proposals that may arise in future designs. It was concluded that auscultation simulators have advanced from basic mechanical models to electronic devices with digitized sounds and artificial intelligence systems that allow the simulation of different pathologies. However, limitations were identified in the clinical validation of some commercial devices, suggesting the need for a greater correlation between patented developments and their implementation in educational and hospital environments.
The review shows an 8% increase in the implementation of devices used for cardiac auscultation with artificial intelligence (AI) compared to traditional models (see Figure 10). This trend suggests a move toward the integration of more sophisticated technologies, which can improve diagnostic accuracy and the detection capacity of a wide range of cardiac pathologies.
On the other hand, the patents reviewed show an emphasis on the integration of new technologies, such as different sensors, wireless connectivity, and augmented reality. However, many of these innovations have not been extensively evaluated in academic studies, which generates uncertainty about their real impact on the practical teaching of auscultation. In addition, it is noted that several patents focus on technical improvements without addressing key aspects such as affordability and compatibility with stethoscopes used in clinical practice. Another important finding is the gap between technological innovation and its adoption in medical training. Despite the increasing sophistication of simulators, their availability in teaching institutions remains limited, mainly due to production and acquisition costs. In addition, 85% of the studies analyzed reported ≥90% compliance with ISO 13485:2016. Medical devices–Quality management systems–Requirements for regulatory purposes. International Organization for Standardization (ISO): Geneva, Switzerland, 2016. reflecting strong alignment with international regulatory standards for medical devices (see Table 3).
If the simulator complies with ISO 13485, it can be guaranteed that heart sounds are reproduced accurately and at the correct points. This improves auditory and anatomical learning, which are key to clinical auscultation, but it does not assess whether students are learning, only that the simulator is well designed, safe, and functions as it should.
This suggests that future research should focus on the development of low-cost devices, taking advantage of technologies such as 3D printing and open-source software.
Although technological advances in auscultation simulators have been made, one of the main barriers to their adoption in medical training is the transition of these devices from the academic to the clinical setting. While current simulators have proven to be effective in controlled training scenarios, their integration in hospitals and clinics remains limited due to several factors, such as production cost, lack of trained personnel to operate them, and the difficulty of integrating these technologies into existing clinical systems.
Currently, many devices with integrated artificial intelligence are in the early stages of development or validation. Although some have proven effective in controlled environments, their rigorous clinical validation is still limited and poses a challenge, especially given the need for multicenter studies with large samples and standardized protocols. In addition, regulatory criteria vary between countries, complicating their widespread clinical adoption.
It is critical to address these challenges through the creation of simulators that are more accessible and easier to implement in the hospital setting. One possible solution would be the development of simulators that can be directly integrated with hospital patient management systems, allowing for better teaching and real-time assessment of auscultation skills. This would not only improve the quality of training but would also contribute to better preparation of healthcare professionals when facing real clinical situations. The effectiveness of the simulator often depends on the human instructor guiding the session. Without an appropriate teaching strategy, even high-fidelity simulators can lead to poor or incorrect learning.
The predominance of multiregional simulators could suggest an advantage in terms of educational versatility. However, our analysis suggests that this breadth of coverage may be accompanied by less specialization in the reproduction of heart sounds. In contrast, simulators designed exclusively for cardiac auscultation tend to have superior acoustic fidelity and a deeper focus on auscultation points, which may specifically benefit cardiology training.
One area that could significantly benefit the development of auscultation simulators is the active involvement of end users in the design process. Involving physicians, medical students, nurses, and other healthcare professionals in the design and testing phases of simulators allows direct feedback on the usability, educational effectiveness, and areas for improvement of the devices. This collaborative approach helps identify practical issues that may not be evident in the initial design, such as the ergonomics of the simulator or the clarity of the user interfaces.
4.1. Structural Elements of the Simulation Mannequin
According to the selected patents and scientific documentation, the design of the patient simulator mannequins consists of a realistic anatomical torso, which must accurately represent the human thoracic anatomy, including anatomical references such as the sternum, ribs and intercostal spaces. It should also have well-defined auscultation points to ensure proper practice (see Figure 13).
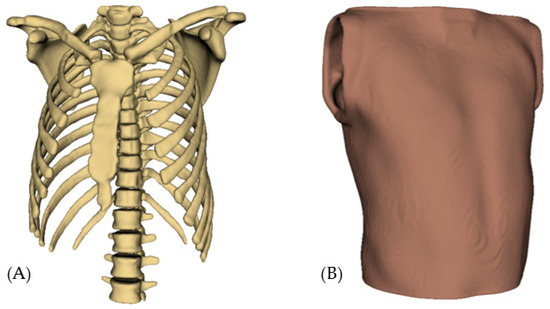
Figure 13.
Basic structure of the mannequin simulator: (A) Thorax; (B) Skin.
4.2. Types of Materials
In the patents reviewed, various types of materials were identified as commonly used in medical simulation mannequins. For skin and soft tissues, materials such as medical-grade silicone are employed due to their flexibility and texture, which closely resemble human skin. This provides greater elasticity and a more realistic tactile experience. Such properties are crucial in medical training devices, as they can enhance the transmission of sound through the mannequin’s surface.
As for the internal structure and support, rigid plastics are commonly used because of their strength and ease of molding. Notably, at least 36% of the devices examined in the patent review incorporate both medical-grade silicone and rigid plastics, highlighting their widespread adoption in the design and manufacturing of medical simulators (see Figure 9).
To illustrate the aforementioned material applications, two renderings generated from a proprietary design proposal are presented. Image (A) shows the external surface, which simulates skin and soft tissues using medical-grade silicone, highlighting its realistic texture, transparency, and flexibility. These visual renderings serve to exemplify how the selected materials contribute to the functionality and realism of the medical simulation mannequin. Image (B) shows the internal structure of the mannequin, highlighting the use of rigid plastics to provide structural support and maintain anatomical integrity (see Figure 14).
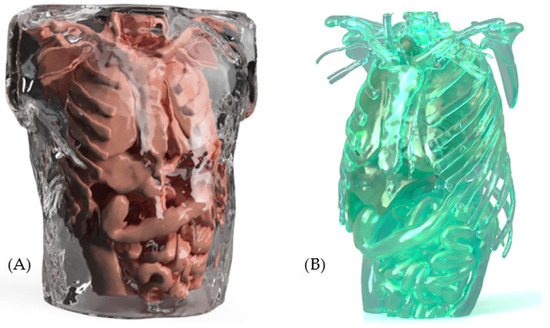
Figure 14.
Type of material: (A) External structure; (B) Internal structure.
4.3. Types of Materials for Sound Simulation
For sound distribution and amplification, acrylic or polycarbonate plates are used to function as resonance chambers, allowing vibrations to propagate uniformly inside the mannequin. These materials help to avoid distortions and improve sound perception at the correct auscultation points. In addition, some simulator models incorporate silicone gel or acoustic foams to control sound absorption and reduce unwanted interference within the mannequin structure. These materials allow the clarity and direction of sound to be adjusted to make the user experience as realistic as possible (see Figure 15).
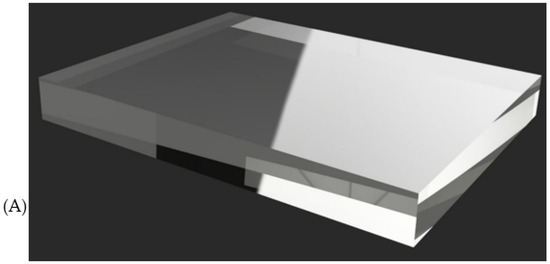
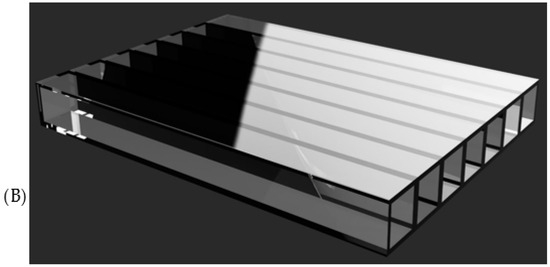
Figure 15.
Types of resonance chamber material used: (A) acrylic; (B) polycarbonate.
From the patents and research articles, it was determined that the choice between acrylic and polycarbonate depends on factors such as mechanical strength and sound transmission quality. While acrylic offers greater rigidity and clarity in the propagation of vibrations, polycarbonate stands out for its high impact resistance and flexibility, making it ideal for more resistant or complex-shaped structures.
In addition, the mannequin design presented in this work has been the subject of an industrial design registration application, specifically the industrial model, which supports the originality and innovation of the proposal. The images included in this article show some of the key technical features of the mannequin, such as the anatomical arrangement. These features were considered in the application with application number MX/f/2025/001145, currently pending before the Mexican Institute of Industrial Property (IMPI). The legal protection seeks to guarantee the authorship of the design and to promote its use in educational contexts, particularly in medical training.
5. Conclusions
Cardiac auscultation simulator mannequins represent an essential tool in medical education, allowing students to develop clinical skills in a controlled and safe environment. Through this review, it has become evident how the combination of acoustic materials, simulation technologies, and ergonomic designs has significantly improved the fidelity and effectiveness of these devices. The results highlight the evolution of these simulators, from basic mechanical models to advanced systems that integrate acoustic optimization materials, electronic feedback, and remote control capabilities.
The review found an 8% increase in the adoption of AI auscultation devices, reflecting a trend toward more accurate and automated technologies. Furthermore, 85% of studies reported ≥90% compliance with ISO 13485, highlighting the priority placed on simulator safety and quality. These advances suggest significant potential for improving diagnostic accuracy and medical education. However, further clinical validation is required to ensure their effectiveness in real-world settings.
Of the 69 patents analyzed, 27 (39.1%) focus exclusively on cardiac auscultation, while 42 (60.9%) cover multiple anatomical regions. When analyzing the citations received and the reported use in educational validation studies, it is observed that multiregional simulators tend to be cited more frequently in studies that integrate comprehensive clinical training. However, cardiac-auscultation-specific simulators show a higher level of detail in their acoustic design and are preferred in research focused on in-depth auscultation skills.
Although the principles behind the technologies remain unchanged, some devices incorporate various technologies and materials to offer a realistic learning experience. They use sound systems with speakers or transducers to reproduce accurate heart tones, along with acoustic materials such as acrylic, polycarbonate, and silicone gel to optimize sound propagation. In addition, many models are compatible with conventional stethoscopes or include electronic versions with simulated pathology settings. Remote connectivity allows instructors to modify parameters in real time, while some advanced devices offer automatic feedback.
Based on the findings, several promising lines of research are identified to strengthen the development of medical simulators. First, it is recommended to promote the integration of artificial intelligence (AI) with real-time feedback systems, which would allow for dynamic adaptation of the simulator to user responses and automated performance evaluation. In addition, there is a clear need to move toward the standardization of validation protocols, which would allow for the comparison of educational effectiveness between different simulators under common and reproducible criteria. Finally, given the growing importance of remote medical care, it is suggested that the use of simulators in telemedicine training contexts be explored, particularly for training clinical skills through virtual platforms that simulate real remote consultation scenarios.
The contribution of these results focuses on the technologies and techniques employed and the accessibility and standardization of these simulators, which opens the door to future research and improvements in their development. In addition, this work contributes to the formulation of the design presented in Section 4.1, Section 4.2 and Section 4.3 as a prototype of a patient simulator mannequin for the practice of cardiac auscultation.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/bioengineering12070731/s1, Table S1: Prisma 2020 Checklist.
Author Contributions
C.R.-M. developed the practical aspects of this research, exhaustive work on reviewing and editing; L.A.Z.-A. provided the original schematic and supervised this research; G.M.C.-M., J.J.R.-L., J.Z.-L. and Á.E.B.-G. reviewed and corrected this document. All authors have read and agreed to the published version of the manuscript.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors. The authors covered the publication costs personally. All development and analysis were conducted using open-source software.
Acknowledgments
The authors would like to thank the faculty and research staff at the School of Medicine, Autonomous University of Mexico State for their guidance and support during the preparation of this systematic review.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- World Health Organization. Cardiovascular Diseases (CVDs). Available online: https://www.who.int/news-room/fact-sheets/detail/cardiovascular-diseases-(cvds) (accessed on 17 February 2025).
- INEGI. Registered Death Statistics January–June 2022 (Preliminary). Available online: https://www.inegi.org.mx/contenidos/saladeprensa/boletines/2023/DR/DR-Ene-jun2022.pdf (accessed on 17 February 2025). (In Spanish).
- Secretary of Health. Timely Care, Essential to Prevent and Treat Cardiovascular Diseases. Available online: https://www.gob.mx/salud/es/articulos/atencion-oportuna-fundamental-para-prevenir-y-atender-enfermedades-cardiovasculares?idiom=es (accessed on 17 February 2025).
- Rosale, C.; Ortega, J. Natural History of Disease, Levels of Prevention, and Epidemiological Chain. Available online: https://www.gob.mx/cms/uploads/attachment/file/811046/0007734_Tema_2_Subtema_1_Historia_Ntural_de_Enfermedad_-_Jos__Carlos_Rosales_Ortega.pdf (accessed on 31 May 2025).
- Nassralla, M.; Zein, Z.E.; Hajj, H. Classification of normal and abnormal heart sounds. In Proceedings of the 2017 Fourth International Conference on Advances in Biomedical Engineering (ICABME), Beirut, Lebanon, 19–21 October 2017; pp. 1–4. [Google Scholar]
- Abo-Zahhad, M.; Ahmed, S.M.; Abbas, S.N. Biometric authentication based on PCG and ECG signals: Present status and future directions. Signal Image Video Process. 2014, 8, 739–751. [Google Scholar] [CrossRef]
- Chakir, F.; Jilbab, A.; Nacir, C.; Hammouch, A. Recognition of cardiac abnormalities from synchronized ECG and PCG signals. Phys. Eng. Sci. Med. 2020, 43, 673–677. [Google Scholar] [CrossRef] [PubMed]
- Gaba, D.M. The future vision of simulation in health care. Qual. Saf. Health Care 2004, 13 (Suppl. 1), i2–i10. [Google Scholar] [CrossRef]
- Riancho, J.; Maestre, J.M.; del Moral, I.; Riancho, J.A. Simulación clínica de alto realismo: Una experiencia en el pregrado. Educ. méd. 2012, 15, 109–115. Available online: http://scielo.isciii.es/scielo.php?script=sci_arttext&pid=S1575-18132012000200009&lng=es (accessed on 22 April 2025). [CrossRef]
- Ruiz-Parra, A.I.; Angel-Müller, E.; Guevara, O. La simulación clínica y el aprendizaje virtual. Tecnologías complementarias para la educación médica. Rev. Fac. Med. 2009, 57, 67–79. [Google Scholar]
- Vázquez-Mata, G. Realidad virtual y simulación en el entrenamiento de los estudiantes de medicina. Educ. Med. 2008, 11 (Suppl. 1), 29–31. Available online: http://scielo.isciii.es/scielo.php?script=sci_arttext&pid=S1575-18132008000500006&lng=es (accessed on 17 February 2025).
- Wei, J.; Sun, W. Auscultation Device for Cardiovascular Medicine Department. CN 213787491 U, 27 July 2021. Available online: https://www.lens.org/lens/patent/027-285-055-151-255/frontpage (accessed on 17 February 2025).
- Wu, Z.; Li, H.; Qiu, L.; Huang, X. Auscultation Position Indication Method and Device. WO 2022068650: A1, 9 December 2022. Available online: https://patents.google.com/patent/WO2022068650A1/en (accessed on 17 February 2025).
- Vernalis, M.; Booth, B.; Martin, S.; Zhou, J.; Islam, M.; Morton, S. Auscultation System. WO 2021/225977: A1, 11 November 2021. Available online: https://www.lens.org/lens/patent/099-754-106-145-295/frontpage (accessed on 17 February 2025).
- Narayan, A. Auscultation System for Guiding a User to Perform Auscultation on a Subject. WO 2021/229600: A1, 18 November 2021. Available online: https://www.lens.org/lens/patent/021-045-798-247-732/frontpage (accessed on 17 February 2025).
- Xu, H.; Chen, Z. Auscultation Training Examination Model. CN216647634U, 27 December 2022. Available online: https://patents.google.com/patent/CN216647634U/en (accessed on 17 February 2025).
- Gideon, E.; Gideon, B. Biofeedback Apparatus and Method. US12011314: B2, 18 June 2024. Available online: https://patents.google.com/patent/US12011314B2/en (accessed on 17 February 2025).
- Myllylä, T.; Zienkiewicz, A. Biosignal Measurement Apparatus and Method. US 2022/0031183: A1, 3 February 2022. Available online: https://www.lens.org/lens/patent/107-939-788-088-695/frontpage (accessed on 17 February 2025).
- Peters, L. Cardiac Health Assessment Systems and Methods. US 11234630 B2, 1 February 2022. Available online: https://www.lens.org/lens/patent/111-713-653-913-320/frontpage (accessed on 17 February 2025).
- Cong, Y.; Xiong, K.; Hao, A.; Zhao, Y. Cardiopulmonary Auscultation Virtual Simulation Training System and Method. CN117576969A, 20 February 2024. Available online: https://patents.google.com/patent/CN117576969A/en (accessed on 17 February 2025).
- Wang, D.; Du, X.; Huang, X. Cardiopulmonary Percussion Model For Teaching. CN213904684U, 6 August 2021. Available online: https://patents.google.com/patent/CN213904684U/en (accessed on 17 February 2025).
- Liugen, X.; Changbian, L. Cardiopulmonary Simultaneous Multidirectional Auscultation Anthropomorphic Dummy. CN211827841U, 30 October 2020. Available online: https://patents.google.com/patent/CN211827841U/en (accessed on 17 February 2025).
- Seunghyun, L. Chest Wall Oscillation System with Digital Auscultation. US 11432991 B2, 6 September 2022. Available online: https://www.lens.org/lens/patent/042-095-533-796-16X/frontpage (accessed on 17 February 2025).
- Chengyuan, K. Comprehensive Delivery Mother-Child First-Aid Model. CN214428214U, 19 October 2021. Available online: https://patents.google.com/patent/CN214428214U/en (accessed on 17 February 2025).
- Binghua, J. Comprehensive Skill Training Simulator. CN219872652U, 20 October 2023. Available online: https://patents.google.com/patent/CN219872652U/en (accessed on 17 February 2025).
- Sepehri, A.; Gharehbaghi, A. Device for Automated Screening of Congenital Heart Diseases. EP 2249707 B1, 30 March 2022. Available online: https://www.lens.org/lens/patent/168-707-107-725-565/frontpage (accessed on 17 February 2025).
- Herzog, D.; Ackerman, W. Diagnostic Simulator. US 10542892 B2, 28 January 2020. Available online: https://www.lens.org/lens/patent/166-559-727-867-084/frontpage (accessed on 17 February 2025).
- Alsaliem, S.; Alsaliem, T.; Alsaliem, E. Digital Stethoscope for Diagnosing Cardiopulmonary Pathologies. MD1702Y, 31 July 2024. Available online: https://patents.google.com/patent/MD1702Y/en (accessed on 17 February 2025).
- Knothe, M.; Lim, M. Dynamic Body Part Simulator. WO 2022/224220 A1, 27 October 2022. Available online: https://www.lens.org/lens/patent/145-320-274-748-130/frontpage (accessed on 17 February 2025).
- Lee, M.; Lee, J. Electronic Device for Auscultation and Method of Operation Thereof. US 2024/0341716 A1, 17 October 2024. Available online: https://www.lens.org/lens/patent/105-934-436-885-492/frontpage (accessed on 17 February 2025).
- Sun, Y.; Feng, S.; Li, M.; Pan, B.; Liu, F.; Zhou, Q. Electronic Standardized Human Body Model System for Emergency Training and Examination. CN112530256A, 19 March 2021. Available online: https://patents.google.com/patent/CN112530256A/en (accessed on 17 February 2025).
- Liu, F.; Cai, Y.; Teng, J. Electronic Standardized Infant Model System for Emergency Training And Examination. CN216719316U, 10 June 2022. Available online: https://patents.google.com/patent/CN216719316U/en (accessed on 17 February 2025).
- Wang, Y.; Zhang, J.; Pan, Y.; Ma, Y.; Tian, Y.; Wu, S. First-Aid Skill Training Model with Airway Management Function. CN219039911U, 16 May 2023. Available online: https://patents.google.com/patent/CN219039911U/en (accessed on 17 February 2025).
- Li, J.; Ge, H.; Yin, Z.; Cao, X.; Yang, Y. Heart Sound Deep Learning Heart Disease Prediction Method and System for Auscultation Teaching. CN 118490200 A, 27 September 2024. Available online: https://www.lens.org/lens/patent/034-414-939-921-595/frontpage (accessed on 17 February 2025).
- Liugen, X.; Changbian, L. Heart-Variable Simulation Device for Cardiopulmonary Auscultation Palpation. CN211928884U, 13 November 2020. Available online: https://patents.google.com/patent/CN211928884U/en (accessed on 17 February 2025).
- Yao, W.; Chi, M.; Hao, L.; Chengtai, X.; Qiang, W.; Zhaoshuang, D.; Guidong, L. High-simulation multifunctional wireless Human Body Vital Sign Examination Training Model. CN114093230A, 25 February 2022. Available online: https://patents.google.com/patent/CN114093230A/en (accessed on 17 February 2025).
- Tao, L. Human Pulse and Heartbeat Simulator. CN220439095U, 2 February 2024. Available online: https://patents.google.com/patent/CN220439095U/en (accessed on 17 February 2025).
- Chou, Y.; Su, W.; Lin, H. Integrated Sensing Device for Heart Sound and ECG Signals. US 2024/0188836 A1, 13 July 2024. Available online: https://www.lens.org/lens/patent/069-735-977-375-57X/frontpage (accessed on 17 February 2025).
- Li, S.; Hao, A.; Zhao, Q. Modeling Method, Apparatus, Device and Storage Medium of Dynamic Cardiovascular System. US 2020/0357162 A1, 12 November 2020. Available online: https://www.lens.org/lens/patent/169-796-594-297-396/frontpage (accessed on 17 February 2025).
- Tsai, K.; Kung, S.; Lan, S.; Chang, Y. Multi-Mic Sound Collector and System and Method for Sound Localization. WO 2019/094399 A1, 16 May 2020. Available online: https://www.lens.org/lens/patent/051-256-624-146-315/frontpage (accessed on 17 February 2025).
- Jovanov, E.; Filipovic, N. Multi-Modal Heart Diagnostic System and Method. US 11872080 B1, 16 January 2024. Available online: https://www.lens.org/lens/patent/112-336-811-234-143/frontpage (accessed on 17 February 2025).
- Shi, L.; Zhang, L.; Zhao, T. Multi-Parameter Simulator. CN 221279137 U, 5 July 2024. Available online: https://www.lens.org/lens/patent/010-578-937-464-466/frontpage (accessed on 17 February 2025).
- Huang, H.; Lee, C.; Yang, C.; Liu, H.; Huang, N. Noninvasive Arterial Condition Detecting Method, System, and Non-Transitory Computer Readable Storage Medium. US 10271737 B2, 30 April 2020. Available online: https://www.lens.org/lens/patent/134-570-038-041-427/frontpage (accessed on 17 February 2025).
- Matthews, D.; Liu, X.; Bi, S. Portable Heart Motion Monitor. US 11116416 B2, 14 September 2021. Available online: https://www.lens.org/lens/patent/190-026-799-936-614/frontpage (accessed on 17 February 2025).
- Liu, Y.; Xia, L.; Pei, Z. PPG Pulse Wave Simulator. CN 113729669 A, 3 December 2021. Available online: https://www.lens.org/lens/patent/164-039-883-261-736/frontpage (accessed on 17 February 2025).
- Cao, Z.; Guo, Z.; Xing, C. Pregnant Woman Four-Step Palpation Simulation Teaching Model. CN217113605U, 2 August 2021. Available online: https://patents.google.com/patent/CN217113605U/en (accessed on 17 February 2025).
- Huang, S.; Su, F.; Zhao, J.; Sun, Y.; An, K.; Liu, Y.; Zhang, S. Pulse Simulator and Simulation Method Thereof. CN 110755054 A, 7 February 2020. Available online: https://www.lens.org/lens/patent/124-928-602-190-142/frontpage (accessed on 17 February 2025).
- Zhuang, S.; Ye, F. Pulse Wave Conduction Parameter Measuring Method and Pulse Wave Conduction Parameter Processing Device. US 2021/0127991 A1, 6 May 2021. Available online: https://www.lens.org/lens/patent/160-091-520-413-748/frontpage (accessed on 17 February 2025).
- Bercoff, J.; Cazeau, S. Real-Time Adaptation of a Personalized Heart Model. WO 2023/030642 A1, 9 March 2023. Available online: https://www.lens.org/lens/patent/092-272-370-635-350/frontpage (accessed on 17 February 2025).
- Nallathambi, G.; Alexander, B.; Selvaraj, N. Screening Device, Method, and System for Structural Heart Disease. US 11534108 B2, 27 December 2022. Available online: https://www.lens.org/lens/patent/031-617-215-015-096/frontpage (accessed on 17 February 2025).
- Wilson, J.; Banerjee, A.; Poon, T.; Mohammed, J. Simulating Clinical Trials Using Whole Body Digital Twin Technology. WO 2023/064304 A1, 11 October 2023. Available online: https://www.lens.org/lens/patent/071-020-483-645-077/frontpage (accessed on 17 February 2025).
- Duan, Y.; Zhou, Q.; Liao, T.; Qin, J.; Wei, S.; Tang, S. Simulation Defibrillator Teaching Equipment with a Cardio-Pulmonary Resuscitation Display Function. CN217133887U, 8 August 2022. Available online: https://patents.google.com/patent/CN217133887U/en (accessed on 17 February 2025).
- Liugen, X.; Changbian, L. Simulation Device for Palpation of Variable Lungs Through Cardiopulmonary Auscultation. CN211827843U, 30 October 2020. Available online: https://patents.google.com/patent/CN211827843U/en (accessed on 17 February 2025).
- Grethe, H. Simulation Doll. CA3213961A1, 6 October 2021. Available online: https://patents.google.com/patent/CA3213961A1/en (accessed on 17 February 2025).
- Shamilov, E.; Rubinstein, V.; Eliyahu, S. Simulation of Heart Pacing for Modeling Arrhythmia. US 11482338 B2, 25 October 2022. Available online: https://www.lens.org/lens/patent/134-923-314-122-559/frontpage (accessed on 17 February 2025).
- Balkizov, Z.; Kostyushov, Y.; Bushuyev, V.; Dudarev, D.; Isayev, A. Simulation System for Abdominal Cavity Examination. WO2024102018A1, 16 May 2022. Available online: https://patents.google.com/patent/WO2024102018A1/en (accessed on 17 February 2025).
- Atashbar, M.; Narakathu, B.; Zhang, X.; Maddipatla, D. Stethographic Device. US 2019/0298269 A1, 3 October 2022. Available online: https://www.lens.org/lens/patent/113-509-295-228-562/frontpage (accessed on 17 February 2025).
- Jingtan, Q. Stethoscope Dummy. CN215341480U, 28 December 2021. Available online: https://patents.google.com/patent/CN215341480U/en (accessed on 17 February 2025).
- Elhilali, M.; Kala, A. System and Method for Determining an Auscultation Quality Metric. US 2023/0240641 A1, 3 August 2023. Available online: https://www.lens.org/lens/patent/011-692-157-329-592/frontpage (accessed on 17 February 2025).
- Pak, H.; Lee, Y. System and Method for Evaluating Effects of Antiarrhythmic Agent. US 10925546 B2, 23 February 2021. Available online: https://www.lens.org/lens/patent/149-137-995-637-836/frontpage (accessed on 17 February 2025).
- Hayes, M. System and Method for Medical Simulation. EP 4231908 A4, 8 May 2024. Available online: https://www.lens.org/lens/patent/082-605-665-635-905/frontpage (accessed on 17 February 2025).
- Starkov, P. System for Recording Chest Signals and Method Using Said System. EP 3694416 B1, 20 July 2022. Available online: https://www.lens.org/lens/patent/160-359-368-900-748/frontpage (accessed on 17 February 2025).
- Andino, J.; Spohn, A. System, Device and Method for Automated Auscultation. US 2024/0000381 A1, 4 January 2024. Available online: https://www.lens.org/lens/patent/045-584-082-742-133/frontpage (accessed on 17 February 2025).
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ 2021, 372, n71. [Google Scholar] [CrossRef]
- Anderson, C.; Park, S. Systems and Methods for Determination of Pulse Arrival Time with Wearable Electronic Devices. WO 2024/049973 A1, 7 March 2024. Available online: https://www.lens.org/lens/patent/142-183-749-411-228/frontpage (accessed on 17 February 2025).
- Potes, B.; Gupta, P.; Moses, K. Systems and Methods for Determining Filtered Cardiac Output. WO 2024/206178 A1, 3 October 2024. Available online: https://www.lens.org/lens/patent/013-242-856-645-660/frontpage (accessed on 17 February 2025).
- Thiagarajan, A. Systems and Methods for Measuring Patient Vital Signs. US 2022/0061666 A1, 3 March 2022. Available online: https://www.lens.org/lens/patent/183-006-071-105-461/frontpage (accessed on 17 February 2025).
- Freeman, G.; Volpe, S.; Stever, T.; Kaib, T. Systems and Methods for Testing a Medical Device. US 2023/0293022 A1, 21 September 2023. Available online: https://www.lens.org/lens/patent/167-051-777-722-253/frontpage (accessed on 17 February 2025).
- Tegen, S.; Shumway, A. Systems, Devices, and/or Methods for Managing Health. US 2020/0037904 A1, 6 February 2020. Available online: https://www.lens.org/lens/patent/164-022-276-681-279/frontpage (accessed on 17 February 2025).
- Qiang, C. Teaching Device for Learning Four Palpation and Fetal Heart Auscultation. CN215376717U, 31 December 2021. Available online: https://patents.google.com/patent/CN215376717U/en (accessed on 17 February 2025).
- Guozhong, L. Teaching is with Simulating People’s Belly Palpation Analogue Means. CN214955598U, 30 November 2021. Available online: https://patents.google.com/patent/CN214955598U/en (accessed on 17 February 2025).
- Hao, A.; Cong, Y.; Zhao, Y.; Xiong, K. Trachea Cannula Simulation Training Method and System. CN115346411A, 15 November 2022. Available online: https://patents.google.com/patent/CN115346411A/en (accessed on 17 February 2025).
- Rui, S.; Jing, T.; Jianbo, H.; He, Z. Training Anthropomorphic Dummy of Hypothermia War Wound. CN114333525A, 12 April 2022. Available online: https://patents.google.com/patent/CN114333525A/en (accessed on 17 February 2025).
- Megawarne, J.; Jacobs, T. Training Manikins. GB2628184A, 18 September 2024. Available online: https://patents.google.com/patent/GB2628184A/en (accessed on 17 February 2025).
- Isaacson, S.; Harding, W.; Bell-Cares, A.; Christiansen, N.; Williams, B.; Larson, C.; Knutson, R.; Devenport, A.; Taylor, D. Vein Simulator System. US 2022/0383777 A1, 1 December 2022. Available online: https://www.lens.org/lens/patent/083-137-223-203-154/frontpage (accessed on 17 February 2025).
- Jiangyun, K. Vital Sign Comprehensive Simulator Convenient to Install. CN 211630650 U, 2 October 2020. Available online: https://www.lens.org/lens/patent/178-045-109-496-914/frontpage (accessed on 17 February 2025).
- Ogawa, S. Vital Sign Measurement Device. US 2020/0297225 A1, 24 September 2020. Available online: https://www.lens.org/lens/patent/149-004-299-418-526/frontpage (accessed on 17 February 2025).
- Rimmer, P.; Alton, P.; Morley-Alton, J.; Levi, S. Wearable Auscultation Device. US 2024/0057963 A1, 22 February 2024. Available online: https://www.lens.org/lens/patent/078-214-736-503-158/frontpage (accessed on 17 February 2025).
- Yao-Sheng, C.; Wei-Sheng, S.; Hsiao-Yi, L. Wearable Heart Sound Detection System and Method Thereof. US 2023/0309949 A1, 5 October 2023. Available online: https://www.lens.org/lens/patent/070-996-528-913-409/frontpage (accessed on 17 February 2025).
- Qiang, M.; Wenjing, G.; Xiaoli, S. Wireless Intelligent Cardiopulmonary Auscultation Anthropomorphic Dummy. CN217740068U, 4 November 2022. Available online: https://patents.google.com/patent/CN217740068U/en (accessed on 17 February 2025).
- Bai, F.; Hu, Q.; Yao, X.; Cheng, M.; Zhao, L.; Xu, L. A prospective comparative study on bladder volume measurement with portable ultrasound scanner and CT simulator in pelvic tumor radiotherapy. Phys. Eng. Sci. Med. 2024, 47, 87–97. [Google Scholar] [CrossRef]
- Manzanares, A.; Camblor, Á.; Romero-Arenas, S.; Segado, F.; Gil-Arias, A. Adapted sailing teaching methodology using vsail-trainer simulator as rehabilitation therapy. A feasibility study J. Spinal Cord Med. 2023, 47, 960–967. [Google Scholar] [CrossRef]
- Leijte, E.; De Blaauw, I.; Rosman, C.; Botden, S.M.B.I. Assessment of validity evidence for the RobotiX robot assisted surgery simulator on advanced suturing tasks. BMC Surg. 2020, 20, 183. [Google Scholar] [CrossRef]
- Huri, G.; Gülşen, M.R.; Karmiş, E.B.; Karagüven, D. Cadaver versus simulator-based arthroscopic training in shoulder surgery. Turk. J. Med. Sci. 2021, 51, 1179–1190. [Google Scholar] [CrossRef] [PubMed]
- Fu, Y.; Cavuoto, L.; Qi, D.; Panneerselvam, K.; Arikatla, V.S.; Enquobahrie, A.; De, S.; Schwaitzberg, S.D. Characterizing the learning curve of a virtual intracorporeal suturing simulator VBLaST-SS©. Surg. Endosc. 2020, 34, 3135–3144. [Google Scholar] [CrossRef] [PubMed]
- Massone, C.; Stanganelli, I.; Ingordo, V.; Ferrara, G.; Brunasso, A.M.G.; Siri‖, G.; Casazza, S.; Gnone, M.; Pizzichetta, M.A.; Giovanni, B.; et al. Clinicopathologic and Dermoscopic Features of 20 Cases of Spark’s Nevus, a Dermoscopic Simulator of Melanoma. Am. J. Dermatopathol. 2023, 45, 153–162. [Google Scholar] [CrossRef] [PubMed]
- Geissler, M.E.; Bereuter, J.P.; Geissler, R.B.; Bökkerink, G.M.J.; Egen, L.; Kowalewski, K.F.; Haney, C. Comparison of distance versus in-person laparoscopy training using a low-cost laparoscopy simulator-a randomized controlled multi-center trial. Surg. Endosc. 2024, 38, 6527–6540. [Google Scholar] [CrossRef]
- Lee, J.E.; Yu, J.H.; Lee, S.K.; Lee, J.H.; Jung, H.J. Comparison of medical students’ perceptions of patient safety: Focusing on simulation training using a high-fidelity simulator. PLoS ONE 2024, 19, e0304883. [Google Scholar] [CrossRef]
- Suzuki, H.; Furuya, J.; Matsubara, C.; Aoyagi, M.; Shirobe, M.; Sato, Y.; Tohara, H.; Minakuchi, S. Comparison of the Amount of Used and the Ease of Oral Care between Liquid and Gel-Type Oral Moisturizers Used with an Oral Care Simulators. Int. J. Environ. Res. Public Health 2022, 19, 8158. [Google Scholar] [CrossRef]
- Hendrickx, D.M.; Sousa, J.D.; Libin, P.J.K.; Delva, W.; Liesenborgs, J.; Hens, N.; Müller, V.; Vandamme, A.-M. Comparison of two simulators for individual based models in HIV epidemiology in a population with HSV 2 in Yaoundé (Cameroon). Sci. Rep. 2021, 11, 14696. [Google Scholar] [CrossRef]
- Yoshimura, T.; Colley, N.; Komizunai, S.; Ninomiya, S.; Kanai, S.; Konno, A.; Yasuda, K.; Taguchi, H.; Hashimoto, T.; Shimizu, S.; et al. Construction of a detachable artificial trachea model for three age groups for use in an endotracheal suctioning training environment simulator. PLoS ONE 2021, 16, e0249010. [Google Scholar] [CrossRef]
- Bhattacharya, S.; Devos, H.; Lemke, C.; Branstetter, C.; Jenkins, R.; Rooker, J.; Kranick, M.; Patel, N.; Gibson, R.; Diaz, J.; et al. Correlation between visuo-cognitive tests and simulator performance of commercial drivers in the United States. Accid. Anal. Prev. 2023, 184, 106994. [Google Scholar] [CrossRef]
- Silva, V.C.; Dias, A.S.; Greve, J.M.D.; Davis, C.L.; Soares, A.L.d.S.; Brech, G.C.; Ayama, S.; Jacob-Filho, W.; Busse, A.L.; de Biase, M.E.M.; et al. Crash Risk Predictors in Older Drivers: A Cross-Sectional Study Based on a Driving Simulator and Machine Learning Algorithms. Int. J. Environ. Res. Public Health 2023, 20, 4212. [Google Scholar] [CrossRef]
- Larraga-García, B.; Castañeda López, L.; Rubio Bolívar, F.J.; Quintana-Díaz, M.; Gutiérrez, Á. Design and Development of an Interactive Web-Based Simulator for Trauma Training: A Pilot Study. J. Med. Syst. 2021, 45, 96. [Google Scholar] [CrossRef] [PubMed]
- Sánchez-Vásquez, U.; Daniel-Guerrero, A.B.; Méndez-Gutiérrez, E.; Morales-López, S.; Tovar-Lozano, I.I.; Martínez-Rodríguez, M.A.; Uribe-Campos, I.E. Design, assembly and validation of a low-cost, high-fidelity simulator for heart exploration. Gac. Med. Mex. 2021, 157, 24–28. [Google Scholar] [CrossRef] [PubMed]
- Fu, T.T.; Wang, X.J.; Xiao, S.Q.; Fan, L. Development and evaluation of a PICC virtual simulator in neonatal nursing: A randomized controlled trial. Nurse Educ. Today 2024, 141, 106306. [Google Scholar] [CrossRef]
- Iwata, M.; Iwamoto, K.; Kambe, D.; Tachibana, N.; Ando, M.; Ozaki, N. Development and validation of a driving simulator for evaluating the residual effects of drugs on driving performance—Sensitivity analysis using zopiclone as a positive control: Study Protocol Clinical Trial (SPIRIT Compliant). Medicine 2020, 99, e19395. [Google Scholar] [CrossRef]
- Yau, S.Y.; Chang, Y.C.; Wu, M.Y.; Liao, S.C. Does seniority always correlate with simulated intubation performance? Comparing endotracheal intubation performance across medical students, residents, and physicians using a high-fidelity simulator. PLoS ONE 2021, 16, e0257969. [Google Scholar] [CrossRef]
- Kliem, P.S.C.; Tisljar, K.; Grzonka, P.; Berger, S.; Amacher, S.A.; De Marchis, G.M.; Dittrich, T.D.; Hunziker, S.; Rüegg, S.; Bassetti, S.; et al. Effects of a scoring aid on Glasgow coma score assessment and physicians’ comprehension: A simulator-based randomized clinical trial. J. Neurol. 2024, 272, 57. [Google Scholar] [CrossRef]
- Kagaya, Y.; Tabata, M.; Arata, Y.; Kameoka, J.; Ishii, S. Employment of color Doppler echocardiographic video clips in a cardiac auscultation class with a patient cardiology simulator: Discrepancy between students’ satisfaction and learning. BMC Med. Educ. 2021, 21, 600. [Google Scholar] [CrossRef]
- Hilleke, S.; Wiener, R.; Frisch, A.; Scheel, M. Endovascular Simulator Training and Shadowing in Interventional Radiology: A Comparison of Two Teaching Methods in the Curricular Training of Medical Students. Cardiovasc. Interv. Radiol. 2024, 47, 1540–1546. [Google Scholar] [CrossRef]
- Scott-Watson, M.; Thornhill, C.; Bhattacharyya, R.; Spencer, S.J. Evaluating the effectiveness of a low fidelity, easily available simulator to teach basic arthroscopy skills to novice learners: A prospective cohort study. Knee 2024, 47, 129–138. [Google Scholar] [CrossRef]
- von Bernstorff, M.; Bausenhart, F.; Rapp, J.; Feierabend, M.; Ipach, I.; Hofmann, U.K. Evaluation of braking performances of patients with osteoarthritis of the knee or hip: Are there alternatives to a brake simulator? Acta Orthop. Traumatol. Turc. 2021, 55, 42–47. [Google Scholar] [CrossRef]
- Maita, H.; Kobayashi, T.; Akimoto, T.; Hirano, T.; Osawa, H.; Kato, H. Evaluation of simulation-based ultrasound education using a bladder simulator for medical students in Japan: A prospective observational study. J. Med. Ultrason. 2023, 50, 73–80. [Google Scholar] [CrossRef] [PubMed]
- Rzeźniczek, P.; Lipiak, A.; Bilski, B.; Laudańska-Krzemińska, I.; Cybulski, M.; Chawłowska, E. Exploring the participant-related determinants of simulator sickness in a physical motion car rollover simulation as measured by the simulator sickness questionnaire. Int. J. Environ. Res. Public Health 2020, 17, 7044. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Lin, J.; Wu, S.; Zhu, Y.; Zou, Y.; Zhang, Q.; Li, Z.; Zhuo, Y.; Li, Y. Eyesi direct ophthalmoscope simulator: An effective training tool for medical undergraduates. BMC Med. Educ. 2024, 24, 783. [Google Scholar] [CrossRef] [PubMed]
- Jaud, C.; Salleron, J.; Cisse, C.; Angioi-Duprez, K.; Berrod, J.P.; Conart, J.B. EyeSi Surgical Simulator: Validation of a proficiency-based test for assessment of vitreoretinal surgical skills. Acta Ophthalmol. 2021, 99, 783. [Google Scholar] [CrossRef]
- Kengen, B.; IJgosse, W.M.; van Goor, H.; Luursema, J.M. Fast or safe? The role of impulsiveness in laparoscopic simulator performance. Am. J. Surg. 2020, 220, 914–919. [Google Scholar] [CrossRef]
- Kagaya, Y.; Tabata, M.; Arata, Y.; Kameoka, J.; Ishii, S. Feasibility and effectiveness of cardiac auscultation training with a cardiology patient simulator for first-year medical students. J. Cardiol. 2022, 80, 462–468. [Google Scholar] [CrossRef]
- Pinto, L.; Paulo-de-Sousa, C.; Ayres-de-Campos, D. Impact of a simulator-based training program on the success rate of external cephalic version. Eur. J. Obstet. Gynecol. Reprod. Biol. 2024, 301, 60–63. [Google Scholar] [CrossRef]
- Sønderup, M.; Gustafsson, A.; Konge, L.; Jacobsen, M.E. Intraoperative fluoroscopy skills in distal radius fracture surgery: Valid and reliable assessment on a novel immersive virtual reality simulator. Acta Orthop. 2024, 95, 477–484. [Google Scholar] [CrossRef]
- Ghazanfar, S.; Qureshi, S.; Zubair, M.; Safdar, Y.; Leghari, A.A.; Quraishy, M.S. Is laparoscopic experience helpful in simulator based robotic training in general surgery? J. Pak. Med. Assoc. 2021, 71, 2198–2202. [Google Scholar] [CrossRef]
- Ducloyer, J.-B.; Poinas, A.; Duchesne, L.; Caillet, P.; Lejus-Bourdeau, C.; Le Meur, G.; Weber, M.; Ivan, C.; Limousin, N.; Desmidt, T.; et al. Learning curves of novice residents on cataract surgery simulator: The E3CAPS pedagogic study. BMC Med. Educ. 2024, 24, 1078. [Google Scholar] [CrossRef]
- Ono, Y.; Shinohara, K.; Shimada, J.; Inoue, S.; Kotani, J. Lower maximum forces on oral structures when using gum-elastic bougie than when using endotracheal tube and stylet during both direct and indirect laryngoscopy by novices: A crossover study using a high-fidelity simulator. BMC Emerg. Med. 2020, 20, 34. [Google Scholar] [CrossRef] [PubMed]
- Ock, J.; Gwon, E.; Kim, D.-H.; Kim, S.-H.; Kim, N. Patient-specific and hyper-realistic phantom for an intubation simulator with a replaceable difficult airway of a toddler using 3D printing. Sci. Rep. 2020, 10, 10631. [Google Scholar] [CrossRef] [PubMed]
- Motov, S.; Santander, X.; Stengel, F.C.; Mohme, M.; Schwake, M.; Zoia, C.; Butenschoen, V.M.; Bauer, M.; Lippa, L.; Belo, D.; et al. Sequential adaptive e-learning and hands-on simulator training for unilateral biportal endoscopy (UBE) of the lumbar spine—Results from an EANS Young Neurosurgeons hands-on course. Acta Neurochir. 2024, 166, 458. [Google Scholar] [CrossRef] [PubMed]
- Grethlein, D.; Winston, F.K.; Walshe, E.; Tanner, S.; Kandadai, V.; Ontañón, S. Simulator pre-screening of underprepared drivers prior to licensing on-road examination: Clustering of virtual driving test time series Data. J. Med. Internet Res. 2020, 22, e13995. [Google Scholar] [CrossRef]
- Jokinen, E.; Mikkola, T.S.; Härkki, P. Simulator training and residents’ first laparoscopic hysterectomy: A randomized controlled trial. Surg. Endosc. 2020, 34, 4874–4882. [Google Scholar] [CrossRef]
- Weimer, J.M.; Sprengart, F.M.; Vieth, T.; Göbel, S.; Dionysopoulou, A.; Krüger, R.; Beer, J.; Weimer, A.M.; Buggenhagen, H.; Kloeckner, R.; et al. Simulator training in focus assessed transthoracic echocardiography (FATE) for undergraduate medical students: Results from the FateSim randomized controlled trial. BMC Med. Educ. 2025, 25, 21. [Google Scholar] [CrossRef]
- Pinto, L.O.A.D.; Silva, R.C.; Junior, H.C.F.d.S.; Bentes, L.G.d.B.; Otake, M.I.T.; de Bacelar, H.P.H.; Kietzer, K.S. Simulators in urology resident’s training in retrograde intrarenal surgery. Acta Cir. Bras. 2024, 39, e394724. [Google Scholar] [CrossRef]
- Qi, D.; Petrusa, E.; Kruger, U.; Milef, N.; Abu-Nuwar, M.R.; Haque, M.; Lim, R.; Jones, D.B.; Turkseven, M.; Demirel, D.; et al. Surgeons With Five or More Actual Cricothyrotomies Perform Significantly Better on a VR Simulator. J. Surg. Res. 2020, 252, 247–254. [Google Scholar] [CrossRef]
- Huang, C.; Wang, Y.Y.; Zhou, J.J.; Liu, Y.F.; He, X. The “warrior” system: A new useful emergency simulator to train clinical pharmacists in emergency medicine. Medicine 2020, 99, e20047. [Google Scholar] [CrossRef]
- Koshmaganbetova, G.; Kurmangalieva, S.; Bazargaliyev, Y.; Zhexenova, A.; Urekeshov, B.; Azhmuratova, M. The effectiveness of training on auscultation of heart with a simulator of cardiology in medical students. Open Access Maced. J. Med. Sci. 2021, 9, 1055–1060. [Google Scholar] [CrossRef]
- Zhang, C.; Bowers, A.R.; Savage, S.W. The Effects of Age, Distraction, and Simulated Central Vision Impairment on Hazard Detection in a Driving Simulator. Optom. Vis. Sci. 2020, 97, 239–248. [Google Scholar] [CrossRef] [PubMed]
- Qi, Z.; Jin, H.; Wang, Q.; Gan, Z.; Xiong, R.; Zhang, S.; Liu, M.; Wang, J.; Ding, X.; Chen, X.; et al. The Feasibility and Accuracy of Holographic Navigation with Laser Crosshair Simulator Registration on a Mixed-Reality Display. Sensors 2024, 24, 896. [Google Scholar] [CrossRef] [PubMed]
- Uhm, D.; Kim, A. Tidal volume according to the 4-point sealing forces of a bag-valve-mask: An adult respiratory arrest simulator-based prospective, descriptive study. BMC Emerg. Med. 2021, 21, 57. [Google Scholar] [CrossRef]
- Leijte, E.; Claassen, L.; Arts, E.; de Blaauw, I.; Rosman, C.; Botden, S.M.B.I. Training benchmarks based on validated composite scores for the RobotiX robot-assisted surgery simulator on basic tasks. J. Robot. Surg. 2021, 15, 69–79. [Google Scholar] [CrossRef]
- Alvarez-Lopez, F.; Maina, M.F.; Saigí-Rubió, F. Use of a low-cost portable 3d virtual reality gesture-mediated simulator for training and learning basic psychomotor skills in minimally invasive surgery: Development and content validity study. J. Med. Internet Res. 2020, 22, e17491. [Google Scholar] [CrossRef]
- Porto, J.T.; Eifler, L.S.; Steffen, L.P.; Rabaioli, G.F.; Tomazzoni, J.M. Use of simulators in video laparoscopic surgery in medical training: A prospective court study with a medicine academic at a university in Southern Brazil. Rev. Col. Bras. Cir. 2020, 47, e20202608. [Google Scholar] [CrossRef]
- Tjong, F.V.Y.; Perrotta, L.; Goette, A.; Duncker, D.; Vernooy, K.; Boveda, S.; Chun, K.-R.J.; Svennberg, E. Utilization of and perceived need for simulators in clinical electrophysiology: Results from an EHRA physician survey. Europace 2024, 26, euae037. [Google Scholar] [CrossRef]
- Bogar, P.Z.; Virag, M.; Bene, M.; Hardi, P.; Matuz, A.; Schlegl, A.T.; Toth, L.; Molnar, F.; Nagy, B.; Rendeki, S.; et al. Validation of a novel, low-fidelity virtual reality simulator and an artificial intelligence assessment approach for peg transfer laparoscopic training. Sci. Rep. 2024, 14, 16702. [Google Scholar] [CrossRef]
- Yaïci, R.; Poirot, J.; Dormegny, L.; Neumann, N.; Bazarya, E.; Solecki, L.; Sauer, A.; Gaucher, D.; Lejay, A.; Thomsen, A.S.; et al. Validity evidence of a new virtual reality simulator for phacoemulsification training in cataract surgery. Sci. Rep. 2024, 14, 25524. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).