Comparison of Tissue Repair with Different Types of Microdissection Tips: A Randomized Histomorphometric Evaluation in Rats
Abstract
1. Introduction
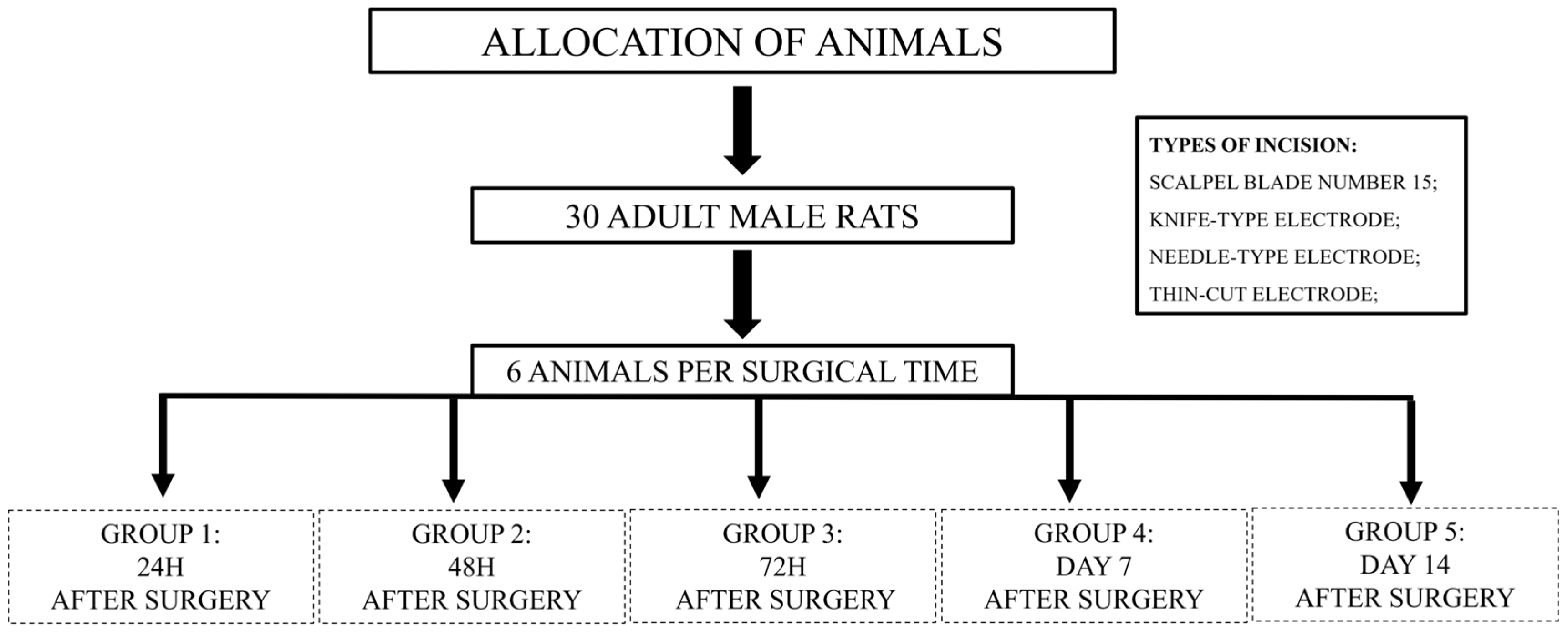
2. Materials and Methods
2.1. Surgical Procedure
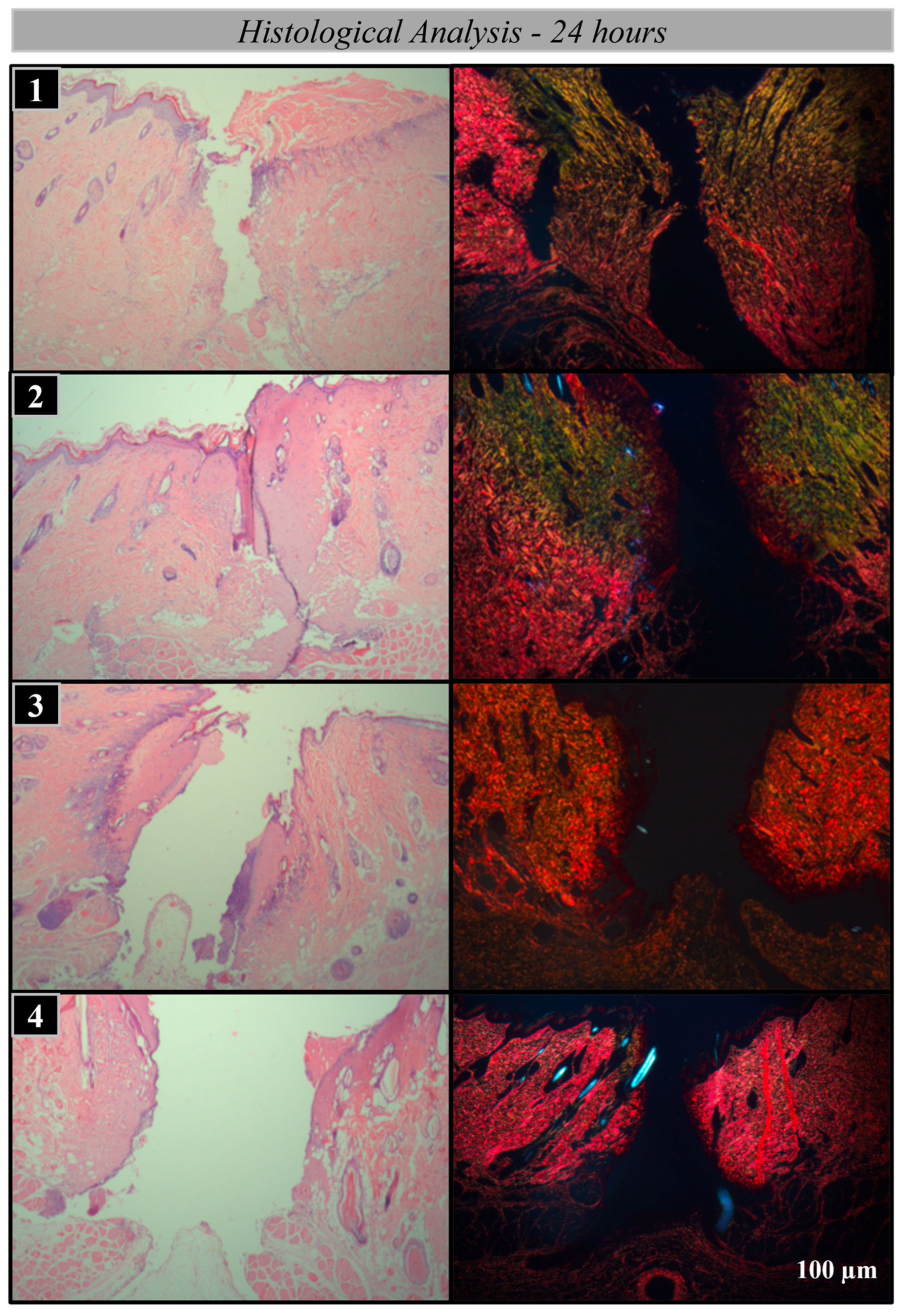
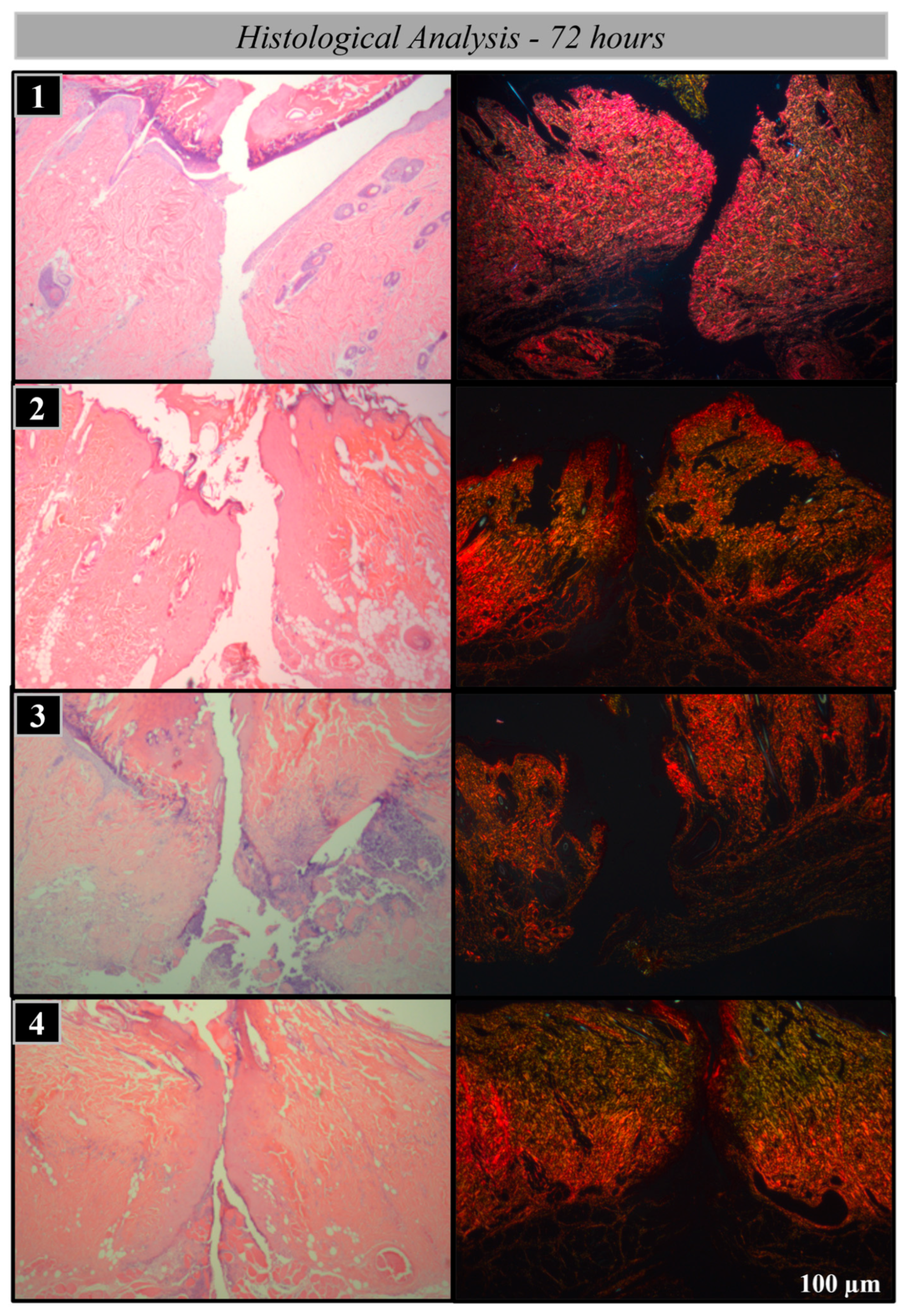
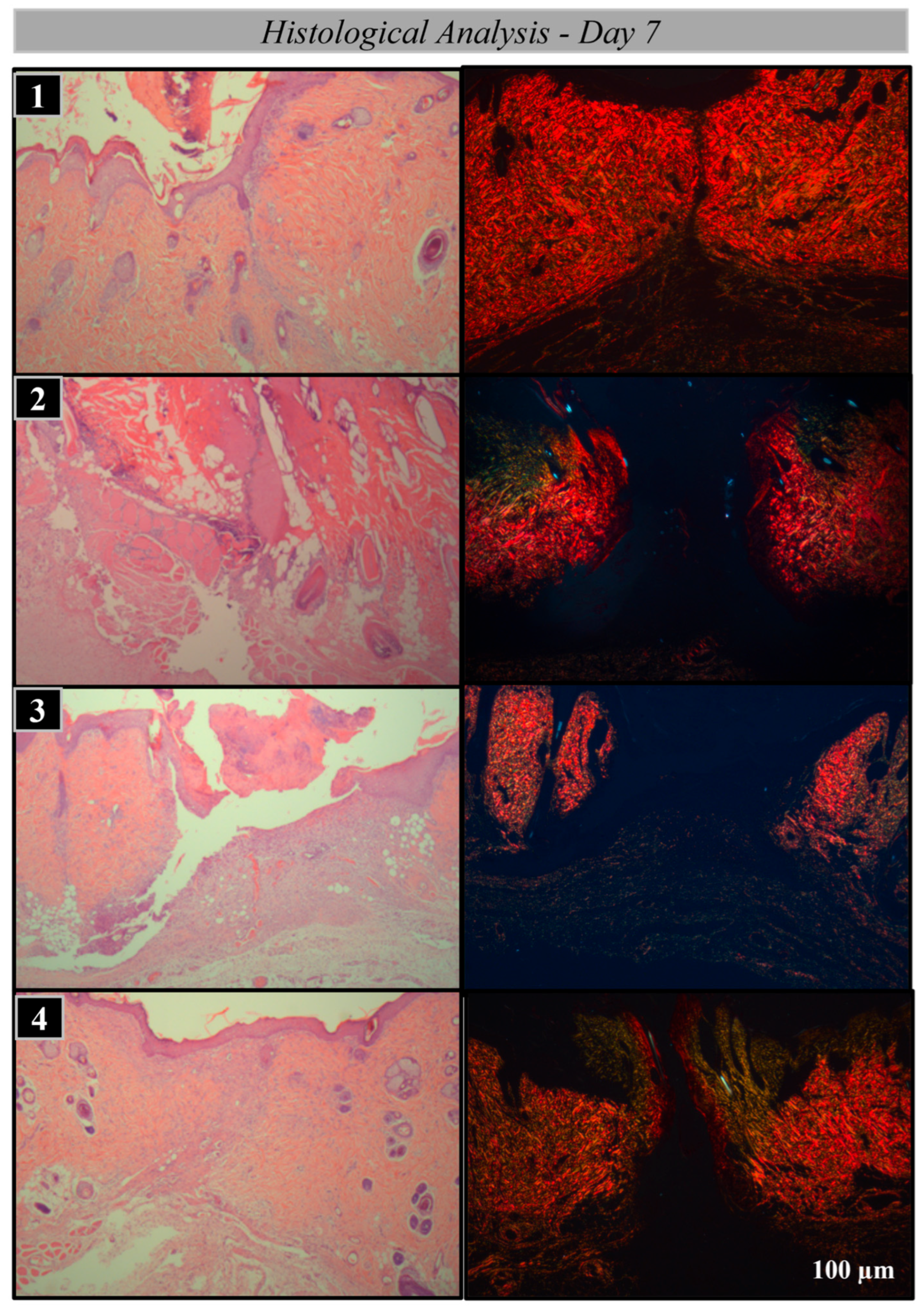
2.2. Histological Processing
2.3. Analysis Methods
2.4. Statistical Analysis
3. Results
Histological and Histomorphometric Analysis
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Mariano, R.C.; Oliveira, M.R.; Silva, L.C.; Ferreira, S.; Garcia Júnior, I.R.; de Carvalho Silva, A. Effect of topical application of chlorhexidine and metronidazole on the tissue repair of palatal wounds of rats: A clinical and histomorphometric study. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. 2015, 119, 505–513. [Google Scholar] [CrossRef] [PubMed]
- Hasar, Z.B.; Ozmeric, N.; Ozdemir, B.; Gökmenoğlu, C.; Baris, E.; Altan, G.; Kahraman, S. Comparison of Radiofrequency and Electrocautery With Conventional Scalpel Incisions. J. Oral Maxillofac. Surg. 2016, 74, 2136–2141. [Google Scholar] [CrossRef]
- Ogawa, R. Keloid and Hypertrophic Scars Are the Result of Chronic Inflammation in the Reticular Dermis. Int. J. Mol. Sci. 2017, 18, 606. [Google Scholar] [CrossRef] [PubMed]
- Singh, D.; Rai, V.; KAgrawal, D. Regulation of Collagen I and Collagen III in Tissue Injury and Regeneration. Cardiol. Cardiovasc. Med. 2023, 7, 5–16. [Google Scholar] [CrossRef]
- Kular, J.K.; Basu, S.; Sharma, R.I. The extracellular matrix: Structure, composition, age-related differences, tools for analysis and applications for tissue engineering. J. Tissue Eng. 2014, 5, 2041731414557112. [Google Scholar] [CrossRef]
- Rittié, L. Cellular mechanisms of skin repair in humans and other mammals. J. Cell Commun. Signal. 2016, 10, 103–120. [Google Scholar] [CrossRef]
- Krafts, K.P. Tissue repair. Organogenesis 2010, 6, 225–233. [Google Scholar] [CrossRef]
- Kumar, P.; Rattan, V.; Rai, S. Comparative evaluation of healing after gingivectomy with electrocautery and laser. J. Oral Biol. Craniofac. Res. 2015, 5, 69–74. [Google Scholar] [CrossRef][Green Version]
- Van Linthout, S.; Miteva, K.; Tschope, C. Crosstalk between fibroblasts and inflammatory cells. Cardiovasc. Res. 2014, 102, 258–269. [Google Scholar] [CrossRef]
- Massarweh, N.N.; Cosgriff, N.; Slakey, D.P. Electrosurgery: History, Principles, and Current and Future Uses. J. Am. Coll. Surg. 2006, 202, 520–530. [Google Scholar] [CrossRef]
- Sharma, R. Safety of Colorado Microdissection Needle (Stryker) for Skin Opening in Craniomaxillofacial Surgery. J. Maxillofac. Oral Surg. 2012, 11, 115–118. [Google Scholar] [CrossRef]
- Chandra, R.; Savitharani, B.; Reddy, A. Comparing the outcomes of incisions made by colorado microdissection needle, electrosurgery tip, and surgical blade during periodontal surgery: A randomized controlled trial. J. Indian Soc. Periodontol. 2016, 20, 616–622. [Google Scholar] [CrossRef]
- Ram, M.; Singh, V.; Kumawat, S.; Kant, V.; Tandan, S.K.; Kumar, D. Bilirubin modulated cytokines, growth factors and angiogenesis to improve cutaneous wound healing process in diabetic rats. Int. Immunopharmacol. 2016, 30, 137–149. [Google Scholar] [CrossRef]
- Aird, L.N.F.; Brown, C.J. Systematic review and meta-analysis of electrocautery versus scalpel for surgical skin incisions. Am. J. Surg. 2012, 204, 216–221. [Google Scholar] [CrossRef]
- Torres-Augusto Neto, R.; Comachio, C.A.; de Almeida, L.C.Q.; de Azambuja Carvalho, P.H.; dos Santos Trento, G.; Pereira-Filho, V.A. Tissue response to different incision tools in animal model. Oral Maxillofac. Surg. 2022, 27, 631–638. [Google Scholar] [CrossRef]
- Kahn, S.; de Oliveira, L.Z.; Dias, A.T.; Fernandes, G.V.d.O. Clinical evaluation and biological understanding of the early step-by-step healing after periodontal microsurgery: A case report with PES analysis comparing initial and 31-day result. J. Adv. Periodontol. Implant. Dent. 2022, 14, 141–145. [Google Scholar] [CrossRef] [PubMed]
- Sabri, H.; Alhachache, S.; Saxena, P.; Dubey, P.; Nava, P.; Rufai, S.H.; Sarkarat, F. Microsurgery in periodontics and oral implantology: A systematic review of current clinical applications and outcomes. Evid.-Based Dent. 2024, 25, 211–212. [Google Scholar] [CrossRef] [PubMed]
- Kahn, S.; Araújo, I.T.E.; Dias, A.T.; de Souza, A.B.; Chambrone, L.; Fernandes, G.V.d.O. Histologic and histomorphometric analysis of connective tissue grafts harvested by the parallel incision method: A pilot randomized controlled trial comparing macro- and microsurgical approaches. Quintessence Int. 2021, 52, 772–778. [Google Scholar]
- Lattouf, R.; Younes, R.; Lutomski, D.; Naaman, N.; Godeau, G.; Senni, K.; Changotade, S. Picrosirius Red Staining: A useful tool to appraise collagen networks in normal and pathological tissues. J. Histochem. Cytochem. 2014, 62, 751–758. [Google Scholar] [CrossRef]
- Carqueville, J.C.; Chesnut, C. Histologic Comparison of Upper Blepharoplasty Skin Excision Using Scalpel Incision Versus Microdissection Electrocautery Needle Tip Versus Continuous Wave CO2 Laser. Dermatol. Surg. 2021, 47, 1376–1378. [Google Scholar] [CrossRef]
- Azevedo, L.H.; de Sousa, S.C.O.M.; Correa, L.; de Paula Eduardo, C.; Dagli, M.L.Z.; Romanos, G.; Migliari, D.A. Mast cell concentration in the wound healing process of incisions made by different instruments. Lasers Med Sci. 2009, 24, 585–590. [Google Scholar] [CrossRef] [PubMed]
- Gáspár, L.; Tóth, J. Comparative study on wound healing in the oral cavity following experimental surgery using a scalpel, electrocauterization and CO2 laser beam. Fogorv. Sz. 1991, 84, 339–343. [Google Scholar] [PubMed]



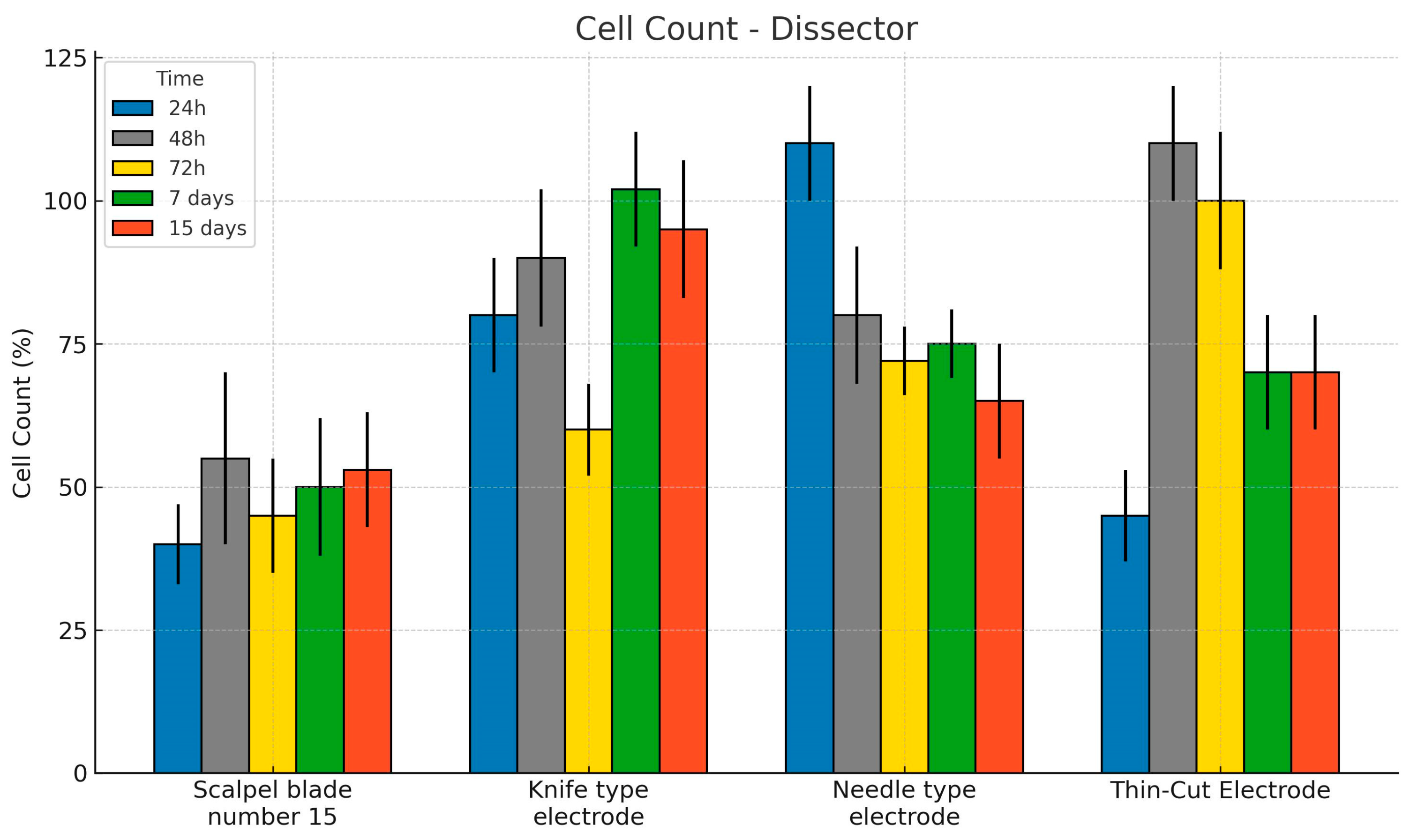

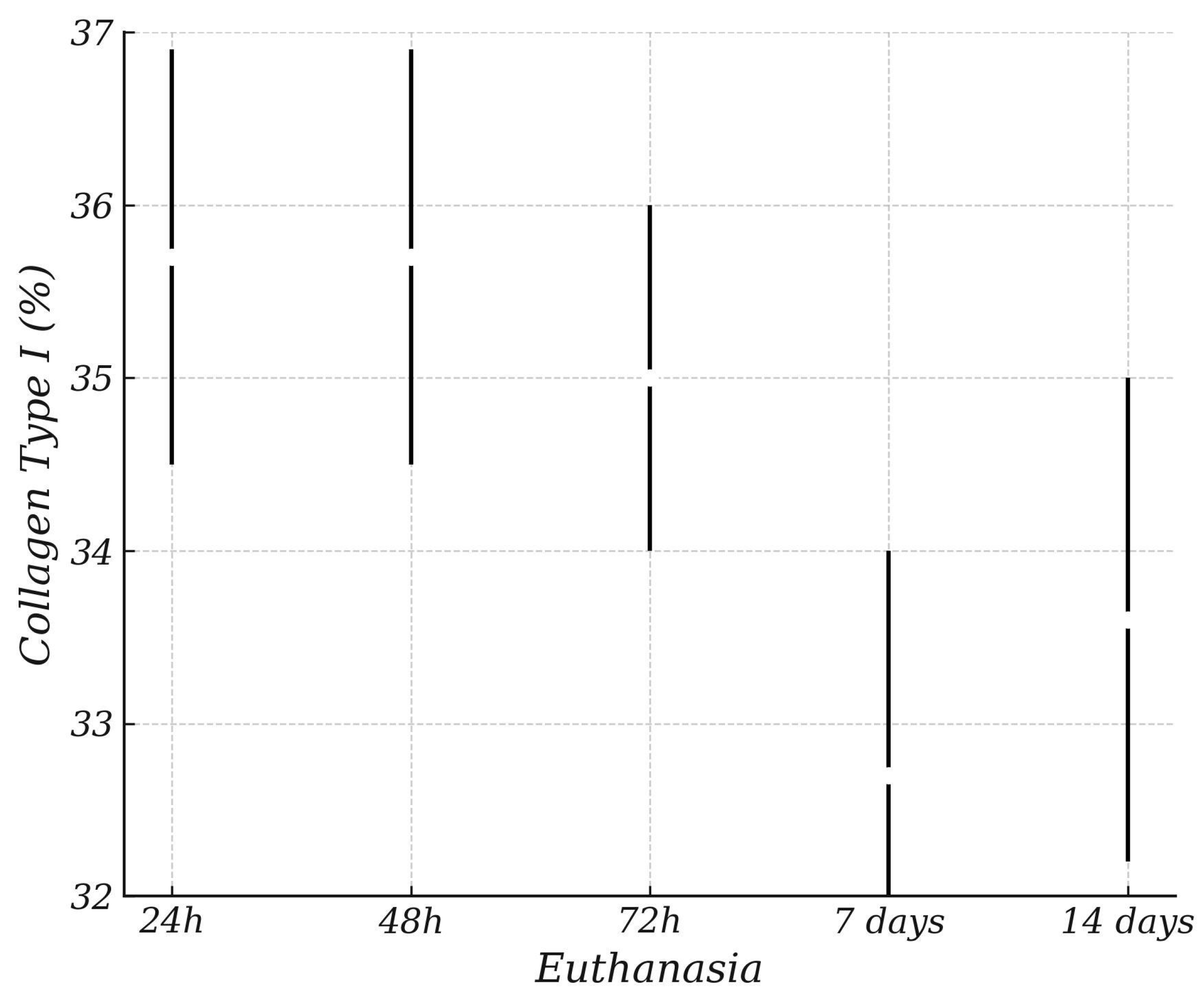

| ANOVA Collagen Type I (%) | Sum of Squares | gl | Mean Square | F | p |
|---|---|---|---|---|---|
| Dissector | 13.8 | 3 | 4.59 | 0.603 | 0.615 |
| Euthanasia | 158.4 | 4 | 39.61 | 5.198 | <001 |
| Dissector * Euthanasia | 95.8 | 12 | 7.99 | 1.048 | 0.412 |
| Waste | 754.3 | 99 | 7.62 |
| Estimated Marginal Means Dissector | ||||
|---|---|---|---|---|
| Confidence Interval 95% | ||||
| Mean | Standard Error | Lower Limit | Superior | |
| Knife-type electrode | 36.5 | 0.860 | 34.8 | 38.2 |
| Needle-type electrode | 35.4 | 0.860 | 33.7 | 37.1 |
| Scalpel blade number 15 | 36.0 | 0.877 | 34.2 | 37.7 |
| Thin-cut electrode | 36.3 | 0.860 | 34.6 | 38.0 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Brunelli, A.L.V.V.; Torres, L.H.S.; Viotto, A.H.A.; Delamura, I.F.; Bassi, A.P.F.; Gabrielli, M.A.C.; Pereira-Filho, V.A. Comparison of Tissue Repair with Different Types of Microdissection Tips: A Randomized Histomorphometric Evaluation in Rats. Bioengineering 2025, 12, 732. https://doi.org/10.3390/bioengineering12070732
Brunelli ALVV, Torres LHS, Viotto AHA, Delamura IF, Bassi APF, Gabrielli MAC, Pereira-Filho VA. Comparison of Tissue Repair with Different Types of Microdissection Tips: A Randomized Histomorphometric Evaluation in Rats. Bioengineering. 2025; 12(7):732. https://doi.org/10.3390/bioengineering12070732
Chicago/Turabian StyleBrunelli, Ana Luiza Vila Verde, Luíz Henrique Soares Torres, Arthur Henrique Alécio Viotto, Izabela Fornazari Delamura, Ana Paula Farnezi Bassi, Marisa Aparecida Cabrini Gabrielli, and Valfrido Antonio Pereira-Filho. 2025. "Comparison of Tissue Repair with Different Types of Microdissection Tips: A Randomized Histomorphometric Evaluation in Rats" Bioengineering 12, no. 7: 732. https://doi.org/10.3390/bioengineering12070732
APA StyleBrunelli, A. L. V. V., Torres, L. H. S., Viotto, A. H. A., Delamura, I. F., Bassi, A. P. F., Gabrielli, M. A. C., & Pereira-Filho, V. A. (2025). Comparison of Tissue Repair with Different Types of Microdissection Tips: A Randomized Histomorphometric Evaluation in Rats. Bioengineering, 12(7), 732. https://doi.org/10.3390/bioengineering12070732






